Sucuk, Turkish-Style Fermented Sausage: Evaluation of the Effect of Bioprotective Starter Cultures on Its Microbiological, Physicochemical, and Chemical Properties
Abstract
1. Introduction
2. Materials and Methods
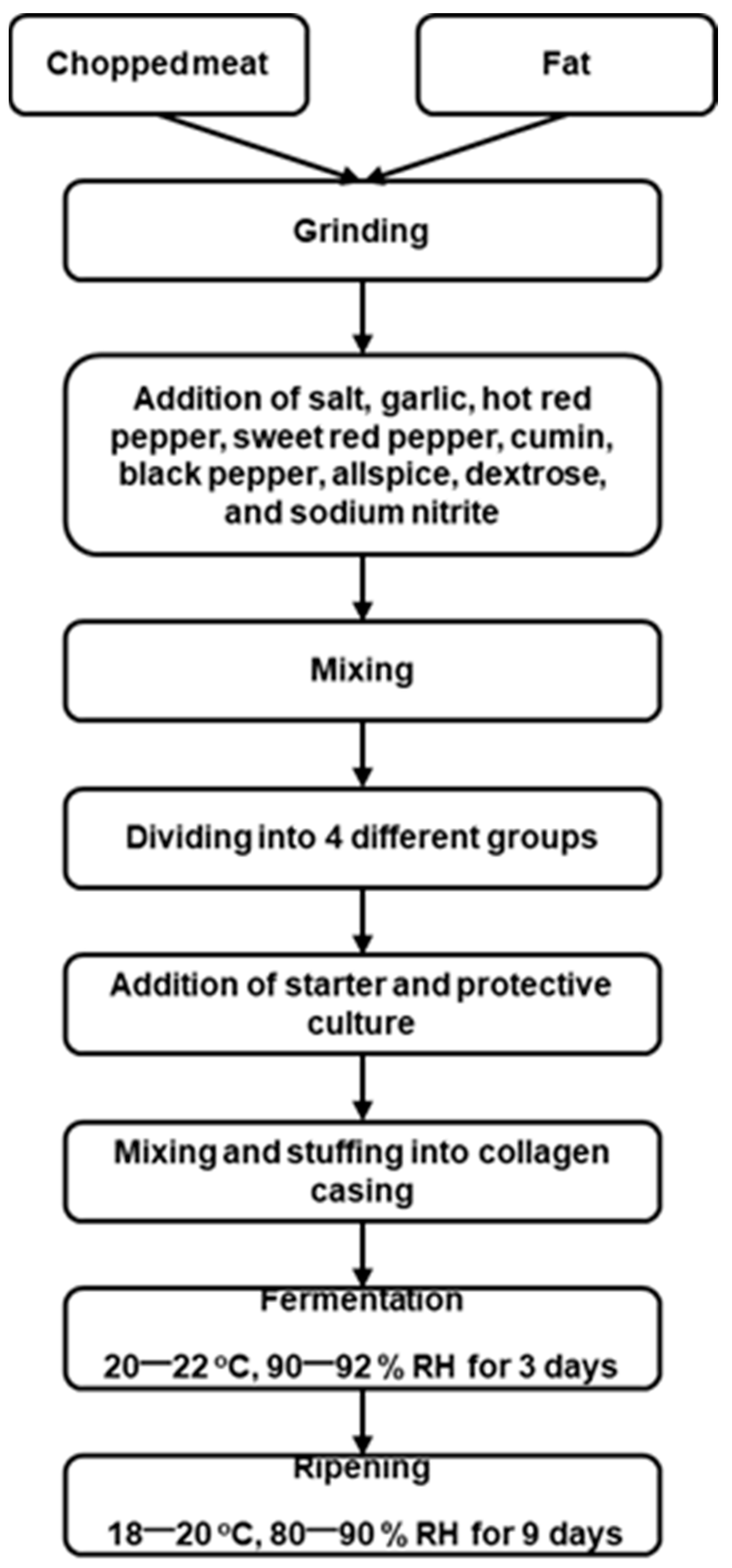
2.1. Sucuk Preparation
2.2. Sampling and Experimental Design
2.3. Microbiological Analyses
2.4. Physicochemical and Chemical Analysis
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results
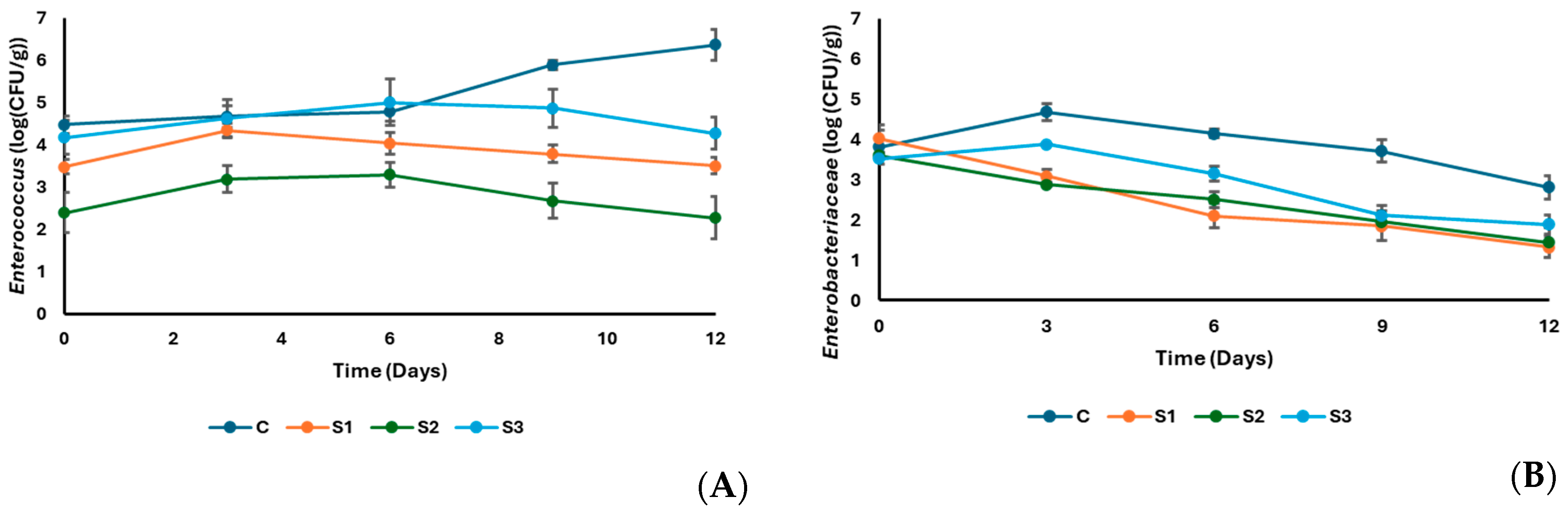
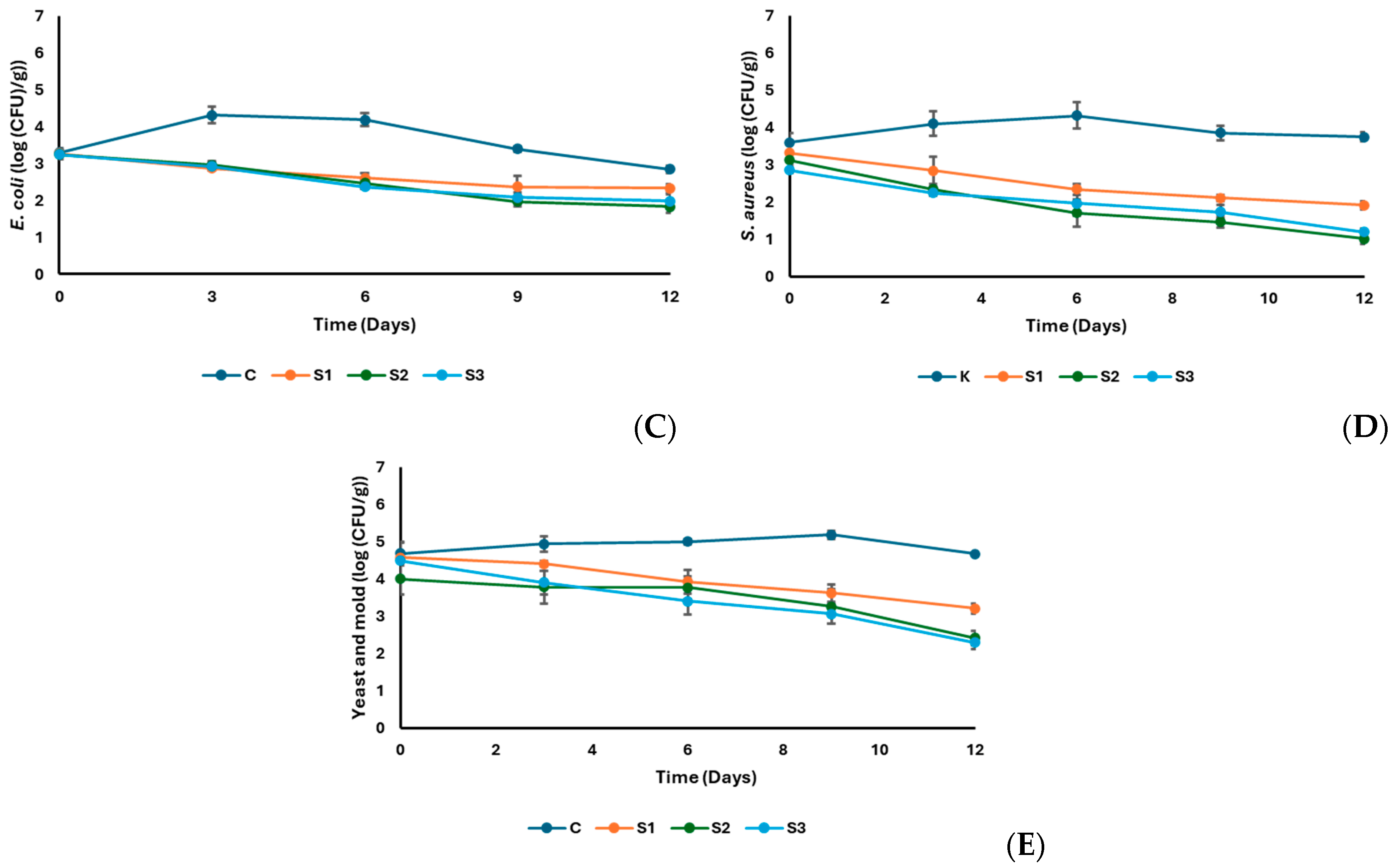
3.1. Microbiological Properties of Sucuk
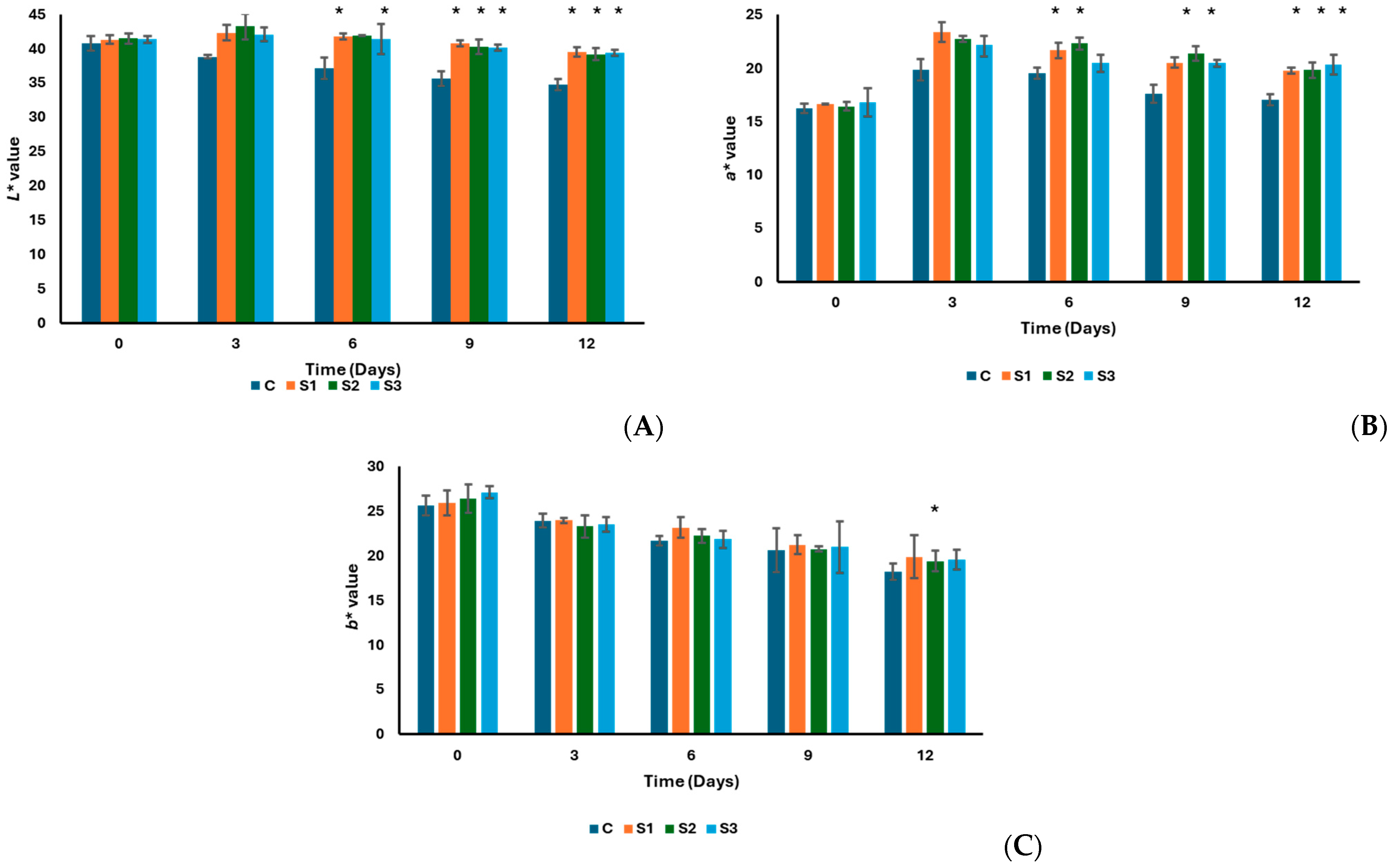
3.2. Sucuk Composition and Physicochemical and Chemical Analysis Results
3.3. Sensory Properties of the Sucuks
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaban, G.; Oral, Z.F.; Kaya, M. “Sucuk”. In Production of Traditional Mediterranean Meat Products; Lorenzo, J.M., Domínguez, R., Pateiro, M., Munekata, P.E., Eds.; Springer Protocols: New York, NY, USA, 2022; pp. 133–142. [Google Scholar]
- Ercoşkun, H.; Özkal, S.G. Kinetics of traditional Turkish sausage quality aspects during fermentation. Food Control 2011, 22, 165–172. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Turkish Food Codex Notification No. 2018/52 on Raw and Processed Meat and Poultry Meat and Their Compounds. 2019. Available online: https://www.fao.org/faolex/results/details/fr/c/LEX-FAOC187829/ (accessed on 25 July 2024).
- Soyer, A. Effect of fat level and ripening temperature on biochemical and sensory characteristics of naturally fermented Turkish sausages (sucuk). Eur. Food Res. Technol. 2005, 221, 412–415. [Google Scholar] [CrossRef]
- Dalmış, Ü.; Soyer, A. Effect of processing methods and starter culture (Staphylococcus xylosus and Pediococcus pentosaceus) on proteolytic changes in Turkish sausages (sucuk) during ripening and storage. Meat Sci. 2008, 80, 345–354. [Google Scholar] [CrossRef]
- Lücke, F.K. Utilization of microbes to process and preserve meat. Meat Sci. 2000, 56, 105–115. [Google Scholar] [CrossRef]
- Kröckel, L. The role of lactic acid bacteria in safety and flavour development of meat and meat products. In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes; Kongo, J.M., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 129–151. [Google Scholar]
- Holzapfel, W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002, 75, 197–212. [Google Scholar] [CrossRef]
- García-Díez, J.; Saraiva, C. Use of starter cultures in foods from animal origin to improve their safety. Int. J. Environ. Res. Public Health 2021, 18, 2544. [Google Scholar] [CrossRef]
- Gençcelep, H.; Kaban, G.; Kaya, M. Effects of starter cultures and nitrite levels on formation of biogenic amines in sucuk. Meat Sci. 2007, 77, 424–430. [Google Scholar] [CrossRef]
- Souza, L.V.; Martins, E.; Moreira, I.M.F.B.; De Carvalho, A.F. Strategies for the development of bioprotective cultures in food preservation. Int. J. Microbiol. 2022, 2022, 6264170. [Google Scholar] [CrossRef]
- Munekata, P.E.; Pateiro, M.; Zhang, W.; Domínguez, R.; Xing, L.; Fierro, E.M.; Lorenzo, J.M. Autochthonous probiotics in meat products: Selection, identification, and their use as starter culture. Microorganisms 2020, 8, 1833. [Google Scholar] [CrossRef]
- Rauta, P.R.; Dhupal, M.; Nayak, B. Screening and characterization of potential probiotic lactic acid bacteria isolated from vegetable waste and fish intestine. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 234–244. [Google Scholar]
- Gálvez, A.; Abriouel, H.; Benomar, N.; Lucas, R. Microbial antagonists to food-borne pathogens and biocontrol. Curr. Opin. Biotechnol. 2010, 21, 142–148. [Google Scholar] [CrossRef]
- Gaggia, F.; Di Gioia, D.; Baffoni, L.; Biavati, B. The role of protective and probiotic cultures in food and feed and their impact in food safety. Trends Food Sci. Technol. 2011, 22, S58–S66. [Google Scholar] [CrossRef]
- Garcia, P.; Rodriguez, L.; Rodriguez, A.; Martinez, B. Food biopreservation: Promising strategies using bacteriocins, bacteriophages and endolysins. Trends Food Sci. Technol. 2010, 21, 373–382. [Google Scholar] [CrossRef]
- Pilevar, Z.; Hosseini, H. Effects of starter cultures on the properties of meat products: A review. Annu. Res. Rev. Biol. 2017, 17, 1–17. [Google Scholar] [CrossRef]
- Pedonese, F.; Torracca, B.; Mancini, S.; Pisano, S.; Turchi, B.; Cerri, D.; Nuvoloni, R. Effect of a Lactobacillus sakei and Staphylococcus xylosus protective culture on Listeria monocytogenes growth and quality traits of Italian fresh sausage (salsiccia) stored at abusive temperature. Ital. J. Anim. Sci. 2020, 19, 1363–1374. [Google Scholar] [CrossRef]
- Hammami, R.; Fliss, I.; Corsetti, A. Editorial: Application of protective cultures and bacteriocins for food biopreservation. Front. Microbiol. 2019, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Settanni, L.; Corsetti, A. Application of bacteriocins in vegetable food biopreservation. Int. J. Food Microbiol. 2008, 121, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Eugenia, D.; Bachmann, C. Use of bioprotective cultures in fish products. Curr. Opin. Food Sci. 2015, 6, 19–23. [Google Scholar]
- Ortiz, S.; López, V.; Garriga, M.; Martínez-Suárez, J.V. Antilisterial effect of two bioprotective cultures in a model system of I berian chorizo fermentation. Int. J. Food Sci. Technol. 2014, 49, 753–758. [Google Scholar] [CrossRef]
- Albano, H.; Pinho, C.; Leite, D.; Barbosa, J.; Silva, J.; Carneiro, L.; Magalhães, R.; Hogg, T.; Teixeira, P. Evaluation of a bacteriocin-producing strain of Pediococcus acidilactici as a biopreservative for “Alheira”, a fermented meat sausage. Food Control 2009, 20, 764–770. [Google Scholar] [CrossRef]
- Zagorec, M.; Champomier-Vergès, M.C. Lactobacillus sakei: A starter for sausage fermentation, a protective culture for meat products. Microorganisms 2017, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Oral, Z.F.Y.; Kaban, G. Effects of autochthonous strains on volatile compounds and technological properties of heat-treated sucuk. Food Biosci. 2021, 43, 101140. [Google Scholar]
- Öz, E.; Kabil, E.; Kaban, G.; Kaya, M. Effect of autochthonous Pediococcus acidilactici on volatile profile and some properties of heat-treated sucuk. J. Food Process. Preserv. 2018, 42, e13752. [Google Scholar] [CrossRef]
- Zdolec, N.; Hadžiosmanović, M.; Kozačinski, L.; Cvrtila, Ž.; Filipović, I.; Škrivanko, M.; Leskovar, K. Microbial and physicochemical succession in fermented sausages produced with bacteriocinogenic culture of Lactobacillus sakei and semi-purified bacteriocin mesenterocin Y. Meat Sci. 2008, 80, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Ahn, D.U.; Olson, D.G.; Jo, C.; Chen, X.; Wu, C.; Lee, J.I. Effect of muscle type, packaging, and irradiation on lipid oxidation, volatile production, and color in raw pork patties. Meat Sci. 1998, 49, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Careri, M.; Mangia, A.; Barbieri, G.; Bouoni, L.; Virgili, R.; Parolari, G. Sensory property relationships to chemical data of Italian-type dry-cured ham. J. Food Sci. 1993, 58, 968–972. [Google Scholar] [CrossRef]
- Arslan, B.; Soyer, A. Effects of chitosan as a surface fungus inhibitor on microbiological, physicochemical, oxidative and sensory characteristics of dry fermented sausages. Meat Sci. 2018, 145, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Akköse, A.; Oğraş, Ş.Ş.; Kaya, M.; Kaban, G. Microbiological, physicochemical and sensorial changes during the ripening of sucuk, a traditional Turkish dry-fermented sausage: Effects of autochthonous strains, sheep tail fat and ripening rate. Fermentation 2023, 9, 558. [Google Scholar] [CrossRef]
- Ockerman, H.W.; Basu, L. Production and consumption of fermented meat products. In Handbook of Fermented Meat and Poultry; Willey Blackwell: West Sussex, UK, 2014; pp. 7–11. [Google Scholar]
- Kaban, G.; Kaya, M. Effect of starter culture on growth of Staphylococcus aureus in sucuk. Food Control 2006, 17, 797–801. [Google Scholar] [CrossRef]
- Aro JM, A.; Nyam-Osor, P.; Tsuji, K.; Shimada, K.I.; Fukushima, M.; Sekikawa, M. The effect of starter cultures on proteolytic changes and amino acid content in fermented sausages. Food Chem. 2010, 119, 279–285. [Google Scholar]
- Rantsiou, K.; Urso, R.; Comi, G.; Cocolin, L. Use of Bacteriocin-Producer Lactobacillus sakei for Fermented Sausages Production. 2005. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=2eadfb34bdddace9b7b8180da40d41526c602527 (accessed on 7 April 2021).
- Talon, R.; Leroy, S.; Lebert, I. Microbial ecosystems of traditional fermented meat products: The importance of indigenous starters. Meat Sci. 2007, 77, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Barbosa, J.; Vendeiro, S.; Mota, A.; Silva, F.; Monteiro, M.J.; Hogg, T.; Gibbs, P.; Teixeira, P. Chemical and microbiological characterization of alheira: A typical Portuguese fermented sausage with particular reference to factors relating to food safety. Meat Sci. 2006, 73, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Gounadaki, A.S.; Skandamis, P.N.; Drosinos, E.H.; Nychas, G.J.E. Microbial ecology of food contact surfaces and products of small-scale facilities producing traditional sausages. Food Microbiol. 2008, 25, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Hugas, M.; Garriga, M.; Aymerich, M.T. Functionality of enterococci in meat products. Int. J. Food Microbiol. 2003, 88, 223–233. [Google Scholar] [CrossRef]
- López, C.; Medina, L.M.; Jordano, R. Occurrence and behavior of Enterobacteriaceae and enterococci in Mediterranean dry sausages during ripening in a pilot-scale chamber. J. Food Prot. 2004, 67, 2812–2814. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Xu, X.; Zhou, G.; Zhu, Z.; Meng, Y.; Sun, Y. Effect of starter cultures on microbial ecosystem and biogenic amines in fermented sausage. Food Control 2010, 21, 444–449. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Gómez, M.; Fonseca, S. Effect of commercial starter cultures on physicochemical characteristics, microbial counts and free fatty acid composition of dry-cured foal sausage. Food Control 2014, 46, 382–389. [Google Scholar] [CrossRef]
- Kaban, G.; Kaya, M. Effects of Lactobacillus plantarum and Staphylococcus xylosus on the quality characteristics of dry fermented sausage “sucuk”. J. Food Sci. 2009, 74, S58–S63. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, L.; Su, R.; Jin, Y. Effects of different starter culture combinations on microbial counts and physico-chemical properties in dry fermented mutton sausages. Food Sci. Nutr. 2019, 7, 1957–1968. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Khalifa, I.; Mesak, M.A.; Lorenzo, J.M.; Farag, M.A. A comprehensive review of the role of microorganisms on texture change, flavor and biogenic amines formation in fermented meat with their action mechanisms and safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 3538–3555. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific opinion on risk-based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Özturk, F.; Halkman, A. Determination of the survival levels of acid-adapted Escherichia coli O157: H7in sucuk (Turkish-type fermented sausage). Turk. J. Vet. Anim. Sci. 2015, 39, 485–492. [Google Scholar] [CrossRef]
- Moon, G.S.; Kim, W.J.; Kim, M.H. Synergistic effects of bacteriocin-producing Pediococcus acidilactici K10 and organic acids on inhibiting Escherichia coli O157: H7 and applications in ground beef. J. Microbiol. Biotechnol. 2002, 12, 936–942. [Google Scholar]
- Tang, K.X.; Shi, T.; Gänzle, M. Effect of starter cultures on taste-active amino acids and survival of pathogenic Escherichia coli in dry fermented beef sausages. Eur. Food Res. Technol. 2018, 244, 2203–2212. [Google Scholar] [CrossRef]
- Wang, X.; Ren, H.; Wang, W.; Zhang, Y.; Bai, T.; Li, J.; Zhu, W. Effects of inoculation of commercial starter cultures on the quality and histamine accumulation in fermented sausages. J. Food Sci. 2015, 80, M377–M384. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Cretenet, M.; Even, S.; Le Loir, Y. Interactions between Staphylococcus aureus and lactic acid bacteria: An old story with new perspectives. Int. J. Food Microbiol. 2009, 131, 30–39. [Google Scholar] [CrossRef]
- Fetsch, A.; Johler, S. Staphylococcus aureus as a foodborne pathogen. Curr. Clin. Microbiol. Rep. 2018, 5, 88–96. [Google Scholar] [CrossRef]
- Wang, X.H.; Ren, H.Y.; Liu, D.Y.; Zhu, W.Y.; Wang, W. Effects of inoculating Lactobacillus sakei starter cultures on the microbiological quality and nitrite depletion of Chinese fermented sausages. Food Control 2013, 32, 591–596. [Google Scholar] [CrossRef]
- Erkmen, O.; Bozkurt, H. Quality characteristics of retailed sucuk (Turkish dry-fermented sausage). Food Technol. Biotechnol. 2004, 42, 63–69. [Google Scholar]
- Kamiloğlu, A.; Kaban, G.; Kaya, M. Effects of autochthonous Lactobacillus plantarum strains on Listeria monocytogenes in sucuk during ripening. J. Food Saf. 2019, 39, e12618. [Google Scholar] [CrossRef]
- Pérez-Alvarez, J.A.; Sayas-Barberá, M.E.; Fernández-López, J.; Aranda-Catalá, V. Physicochemical characteristics of Spanish-type dry-cured sausage. Food Res. Int. 1999, 32, 599–607. [Google Scholar] [CrossRef]
- Bozkurt, H.; Bayram, M. Colour and textural attributes of sucuk during ripening. Meat Sci. 2006, 73, 344–350. [Google Scholar] [CrossRef]
- Candogan, K.; Wardlaw, F.B.; Acton, J.C. Effect of starter culture on proteolytic changes during processing of fermented beef sausages. Food Chem. 2009, 116, 731–737. [Google Scholar] [CrossRef]
- Bañón, S.; Serrano, R.; Bedia, M. Use of Micrococcaceae combined with a low proportion of Lactic Acid Bacteria as a starter culture for salami stuffed in natural casing. CyTA-J. Food 2014, 12, 160–165. [Google Scholar] [CrossRef]
- Casaburi, A.; Di Monaco, R.; Cavella, S.; Toldrá, F.; Ercolini, D.; Villani, F. Proteolytic and lipolytic starter cultures and their effect on traditional fermented sausages ripening and sensory traits. Food Microbiol. 2008, 25, 335–347. [Google Scholar] [CrossRef]
- Basso, A.L.; Picariello, G.; Coppola, R.; Tremonte, P.; Musso, S.S.; Luccia, A.D. Proteolytic activity of Lactobacillus sakei, Lactobacillus farciminis and Lactobacillus plantarum on sarcoplasmic proteins of pork lean. J. Food Biochem. 2004, 28, 195–212. [Google Scholar] [CrossRef]
- Candogan, K.; Acton, J.C. Proteolytic activity of bacterial starter cultures for meat fermentation. J. Muscle Foods 2004, 15, 23–34. [Google Scholar] [CrossRef]
- Sriphochanart, W.; Skolpap, W. Characterization of proteolytic effect of lactic acid bacteria starter cultures on Thai fermented sausages. Food Biotechnol. 2010, 24, 293–311. [Google Scholar] [CrossRef]
- Bedia, M.; Méndez, L.; Bañón, S. Evaluation of different starter cultures (Staphylococci plus Lactic Acid Bacteria) in semi-ripened Salami stuffed in swine gut. Meat Sci. 2011, 87, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, H.; Erkmen, O. Effect of nitrate/nitrite on the quality of sausage (sucuk) during ripening and storage. J. Sci. Food Agric. 2004, 84, 279–286. [Google Scholar] [CrossRef]
- Ansorena, D.; Astiasaran, I. Effect of storage and packaging on fatty acid composition and oxidation in dry fermented sausages made with added olive oil and antioxidants. Meat Sci. 2004, 67, 237–244. [Google Scholar] [CrossRef]
- Šojić, B.V.; Džinić, N.R.; Tomović, V.M.; Ikonić, P.M.; Jokanović, M.R.; Kravić, S.Ž.; Tasić, T.A.; Škaljac, S.B. Effect of starter culture addition on fatty acid profile, oxidative and sensory stability of traditional fermented sausage (Petrovská klobása). Acta Periodica Technol. 2016, 75–81. [Google Scholar] [CrossRef]
- Barriere, C.; Centeno, D.; Lebert, A.; Leroy-Setrin, S.; Berdague, J.L.; Talon, R. Roles of superoxide dismutase and catalase of Staphylococcus xylosus in the inhibition of linoleic acid oxidation. FEMS Microbiol. Lett. 2001, 201, 181–185. [Google Scholar] [CrossRef]
- Leroy, S.; Giammarinaro, P.; Chacornac, J.P.; Lebert, I.; Talon, R. Biodiversity of indigenous staphylococci of naturally fermented dry sausages and manufacturing environments of small-scale processing units. Food Microbiol. 2010, 27, 294–301. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, Z.; Yohannes, K.W.; Yu, Q.; Yang, Z.; Li, H. Functional characteristics of Lactobacillus and yeast single starter cultures in the ripening process of dry fermented sausage. Front. Microbiol. 2021, 11, 611260. [Google Scholar] [CrossRef]
- Wen, R.; Sun, F.; Wang, Y.; Chen, Q.; Kong, B. Evaluation the potential of lactic acid bacteria isolates from traditional beef jerky as starter cultures and their effects on flavor formation during fermentation. LWT 2021, 142, 110982. [Google Scholar] [CrossRef]
- Bozkurt, H.; Erkmen, O. Effects of starter cultures and additives on the quality of Turkish style sausage (sucuk). Meat Sci. 2002, 61, 149–156. [Google Scholar] [CrossRef]
- Scannell, A.G.; Kenneally, P.M.; Arendt, E.K. Contribution of starter cultures to the proteolytic process of a fermented non-dried whole muscle ham product. Int. J. Food Microbiol. 2004, 93, 219–230. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Wang, D.; Gao, F.; Zhang, K.; Tian, J.; Jin, Y. Research update on the impact of lactic acid bacteria on the substance metabolism, flavor, and quality characteristics of fermented meat products. Foods 2022, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Ammor, S.; Dufour, E.; Zagorec, M.; Chaillou, S.; Chevallier, I. Characterization and selection of Lactobacillus sakei strains isolated from traditional dry sausage for their potential use as starter cultures. Food Microbiol. 2005, 22, 529–538. [Google Scholar] [CrossRef]
- Fadda, S.; Vignolo, G.; Oliver, G. Tyramine degradation and tyramine/histamine production by lactic acid bacteria and Kocuria strains. Biotechnol. Lett. 2001, 23, 2015–2019. [Google Scholar] [CrossRef]
- Gandhi, D.; Chanalia, P.; Attri, P.; Dhanda, S. Dipeptidyl peptidase-II from probiotic Pediococcus acidilactici: Purification and functional characterization. Int. J. Biol. Macromol. 2016, 93, 919–932. [Google Scholar] [CrossRef] [PubMed]

| TAMB (log CFU/g) | LAB (log CFU/g) | CNC (log CFU/g) | ||||||||||||||
| DAYS | ||||||||||||||||
| 0 | 3 | 6 | 9 | 12 | 0 | 3 | 6 | 9 | 12 | 0 | 3 | 6 | 9 | 12 | ||
| Groups | C | 5.48 ± 0.20 Aa | 8.78 ± 0.24 Ac | 8.83 ± 0.06 Ac | 8.63 ± 0.12 Ac | 8.22 ± 0.07 Ab | 5.22 ± 0.13 Aa | 7.48 ± 0.16 Ab | 8.53 ± 0.11 Cd | 8.47 ± 0.03 Ad | 8.34 ± 0.04 Ac | 4.17 ± 0.02 Aa | 5.35 ± 0.99 Ab | 5.71 ± 0.97 Cd | 5.77 ± 1.14 Bd | 5.57 ± 1.03 Bbc |
| S1 | 7.05 ± 0.10 Ba | 9.28 ± 0.16 Cb | 9.40 ± 0.13 Cc | 9.29 ± 0.03 Cb | 9.16 ± 0.20 Cb | 6.64 ± 0.08 Ba | 9.31 ± 0.15 Bc | 9.48 ± 0.04 Bd | 9.30 ± 0.14 Cc | 9.11 ± 0.27 Cb | 6.86 ± 0.13 Bb | 7.00 ± 0.12 Bb | 6.90 ± 0.06 Db | 6.85 ± 0.14 Cb | 6.37 ± 0.38 Ca | |
| S2 | 6.95 ± 0.32 Ba | 9.38 ± 0.17 Cc | 9.75 ± 0.35 Dd | 9.08 ± 0.11 Bb | 9.05 ± 0.16 Cb | 6.68 ± 0.07 Ba | 10.03 ± 0.22 Cd | 10.14 ± 0.16 Ad | 9.62 ± 0.27 Dc | 9.25 ± 0.19 Cb | 4.30 ± 0.15 Ab | 4.88 ± 0.17 Ad | 4.68 ± 0.15 Ac | 4.31 ± 0.14 Ab | 3.97 ± 1.12 Aa | |
| S3 | 6.77 ± 0.33 Ba | 9.09 ± 0.39 Bc | 9.32 ± 0.20 Bd | 8.74 ± 0.24 Ab | 8.68 ± 0.17 Bb | 6.80 ± 0.23 Ba | 9.46 ± 0.18 Bd | 9.53 ± 0.10 Bd | 9.02 ± 0.03 Bc | 8.86 ± 0.08 Bb | 4.21 ± 0.09 Aa | 5.14 ± 0.16 Ad | 4.93 ± 0.26 Bc | 4.43 ± 0.16 Ab | 4.22 ± 0.07 Aa | |
| PI (%) | TBARSs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Groups | DAYS | |||||||||
| 0 | 3 | 6 | 9 | 12 | 0 | 3 | 6 | 9 | 12 | |
| C | 10.14 ± 0.35 Aa | 9.83 ± 0.63 Aa | 10.37 ± 0.77 A | 10.86 ± 0.67 Aa | 12.30 ± 0.30 Ab | 0.51 ± 0.04 Aa | 0.66 ± 0.02 Bb | 0.75 ± 0.02 Bc | 0.84 ± 0.04 Bd | 0.98 ± 0.10 Be |
| S1 | 13.51 ± 2.08 Ba | 13.26 ± 0.94 Ba | 14.81 ± 1.57 Bb | 17.14 ± 1.29 Cc | 19.31 ± 1.15 Cd | 0.50 ± 0.04 Aa | 0.54 ± 0.04 Aa | 0.59 ± 0.04 Ab | 0.67 ± 0.03 Ac | 0.62 ± 0.04 Ab |
| S2 | 12.84 ± 0.93 Ba | 13.05 ± 0.90 Ba | 14.93 ± 0.46 Bb | 16.12 ± 0.73 Cc | 17.35 ± 1.24 Bc | 0.51 ± 0.04 Aa | 0.56 ± 0.02 Ab | 0.61 ± 0.03 Abc | 0.59 ± 0.04 Ab | 0.56 ± 0.02 Ab |
| S3 | 13.44 ± 2.06 Ba | 13.49 ± 0.71 Ba | 13.11 ± 0.71 Ba | 14.63 ± 0.26 Ba | 16.24 ± 1.32 Bb | 0.52 ± 0.02 Aa | 0.56 ± 0.02 Ab | 0.58 ± 0.03 Ab | 0.64 ± 0.02 Ac | 0.58 ± 0.03 Ab |
| Raw Samples | Grilled Samples | |||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Odor | Color | Taste | Overall Liking | Odor | Color | Taste | Overall Liking |
| C | 6.89 ± 0.78 a | 6.98 ± 0.47 a | 6.91 ± 0.42 a | 6.83 ± 0.63 a | 7.74 ± 0.39 a | 7.64 ± 0.16 a | 6.96 ± 0.61 a | 6.95 ± 0.51 a |
| S1 | 7.52 ± 0.90 b | 8.44 ± 0.85 c | 7.76 ± 0.45 b | 7.76 ± 0.50 b | 8.47 ± 0.36 b | 8.44 ± 0.59 b | 8.30 ± 0.34 b | 7.84 ± 0.51 b |
| S2 | 7.48 ± 0.74 b | 7.85 ± 0.68 b | 7.52 ± 0.06 b | 7.44 ± 0.40 b | 7.56 ± 0.33 a | 8.37 ± 0.34 b | 7.67 ± 0.11 b | 7.87 ± 0.14 b |
| S3 | 7.63 ± 0.51 b | 7.82 ± 0.34 b | 7.93 ± 0.36 b | 7.89 ± 0.28 b | 7.85 ± 0.13 a | 8.04 ± 0.39 b | 8.00 ± 0.40 b | 8.11 ± 0.39 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz Topcam, M.M.; Arslan, B.; Soyer, A. Sucuk, Turkish-Style Fermented Sausage: Evaluation of the Effect of Bioprotective Starter Cultures on Its Microbiological, Physicochemical, and Chemical Properties. Appl. Microbiol. 2024, 4, 1215-1231. https://doi.org/10.3390/applmicrobiol4030083
Yilmaz Topcam MM, Arslan B, Soyer A. Sucuk, Turkish-Style Fermented Sausage: Evaluation of the Effect of Bioprotective Starter Cultures on Its Microbiological, Physicochemical, and Chemical Properties. Applied Microbiology. 2024; 4(3):1215-1231. https://doi.org/10.3390/applmicrobiol4030083
Chicago/Turabian StyleYilmaz Topcam, Mahide Muge, Betul Arslan, and Ayla Soyer. 2024. "Sucuk, Turkish-Style Fermented Sausage: Evaluation of the Effect of Bioprotective Starter Cultures on Its Microbiological, Physicochemical, and Chemical Properties" Applied Microbiology 4, no. 3: 1215-1231. https://doi.org/10.3390/applmicrobiol4030083
APA StyleYilmaz Topcam, M. M., Arslan, B., & Soyer, A. (2024). Sucuk, Turkish-Style Fermented Sausage: Evaluation of the Effect of Bioprotective Starter Cultures on Its Microbiological, Physicochemical, and Chemical Properties. Applied Microbiology, 4(3), 1215-1231. https://doi.org/10.3390/applmicrobiol4030083







