Interactions of Laser-Induced Thermal Plume with Liquid–Air Interfaces in Straight-Chain Alcohols
Abstract
1. Introduction
2. Materials and Methods
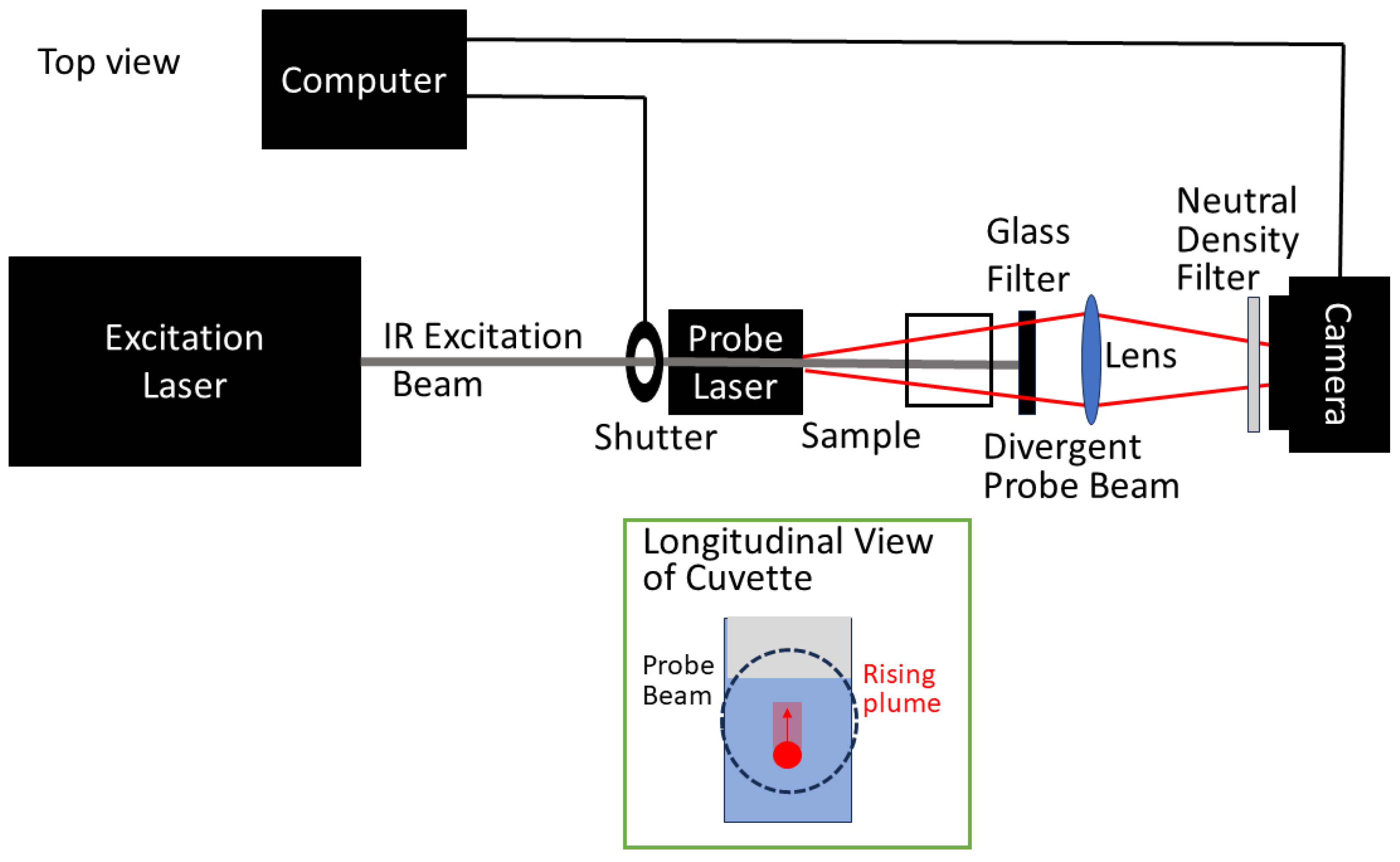
2.1. Experimental Setup
2.2. Sample Preparation
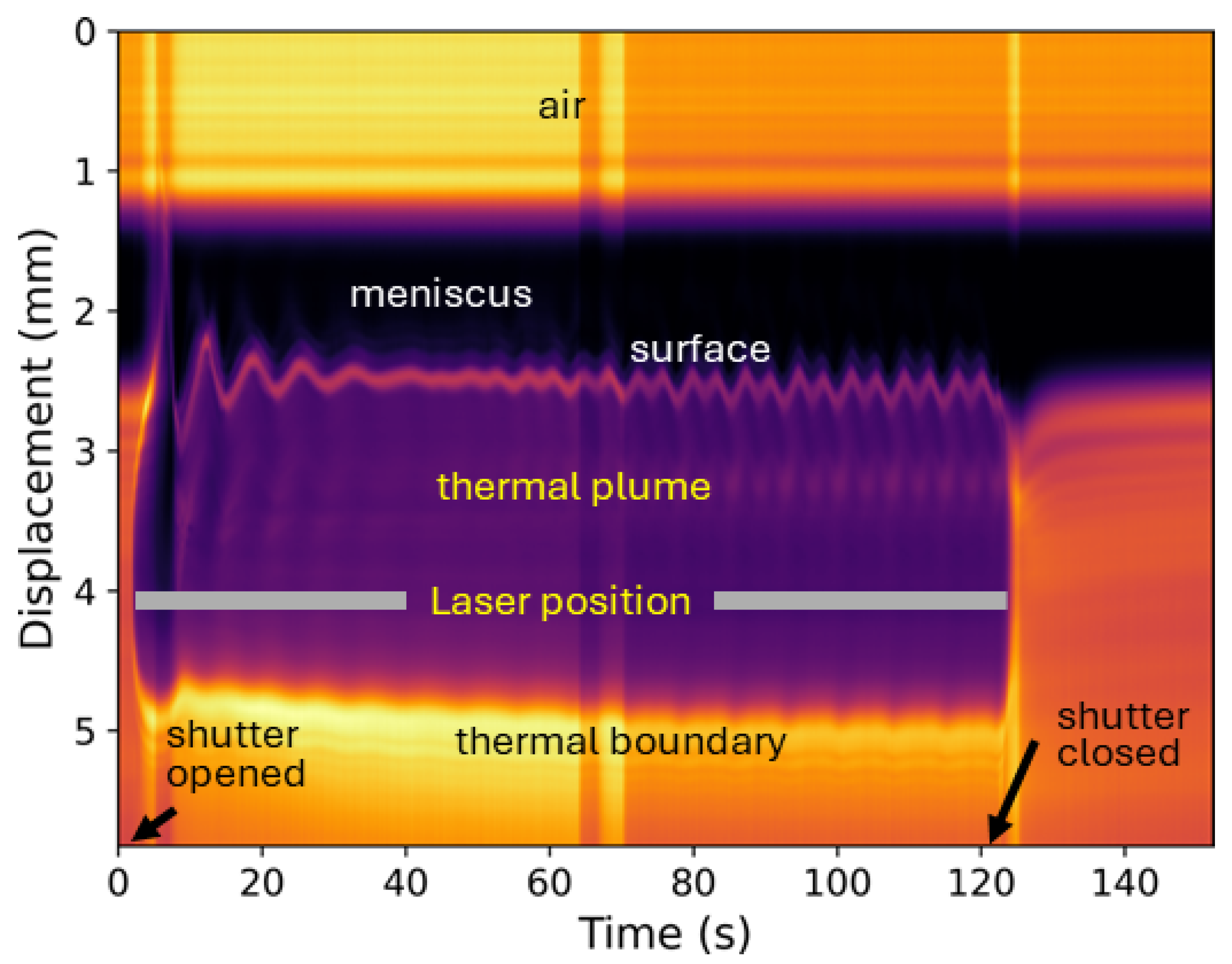
2.3. Data Collection and Analysis
2.4. Comparison with the Experiments of Gouesbet et al. [17,18,19,20]
2.5. Artificial Intelligence
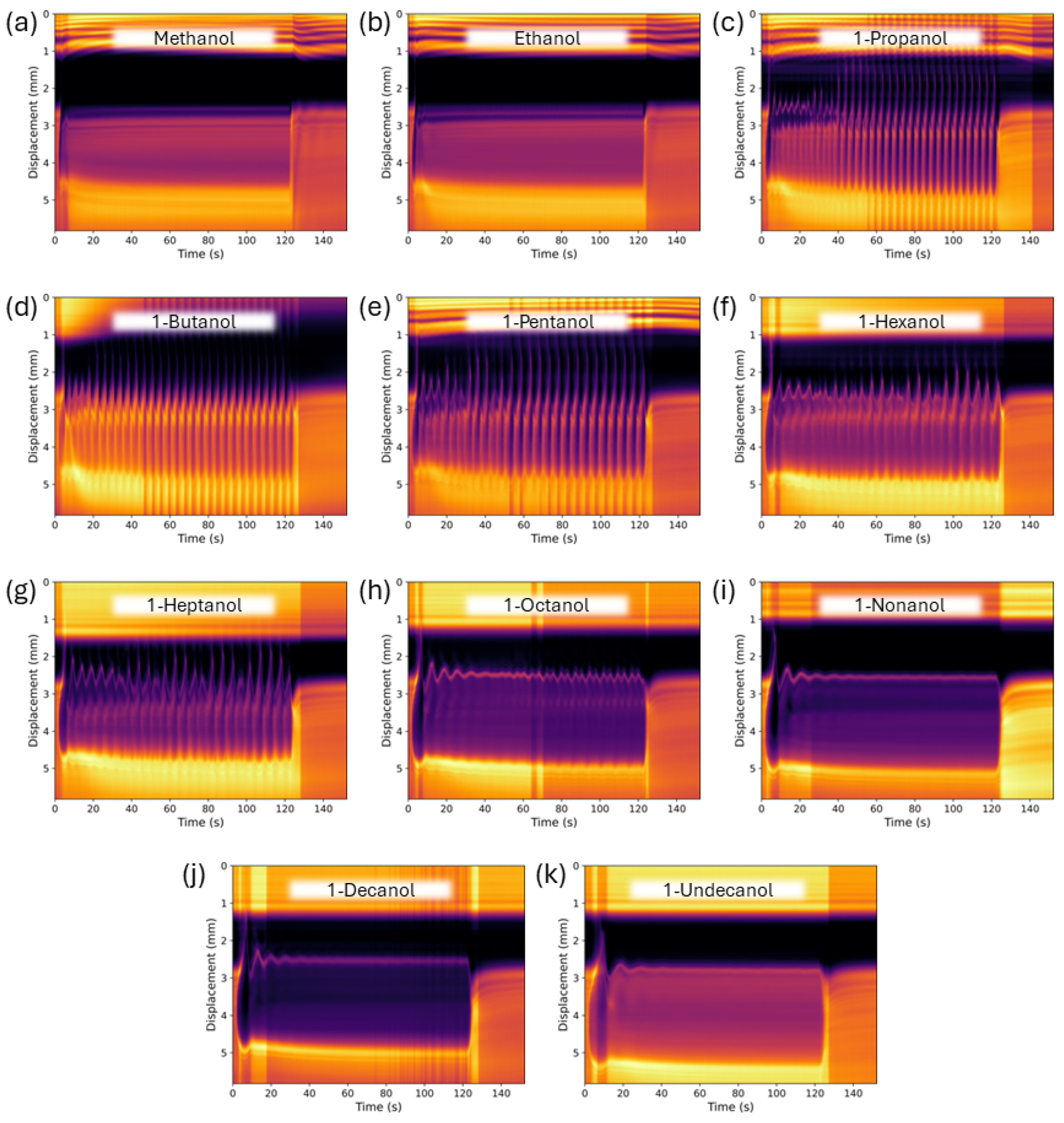
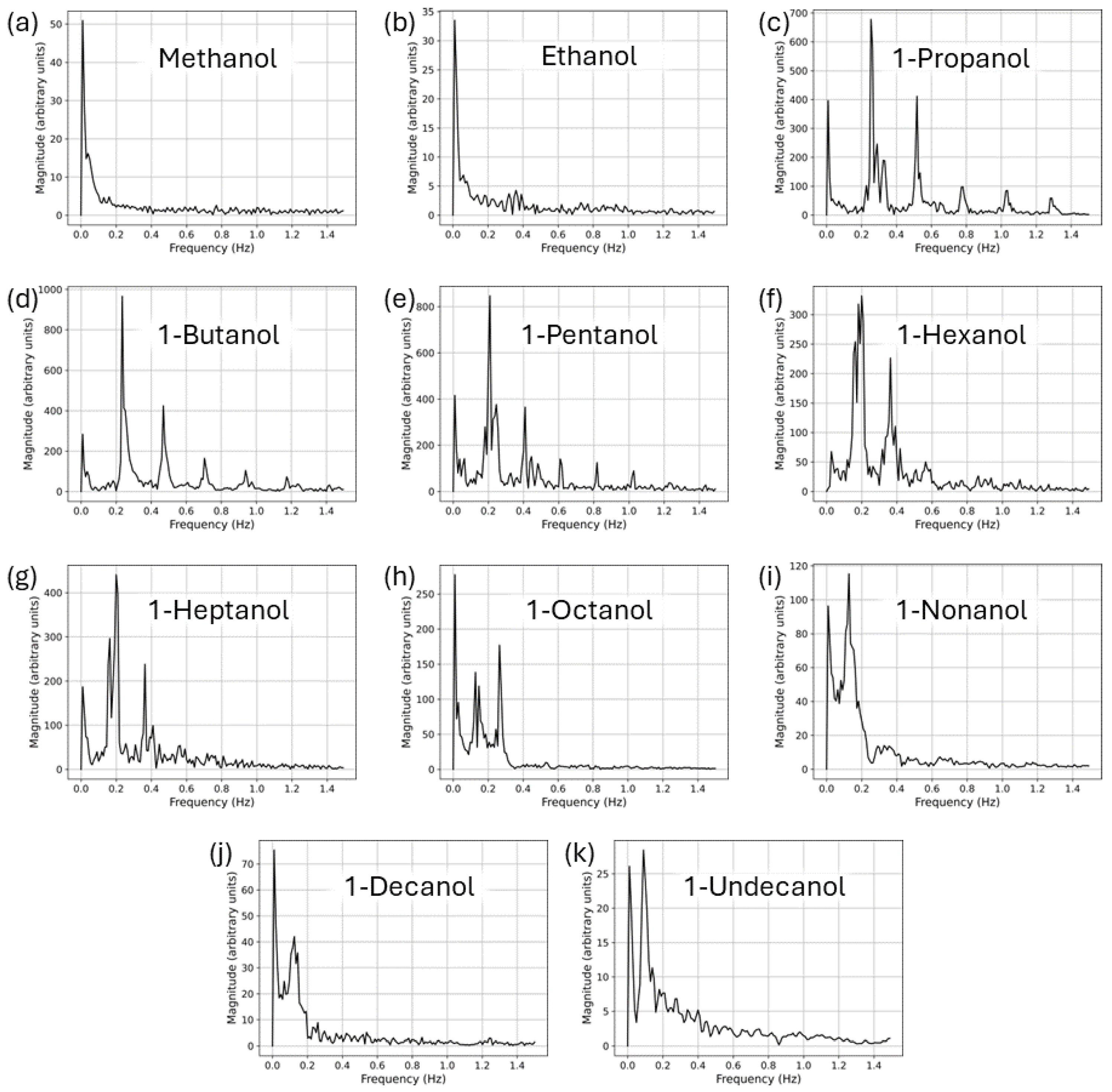
3. Results and Discussion
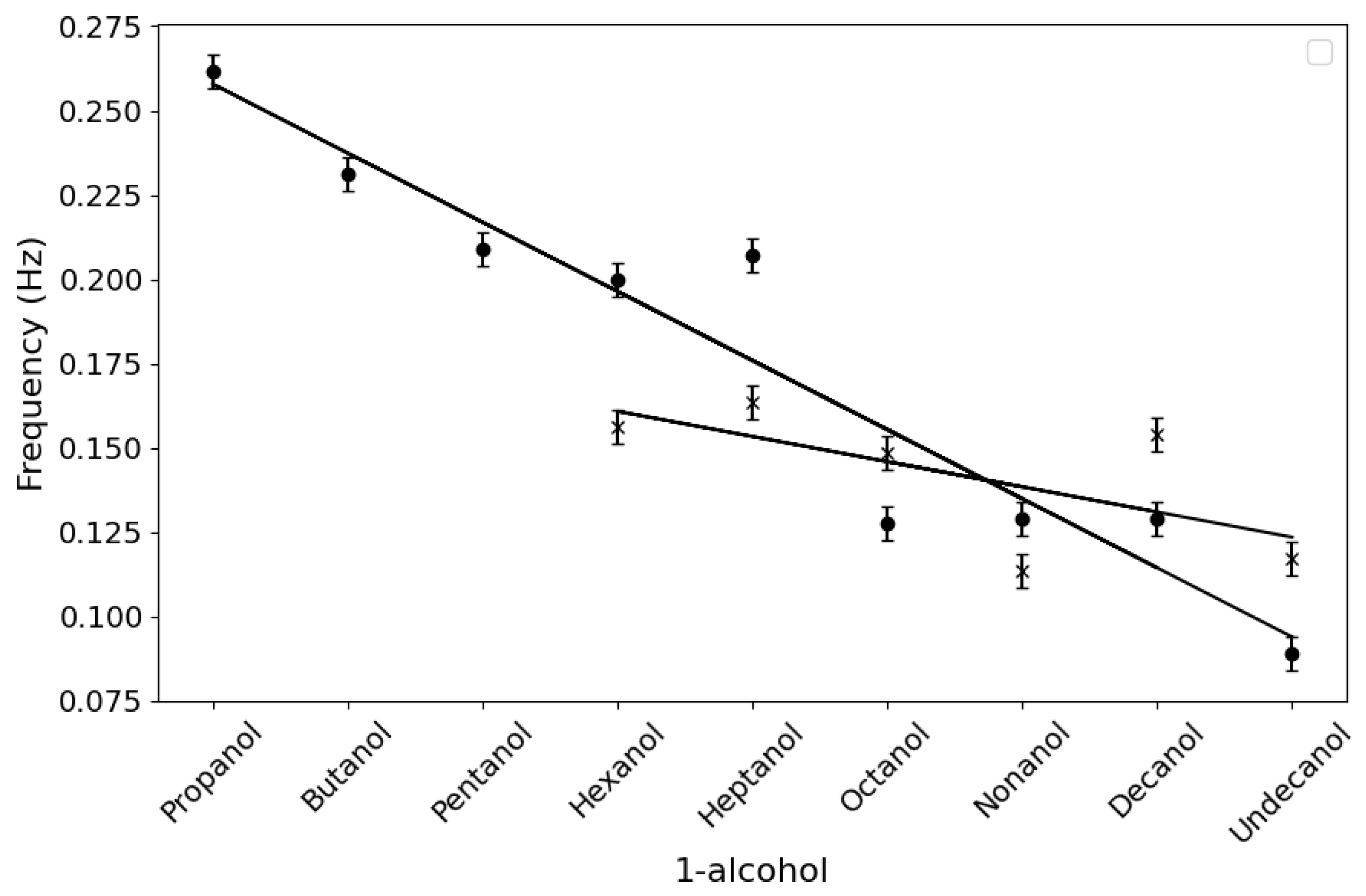
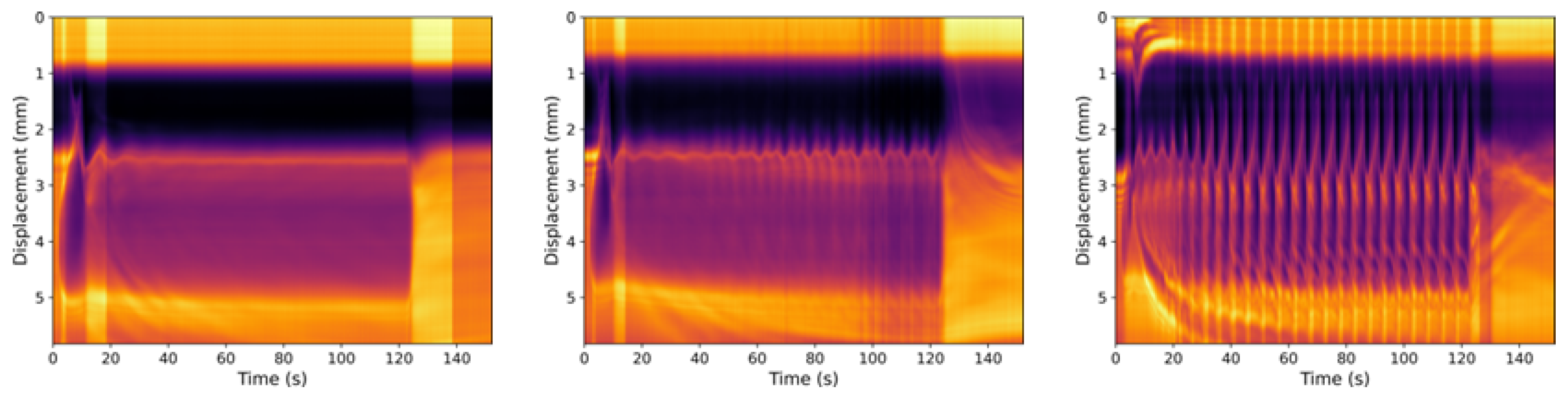
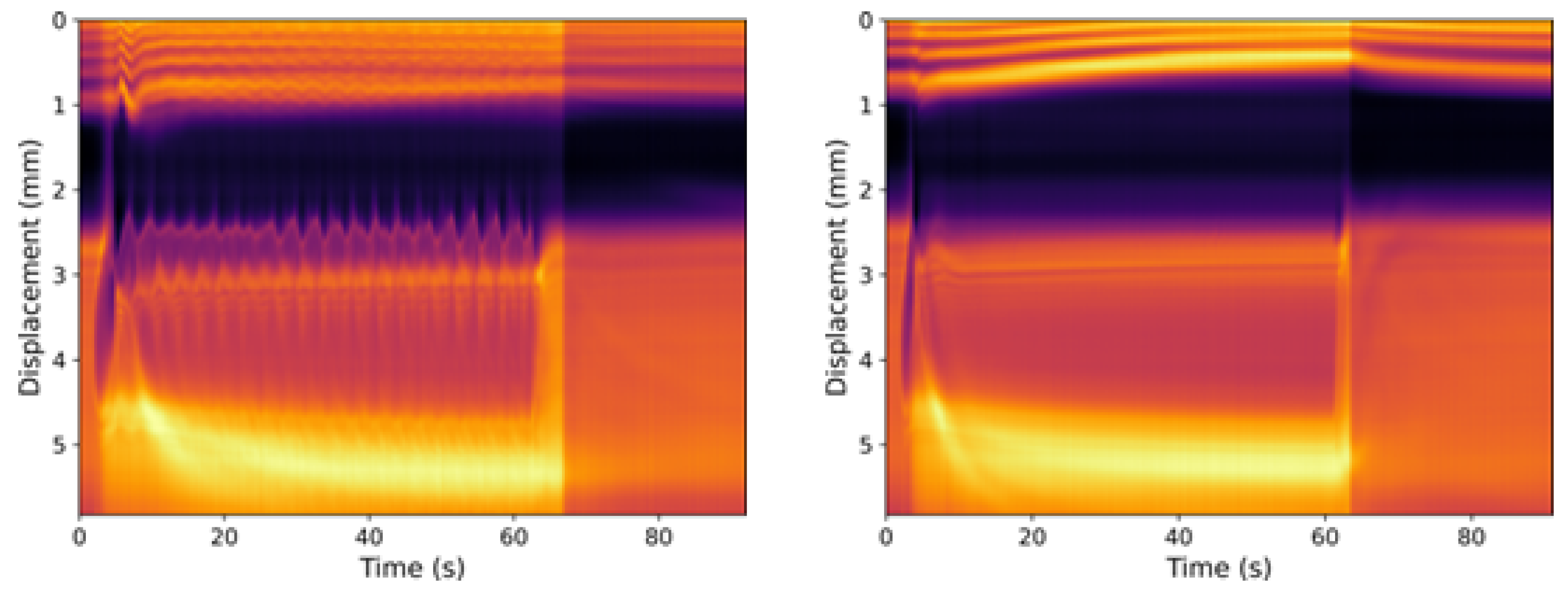
3.1. Neat Straight-Chain Alcohols
3.2. Undecanol–Methanol Mixtures
3.3. Butanol–Methanol Mixtures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, J.P.; Leite, R.C.C.; Moore, R.S.; Porto, S.P.S.; Whinnery, J.R. Long-Transient Effects in Lasers with Inserted Liquid Samples. J. Appl. Phys. 1965, 36, 3–8. [Google Scholar] [CrossRef]
- Singhal, S.; Goswami, D. Thermal Lens Study of NIR Femtosecond Laser-Induced Convection in Alcohols. ACS Omega 2019, 4, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Kumar Rawat, A.; Chakraborty, S.; Kumar Mishra, A.; Goswami, D. Achieving Molecular Distinction in Alcohols with Femtosecond Thermal Lens Spectroscopy. Chem. Phys. 2022, 561, 111596. [Google Scholar] [CrossRef]
- Goswami, D. Intense Femtosecond Optical Pulse Shaping Approaches to Spatiotemporal Control. Front. Chem. 2023, 10, 1006637. [Google Scholar] [CrossRef]
- Rawat, A.K.; Chakraborty, S.; Mishra, A.K.; Goswami, D. Unraveling Molecular Interactions in Binary Liquid Mixtures with Time-Resolved Thermal-Lens-Spectroscopy. J. Mol. Liq. 2021, 336, 116322. [Google Scholar] [CrossRef]
- Singhal, S.; Goswami, D. Unraveling the Molecular Dependence of Femtosecond Laser-Induced Thermal Lens Spectroscopy in Fluids. Analyst 2020, 145, 929–938. [Google Scholar] [CrossRef]
- Khabibullin, V.R.; Usoltseva, L.O.; Galkina, P.A.; Galimova, V.R.; Volkov, D.S.; Mikheev, I.V.; Proskurnin, M.A. Measurement Precision and Thermal and Absorption Properties of Nanostructures in Aqueous Solutions by Transient and Steady-State Thermal-Lens Spectrometry. Physchem 2023, 3, 156–197. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Chen, R.; Zhu, X.; Liao, Q. Simulation on the Dynamic Flow and Heat and Mass Transfer of a Liquid Column Induced by the IR Laser Photothermal Effect Actuated Evaporation in a Microchannel. Int. J. Heat Mass Transf. 2017, 113, 975–983. [Google Scholar] [CrossRef]
- Dominguez Lopez, J.; Gealy, M.W.; Ulness, D.J. Photothermal Imaging of Transient and Steady State Convection Dynamics in Primary Alkanes. Liquids 2023, 3, 371–384. [Google Scholar] [CrossRef]
- Biebighauser, J.; Dominguez Lopez, J.; Strand, K.; Gealy, M.W.; Ulness, D.J. Frustrated-Laser-Induced Thermal Starting Plumes in Fresh and Salt Water. Liquids 2024, 4, 332–351. [Google Scholar] [CrossRef]
- Long, M.E.; Swofford, R.L.; Albrecht, A.C. Thermal Lens Technique: A New Method of Absorption Spectroscopy. Science 1976, 191, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.L.; Swofford, R.L. The Thermal Lens in Absorption Spectroscopy. In Ultrasensitive Laser Spectroscopy; Kliger, D.S., Ed.; Academic Press: New York, NY, USA, 1983. [Google Scholar]
- Fang, H.L.; Swofford, R.L. Highly Excited Vibrational States of Molecules by Thermal Lensing Spectroscopy and the Local Mode Model. II. Normal, Branched, and Cycloalkanes. J. Phys. Chem. 1980, 73, 2607–2617. [Google Scholar] [CrossRef]
- Fang, H.L.; Meister, D.M.; Swofford, R.L. Overtone Spectroscopy of Nonequivalent Methyl C-H Oscillators. Influence of Conformation on Vibrational Overtone Energies. J. Phys. Chem. 1984, 88, 410–416. [Google Scholar] [CrossRef]
- Turner, J.S. Buoyancy Effects in Fluids; Cambridge University Press: Cambridge, UK, 1973. [Google Scholar]
- Jakeman, E.; Pike, E.R.; Vaughan, J.M. Observation of an Oscillating Hydrodynamic Mode in a Laser Scattering Experiment. RRE Newsl. Res. Rev. 1973. [Google Scholar]
- Anthore, R.; Flament, P.; Gouesbet, G.; Rhazi, M.; Weill, M.E. Interaction Between a Laser Beam and Some Liquid Media. Appl. Opt. 1982, 21, 2–4. [Google Scholar] [CrossRef]
- Meunier-Guttin-Cluzel, S.; Maheu, B.; Gouesbet, G. Combined Approaches and Characterizations of Experimental Chaotic Attractors in Thermal Lensing. Physica D 1992, 58, 423–440. [Google Scholar] [CrossRef]
- Gouesbet, G.; Lefort, E. Dynamical States and Bifurcations of a Thermal Lens Using Spectral Analysis. Phys. Rev. A 1988, 37, 4903–4915. [Google Scholar] [CrossRef]
- Weill, M.E.; Rhazi, M.; Gouesbet, G. Experimental Investigation of Oscillatory Phenomena Produced by a Hot Wire Located Near and Below a Free Surface. J. Phys. 1985, 46, 1501–1506. [Google Scholar] [CrossRef]
- Oakes, S.; Anderson, R.W.; Rapelji, A.; Gealy, M.W.; Ulness, D.J. Stable oscillations in laser-induced thermal plume—Surface interaction in several molecular liquids. Physica D, 2024; submitted. [Google Scholar]
- Schlichting, H. Boundary Layer Theory, 7th ed.; McGraw-Hill: New York, NY, USA, 1979. [Google Scholar]
- Rott, N. Unsteady Viscous Flow in the Vicinity of a Stagnation Point. Q. Appl. Math. 1956, 13, 444–451. [Google Scholar] [CrossRef]
- Butt, H.-J.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- ChatGPT 4.0. Available online: https://chatgpt.com/ (accessed on 1 May 2024).
- De Groot, S.R.; Mazur, P. Non-Equilibrium Thermodynamics; Dover Publications: New York, NY, USA, 1984. [Google Scholar]
- Giglio, M.; Vendramini, A. Thermal Lens Effect in a Binary Liquid Mixture: A New Effect. Appl. Phys. Lett. 1974, 25, 555–557. [Google Scholar] [CrossRef]
- Cabrera, H.; Sira, E.; Rahn, K.; García-Sucre, M. A Thermal Lens Model Including the Soret Effect. Appl. Phys. Lett. 2009, 94, 051103. [Google Scholar] [CrossRef]
- Polyakov, P.; Wiegand, S. Investigation of the Soret effect in aqueous and non-aqueous mixtures by the thermal lens technique. Phys. Chem. Chem. Phys. 2009, 11, 864–871. [Google Scholar] [CrossRef] [PubMed][Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, R.W.; Anderson, A.I.; Gealy, M.W.; Ulness, D.J. Interactions of Laser-Induced Thermal Plume with Liquid–Air Interfaces in Straight-Chain Alcohols. Liquids 2024, 4, 732-743. https://doi.org/10.3390/liquids4040041
Anderson RW, Anderson AI, Gealy MW, Ulness DJ. Interactions of Laser-Induced Thermal Plume with Liquid–Air Interfaces in Straight-Chain Alcohols. Liquids. 2024; 4(4):732-743. https://doi.org/10.3390/liquids4040041
Chicago/Turabian StyleAnderson, Reese W., Allison I. Anderson, Mark W. Gealy, and Darin J. Ulness. 2024. "Interactions of Laser-Induced Thermal Plume with Liquid–Air Interfaces in Straight-Chain Alcohols" Liquids 4, no. 4: 732-743. https://doi.org/10.3390/liquids4040041
APA StyleAnderson, R. W., Anderson, A. I., Gealy, M. W., & Ulness, D. J. (2024). Interactions of Laser-Induced Thermal Plume with Liquid–Air Interfaces in Straight-Chain Alcohols. Liquids, 4(4), 732-743. https://doi.org/10.3390/liquids4040041







