Microalgae-Based Functional Foods: A Blue-Green Revolution in Sustainable Nutrition and Health
Abstract
1. Introduction
2. Challenges in Microalgae Cultivation and Bioprocessing
2.1. Species Diversity in Microalgae Cultivation
2.2. Microalgae Cultivation Under Various Trophic Conditions
2.3. Factors Influencing Microalgae Production and Commercial Feasibility
2.4. Biomass Recovery Methods and Their Challenges
3. Bioactive Components of Microalgal Biomass
3.1. Polysaccharides
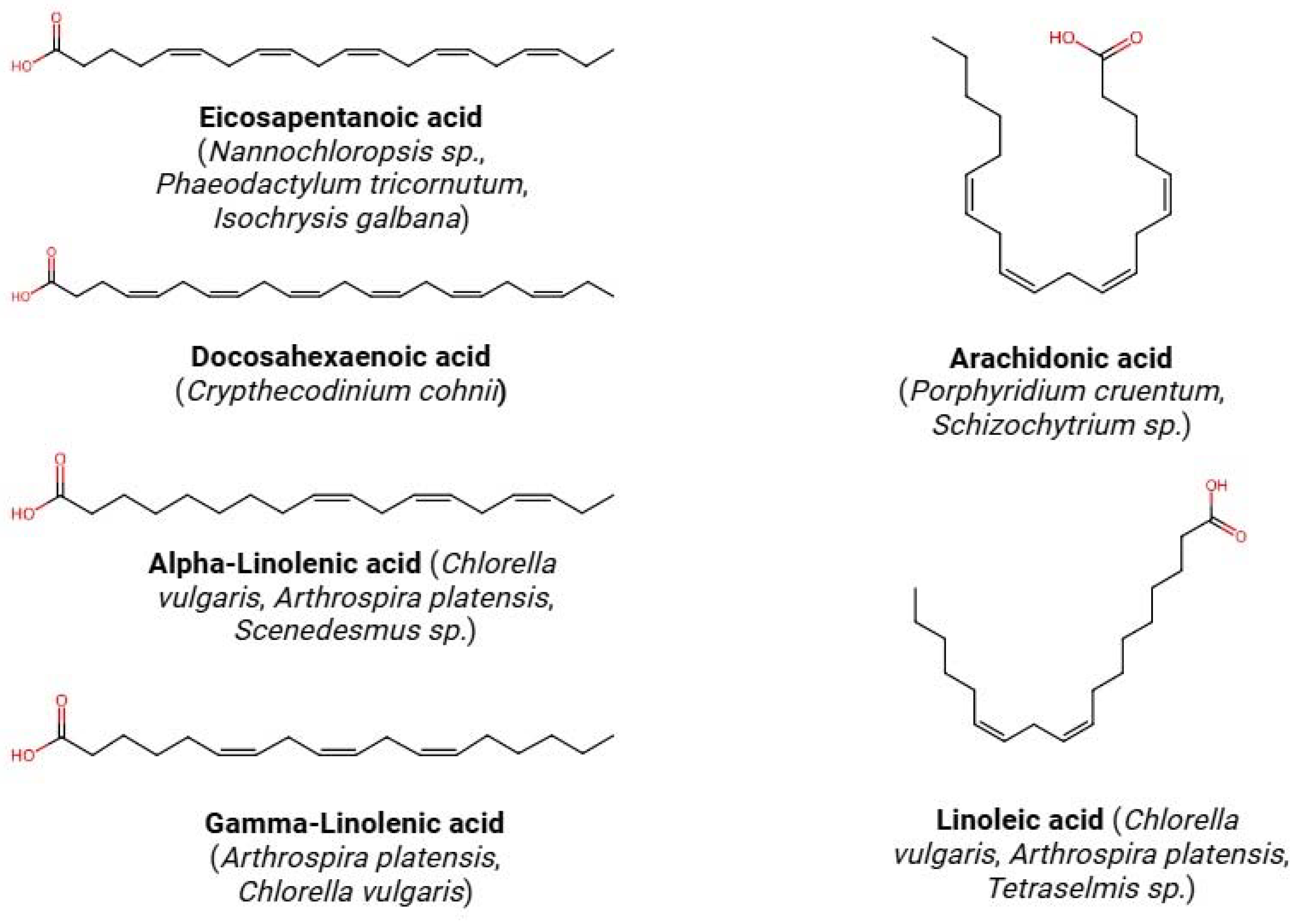
3.2. Fatty Acids
3.3. Proteins, Peptides, and Amino Acids
3.4. Pigments and Vitamins
3.5. Antioxidants
4. Microalgal Incorporation into Foods with Potential Health Benefits
4.1. Microalgal PUFA Incorporated into Different Foods
4.2. Microalgae Incorporated into Dairy and Probiotic Products
4.3. Microalgae Incorporated into Pasta, Baked Goods, Condiments, and Beverages
5. Absorption and Availability of Bioactive Components from Microalgae
5.1. Advanced Strategies to Improve Bioavailability
5.1.1. Microencapsulation
5.1.2. Lipid Structural Modification
5.1.3. Fermentation
6. Challenges in Food Formulation with Microalgae
6.1. Challenges for Sensory Qualities of Food with Microalgae
6.2. Food Safety and Risk Factors
6.3. Challenges in Maintaining Consistent Nutritional Value of Microalgae
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance Toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Liu, K. Arthrospira platensis as Future Food: A Review on Functional Ingredients, Bioactivities and Application in the Food Industry. Int. J. Food Sci. Technol. 2024, 59, 1197–1212. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Domínguez Díaz, L.; Fernández-Ruiz, V.; Cámara, M. The Frontier Between Nutrition and Pharma: The International Regulatory Framework of Functional Foods, Food Supplements and Nutraceuticals. Crit. Rev. Food Sci. Nutr. 2020, 60, 1738–1746. [Google Scholar] [CrossRef]
- Zazzara, M.B.; Palmer, K.; Vetrano, D.L.; Carfì, A.; Graziano, O. Adverse Drug Reactions in Older Adults: A Narrative Review of the Literature. Eur. Geriatr. Med. 2021, 12, 463–473. [Google Scholar] [CrossRef]
- Jędrusek-Golińska, A.; Górecka, D.; Buchowski, M.; Wieczorowska-Tobis, K.; Gramza-Michałowska, A.; Szymandera-Buszka, K. Recent Progress in the Use of Functional Foods for Older Adults: A Narrative Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 835–856. [Google Scholar] [CrossRef]
- Donato, K.; Medori, M.C.; Stuppia, L.; Beccari, T.; Dundar, M.; Marks, R.S.; Michelini, S.; Borghetti, E.; Zuccato, C.; Seppilli, L.; et al. Unleashing the Potential of Biotechnology for Sustainable Development. Eur. Rev. Med. Pharmacol. Sci. 2023, 27 (Suppl. S6), 100–113. [Google Scholar]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Sgroi, F.; Sciortino, C.; Baviera-Puig, A.; Modica, F. Analyzing Consumer Trends in Functional Foods: A Cluster Analysis Approach. J. Agric. Food Res. 2024, 15, 101041. [Google Scholar] [CrossRef]
- Onyeaka, H.; Nwaiwu, O.; Obileke, K.; Miri, T.; Al-Sharify, Z.T. Global Nutritional Challenges of Reformulated Food: A Review. Food Sci. Nutr. 2023, 11, 2483–2499. [Google Scholar] [CrossRef]
- Albertsen, L.; Wiedmann, K.P.; Schmidt, S. The Impact of Innovation-Related Perception on Consumer Acceptance of Food Innovations—Development of an Integrated Framework of the Consumer Acceptance Process. Food Qual. Prefer. 2020, 84, 103958. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Gohara-Beirigo, A.K.; Matsudo, M.C.; Cezare-Gomes, E.A.; de Carvalho, J.C.M.; Danesi, E.D.G. Microalgae Trends Toward Functional Staple Food Incorporation: Sustainable Alternative for Human Health Improvement. Trends Food Sci. Technol. 2022, 125, 185–199. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Galasso, C.; Gentile, A.; Orefice, I.; Ianora, A.; Bruno, A.; Noonan, D.M.; Sansone, C.; Albini, A.; Brunet, C. Microalgal Derivatives as Potential Nutraceutical and Food Supplements for Human Health: A Focus on Cancer Prevention and Interception. Nutrients 2019, 11, 1226. [Google Scholar] [CrossRef]
- Nayaka, S.; Toppo, K.; Verma, S. Adaptation in Algae to Environmental Stress and Ecological Conditions. In Plant Adaptation Strategies in Changing Environment; Springer: Cham, Switzerland, 2017; pp. 103–115. [Google Scholar]
- Buono, S.; Langellotti, A.L.; Martello, A.; Rinna, F.; Fogliano, V. Functional Ingredients from Microalgae. Food Funct. 2014, 5, 1669–1685. [Google Scholar] [CrossRef]
- Aishvarya, V.; Jena, J.; Pradhan, N.; Panda, P.K.; Sukla, L.B. Microalgae: Cultivation and Application. In Environmental Microbial Biotechnology; Springer: Singapore, 2015; pp. 289–311. [Google Scholar]
- Algae Novel Food. Available online: https://www.algae-novel-food.com (accessed on 30 March 2025).
- Chemsafe Consulting. Microalgae-Based Novel Foods in EU. Available online: https://www.chemsafe-consulting.com/en/2024/07/16/microalgae-based-novel-foods-in-eu/ (accessed on 30 March 2025).
- Yan, N.; Fan, C.; Chen, Y.; Hu, Z. The Potential for Microalgae as Bioreactors to Produce Pharmaceuticals. Int. J. Mol. Sci. 2016, 17, 962. [Google Scholar] [CrossRef]
- Barra, L.; Chandrasekaran, R.; Corato, F.; Brunet, C. The Challenge of Ecophysiological Biodiversity for Biotechnological Applications of Marine Microalgae. Mar. Drugs 2014, 12, 1641–1675. [Google Scholar] [CrossRef]
- Renganathan, P.; Gaysina, L.A.; Jaime, R.; Carlos, J.; Omar, E. Phycoremediated Microalgae and Cyanobacteria Biomass as Biofertilizer for Sustainable Agriculture: A Holistic Biorefinery Approach to Promote Circular Bioeconomy. Biomass 2024, 4, 1047–1077. [Google Scholar] [CrossRef]
- Gatamaneni, B.L.; Orsat, V.; Lefsrud, M. Factors Affecting Growth of Various Microalgal Species. Environ. Eng. Sci. 2018, 35, 1037–1048. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.K.; Dunn, G.P.; Coats, E.R.; Newby, D.T.; Feris, K.P. Algal Diversity and Traits Predict Biomass Yield and Grazing Resistance in Wastewater Cultivation. J. Appl. Phycol. 2019, 31, 2323–2334. [Google Scholar] [CrossRef]
- Stockenreiter, M.; Graber, A.K.; Haupt, F.; Stibor, H. The Effect of Species Diversity on Lipid Production by Microalgal Communities. J. Appl. Phycol. 2012, 24, 45–54. [Google Scholar] [CrossRef]
- Hossain, N.; Zaini, J.; Mahlia, T.M.I. Life Cycle Assessment, Energy Balance and Sensitivity Analysis of Bioethanol Production from Microalgae in a Tropical Country. Renew. Sustain. Energy Rev. 2019, 115, 109371. [Google Scholar] [CrossRef]
- Olguín, E.J.; Melo, F.J.; Cruz, L.; De Philippis, R.; Adessi, A. Microalgae-Based Biorefineries: Challenges and Future Trends to Produce Carbohydrate-Enriched Biomass, High-Added Value Products and Bioactive Compounds. Biology 2022, 11, 1146. [Google Scholar] [CrossRef]
- Castillo, T.; Ramos, D.; García-Beltrán, T.; Brito-Bazan, M.; Galindo, E. Mixotrophic Cultivation of Microalgae: An Alternative to Produce High-Value Metabolites. Biochem. Eng. J. 2021, 176, 108183. [Google Scholar] [CrossRef]
- Smith, J.P.; Hughes, A.D.; McEvoy, L.; Day, J.G. Tailoring of the Biochemical Profiles of Microalgae by Employing Mixotrophic Cultivation. Bioresour. Technol. Rep. 2020, 9, 100321. [Google Scholar]
- Ansari, F.A.; Ravindran, B.; Gupta, S.K.; Nasr, M.; Rawat, I.; Bux, F. Techno-Economic Estimation of Wastewater Phycoremediation and Environmental Benefits Using Scenedesmus obliquus Microalgae. J. Environ. Manag. 2019, 240, 293–302. [Google Scholar] [CrossRef]
- AlMomani, F.A.; Örmeci, B. Performance of Chlorella vulgaris, Neochloris oleoabundans, and Mixed Indigenous Microalgae for Treatment of Primary Effluent, Secondary Effluent, and Centrate. Ecol. Eng. 2016, 95, 280–289. [Google Scholar] [CrossRef]
- Dubey, K.K.; Kumar, S.; Dixit, D.; Kumar, P.; Kumar, D.; Jawed, A.; Haque, S. Implication of Industrial Waste for Biomass and Lipid Production in Chlorella minutissima under Autotrophic, Heterotrophic, and Mixotrophic Growth Conditions. Appl. Biochem. Biotechnol. 2015, 176, 1581–1595. [Google Scholar] [CrossRef]
- Walls, L.E.; Velasquez-Orta, S.B.; Romero-Frasca, E.; Leary, P.; Noguez, I.Y.; Ledesma, M.T.O. Non-Sterile Heterotrophic Cultivation of Native Wastewater Yeast and Microalgae for Integrated Municipal Wastewater Treatment and Bioethanol Production. Biochem. Eng. J. 2019, 151, 107319. [Google Scholar] [CrossRef]
- Lin, T.S.; Wu, J.Y. Effect of Carbon Sources on Growth and Lipid Accumulation of Newly Isolated Microalgae Cultured under Mixotrophic Condition. Bioresour. Technol. 2015, 184, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Sajadian, S.F.; Morowvat, M.H.; Ghasemi, Y. Investigation of Autotrophic, Heterotrophic, and Mixotrophic Modes of Cultivation on Lipid and Biomass Production in Chlorella vulgaris. Nat. J. Physiol. Pharm. Pharmacol. 2018, 8, 594–599. [Google Scholar] [CrossRef]
- Yeesang, C.; Cheirsilp, B. Low-Cost Production of Green Microalga Botryococcus braunii Biomass with High Lipid Content through Mixotrophic and Photoautotrophic Cultivation. Appl. Biochem. Biotechnol. 2014, 174, 116–129. [Google Scholar] [CrossRef]
- Trabelsi, L.; Ben Ouada, H.; Zili, F.; Mazhoud, N.; Ammar, J. Evaluation of Arthrospira platensis Extracellular Polymeric Substances Production in Photoautotrophic, Heterotrophic, and Mixotrophic Conditions. Folia Microbiol. 2012, 58, 39–45. [Google Scholar] [CrossRef]
- Sen, R.; Martin, G.J. Glycerol and Nitrate Utilisation by Marine Microalgae Nannochloropsis salina and Chlorella sp. and Associated Bacteria During Mixotrophic and Heterotrophic Growth. Algal Res. 2018, 33, 298–309. [Google Scholar]
- Gim, G.H.; Kim, J.K.; Kim, H.S.; Kathiravan, M.N.; Yang, H.; Jeong, S.H.; Kim, S.W. Comparison of Biomass Production and Total Lipid Content of Freshwater Green Microalgae Cultivated under Various Culture Conditions. Bioprocess Biosyst. Eng. 2014, 37, 99–106. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Bhatnagar, M.; Chinnasamy, S.; Das, K.C. Chlorella minutissima—A Promising Fuel Alga for Cultivation in Municipal Wastewaters. Appl. Biochem. Biotechnol. 2010, 161, 523–536. [Google Scholar] [CrossRef]
- Sforza, E.; Cipriani, R.; Morosinotto, T.; Bertucco, A.; Giacometti, G.M. Excess CO₂ Supply Inhibits Mixotrophic Growth of Chlorella protothecoides and Nannochloropsis salina. Bioresour. Technol. 2012, 104, 523–529. [Google Scholar] [CrossRef]
- Li, T.; Kirchhoff, H.; Gargouri, M.; Feng, J.; Cousins, A.B.; Pienkos, P.T.; Chen, S. Assessment of Photosynthesis Regulation in Mixotrophically Cultured Microalga Chlorella sorokiniana. Algal Res. 2016, 19, 30–38. [Google Scholar] [CrossRef]
- Mirzaie, M.; Ma, K.M.; Mousavi, S.M.; Ghobadian, B. Investigation of Mixotrophic, Heterotrophic, and Autotrophic Growth of Chlorella vulgaris under Agricultural Waste Medium. Prep. Biochem. Biotechnol. 2016, 46, 150–156. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, S.; Li, A.; Liu, P.; Wang, M. Effects of Trophic Modes, Carbon Sources, and Salinity on the Cell Growth and Lipid Accumulation of Tropic Ocean Oilgae Strain Desmodesmus sp. WC08. Appl. Biochem. Biotechnol. 2016, 180, 452–463. [Google Scholar] [CrossRef]
- Gim, G.H.; Ryu, J.; Kim, M.J.; Kim, P.I.; Kim, S.W. Effects of Carbon Source and Light Intensity on the Growth and Total Lipid Production of Three Microalgae under Different Culture Conditions. J. Ind. Microbiol. Biotechnol. 2016, 43, 605–616. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Park, Y.; Kim, S.; Cho, S.; Yu, J.; Lee, T. Comparison of Trophic Modes to Maximize Biomass and Lipid Productivity of Micractinium inermum NLP-F014. Biotechnol. Bioprocess Eng. 2018, 23, 238–245. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, W.; Mao, X.; Li, Y.; Wu, T.; Chen, F. High-Value Biomass from Microalgae Production Platforms: Strategies and Progress Based on Carbon Metabolism and Energy Conversion. Biotechnol. Biofuels 2018, 11, 227. [Google Scholar]
- Kang, S.; Heo, S.; Lee, J.H. Techno-Economic Analysis of Microalgae-Based Lipid Production: Considering Influences of Microalgal Species. Ind. Eng. Chem. Res. 2018, 58, 944–955. [Google Scholar] [CrossRef]
- Kadir, W.N.A.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T. Harvesting and Pre-Treatment of Microalgae Cultivated in Wastewater for Biodiesel Production: A Review. Energy Convers. Manag. 2018, 171, 1416–1429. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, H.; Cheng, P.; Chang, T.; Chen, P.; Zhou, C.; Ruan, R. Development of Integrated Culture Systems and Harvesting Methods for Improved Algal Biomass Productivity and Wastewater Resource Recovery—A Review. Sci. Total Environ. 2020, 746, 141039. [Google Scholar] [CrossRef]
- Heasman, M.; Diemar, J.; O’Connor, W.; Sushames, T.; Foulkes, L. Development of Extended Shelf-Life Microalgae Concentrate Diets Harvested by Centrifugation for Bivalve Molluscs—A Summary. Aquacult. Res. 2000, 31, 637–659. [Google Scholar]
- Christenson, L.; Sims, R. Production and Harvesting of Microalgae for Wastewater Treatment, Biofuels, and Bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef]
- Liu, X.Y.; Hong, Y. Microalgae-Based Wastewater Treatment and Recovery with Biomass and Value-Added Products: A Brief Review. Curr. Pollut. Rep. 2021, 7, 227–245. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, L.; Zang, X.; Cheng, S.; Zhang, X. Effect of Shear Rate on Floc Characteristics and Concentration Factors for the Harvesting of Chlorella vulgaris Using Coagulation-Flocculation-Sedimentation. Sci. Total Environ. 2019, 688, 811–817. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X. Microalgal Harvesting Using Foam Flotation: A Critical Review. Biomass Bioenergy 2018, 120, 176–188. [Google Scholar] [CrossRef]
- El-Moustaqim, K.; El Bakraoui, H.; Mabrouki, J.; Fouad, Y.; Slaoui, M.; Hmouni, D.; Benyeogor, M.S.; Igbigbi, T.L. Combination of Microalgae Method, Decantation, and Filtration for Domestic Wastewater Treatment. Sustainability 2023, 15, 16110. [Google Scholar] [CrossRef]
- Lv, K.; Yuan, Q.; Li, H.; Li, T.; Ma, H.; Gao, C.; Zhao, L. Chlorella pyrenoidosa Polysaccharides as a Prebiotic to Modulate Gut Microbiota: Physicochemical Properties and Fermentation Characteristics in Vitro. Foods 2022, 11, 725. [Google Scholar] [CrossRef]
- Fayad, N.; Yehya, T.; Audonnet, F.; Vial, C. Harvesting of Microalgae Chlorella vulgaris Using Electro-Coagulation-Flocculation in the Batch Mode. Algal Res. 2017, 25, 1–11. [Google Scholar] [CrossRef]
- Liu, P.; Wang, T.; Yang, Z.; Hong, Y.; Hou, Y. Long-Chain Poly-Arginine Functionalized Porous Fe₃O₄ Microspheres as Magnetic Flocculant for Efficient Harvesting of Oleaginous Microalgae. Algal Res. 2017, 27, 99–108. [Google Scholar] [CrossRef]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.Z.; Hossain, M.M. A Comprehensive Review on Conventional and Biological-Driven Heavy Metals Removal From Industrial Wastewater. Environ Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Vervaeren, H.; Desmet, S.; Boon, N. Bioflocculation of microalgae and bacteria combined with flue gas to improve sewage treatment. New Biotechnol. 2011, 29, 23–31. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Biology of microalgae. In Microalgae in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2018; pp. 23–72. [Google Scholar]
- Sidari, R.; Tofalo, R. A comprehensive overview on microalgal-fortified/based food and beverages. Food Rev. Int. 2019, 35, 778–805. [Google Scholar] [CrossRef]
- Zarekarizi, A.; Hoffmann, L.; Burritt, D. Approaches for the sustainable production of fucoxanthin, a xanthophyll with potential health benefits. J. Appl. Phycol. 2019, 31, 281–299. [Google Scholar] [CrossRef]
- Fu, W.; Nelson, D.R.; Yi, Z.; Xu, M.; Khraiwesh, B.; Jijakli, K.; Chaiboonchoe, A.; Alzahmi, A.; Al-Khairy, D.; Brynjolfsson, S.; et al. Bioactive compounds from microalgae: Current development and prospects. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 54, pp. 199–225. [Google Scholar]
- Vaz, S.; Vieira Costa, J.A. Biologically active metabolites synthesized by microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar]
- Marjanović, B.; Benković, M.; Jurina, T.; Sokač Cvetnić, T.; Valinger, D.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Bioactive compounds from Spirulina spp.—Nutritional value, extraction, and application in the food industry. Separations 2024, 11, 257. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Mendes, A.R.; Spínola, M.P.; Lordelo, M.; Prates, J.A. Chemical compounds, bioactivities, and applications of Chlorella vulgaris in food, feed and medicine. Appl. Sci. 2023, 14, 10810. [Google Scholar] [CrossRef]
- Eakpetch, P. Maximising the Nutritional Value of Microalgae (Chlamydomonas reinhardtii) Through Cultivation and Downstream Bioprocessing. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2019. Available online: https://eprints.nottingham.ac.uk/57061/ (accessed on 30 March 2025).
- Darwish, R.; Gedi, M.A.; Akepach, P.; Assaye, H.; Zaky, A.S.; Gray, D.A. Chlamydomonas reinhardtii is a potential food supplement with the capacity to outperform Chlorella and Spirulina. Appl. Sci. 2019, 10, 6736. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Tertychnaya, T.N.; Manzhesov, V.I.; Andrianov, E.A.; Yakovleva, S.F. New aspects of application of microalgae Dunaliella salina in the formula of enriched bread. IOP Conf. Ser. Earth Environ. Sci. 2020, 422, 012021. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef]
- Rebolloso-Fuentes, M.M.; Navarro-Pérez, A.; García-Camacho, F.; Ramos-Miras, J.J.; Guil-Guerrero, J.L. Biomass nutrient profiles of the microalga Nannochloropsis. J. Agric. Food Chem. 2001, 49, 2966–2972. [Google Scholar] [CrossRef]
- Gundersen, E.; Jakobsen, J.; Holdt, S.L.; Jacobsen, C. Nannochloropsis oceanica as a source of bioactive compounds: Mapping the effects of cultivation conditions on biomass productivity and composition using response surface methodology. Mar. Drugs 2024, 22, 505. [Google Scholar] [CrossRef]
- Martínez, R.; García-Beltrán, A.; Kapravelou, G.; Mesas, C.; Cabeza, L.; Perazzoli, G.; Guarnizo, P.; Rodríguez-López, A.; Vallejo, R.A.; Galisteo, M.; et al. In vivo nutritional assessment of the microalga Nannochloropsis gaditana and evaluation of the antioxidant and antiproliferative capacity of its functional extracts. Mar. Drugs 2022, 20, 318. [Google Scholar] [CrossRef]
- Oslan, S.N.; Tan, J.S.; Oslan, S.N.; Matanjun, P.; Mokhtar, R.A.; Shapawi, R.; Huda, N. Haematococcus pluvialis as a potential source of astaxanthin with diverse applications in industrial sectors: Current research and future directions. Molecules 2020, 26, 6470. [Google Scholar] [CrossRef]
- Wu, J.Y.; Tso, R.; Teo, H.S.; Haldar, S. The utility of algae as sources of high value nutritional ingredients, particularly for alternative/complementary proteins to improve human health. Front. Nutr. 2023, 10, 1277343. [Google Scholar] [CrossRef]
- da Silva Gorgônio, C.M.; Aranda, D.A.; Couri, S. Morphological and chemical aspects of Chlorella pyrenoidosa, Dunaliella tertiolecta, Isochrysis galbana and Tetraselmis gracilis microalgae. Nat. Sci. 2013, 5, 783–791. [Google Scholar]
- Lu, X.; Yang, S.; He, Y.; Zhao, W.; Nie, M.; Sun, H. Nutritional value and productivity potential of the marine microalgae Nitzschia laevis, Phaeodactylum tricornutum and Isochrysis galbana. Mar. Drugs 2024, 22, 386. [Google Scholar] [CrossRef]
- Paterson, S.; Gómez-Cortés, P.; Hernández-Ledesma, B. Bioactivity and digestibility of microalgae Tetraselmis sp. and Nannochloropsis sp. as basis of their potential as novel functional foods. Nutrients 2023, 15, 477. [Google Scholar] [CrossRef]
- Fithriani, D.; Melanie, S. Vitamin and mineral content of microalgae Phorpyridium and Chlorella and development prospects for food raw materials in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2022, 1034, 012043. [Google Scholar] [CrossRef]
- Tsvetanova, F.; Yankov, D. Bioactive compounds from red microalgae with therapeutic and nutritional value. Microorganisms 2022, 10, 2290. [Google Scholar] [CrossRef]
- López, C.V.; García, M.D.; Fernández, F.G.; Bustos, C.S.; Chisti, Y.; Sevilla, J.M. Protein measurements of microalgal and cyanobacterial biomass. Bioresour. Technol. 2010, 101, 7587–7591. [Google Scholar] [CrossRef]
- Gong, Y.; Bandara, T.; Huntley, M.; Johnson, Z.I.; Dias, J.; Dahle, D.; Sørensen, M.; Kiron, V. Microalgae Scenedesmus sp. as a potential ingredient in low fishmeal diets for Atlantic salmon (Salmo salar L.). Aquaculture 2019, 501, 455–464. [Google Scholar] [CrossRef]
- Brown, M.R. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1991, 145, 79–99. [Google Scholar] [CrossRef]
- Ebbing, T.; Kopp, L.; Frick, K.; Simon, T.; Würtz, B.; Pfannstiel, J.; Bischoff, S.C.; Tovar, G.E. Exploring Phaeodactylum tricornutum for nutraceuticals: Cultivation techniques and neurotoxin risk assessment. Mar. Drugs 2025, 23, 58. [Google Scholar] [CrossRef]
- Uzlaşır, T.; Selli, S.; Kelebek, H. Spirulina platensis and Phaeodactylum tricornutum as sustainable sources of bioactive compounds: Health implications and applications in the food industry. Future Postharvest Food 2024, 1, 34–46. [Google Scholar] [CrossRef]
- Vidé, J.; Virsolvy, A.; Romain, C.; Ramos, J.; Jouy, N.; Richard, S.; Cristol, J.P.; Gaillet, S.; Rouanet, J.M. Dietary Silicon-Enriched Spirulina Improves Early Atherosclerosis Markers in Hamsters on a High-Fat Diet. Nutrients 2015, 31, 1148–1154. [Google Scholar] [CrossRef]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef]
- Sandgruber, F.; Höger, A.L.; Kunze, J.; Schenz, B.; Griehl, C.; Kiehntopf, M.; Kipp, K.; Kühn, J.; Stangl, G.I.; Lorkowski, S.; et al. Impact of Regular Intake of Microalgae on Nutrient Supply and Cardiovascular Risk Factors: Results from the NovAL Intervention Study. Nutrients 2023, 15, 1645. [Google Scholar] [CrossRef]
- Nascimento, T.C.; Cazarin, C.B.B.; Marostica, M.R., Jr.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Microalgae Carotenoids Intake: Influence on Cholesterol Levels, Lipid Peroxidation and Antioxidant Enzymes. Food Res. Int. 2020, 128, 108770. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Trepiana, J.; González-Arceo, M.; Aguirre, L.; Milton-Laskibar, I.; González, M.; Eseberri, I.; Fernández-Quintela, A.; Portillo, M.P. Anti-Obesity Effects of Microalgae. Int. J. Mol. Sci. 2019, 21, 41. [Google Scholar] [CrossRef]
- Parameswari, R.P.; Lakshmi, T. Microalgae as a Potential Therapeutic Drug Candidate for Neurodegenerative Diseases. J. Biotechnol. 2022, 358, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Ahda, M.; Suhendra; Permadi, A. Spirulina platensis Microalgae as High Protein-Based Products for Diabetes Treatment. Food Rev. Int. 2024, 40, 1796–1804. [Google Scholar] [CrossRef]
- Fields, F.J.; Lejzerowicz, F.; Schroeder, D.; Ngoi, S.M.; Tran, M.; McDonald, D.; Jiang, L.; Chang, J.T.; Knight, R.; Mayfield, S. Effects of the Microalgae Chlamydomonas on Gastrointestinal Health. J. Funct. Foods 2020, 65, 103738. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, J.; Yan, B.; Zhang, N.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. Evaluation of Microalgae on Preventing Bone Loss in C57BL/6J Mice Induced by Hindlimb Suspension. Food Front. 2023, 4, 1311–1323. [Google Scholar] [CrossRef]
- Kim, E.A.; Kang, N.; Heo, S.Y.; Oh, J.Y.; Lee, S.H.; Cha, S.H.; Kim, W.K.; Heo, S.J. Antioxidant, Antiviral, and Anti-Inflammatory Activities of Lutein-Enriched Extract of Tetraselmis Species. Mar. Drugs 2023, 21, 369. [Google Scholar] [CrossRef]
- Lee, J.Y.; Wong, C.Y.; Koh, R.Y.; Lim, C.L.; Kok, Y.Y.; Chye, S.M. Natural Bioactive Compounds from Macroalgae and Microalgae for the Treatment of Alzheimer’s Disease: A Review. Yale J. Biol. Med. 2024, 97, 205. [Google Scholar] [CrossRef]
- Olabi, A.G.; Shehata, N.; Sayed, E.T.; Rodriguez, C.; Anyanwu, R.C.; Russell, C.; Abdelkareem, M.A. Role of Microalgae in Achieving Sustainable Development Goals and Circular Economy. Sci. Total Environ. 2023, 854, 158689. [Google Scholar] [CrossRef]
- Alavijeh, R.S.; Karimi, K.; Wijffels, R.H.; van Den Berg, C.; Eppink, M. Combined Bead Milling and Enzymatic Hydrolysis for Efficient Fractionation of Lipids, Proteins, and Carbohydrates of Chlorella vulgaris Microalgae. Bioresour. Technol. 2020, 309, 123321. [Google Scholar] [CrossRef]
- Bernaerts, T.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The Potential of Microalgae and Their Biopolymers as Structuring Ingredients in Food: A Review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef]
- Qi, J.; Kim, S.M. Characterization and Immunomodulatory Activities of Polysaccharides Extracted from Green Alga Chlorella ellipsoidea. Int. J. Biol. Macromol. 2017, 95, 106–114. [Google Scholar] [CrossRef]
- Chen, P.B.; Wang, H.C.; Liu, Y.W.; Lin, S.H.; Chou, H.N.; Sheen, L.Y. Immunomodulatory Activities of Polysaccharides from Chlorella pyrenoidosa in a Mouse Model of Parkinson’s Disease. J. Funct. Foods 2014, 11, 103–113. [Google Scholar] [CrossRef]
- Albar, S.A. Dietary Omega-6/Omega-3 Polyunsaturated Fatty Acid (PUFA) and Omega-3 Are Associated with General and Abdominal Obesity in Adults: UK National Diet and Nutritional Survey. Cureus 2022, 14, e30542. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Aflalo, C.; Bernard, O. Microalgal Lipids: A Review of Lipids Potential and Quantification for 95 Phytoplankton Species. Biomass Bioenergy 2021, 150, 106108. [Google Scholar] [CrossRef]
- Katiyar, R.; Arora, A. Health Promoting Functional Lipids from Microalgae Pool: A Review. Algal Res. 2020, 46, 101800. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.S. Omega-3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits—A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.C.; Barrio, R.J. Producing Omega-3 Polyunsaturated Fatty Acids: A Review of Sustainable Sources and Future Trends for the EPA and DHA Market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Gonzalez-Soto, M.; Mutch, D.M. Diet Regulation of Long-Chain PUFA Synthesis: Role of Macronutrients, Micronutrients, and Polyphenols on Δ-5/Δ-6 Desaturases and Elongases 2/5. Adv. Nutr. 2021, 12, 980–994. [Google Scholar] [CrossRef]
- Barry, A.R.; Dixon, D.L. Omega-3 Fatty Acids for the Prevention of Atherosclerotic Cardiovascular Disease. Pharmacotherapy 2021, 41, 1056–1065. [Google Scholar] [CrossRef]
- Yorek, M.A. The Potential Role of Fatty Acids in Treating Diabetic Neuropathy. Curr. Diabetes Rep. 2018, 18, 86. [Google Scholar] [CrossRef]
- Devers, P.M.; Brown, W.M. Fatty Acid Profiling. In Textbook of Natural Medicine, 5th ed.; Pizzorno, J.E., Murray, M.T., Eds.; Churchill Livingstone: London, UK, 2020; pp. 127–133.e2. [Google Scholar]
- Shanab, S.M.; Hafez, R.M.; Fouad, A.S. A Review on Algae and Plants as Potential Sources of Arachidonic Acid. J. Adv. Res. 2018, 11, 3–13. [Google Scholar] [CrossRef]
- Chaves, H.; Singh, R.B.; Khan, S.; Wilczynska, A.; Takahashi, T. High Omega-6/Omega-3 Fatty Acid Ratio Diets and Risk of Noncommunicable Diseases: Is the Tissue the Main Issue? In The Role of Functional Food Security in Global Health; Academic Press: Cambridge, MA, USA, 2019; pp. 217–259. [Google Scholar]
- Sun, K.; Meesapyodsuk, D.; Qiu, X. Biosynthetic Mechanisms of Omega-3 Polyunsaturated Fatty Acids in Microalgae. J. Biol. Chem. 2024, 300, 9. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kate, B.N.; Banerjee, U.C. Bioactive Compounds from Cyanobacteria and Microalgae: An Overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef]
- Zvi, C.; Colin, R. Single Cell Oils, Microbial and Algal Oils; AOCS Press: Urbana, IL, USA, 2010. [Google Scholar]
- Xia, Y.; Zhang, Y.T.; Sun, J.Y.; Huang, H.; Zhao, Q.; Ren, L.J. Strategies for Enhancing Eicosapentaenoic Acid Production: From Fermentation to Metabolic Engineering. Algal Res. 2020, 51, 102038. [Google Scholar] [CrossRef]
- Pereira, A.M.; Lisboa, C.R.; Costa, J.A.V. High Protein Ingredients of Microalgal Origin: Obtainment and Functional Properties. Innov. Food Sci. Emerg. Technol. 2018, 47, 187–194. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; Coimbra, J.S.D.R.; Leite, M.D.O.; Albino, L.F.T.; Martins, M.A. Microalgae Proteins: Production, Separation, Isolation, Quantification, and Application in Food and Feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. [Google Scholar] [CrossRef]
- Fasolin, L.H.; Pereira, R.N.; Pinheiro, A.C.; Martins, J.T.; Andrade, C.C.P.; Ramos, O.L.; Vicente, A.A. Emergent Food Proteins–Towards Sustainability, Health and Innovation. Food Res. Int. 2019, 125, 108586. [Google Scholar] [CrossRef]
- Grossmann, L.; Hinrichs, J.; Weiss, J. Solubility and Aggregation Behavior of Protein Fractions from the Heterotrophically Cultivated Microalga Chlorella protothecoides. Food Res. Int. 2019, 116, 283–290. [Google Scholar] [CrossRef]
- Callejo-López, J.A.; Ramírez, M.; Cantero, D.; Bolívar, J. Versatile Method to Obtain Protein-and/or Amino Acid-Enriched Extracts from Fresh Biomass of Recalcitrant Microalgae Without Mechanical Pretreatment. Algal Res. 2020, 50, 102010. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Lee, S.Y.; Zhu, L.; Show, P.L. Enhanced Microalgal Protein Extraction and Purification Using Sustainable Microwave-Assisted Multiphase Partitioning Technique. Chem. Eng. J. 2019, 367, 1–8. [Google Scholar] [CrossRef]
- Buchmann, L.; Bertsch, P.; Böcker, L.; Krähenmann, U.; Fischer, P.; Mathys, A. Adsorption Kinetics and Foaming Properties of Soluble Microalgae Fractions at the Air/Water Interface. Food Hydrocoll. 2019, 97, 105182. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L.; Hosokawa, M.; Miyashita, K. Effect of Spirulina Lipids on High-Fat and High-Sucrose Diet Induced Obesity and Hepatic Lipid Accumulation in C57BL/6J Mice. J. Funct. Foods 2020, 65, 103741. [Google Scholar] [CrossRef]
- Garcia, E.S.; Van Leeuwen, J.; Safi, C.; Sijtsma, L.; Eppink, M.H.; Wijffels, R.H.; van den Berg, C. Selective and Energy Efficient Extraction of Functional Proteins from Microalgae for Food Applications. Bioresour. Technol. 2018, 268, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Phong, W.N.; Show, P.L.; Ling, T.C.; Juan, J.C.; Ng, E.P.; Chang, J.S. Mild Cell Disruption Methods for Bio-Functional Proteins Recovery from Microalgae—Recent Developments and Future Perspectives. Algal Res. 2018, 31, 506–516. [Google Scholar] [CrossRef]
- Koyande, A.K.; Tanzil, V.; Dharan, H.M.; Subramaniam, M.; Robert, R.N.; Lau, P.L.; Khoiroh, I.; Show, P.L. Integration of Osmotic Shock-Assisted Liquid Biphasic System for Protein Extraction from Microalgae Chlorella vulgaris. Biochem. Eng. J. 2020, 157, 107532. [Google Scholar]
- Ejike, C.E.; Collins, S.A.; Balasuriya, N.; Swanson, A.K.; Mason, B.; Udenigwe, C.C. Prospects of Microalgae Proteins in Producing Peptide-Based Functional Foods for Promoting Cardiovascular Health. Trends Food Sci. Technol. 2017, 59, 30–36. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Basheer, S.; Huo, S.; Zhu, F.; Qian, J.; Xu, L.; Cui, F.; Zou, B. Microalgae in Human Health and Medicine. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Cham, Switzerland, 2020; pp. 149–174. [Google Scholar]
- Molina, A.K.; Corrêa, R.C.; Prieto, M.A.; Pereira, C.; Barros, L. Bioactive Natural Pigments’ Extraction, Isolation, and Stability in Food Applications. Molecules 2023, 28, 1200. [Google Scholar] [CrossRef]
- Renganathan, P.; Puente, E.O.R.; Sukhanova, N.V.; Gaysina, L.A. Hydroponics with Microalgae and Cyanobacteria: Emerging Trends and Opportunities in Modern Agriculture. BioTech 2024, 13, 27. [Google Scholar] [CrossRef]
- McGee, D.; Archer, L.; Fleming, G.T.; Gillespie, E.; Touzet, N. The Effect of Nutrient and Phytohormone Supplementation on the Growth, Pigment Yields, and Biochemical Composition of Newly Isolated Microalgae. Process Biochem. 2020, 92, 61–68. [Google Scholar] [CrossRef]
- Camacho, F.; Macedo, A.; Malcata, F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef]
- Matos, Â.P. The Impact of Microalgae in Food Science and Technology. J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Z.; Ding, Y.; Geng, Y.; Li, Y. Enhancing the Production of Astaxanthin by Mixotrophic Cultivation of Haematococcus pluvialis in Open Raceway Ponds. Aquacult. Int. 2020, 28, 625–638. [Google Scholar] [CrossRef]
- Prabakaran, G.; Sampathkumar, P.; Kavisri, M.; Moovendhan, M. Extraction and Characterization of Phycocyanin from Spirulina platensis and Evaluation of Its Anticancer, Antidiabetic, and Anti-Inflammatory Effect. Int. J. Biol. Macromol. 2020, 153, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, D.P.; Menegol, T.; Rech, R.; Mercali, G.D.; Marczak, L.D.F. Carotenoid and Lipid Extraction from Heterochlorella luteoviridis Using Moderate Electric Field and Ethanol. Process Biochem. 2016, 51, 1636–1643. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, Characterization, and Antioxidant Activity of Fucoxanthin from the Marine Diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
- Ma, P.; Huang, R.; Jiang, J.; Ding, Y.; Li, T.; Ou, Y. Potential Use of C-Phycocyanin in Non-Alcoholic Fatty Liver Disease. Biochem. Biophys. Res. Commun. 2020, 526, 906–912. [Google Scholar] [CrossRef]
- Sui, Y.; Mazzucchi, L.; Acharya, P.; Xu, Y.; Morgan, G.; Harvey, P.J. A Comparison of β-Carotene, Phytoene and Amino Acids Production in Dunaliella salina DF 15 (CCAP 19/41) and Dunaliella salina CCAP 19/30 Using Different Light Wavelengths. Foods 2021, 10, 2824. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Nazih, H.; Bard, J.M. Microalgae in Human Health: Interest as a Functional Food. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 211–226. [Google Scholar]
- Sheikhzadeh, N.; Tayefi-Nasrabadi, H.; Khani Oushani, A.; Najafi Enferadi, M.H. Effects of Haematococcus pluvialis Supplementation on Antioxidant System and Metabolism in Rainbow Trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2012, 38, 413–419. [Google Scholar] [CrossRef]
- Zisis, F.; Kyriakaki, P.; Satolias, F.F.; Mavrommatis, A.; Simitzis, P.E.; Pappas, A.C.; Surai, P.F.; Tsiplakou, E. The Effect of Dietary Inclusion of Microalgae Schizochytrium spp. on Ewes’ Milk Quality and Oxidative Status. Foods 2022, 11, 2950. [Google Scholar] [CrossRef]
- Dhandwal, A.; Bashir, O.; Malik, T.; Salve, R.V.; Dash, K.K.; Amin, T.; Shams, R.; Wani, A.W.; Shah, Y.A. Sustainable microalgal biomass as a potential functional food and its applications in the food industry: A comprehensive review. Environ. Sci. Pollut. Res. 2024, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lousada Falcão, R.; Pinheiro, V.; Ribeiro, C.; Sousa, I.; Raymundo, A.; Nunes, M.C. Nutritional improvement of fresh cheese with microalga Chlorella vulgaris: Impact on composition, structure and sensory acceptance. Food Technol. Biotechnol. 2023, 61, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Çelekli, A.; Özbal, B.; Bozkurt, H. Challenges in functional food products with the incorporation of some microalgae. Foods 2024, 13, 725. [Google Scholar] [CrossRef]
- Souza Celente, G.; Rizzetti, T.M.; Sui, Y.; de Souza Schneider, R.D.C. Potential use of microalga Dunaliella salina for bioproducts with industrial relevance. Biomass Bioenergy 2022, 167, 106647. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Wang, W.; Chen, Z.; Li, C.; Wu, H.; Wu, H.; Xiang, W. A novel three-step extraction strategy for high-value products from red algae Porphyridium purpureum. Foods 2021, 10, 2164. [Google Scholar] [CrossRef]
- Moser, G.A.O.; Barrera-Alba, J.J.; Ortega, M.J.; Alves-de-Souza, C.; Bartual, A. Comparative characterization of three Tetraselmis chui (Chlorophyta) strains as sources of nutraceuticals. J. Appl. Phycol. 2022, 34, 821–835. [Google Scholar] [CrossRef]
- Nova, P.; Martins, A.P.; Teixeira, C.; Abreu, H.; Silva, J.G.; Silva, A.M.; Freitas, A.C.; Gomes, A.M. Foods with microalgae and seaweeds fostering consumers’ health: A review on scientific and market innovations. J. Appl. Phycol. 2020, 32, 1789–1802. [Google Scholar] [CrossRef]
- Gouveia, L.; Batista, A.P.; Miranda, A.; Empis, J.; Raymundo, A. Chlorella vulgaris biomass used as a coloring source in traditional butter cookies. Innov. Food Sci. Emerg. Technol. 2007, 8, 433–436. [Google Scholar] [CrossRef]
- Qazi, W.M.; Ballance, S.; Kousoulaki, K.; Uhlen, A.K.; Kleinegris, D.M.; Skjånes, K.; Rieder, A. Protein enrichment of wheat bread with microalgae: Microchloropsis gaditana, Tetraselmis chui, and Chlorella vulgaris. Foods 2021, 10, 3078. [Google Scholar] [CrossRef]
- Lucas, B.F.; de Morais, M.G.; Santos, T.D.; Costa, J.A.V. Spirulina for snack enrichment: Nutritional, physical, and sensory evaluations. LWT 2018, 90, 270–276. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Abdo, S.M.; Hussein, A.M. Microalgae Dunaliella salina for use as a food supplement to improve pasta quality. Int. J. Pharm. Sci. Rev. Res. 2017, 46, 45–51. [Google Scholar]
- Matos, J.; Afonso, C.; Cardoso, C.; Serralheiro, M.L.; Bandarra, N.M. Yogurt enriched with Isochrysis galbana: An innovative functional food. Foods 2021, 10, 1458. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.; Thorpe, G.; Winstanley, L.; Abdelhamid, A.S.; Hooper, L. Omega-3, omega-6, and total dietary polyunsaturated fat on cancer incidence: Systematic review and meta-analysis of randomized trials. Br. J. Cancer 2020, 122, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: Systematic review and meta-analysis of randomized controlled trials. BMJ 2019, 366, l4697. [Google Scholar] [CrossRef]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega-3 and omega-6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef]
- Abdelhamid, A.; Hooper, L.; Sivakaran, R.; Hayhoe, R.P.; Welch, A. The relationship between omega-3, omega-6 and total polyunsaturated fat and musculoskeletal health and functional status in adults: A systematic review and meta-analysis of RCTs. Calcif. Tissue Int. 2019, 105, 353–372. [Google Scholar] [CrossRef]
- Babuskin, S.; Krishnan, K.R.; Saravana Babu, P.A.; Sivarajan, M.; Sukumar, M. Functional foods enriched with marine microalga Nannochloropsis oculata as a source of ω-3 fatty acids. Food Technol. Biotechnol. 2014, 52, 292–299. [Google Scholar]
- Valencia, I.; Ansorena, D.; Astiasarán, I. Development of dry fermented sausages rich in docosahexaenoic acid with oil from the microalgae Schizochytrium sp.: Influence on nutritional properties, sensorial quality and oxidation stability. Food Chem. 2007, 104, 1087–1096. [Google Scholar] [CrossRef]
- Chee, C.P.; Djordjevic, D.; Faraji, H.; Decker, E.A.; Hollender, R.; McClements, D.J.; Peterson, D.G.; Roberts, R.F.; Coupland, J.N. Sensory properties of vanilla and strawberry flavored ice cream supplemented with omega-3 fatty acids. J. Dairy Sci. 2007, 90, 1089–1095. [Google Scholar]
- Ścieszka, S.; Klewicka, E. Algae in food: A general review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3538–3547. [Google Scholar] [CrossRef]
- Tohamy, M.M.; Ali, M.A.; Shaaban, H.A.G.; Mohamad, A.G.; Hasanain, A.M. Production of functional spreadable processed cheese using Chlorella vulgaris. Acta Sci. Pol. Technol. Aliment. 2018, 17, 347–358. [Google Scholar] [PubMed]
- Hanjabam, M.D.; Zynudheen, A.A.; Ninan, G.; Panda, S. Seaweed as an ingredient for nutritional improvement of fish jerky. J. Food Process. Preserv. 2017, 41, e12845. [Google Scholar] [CrossRef]
- Robertson, R.C.; Mateo, M.R.G.; O’Grady, M.N.; Guihéneuf, F.; Stengel, D.B.; Ross, R.P.; Fitzgerald, G.F.; Kerry, J.P.; Stanton, C. An assessment of the techno-functional and sensory properties of yoghurt fortified with a lipid extract from the microalga Pavlova lutheri. Innov. Food Sci. Emerg. Technol. 2016, 37, 237–246. [Google Scholar] [CrossRef]
- Lane, K.E.; Li, W.; Smith, C.; Derbyshire, E. The bioavailability of an omega-3-rich algal oil is improved by nanoemulsion technology using yogurt as a food vehicle. Int. J. Food Sci. Technol. 2014, 49, 1264–1271. [Google Scholar] [CrossRef]
- Ak, B.; Avsaroglu, E.; Isik, O.; Özyurt, G.; Kafkas, E.; Etyemez, M. Nutritional and physicochemical characteristics of bread enriched with microalgae Spirulina platensis. Int. J. Eng. Res. Appl. 2016, 6, 37–42. [Google Scholar]
- Hyun-Jae, C. Quality characteristics of white bread added with Chlorella powder. Korean J. Food Preserv. 2006, 13, 465–471. [Google Scholar]
- Uribe-Wandurraga, Z.N.; Igual, M.; Reino-Moyón, J.; García-Segovia, P.; Martínez-Monzó, J. Effect of microalgae (Arthrospira platensis and Chlorella vulgaris) addition on 3D printed cookies. Food Biophys. 2021, 16, 27–39. [Google Scholar] [CrossRef]
- Hossain, A.M.; Brennan, M.A.; Mason, S.L.; Guo, X.; Zeng, X.A.; Brennan, C.S. The effect of astaxanthin-rich microalgae Haematococcus pluvialis and wholemeal flours incorporation in improving the physical and functional properties of cookies. Foods 2017, 6, 57. [Google Scholar] [CrossRef]
- Şahin, O.I. Functional and sensorial properties of cookies enriched with Spirulina and Dunaliella biomass. J. Food Sci. Technol. 2020, 57, 3639–3646. [Google Scholar] [CrossRef]
- Bosnea, L.; Terpou, A.; Pappa, E.; Kondyli, E.; Mataragas, M.; Markou, G.; Katsaros, G. Incorporation of Spirulina platensis on traditional Greek soft cheese with respect to its nutritional and sensory perspectives. Proceedings 2020, 5, 46. [Google Scholar]
- Özbal, B.; Çelekli, A.; Gün, D.; Bozkurt, H. Effect of Arthrospira platensis incorporation on nutritional and sensory attributes of white chocolate. Int. J. Gastron. Food Sci. 2022, 28, 100544. [Google Scholar] [CrossRef]
- Jorgensen, J. Fermentation Process for Producing Alcoholic Beverages from Microalgae. U.S. Patent 3,389,998, 25 June 1968. [Google Scholar]
- Raymundo, A.; Gouveia, L.; Batista, A.P.; Empis, J.; Sousa, I. Fat mimetic capacity of Chlorella vulgaris biomass in oil-in-water food emulsions stabilized by pea protein. Food Res. Int. 2005, 38, 961–965. [Google Scholar] [CrossRef]
- Gouveia, L.; Raymundo, A.; Batista, A.P.; Sousa, I.; Empis, J. Chlorella vulgaris and Haematococcus pluvialis biomass as colouring and antioxidant in food emulsions. Eur. Food Res. Technol. 2006, 222, 362–367. [Google Scholar] [CrossRef]
- Gouveia, L.; Batista, A.P.; Raymundo, A.; Bandarra, N. Spirulina maxima and Diacronema vlkianum microalgae in vegetable gelled desserts. Nutr. Food Sci. 2008, 38, 492–501. [Google Scholar] [CrossRef]
- Batista, A.P.; Nunes, M.C.; Fradinho, P.; Gouveia, L.; Sousa, I.; Raymundo, A.; Franco, J.M. Novel foods with microalgal ingredients–Effect of gel setting conditions on the linear viscoelasticity of Spirulina and Haematococcus gels. J. Food Eng. 2012, 110, 182–189. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations; Food and Drug Administration (FDA): Silver Spring, MD, USA, 2003; Available online: https://www.gmp-compliance.org/files/guidemgr/ucm154838.pdf (accessed on 14 February 2025).
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Řezanka, T.; Lukavský, J.; Nedbalová, L.; Sigler, K. Production of structured triacylglycerols from microalgae. Phytochemistry 2014, 104, 95–104. [Google Scholar] [CrossRef]
- Kagan, M.L.; West, A.L.; Zante, C.; Calder, P.C. Acute appearance of fatty acids in human plasma—A comparative study between polar-lipid rich oil from the microalgae Nannochloropsis oculata and krill oil in healthy young males. Lipids Health Dis. 2013, 12, 102. [Google Scholar] [CrossRef]
- Hulatt, C.J.; Wijffels, R.H.; Bolla, S.; Kiron, V. Production of fatty acids and protein by Nannochloropsis in flat-plate photobioreactors. PLoS ONE 2017, 12, e0170440. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Comprehen. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef]
- Mello-Sampayo, C.; Paterna, A.; Polizzi, A.; Duarte, D.; Batista, I.; Pinto, R.; Gonçalves, P.; Raymundo, A.; Batista, A.P.; Gouveia, L.; et al. Evaluation of marine microalga Diacronema vlkianum biomass fatty acid assimilation in Wistar rats. Molecules 2017, 22, 1097. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, X.; Fan, C.; Wu, W.; Zhang, W.; Wang, Y. Health benefits, food applications, and sustainability of microalgae-derived N-3 PUFA. Foods 2022, 11, 1883. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, K.M.; Abrams, S.A.; Heird, W.C. Docosahexaenoic acid (DHA) supplementation of orange juice increases plasma phospholipid DHA content of children. J. Am. Diet. Assoc. 2009, 109, 708–712. [Google Scholar] [CrossRef]
- Fabregas, J.; Herrero, C. Vitamin content of four marine microalgae. Potential use as source of vitamins in nutrition. J. Ind. Microbiol. Biotechnol. 1990, 5, 259–263. [Google Scholar] [CrossRef]
- Škrovánková, S. Seaweed vitamins as nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 357–369. [Google Scholar]
- Goh, L.P.; Loh, S.P.; Fatimah, M.Y.; Perumal, K. Bioaccessibility of carotenoids and tocopherols in marine microalgae, Nannochloropsis sp. and Chaetoceros sp. Malays. J. Nutr. 2009, 15, 77–86. [Google Scholar]
- Failla, M.L.; Huo, T.; Thakkar, S.K. In vitro screening of relative bioaccessibility of carotenoids from foods. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 200–203. [Google Scholar]
- Gille, A.; Trautmann, A.; Posten, C.; Briviba, K. Bioaccessibility of carotenoids from Chlorella vulgaris and Chlamydomonas reinhardtii. Int. J. Food Sci. Nutr. 2016, 67, 507–513. [Google Scholar] [CrossRef]
- Tang, G.; Suter, P.M. Vitamin A, nutrition, and health values of algae: Spirulina, Chlorella, and Dunaliella. J. Pharm. Nutr. Sci. 2011, 1, 111–118. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M.; Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis: Effects on astaxanthin recovery and implications for bioavailability. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar] [CrossRef]
- Okada, Y.; Ishikura, M.; Maoka, T. Bioavailability of astaxanthin in Haematococcus algal extract: The effects of timing of diet and smoking habits. Biosci. Biotechnol. Biochem. 2009, 73, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lim, H.R.; Khoo, K.S.; Ng, H.S.; Cai, Y.; Wang, J.; Chan, A.T.Y.; Show, P.L. An integration study of microalgae bioactive retention: From microalgae biomass to microalgae bioactives nanoparticle. Food Chem. Toxicol. 2021, 158, 112607. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Hwang, K.T.; Hwang, S.; Choi, K.Y.; Park, Y.J.; Choi, J.H.; Truong, T.Q.; Kim, S.M. Nanoencapsulation enhances the bioavailability of fucoxanthin in microalga Phaeodactylum tricornutum extract. Food Chem. 2023, 403, 134348. [Google Scholar] [CrossRef]
- Djihad, N.; Oukil, N.F.; Hamid, S.; Attia, A.; Petronilho, S. Microencapsulation of a green microalga (Chlorella vulgaris) by complex coacervation for its valuation as a feasible ingredient in pear snacks. Algal Res. 2024, 83, 103727. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, X.; McClements, D.J.; Cao, Y.; Xiao, H. Enhancement of phytochemical bioaccessibility from plant-based foods using excipient emulsions: Impact of lipid type on carotenoid solubilization from spinach. Food Funct. 2018, 9, 4352–4365. [Google Scholar] [CrossRef]
- Demarco, M.; de Moraes, J.O.; Matos, Â.P.; Derner, R.B.; de Farias Neves, F.; Tribuzi, G. Digestibility, bioaccessibility, and bioactivity of compounds from algae. Trends Food Sci. Technol. 2022, 121, 114–128. [Google Scholar] [CrossRef]
- Bürck, M.; Lemes, A.C.; Egea, M.B.; Braga, A.R. Exploring the potential and challenges of fermentation in creating foods: A spotlight on microalgae. Fermentation 2024, 10, 649. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T.; Shuit, S.H. Insights into the microalgae cultivation technology and harvesting process for biofuel production: A review. Renew. Sustain. Energy Rev. 2019, 115, 109361. [Google Scholar] [CrossRef]
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Chen, C.; Tang, T.; Shi, Q.; Zhou, Z.; Fan, J. The potential and challenge of microalgae as promising future food sources. Trends Food Sci. Technol. 2022, 126, 99–112. [Google Scholar] [CrossRef]
- Beheshtipour, H.; Mortazavian, A.M.; Haratian, P.; Darani, K.K. Effects of Chlorella vulgaris and Arthrospira platensis addition on viability of probiotic bacteria in yogurt and its biochemical properties. Eur. Food Res. Technol. 2012, 235, 719–728. [Google Scholar] [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Gyenis, B.; Szigeti, J.F.; Ásványi-Molnár, N.; Varga, L. Use of dried microalgal biomasses to stimulate acid production and growth of Lactobacillus plantarum and Enterococcus faecium in milk. Acta Agrar. Kaposv. 2005, 9, 53–59. [Google Scholar]
- Hosseinkhani, N.; McCauley, J.I.; Ralph, P.J. Key challenges for the commercial expansion of ingredients from algae into human food products. Algal Res. 2022, 64, 102696. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as functional ingredients in savory food products: Application to wheat crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef]
- Marco, E.R.; Steffolani, M.E.; Martínez, C.S.; León, A.E. Effects of Spirulina biomass on the technological and nutritional quality of bread wheat pasta. LWT Food Sci. Technol. 2014, 58, 102–108. [Google Scholar] [CrossRef]
- New-Look Microalgae: Newly Approved Chlorella vulgaris Powders Accentuates Ice Creams, Shakes, Cakes and Pasta. Available online: https://www.foodingredientsfirst.com/news/new-look-microalgae-newly-approved-Chlorella-vulgaris-powders-accentuates-ice-creams-shakes-and-pasta.html (accessed on 30 March 2025).
- Li, S.; Ji, L.; Chen, C.; Zhao, S.; Sun, M.; Gao, Z.; Wu, H.; Fan, J. Efficient accumulation of high-value bioactive substances by carbon to nitrogen ratio regulation in marine microalgae Porphyridium purpureum. Bioresour. Technol. 2020, 309, 123362. [Google Scholar] [CrossRef]
- Markou, G.; Ilkiv, B.; Brulé, M.; Antonopoulos, D.; Chakalis, L.; Arapoglou, D.; Chatzipavlidis, I. Methane production through anaerobic digestion of residual microalgal biomass after the extraction of valuable compounds. Biomass Convers. Biorefin. 2022, 12, 419–426. [Google Scholar] [CrossRef]
- Wu, G.; Zhuang, D.; Chew, K.W.; Ling, T.C.; Khoo, K.S.; Van Quyen, D.; Feng, S.; Show, P.L. Current status and future trends in removal, control, and mitigation of algae food safety risks for human consumption. Molecules 2022, 27, 6633. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin, and phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae encapsulation systems for food, pharmaceutical and cosmetics applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-derived pigments for the food industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Barros, L.; Prieto, M.A.; Cassani, L. Recent progress in understanding the impact of food processing and storage on the structure–activity relationship of fucoxanthin. Foods 2023, 12, 3167. [Google Scholar] [CrossRef]
- Shkolnikov Lozober, H.; Okun, Z.; Parvari, G.; Shpigelman, A. The effect of storage and pasteurization (thermal and high-pressure) conditions on the stability of phycocyanobilin and phycobiliproteins. Antioxidants 2023, 12, 568. [Google Scholar] [CrossRef]
- Martínez-Delgado, A.A.; Khandual, S.; Villanueva–Rodríguez, S.J. Chemical stability of astaxanthin integrated into a food matrix: Effects of food processing and methods for preservation. Food Chem. 2017, 225, 23–30. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Muylaert, K.; Eeckhout, M.; Ruyssen, T.; Foubert, I. Influence of drying and storage on lipid and carotenoid stability of the microalga Phaeodactylum tricornutum. J. Agric. Food Chem. 2011, 59, 11063–11069. [Google Scholar] [CrossRef]
- Gheysen, L.; Bernaerts, T.; Bruneel, C.; Goiris, K.; Van Durme, J.; Van Loey, A.; De Cooman, L.; Foubert, I. Impact of processing on n-3 LC-PUFA in model systems enriched with microalgae. Food Chem. 2018, 268, 441–450. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Y.; Fan, C.; Hong, H.; Wu, W.; Zhang, W.; Wang, Y. Production, processing, and protection of microalgal n-3 PUFA-rich oil. Foods 2022, 11, 1215. [Google Scholar] [CrossRef]
- Lane, K.E.; Zhou, Q.; Robinson, S.; Li, W. The composition and oxidative stability of vegetarian omega-3 algal oil nanoemulsions suitable for functional food enrichment. J. Sci. Food Agric. 2020, 100, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Lisboa, C.R.; Santos, T.D.; Costa, J.A. Bioactive stability of microalgal protein hydrolysates under food processing and storage conditions. J. Food Sci. Technol. 2019, 56, 4543–4551. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Liu, H.; Yang, S.; Sun, Z.; Sun, L.; Wang, L. Manufacturing processes, additional nutritional value and versatile food applications of fresh microalgae Spirulina. Front. Nutr. 2024, 11, 1455553. [Google Scholar] [CrossRef]



| Species | Autotropic (g L−1) | Heterotrophic (g L−1) | Mixotrophic (g L−1) | Substrate | Reference |
|---|---|---|---|---|---|
| Arthrospira platensis | 0.51 | 0.39 | 0.80 | Glucose | [39] |
| Botryococcus braunii | 1.14 | 1.75 | 2.46 | Glucose | [38] |
| Chlorella sp. | 0.22 | 0.18 | 0.37 | Glycerol | [40] |
| Chlorella sp. | 1.3 | 2.5 | 2.7 | Glucose | [41] |
| Chlorella sp. | 0.22 | 0.17 | 0.45 | Sucrose | [36] |
| Chlorella minutissima | 0.45 | 1.02 | 0.89 | Glucose | [34] |
| C. minutissima | 0.899 | 0.364 | 0.630 | Acetate | [42] |
| 0.899 | 0.328 | 1.007 | Citrate | ||
| 0.899 | 0.656 | 1.164 | Sucrose | ||
| 0.899 | 0.740 | 2.456 | Glucose | ||
| 0.899 | - | 0.120 | Propionate | ||
| Chlorella protothecoides | 0.52 | 1.10 | 2.67 | Glycerol | [43] |
| Chlorella sorokiniana | 1.70 | 2.78 | 4.57 | Glucose | [44] |
| Chlorella vulgaris | 2.47 | 1.52 | 3.91 | Glucose | [37] |
| C. vulgaris | 1.08 | 0.40 | 2.62 | Cane molasses | [45] |
| Desmodesmus sp. | 1.83 | 0.58 | 0.79 | Glucose | [46] |
| Desmodesmus salina | 0.59 | 0.90 | 1.16 | Glucose | [47] |
| Isochrysis galbana | 0.56 | 0.83 | 0.89 | Glucose | |
| Nannochloropsis oculata | 0.54 | 1.46 | 1.69 | Glucose | |
| Scenedesmus obliquus | 1.1 | 2.2 | 2.3 | Glucose | |
| Micractinium inermum | 5.00 | 0.21 | 5.30 | Glucose and acetate | [48] |
| Microalgae Species | Proteins (% DW) | Carbohydrates (% DW) | Lipids (% DW) | Vitamins | Minerals | Bioactive Compounds | References |
|---|---|---|---|---|---|---|---|
| Arthrospira spp. | 50–70 | 10–20 | 5–10 | B1, B2, B12, E | Fe, Ca, Mg, Zn, P, K | Phycocyanin, C-phycocyanin, flavonoids, phenolic acids, PUFAs (n-3) fatty acids, oleic acid, linolenic acid, palmitoleic acid, β-carotene, lutein, zeaxanthin, tocopherols (vitamin E), neophytadiene, and phytol | [68,69] |
| Chlorella vulgaris | 43–61 | 12–26 | 5–58 | B₁, B₂, B₃, provitamin A | Fe, Ca, Mg | Lutein, beta-carotene, polyphenols, phytosterols, and sulphated polysaccharides | [70,71,72] |
| Chlamydomonas reinhardtii | 41.4–46.9 | 21.5–27.8 | 13.2–24.7 | C, provit-amin A | Ca, Mg, P, K, Fe, Se | Beta-carotene, lutein, retinol, chlorophylls a and b, and polysaccharides | [73,74] |
| Dunaliella salina | 34–57 | 14–33 | 6–14 | A, B12, C, E | Ca, P, Fe | β-Carotene and astaxanthin | [71,75,76] |
| Nannochloropsis sp. | 28.8–40 | 27–37.6 | 18.4–28 | B12, C, E, K | Ca, P, Na, Mg, Zn, Fe | Carotenoids (β-carotene, astaxanthin, canthaxanthin, violaxanthin, and zeaxanthin), polyphenols, and chlorophylls | [77,78,79,80] |
| Haemato-coccus pluvialis | 29–45 | 15–17 | 20–25 | A, E | Fe, Ca, Mg, P | Astaxanthin, lutein, β-carotene, and tocopherols | [81,82] |
| Isochrysis galbana | 27–43.29 | 25.40–34 | 10.95–25.3 | B1, B12, C, E | Fe, Ca, Mg, P | Fucoxanthin and DHA | [82,83,84] |
| Tetraselmis sp. | 13–48 | 6.4–36.2 | 37–60 | A, B1, B2, B6, B12, C, E | Ca, Mg, P, K, Na, S | β-carotene and lutein | [85] |
| Porphyridium cruentum | 28–39 | 40–57 | 5–14 | B1, B2, B6, C, E | K, Ca, P, Mg, Fe, Zn, Se | Sulfated polysaccharides, phycoerythrin, phycocyanin, ARA, and EPA | [71,86,87] |
| Scenedesmus sp. | 31–56 | 10–48 | 5–15 | B1, B2, C, E | K, P, Mg, Fe, Zn | Carotenoids, antioxidants, and phenolic compounds | [71,75,88,89] |
| Phaeodac-tylum tri-cornutum | 29–43.29 | 14.85–40 | 35–45 | B1, B12, C, E | Ca, Fe, Zn | Fucoxanthin, phenolic compounds (4-hydroxybenzaldehyde, ferulic acid, and caffeic acid), and chrysolaminarin | [77,84,90,91,92] |
| Microalgae | Metabolite | Concentration | Bioactive Effect | References |
|---|---|---|---|---|
| Scenedesmus obliquus | Carotenoids | 0.25–2.5 mg·kg−1 body weight | Antioxidant activity, reduction in lipid peroxidation | [96] |
| Haematococcus pluvialis | Astaxanthin | 1.95–2.75% | Antibacterial, anticancer, anti-inflammatory, antioxidative, neuroprotective, antimicrobial | [143] |
| Arthrospira platensis | Phycocyanin | 100–500 μL ml−1 | Anti-inflammation, antidiabetic, anticancer | [144] |
| Heterochlorella luteoviridis | Zeaxanthin | 0.244 mg g−1 | Improved eye health, antidiabetic | [145] |
| Odontella sp. | Fucoxanthin | 5.13 mg g−1 | Antioxidative, anticancer, anti-cholesterol, antidiabetic, antitumor | [146] |
| A. platensis | Phycocyanin | 5 μM | Reduction in fat accumulation in the liver caused by nonalcoholic fatty liver disease | [147] |
| Dunaliella salina | β-carotene | 0.01–15.0 g L−1 | Anticancer, antioxidative, antihypertensive, neuroprotective, protection against macular degeneration, anti-cholesterol | [148] |
| Product | Microalgae | Biomass Addition | Benefits | References |
|---|---|---|---|---|
| Butter cookies | Chlorella vulgaris | 0.5–3.0% (w/w) | Techno-functional properties | [160] |
| Bread | Microchloropsis gaditana Tetraselmis chuii C. vulgaris | - | Protein enrichment, techno-functional properties | [161] |
| Snacks | Arthrospira platensis | 2.6% (w/w) | Enhanced nutritional value (including proteins, fats, and minerals), physical properties (such as expansion ratio, density, firmness, water absorption and solubility indices, structural composition, and color measurements), and sensory qualities (encompassing aroma, appearance, flavor, consistency, overall likeability, and willingness to purchase) | [162] |
| Pasta | Dunaliella salina | 1–3% (w/w) | Improved proteins, fats, ash, minerals (calcium, iron, magnesium, and potassium), pigments (chlorophyll a, chlorophyll b, and carotene), and unsaturated fatty acids | [163] |
| Yogurt | Isochrysis galbana | 2% (w/w) | Enriched ω3 -polyunsaturated fatty acid contents of oleic, linoleic, α-linolenic acid, stearidonic, and docosahexaenoic acids | [164] |
| Cheese | C. vulgaris | 2–4% (m/v) | Improved nutritional profile, including protein and minerals (Mg, P, S, Cu, Zn, Fe, and Mn); improved bioactivity of antioxidant | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade-Bustamante, G.; Martínez-Ruiz, F.E.; Ortega-García, J.; Renganathan, P.; Gaysina, L.A.; Mahendhiran, M.; Puente, E.O.R. Microalgae-Based Functional Foods: A Blue-Green Revolution in Sustainable Nutrition and Health. Appl. Microbiol. 2025, 5, 39. https://doi.org/10.3390/applmicrobiol5020039
Andrade-Bustamante G, Martínez-Ruiz FE, Ortega-García J, Renganathan P, Gaysina LA, Mahendhiran M, Puente EOR. Microalgae-Based Functional Foods: A Blue-Green Revolution in Sustainable Nutrition and Health. Applied Microbiology. 2025; 5(2):39. https://doi.org/10.3390/applmicrobiol5020039
Chicago/Turabian StyleAndrade-Bustamante, Gabriela, Francisco Eleazar Martínez-Ruiz, Jesus Ortega-García, Prabhaharan Renganathan, Lira A. Gaysina, Muhilan Mahendhiran, and Edgar Omar Rueda Puente. 2025. "Microalgae-Based Functional Foods: A Blue-Green Revolution in Sustainable Nutrition and Health" Applied Microbiology 5, no. 2: 39. https://doi.org/10.3390/applmicrobiol5020039
APA StyleAndrade-Bustamante, G., Martínez-Ruiz, F. E., Ortega-García, J., Renganathan, P., Gaysina, L. A., Mahendhiran, M., & Puente, E. O. R. (2025). Microalgae-Based Functional Foods: A Blue-Green Revolution in Sustainable Nutrition and Health. Applied Microbiology, 5(2), 39. https://doi.org/10.3390/applmicrobiol5020039









