Preparation of Antimony Tin Oxide Thin Film Using Green Synthesized Nanoparticles by E-Beam Technique for NO2 Gas Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of ATO Films Using E-Beam Evaporation
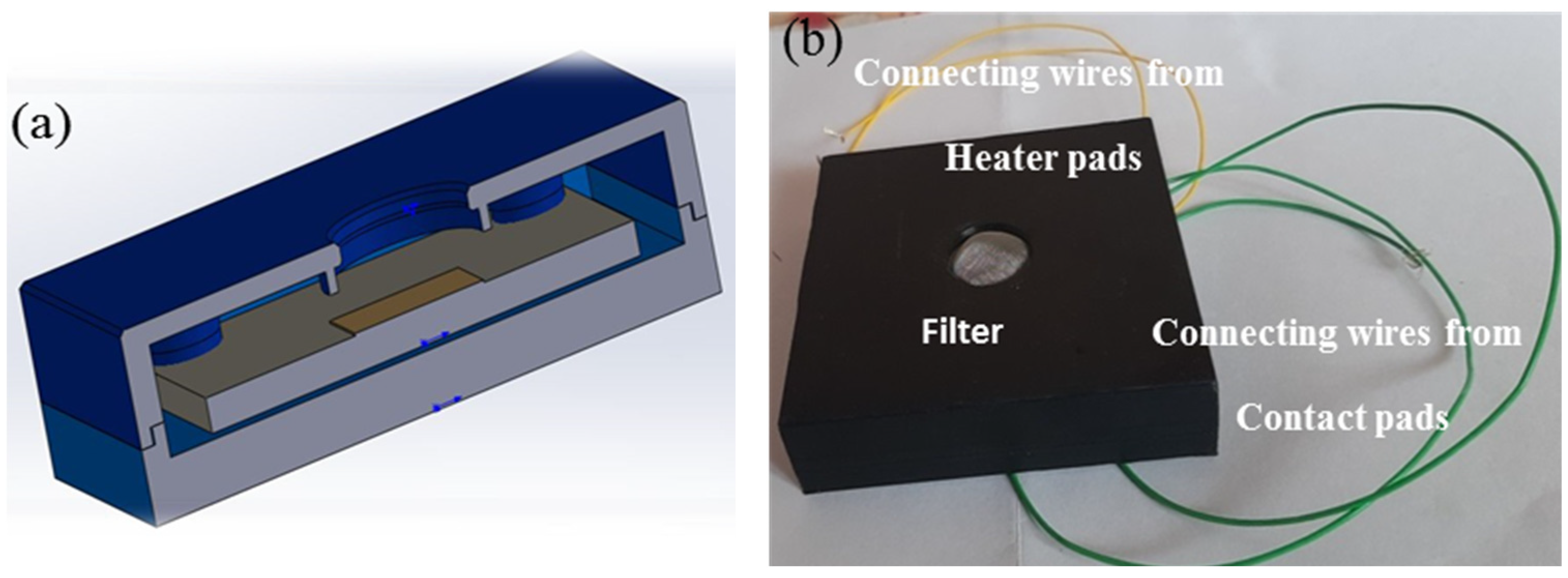
2.2. Fabrication and Packaging of ATO Film-Based NO2 Gas Sensor
2.3. Material Characterization
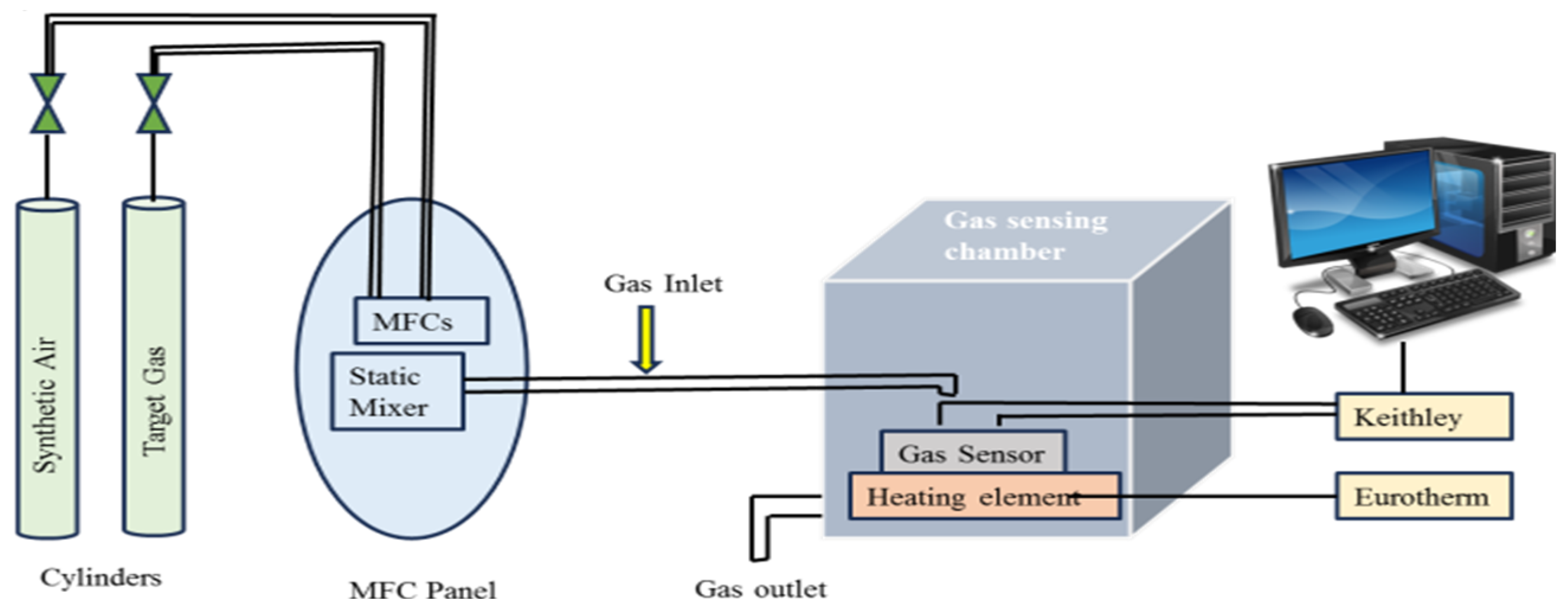
2.4. Gas Sensing Characterization of ATO Thin Films Prepared on IDEs Using Gas Calibration Setup
3. Results and Discussions
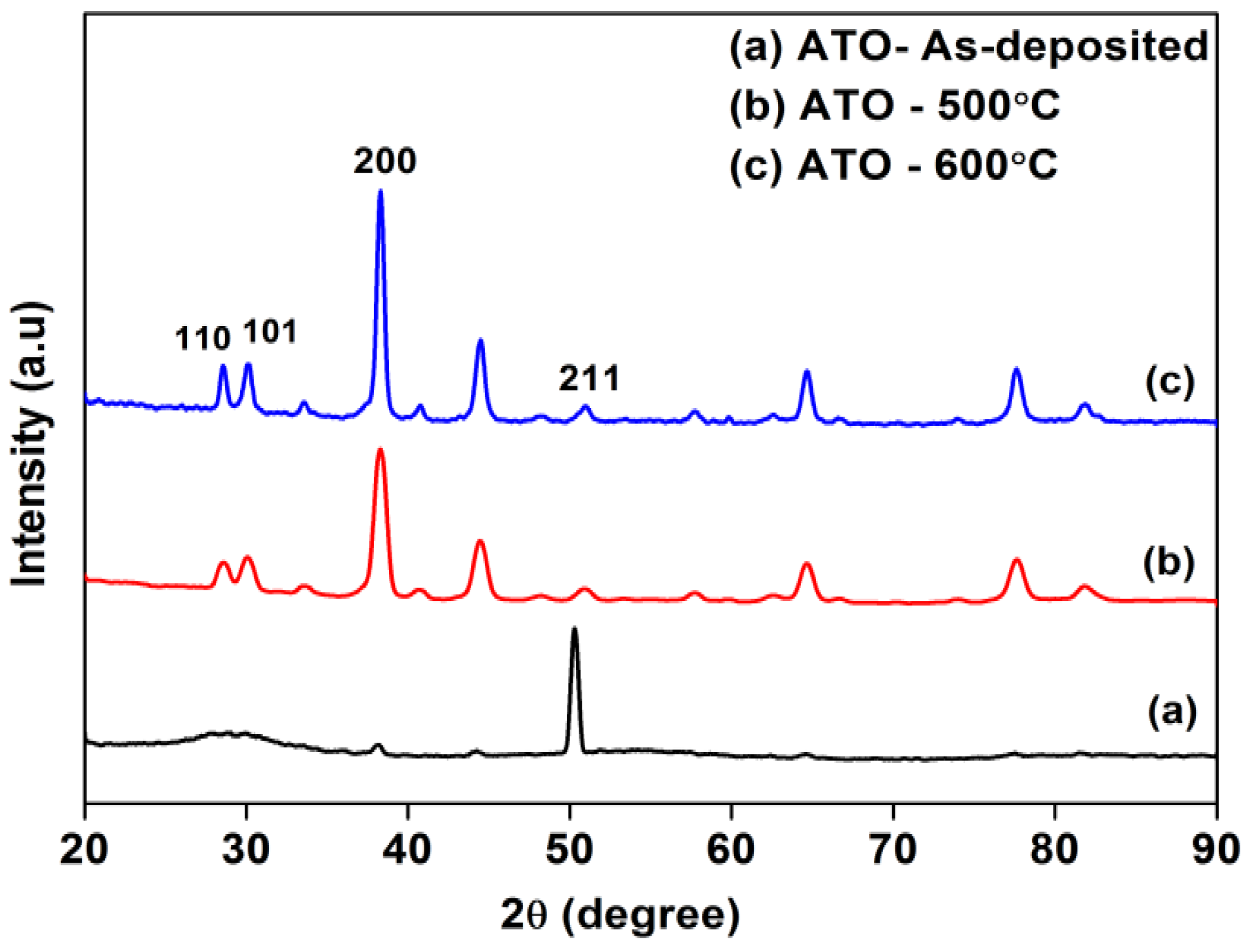
3.1. Structural Analysis
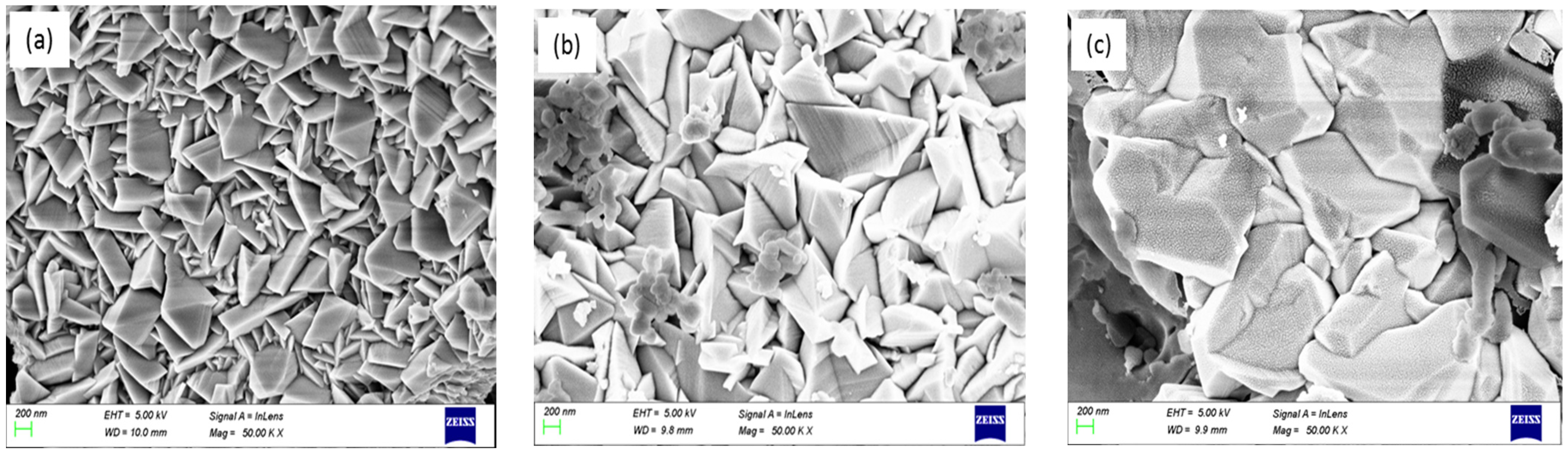
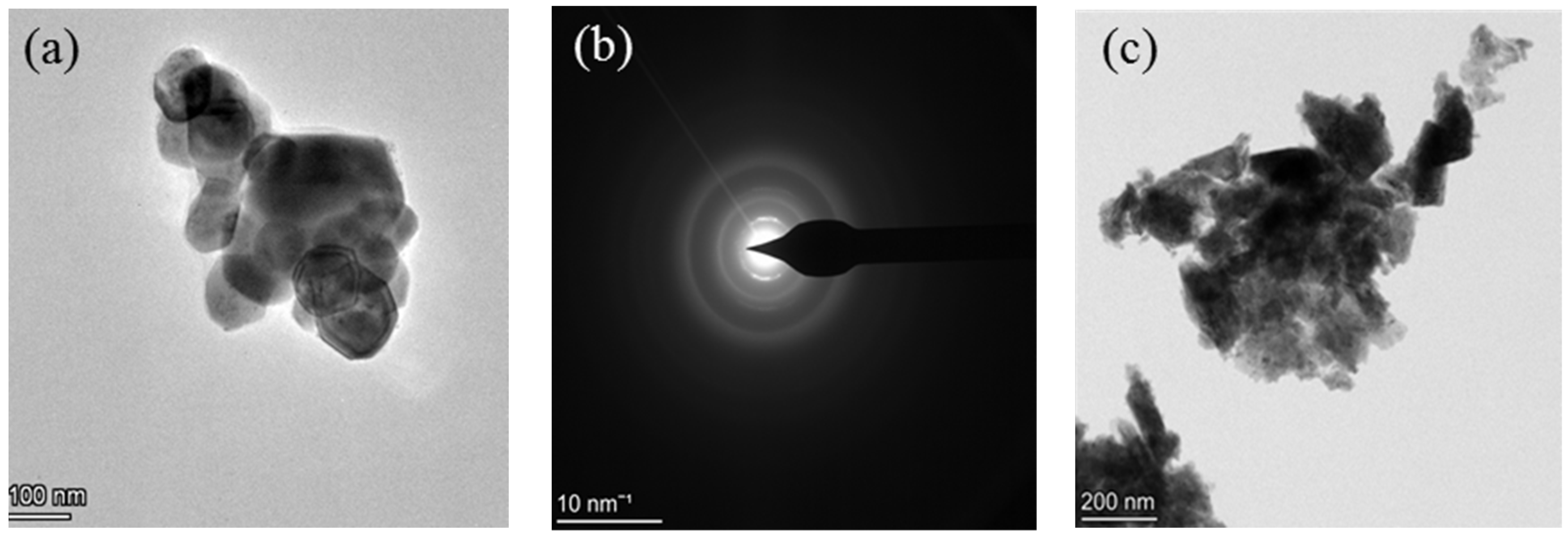
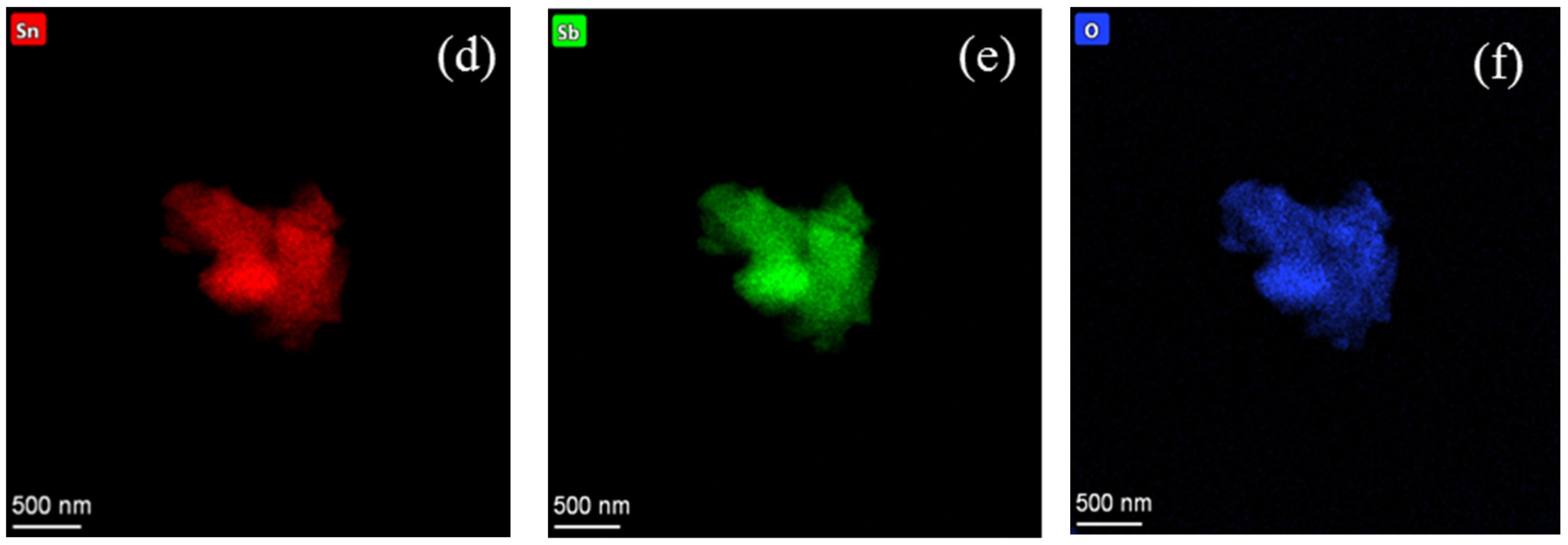
3.2. Surface Morphological Study
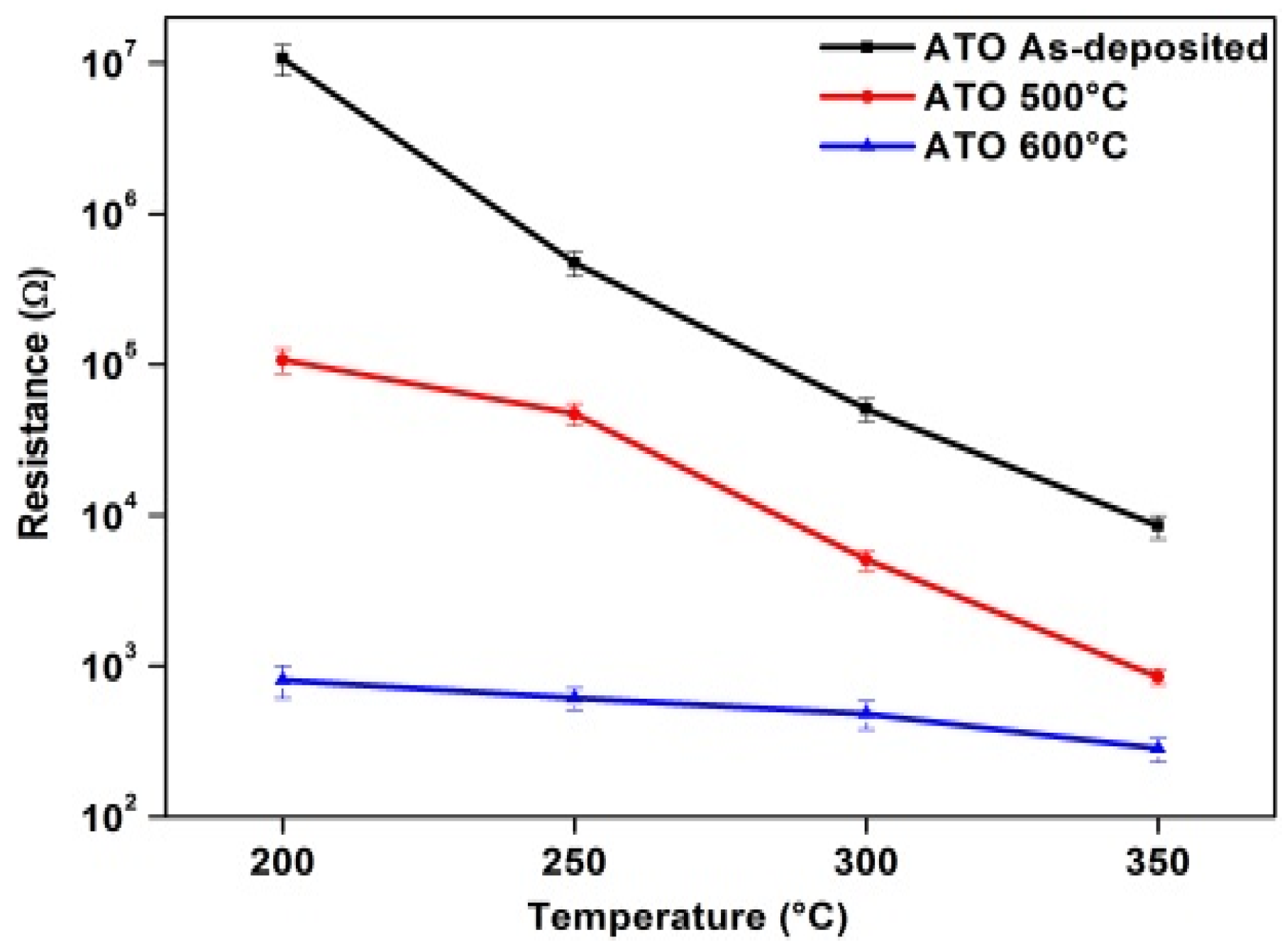
3.3. Electrical Resistance Measurement
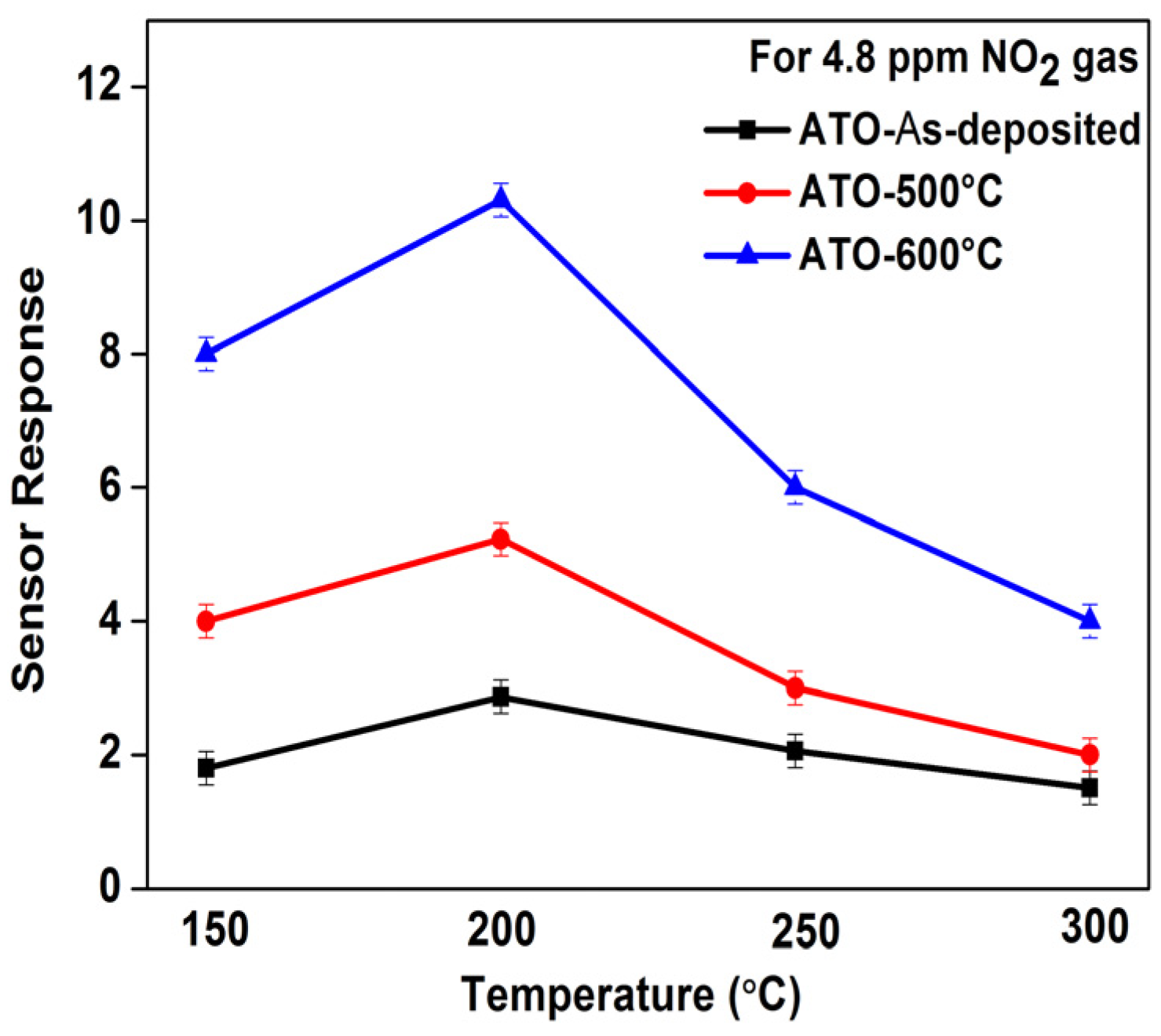
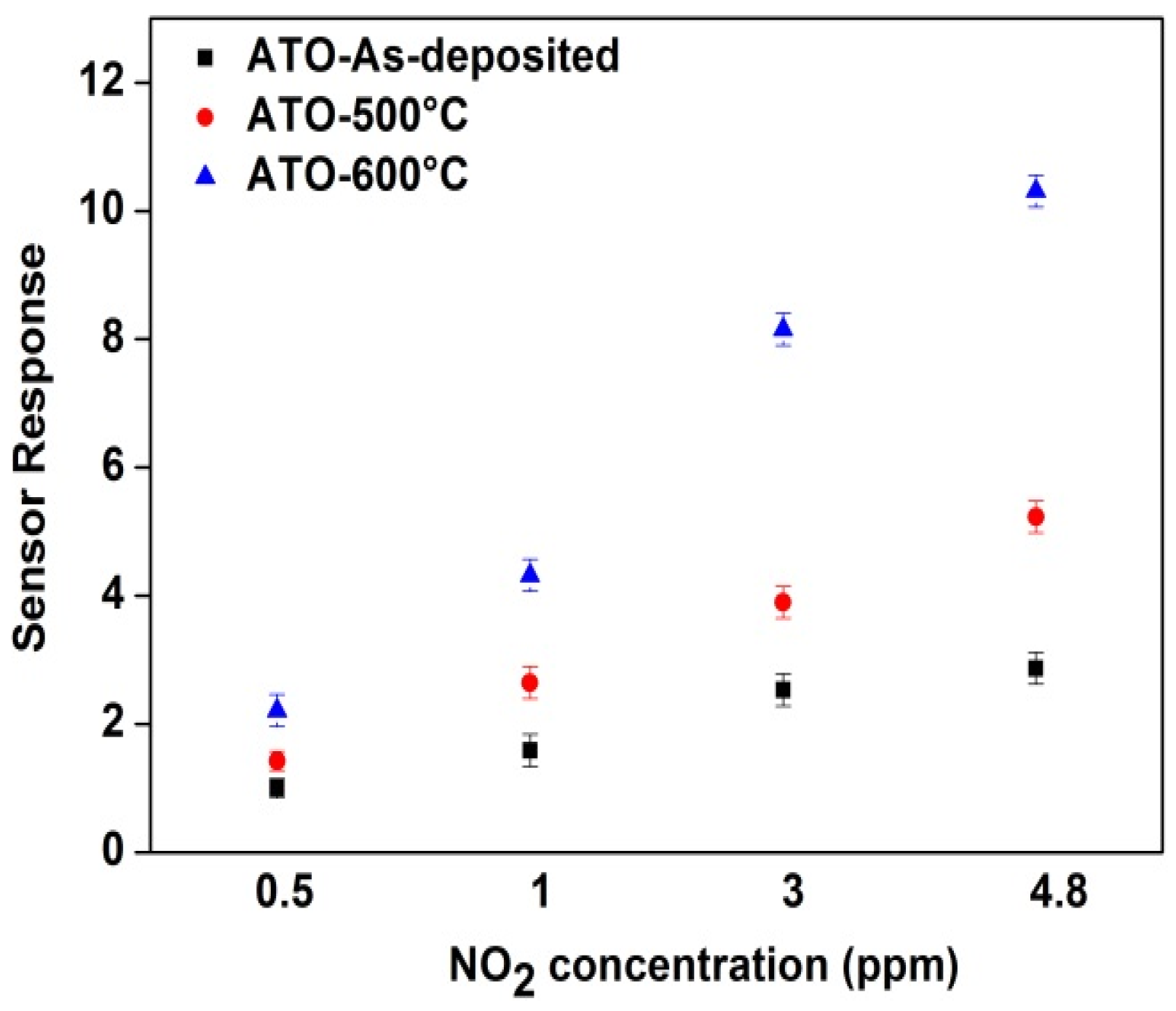
3.4. Gas Sensing Characterization of ATO Films Deposited on Normal IDEs
3.5. Gas Sensing Characterization of Packaged NO2 Gas Sensor
- (i)
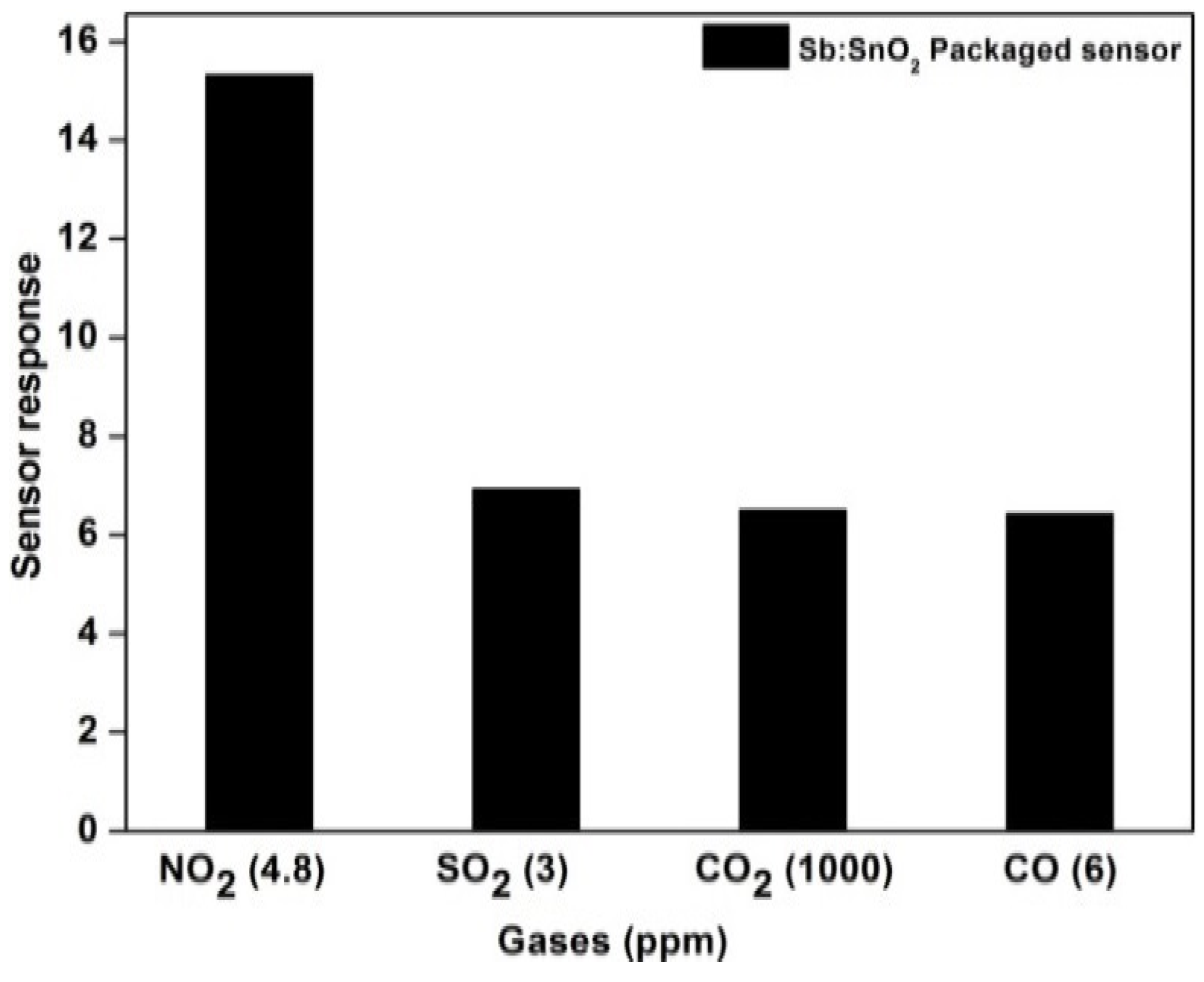
- Cross-sensitivity of packaged sensor: The packaged gas sensor exhibits a response of 15.30 for NO2 gas (4.8 ppm), 6.52 for CO2 gas (1000 ppm), 6.94 for SO2 gas (3.2 ppm), and 6.44 for CO gas (4.8 ppm). The cross-sensitivity of the packaged sensor is shown in Figure 10, and it is observed that the ATO thin film exhibited good selectivity to NO2 gas.
- (ii)
- Stability of packaged sensor: Herein, we recorded the response to 4.8 ppm of NO2 gas at 200 °C for six months. Since the response deviation over 4 months is around 6%, the packed sensor exhibits good stability of response when the film is prepared using target nanoparticles obtained by the green chemistry method. Additionally, the sensor’s response is enhanced by the device’s packaging using IDEs with a microheater.
- (iii)
- Comparative studies: The responses of films coated on a die with a normal IDE and microheater-based IDE for NO2 gas were compared. The film deposited on the die with the IDE and microheater demonstrated an enhanced response to NO2 gas at various concentrations. The surface-based localized heating of the die with the IDE and microheater results in an improved response.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeong, H.S.; Park, M.J.; Kwon, S.H.; Joo, H.J.; Kwon, H.I. Highly sensitive and selective room-temperature NO2 gas- sensing characteristics of SnOX-based p-type thin-film transistor. Sens. Actuators B Chem. 2019, 288, 625–633. [Google Scholar] [CrossRef]
- Sharma, A.; Tomar, M.; Gupta, V. Enhanced response characteristics of SnO2 thin film based NO2 gas sensor integrated with nanoscaled metal oxide clusters. Sens. Actuators B Chem. 2013, 181, 735–742. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Sayed, M.H.; Patil, V.L.; Boshta, M.; Patil, P.S. Gas sensing performance of sprayed NiO thin films toward NO2 gas. J. Alloys Compd. 2021, 885, 160908. [Google Scholar] [CrossRef]
- Boomashri, M.; Perumal, P.; Gunavathy, K.V.; Alkallas, F.H.; Trabelsi, A.B.G.; Shkir, M.; AlFaify, S. Detection of ammonia gas at room temperature through Sb doped SnO2 thin films: Improvement in sensing performance of SnO2. Ceram. Int. 2023, 49, 10096–10106. [Google Scholar] [CrossRef]
- Patil, V.L.; Vanalakar, S.A.; Patil, P.S.; Kim, J.H. Fabrication of nanostructured ZnO thin films based NO2 gas sensor via SILAR technique. Sens. Actuators B Chem. 2017, 239, 1185–1193. [Google Scholar] [CrossRef]
- Tyagi, S.; Chaudhary, M.; Ambedkar, A.K.; Sharma, K.; Gautam, Y.K.; Singh, B.P. Metal oxide nanomaterial-based sensors for monitoring environmental NO 2 and its impact on the plant ecosystem: A review. Sens. Diagn. 2022, 1, 106–129. [Google Scholar] [CrossRef]
- Kim, T.H.; Hasani, A.; Van Quyet, L.; Kim, Y.; Park, S.Y.; Lee, M.G.; Sohn, W.; Nguyen, T.P.; Choi, K.S.; Kim, S.Y.; et al. NO2 sensing properties of porous Au-incorporated tungsten oxide thin films prepared by solution process. Sens. Actuators B Chem. 2019, 286, 512–520. [Google Scholar] [CrossRef]
- Liu, H.; Wan, J.; Fu, Q.; Li, M.; Luo, W.; Zheng, Z.; Cao, H.; Hu, Y.; Zhou, D. Tin oxide films for nitrogen dioxide gas detection at low temperatures. Sens. Actuators B Chem. 2013, 177, 460–466. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Upadhyaya, K.; Ayachit, N.H.; Iyer, N. Quantum Dot–Polymer Composites in Light-Emitting Diode Applications. In Quantum Dots and Polymer Nanocomposites; CRC Press: Boca Raton, FL, USA, 2022; pp. 259–279. [Google Scholar]
- Ingole, S.M.; Navale, S.; Navale, Y.; Bandgar, D.; Stadler, F.; Mane, R.; Ramgir, N.; Gupta, S.; Aswal, D.; Patil, V. Nanostructured tin oxide films: Physical synthesis, characterization, and gas sensing properties. J. Colloid Interface Sci. 2017, 493, 162–170. [Google Scholar] [CrossRef]
- Jaiswal, J.; Singh, P.; Chandra, R. Low-temperature highly selective and sensitive NO2 gas sensors using CdTe-functionalized ZnO filled porous Si hybrid hierarchical nanostructured thin films. Sens. Actuators B Chem. 2021, 327, 128862. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. Antimony toxicity. Int. J. Environ. Res. Public Health 2010, 7, 4267–4277. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, T.; Tepore, A.; Micocci, G.; Serra, A.; Manno, D.; Filippo, E. WO3 gas sensors prepared by thermal oxidization of tungsten. Sens. Actuators B Chem. 2008, 133, 321–326. [Google Scholar] [CrossRef]
- Ani, A.; Poornesh, P.; Antony, A.; Nagaraja, K.K.; Rao, A.; Hegde, G.; Kolesnikov, E.; Shchetinin, I.V.; Kulkarni, S.D.; Petwal, V.C.; et al. An 8 mev electron beam modified in: Zno thin films for co gas sensing towards low concentration. Nanomaterials 2021, 11, 3151. [Google Scholar] [CrossRef] [PubMed]
- Mane, A.A.; Moholkar, A.V. Palladium (Pd) sensitized molybdenum trioxide (MoO3) nanobelts for nitrogen dioxide (NO2) gas detection. Solid-State Electron. 2018, 139, 21–30. [Google Scholar] [CrossRef]
- Chaitra, C.; Kalpana, H.M.; Ananda, C.M.; Lalithamba, H.S. Green synthesis of tin oxide based nanoparticles using Terminalia bellirica seed extract: Impact of operating temperature and antimony dopant on sensitivity for carbon dioxide gas sensing application. Mater. Technol. 2022, 37, 2339–2346. [Google Scholar] [CrossRef]
- Kim, J.; Murdoch, B.J.; Partridge, J.G.; Xing, K.; Qi, D.; Lipton-Duffin, J.; McConville, C.F.; van Embden, J.; Della Gaspera, E. Ultrasonic Spray Pyrolysis of Antimony-Doped Tin Oxide Transparent Conductive Coatings. Adv. Mater. Interfaces 2020, 7, 2000655. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H. Energy conversion and storage devices. In Green Nanomaterials in Energy Conversion and Storage Applications; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 75–93. [Google Scholar]
- Krishnakumar, T.; Jayaprakash, R.; Pinna, N.; Phani, A.; Passacantando, M.; Santucci, S. Structural, optical and electrical characterization of antimony-substituted tin oxide nanoparticles. J. Phys. Chem. Solids 2009, 70, 993–999. [Google Scholar] [CrossRef]
- Zheng, H.; Li, L.; Sun, Z.; Yu, S.; Luo, W. Preferential orientation, microstructure and functional properties of SnO2:Sb thin film: The effects of post-growth annealing. Appl. Surf. Sci. 2016, 362, 230–236. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Vyas, R. A role of integrated microheaters in a microfluidics based point-of-care-testing and beyond for healthcare applications. Appl. Mater. Today 2024, 38, 102225. [Google Scholar] [CrossRef]
- Velmurugan, K.; Kulkarni, M.B.; Gupta, I.; Das, R.; Goel, S.; Nirmal, J. Role of microfluidics in drug delivery. In Microfluidics and Multi Organs on Chip; Springer: Singapore, 2022; pp. 107–133. [Google Scholar]
- Xiong, Y.; Lin, Y.; Wang, X.; Zhao, Y.; Tian, J. Defect engineering on SnO2 nanomaterials for enhanced gas sensing performances. Adv. Powder Mater. 2022, 1, 100033. [Google Scholar] [CrossRef]
- Saeedabad, S.H.; Selopal, G.S.; Rozati, S.M.; Tavakoli, Y.; Sberveglieri, G. From Transparent Conducting Material to Gas-Sensing Application of SnO2:Sb Thin Films. J. Electron. Mater. 2018, 47, 5165–5173. [Google Scholar] [CrossRef]
- Babar, A.R.; Shinde, S.S.; Moholkar, A.V.; Bhosale, C.H.; Kim, J.H.; Rajpure, K.Y. Structural and optoelectronic properties of antimony incorporated tin oxide thin films. J. Alloys Compd. 2010, 505, 416–422. [Google Scholar] [CrossRef]
- Afrouzmehr, M.; Yasrebi, N.; Sheikhi, M.H. Fabrication and characterization of Ag-Decorated indium–tin-oxide nanoparticle based ethanol sensors using an enhanced electrophoretic method. Ceram. Int. 2021, 47, 30504–30513. [Google Scholar] [CrossRef]
- Chen, F.; Li, N.; Shen, Q.; Wang, C.; Zhang, L. Fabrication of transparent conducting ATO films using the ATO sintered targets by pulsed laser deposition. Sol. Energy Mater. Sol. Cells 2012, 105, 153–158. [Google Scholar] [CrossRef]
- Peters, K.; Zeller, P.; Stefanic, G.; Skoromets, V.; Němec, H.; Kužel, P.; Fattakhova-Rohlfing, D. Water-Dispersible Small Monodisperse Electrically Conducting Antimony Doped Tin Oxide Nanoparticles. Chem. Mater. 2015, 27, 1090–1099. [Google Scholar] [CrossRef]
- Eom, N.S.A.; Cho, H.B.; Lim, H.R.; Kim, B.S.; Choa, Y.H. Facile tilted sputtering process (TSP) for enhanced H2S gas response over selectively loading Pt nanoparticles on SnO2 thin films. Sens. Actuators B Chem. 2019, 300, 127009. [Google Scholar] [CrossRef]
- Rudraswamy, S.B.; Bhat, N. Optimization of RF sputtered Ag-Doped BaTiO3-CuO mixed oxide thin film as carbon dioxide sensor for environmental pollution monitoring application. IEEE Sens. J. 2016, 16, 5145–5151. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Wu, T.; Fu, Z.; Zhang, W.; Lian, Z.; Cai, S.; Yang, R. Dual ligand-induced photoelectrochemical sensing by integrating Pt/MoS2 heterostructure and Au polyhedra for sensitive detection of SARS-CoV-2. Sens. Actuators B Chem. 2023, 376, 132970. [Google Scholar] [CrossRef]
- Thomas, A.; Thirumalaisamy, L.; Madanagurusamy, S.; Sivaperuman, K. Incompatibility of pure SnO2 thin films for room-temperature gas sensing application. ACS Omega 2023, 8, 32848–32854. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Z.; Chang, X.; Liu, X.; Hu, Y.; Li, M.; Xu, P.; Pinna, N.; Zhang, J. PdRh-sensitized iron oxide ultrathin film sensors and mechanistic investigation by operando TEM and DFT calculation. Small 2023, 19, 2301485. [Google Scholar] [CrossRef]
- Pan, H.; Zhou, L.; Zheng, W.; Liu, X.; Zhang, J.; Pinna, N. Atomic layer deposition to heterostructures for application in gas sensors. Int. J. Extrem. Manuf. 2023, 5, 022008. [Google Scholar] [CrossRef]

| Investigator | Deposition Method | Thin-Film Material | Operating Temperature | Response | Response/Recovery Time | Reference |
|---|---|---|---|---|---|---|
| Siciliano et al. (2008) | Thermal Evaporation | WO3 | 250 °C | 4 | - | [13] |
| Luís F. da Silva et al. (2015) | E-beam evaporation | SrTi0.85Fe00.15O3 | 260 °C | 4 | 15 s/35 s | [14] |
| Mane et al. (2017) | Chemical Spray Pyrolysis | Pd:MoO3 | 200 °C | 4.5 | 11 s/76 s | [15] |
| This work | E-beam Evaporation | Sb:SnO2 | 200 °C | 16.20 | 285/510 s | - |
| Process Parameters | Values |
|---|---|
| Base pressure | 2 × 10−3 mbar |
| Working pressure | 5 × 10−5 mbar |
| Target-to-substrate distance | 13 cm |
| Substrate temperature | 27 °C |
| Accelerating voltage | 5 KV |
| Beam current | 20 mA |
| Rate of deposition | 0.9 Å/s |
| Film thickness | ~250 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandraiah, C.; Kalpana, H.M.; Ananda, C.M.; Kulkarni, M.B. Preparation of Antimony Tin Oxide Thin Film Using Green Synthesized Nanoparticles by E-Beam Technique for NO2 Gas Sensing. Micro 2024, 4, 401-411. https://doi.org/10.3390/micro4030025
Chandraiah C, Kalpana HM, Ananda CM, Kulkarni MB. Preparation of Antimony Tin Oxide Thin Film Using Green Synthesized Nanoparticles by E-Beam Technique for NO2 Gas Sensing. Micro. 2024; 4(3):401-411. https://doi.org/10.3390/micro4030025
Chicago/Turabian StyleChandraiah, Chaitra, Hullekere Mahadevaiah Kalpana, Challaghatta Muniyappa Ananda, and Madhusudan B. Kulkarni. 2024. "Preparation of Antimony Tin Oxide Thin Film Using Green Synthesized Nanoparticles by E-Beam Technique for NO2 Gas Sensing" Micro 4, no. 3: 401-411. https://doi.org/10.3390/micro4030025
APA StyleChandraiah, C., Kalpana, H. M., Ananda, C. M., & Kulkarni, M. B. (2024). Preparation of Antimony Tin Oxide Thin Film Using Green Synthesized Nanoparticles by E-Beam Technique for NO2 Gas Sensing. Micro, 4(3), 401-411. https://doi.org/10.3390/micro4030025







