Barrier Gesture Relaxation during Vaccination Campaign in France: Modelling Impact of Waning Immunity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model
2.2. Parameters
2.3. Contact Matrices and Public Health Mitigation Strategies
2.4. Calibration
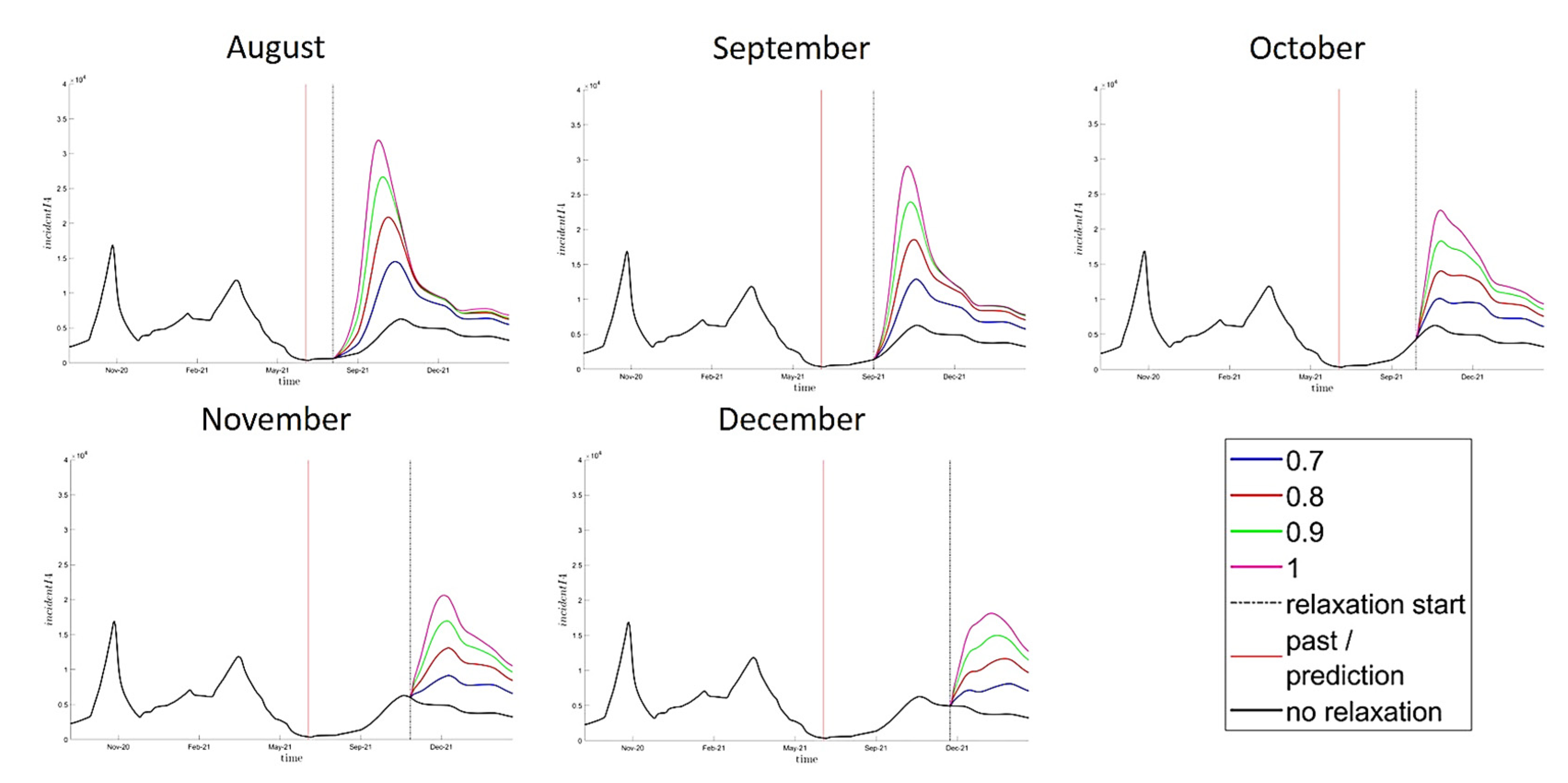
2.5. Barrier Gesture Relaxation Scenarios
2.6. Sensitivity Analyses
3. Results
3.1. Calibration
3.2. Impact of Barrier Gesture Relaxation, Main Analysis
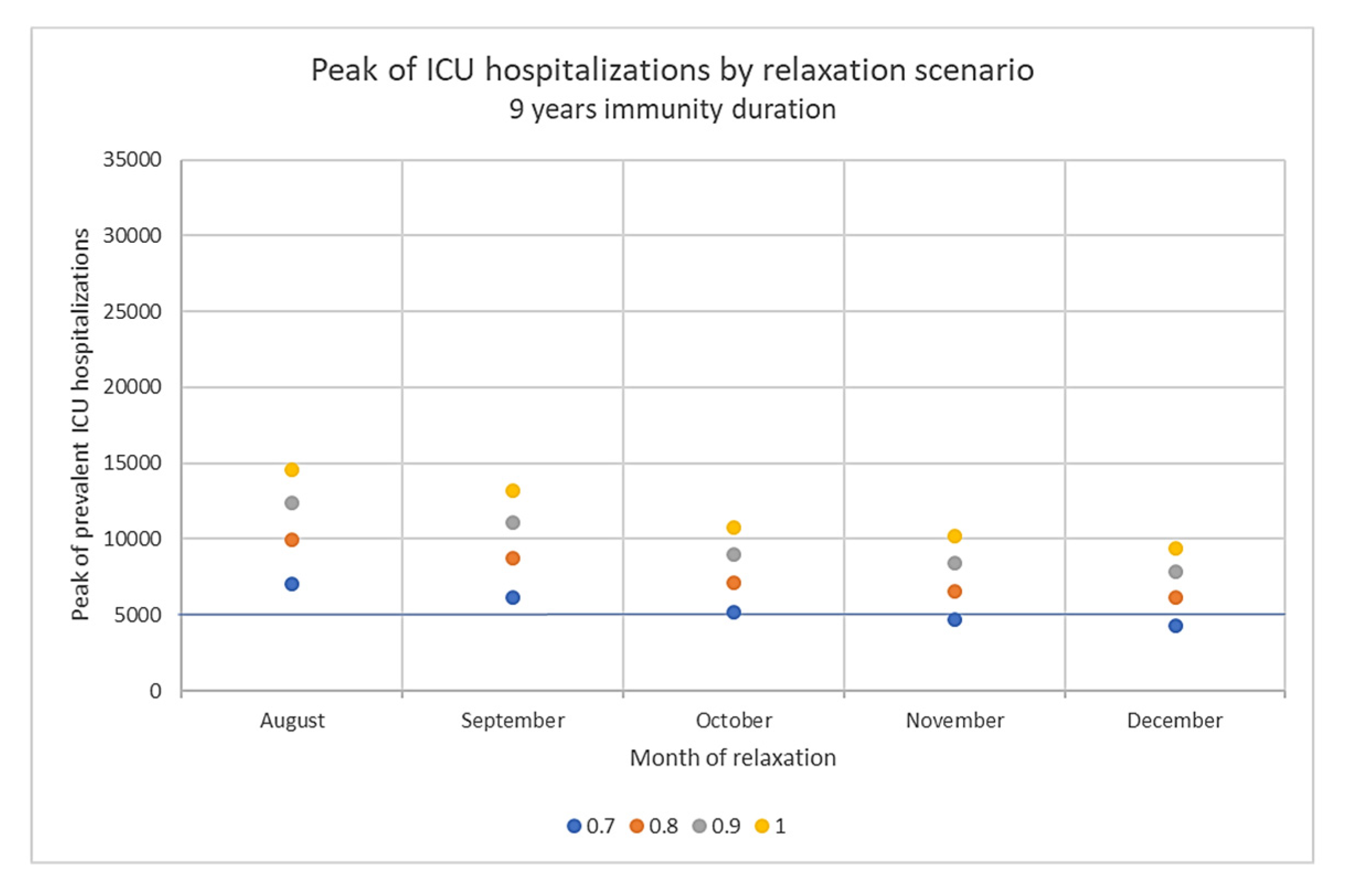
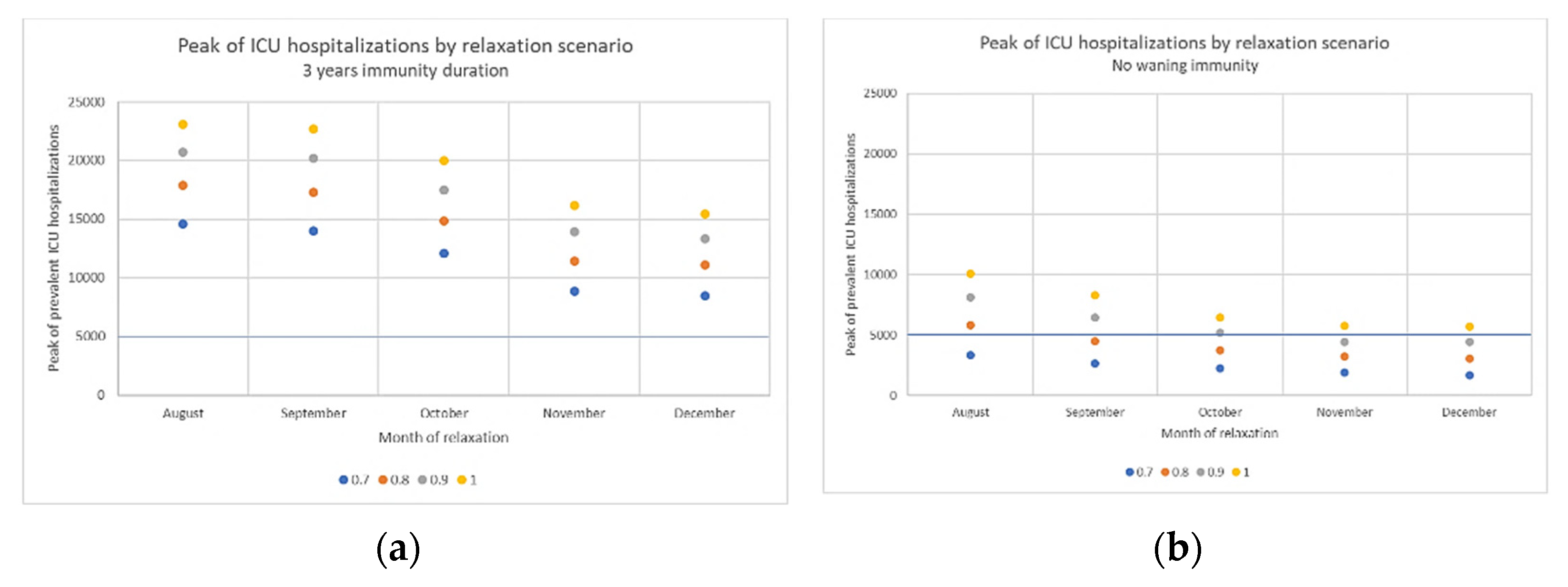
3.3. Impact of Immunity Duration Hypothesis
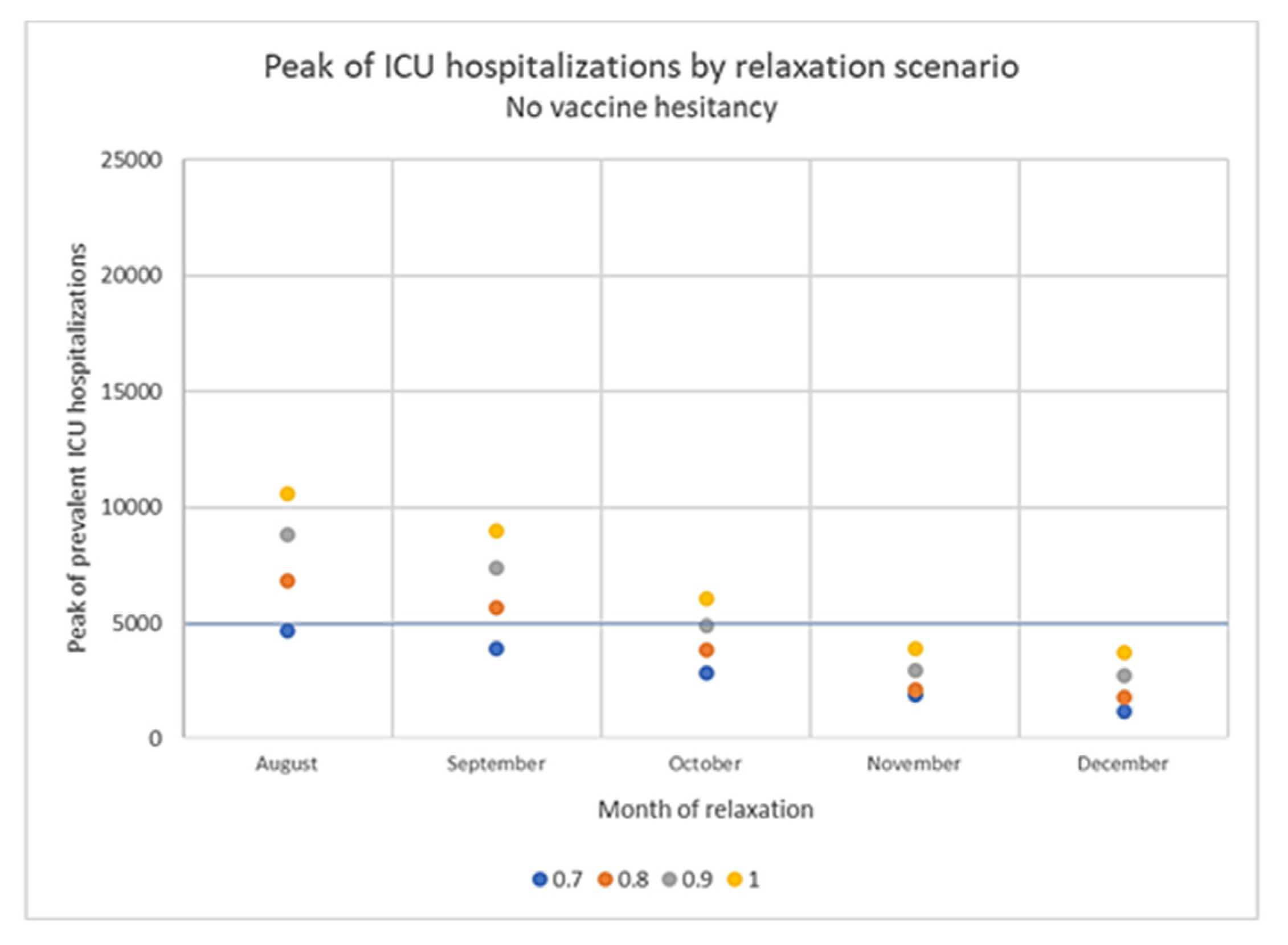
3.4. Impact of Vaccine Hesitancy
3.5. Booster Campaign
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Variants of Concern Data for France Are Publicly Available on
Conflicts of Interest
Appendix A
References
- Kraemer, M.U.G.; Yang, C.-H.; Gutierrez, B.; Wu, C.H.; Klein, B.; Pigott, D.M.; Open COVID-19 Data Working Group; du Plessis, L.; Faria, N.R.; Li, R.; et al. The Effect of Human Mobility and Control Measures on the COVID-19 Epidemic in China. Science 2020, 368, 493–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, S.; Kandula, S.; Shaman, J. Differential Effects of Intervention Timing on COVID-19 Spread in the United States. Sci. Adv. 2020, 6, eabd6370. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Weekly Operational Update on COVID-19. 30 August 2021. Issue No 70. Available online: https://www.who.int/publications/m/item/weekly-operational-update-on-covid-19---30-august-2021 (accessed on 7 October 2021).
- Salje, H.; Kiem, C.T.; Lefrancq, N.; Courtejoie, N.; Bosetti, P.; Paireau, J.; Andronico, A.; Hozé, N.; Richet, J.; Dubost, C.L.; et al. Estimating the Burden of SARS-CoV-2 in France. Science 2020, 369, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.G.; Klepac, P.; Liu, Y.; Prem, K.; Jit, M.; CMMID COVID-19 Working Group; Eggo, R.M. Age-Dependent Effects in the Transmission and Control of COVID-19 Epidemics. Nat. Med. 2020, 26, 1205–1211. [Google Scholar] [CrossRef]
- Davies, N.G.; Kucharski, A.J.; Eggo, R.M.; Gimma, A.; Edmunds, W.J.; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. Effects of Non-Pharmaceutical Interventions on COVID-19 Cases, Deaths, and Demand for Hospital Services in the UK: A Modelling Study. Lancet Public Health 2020, 5, e375–e385. [Google Scholar] [CrossRef]
- Di Domenico, L.; Pullano, G.; Sabbatini, C.E.; Boëlle, P.Y.; Colizza, V. Impact of Lockdown on COVID-19 Epidemic in Île-de-France and Possible Exit Strategies. BMC Med. 2020, 18, 240. [Google Scholar] [CrossRef]
- Collin, A.; Hejblum, B.P.; Vignals, C.; Lehot, L.; Thiebaut, R.; Moireau, P.; Prague, M. Using Population Based Kalman Estimator to Model COVID-19 Epidemic in France: Estimating the Effects of Non-Pharmaceutical Interventions on the Dynamics of Epidemic. MedRxiv 2021. submitted. [Google Scholar] [CrossRef]
- Bubar, K.M.; Reinholt, K.; Kissler, S.M.; Lipsitch, M.; Cobey, S.; Grad, Y.H.; Larremore, D.B. Model-Informed COVID-19 Vaccine Prioritization Strategies by Age and Serostatus. Science 2021, 371, 916–921. [Google Scholar] [CrossRef]
- Harvey, R.A.; Rassen, J.A.; Kabelac, C.A.; Turenne, W.; Leonard, S.; Klesh, R.; Meyer, W.A., 3rd; Kaufman, H.W.; Anderson, S.; Cohen, O.; et al. Association of SARS-CoV-2 Seropositive Antibody Test with Risk of Future Infection. JAMA Intern. Med. 2021, 181, 672–679. [Google Scholar] [CrossRef]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; Warren, F.; et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N. Engl. J. Med. 2021, 384, 533–540. [Google Scholar] [CrossRef]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal Observation and Decline of Neutralizing Antibody Responses in the Three Months Following SARS-CoV-2 Infection in Humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and Immunological Assessment of Asymptomatic SARS-CoV-2 Infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- den Hartog, G.; Vos, E.R.A.; van den Hoogen, L.L.; van Boven, M.; Schepp, R.M.; Smits, G.; van Vliet, J.; Woudstra, L.; Wijmenga-Monsuur, A.J.; van Hagen, C.C.E.; et al. Persistence of Antibodies to SARS-CoV-2 in Relation to Symptoms in a Nationwide Prospective Study. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Jung, J.H.; Rha, M.-S.; Sa, M.; Choi, H.K.; Jeon, J.H.; Seok, H.; Park, D.W.; Park, S.H.; Jeong, H.W.; Choi, W.S.; et al. SARS-CoV-2-Specific T Cell Memory Is Sustained in COVID-19 Convalescent Patients for 10 Months with Successful Development of Stem Cell-like Memory T Cells. Nat. Commun. 2021, 12, 4043. [Google Scholar] [CrossRef]
- Edridge, A.W.D.; Kaczorowska, J.; Hoste, A.C.; Bakker, M.; Klein, M.; Loens, K.; Jebbink, M.F.; Matser, A.; Kinsella, C.M.; Rueda, P.; et al. Seasonal Coronavirus Protective Immunity Is Short-Lasting. Nat. Med. 2020, 26, 1691–1693. [Google Scholar] [CrossRef]
- Sariol, A.; Perlman, S. Lessons for COVID-19 Immunity from Other Coronavirus Infections. Immunity 2020, 53, 248–263. [Google Scholar] [CrossRef]
- Liu, W.; Fontanet, A.; Zhang, P.H.; Zhan, L.; Xin, Z.T.; Baril, L.; Tang, F.; Lv, H.; Cao, W.C. Two-Year Prospective Study of the Humoral Immune Response of Patients with Severe Acute Respiratory Syndrome. J. Infect. Dis. 2006, 193, 792–795. [Google Scholar] [CrossRef] [Green Version]
- Le Bert, N.; Tan, A.; Kunasegaran, K.; Tham, C.Y.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-Specific T Cell Immunity in Cases of COVID-19 and SARS, and Uninfected Controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-Antibody Waning after Second Dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Gonzalo Pérez, M.; Polack, F.P.; Zerbini, C.; et al. Six Month Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. MedRxiv 2021. to be submitted. [Google Scholar] [CrossRef]
- Kissler, S.M.; Tedijanto, C.; Goldstein, E.; Grad, Y.H.; Lipsitch, M. Projecting the Transmission Dynamics of SARS-CoV-2 through the Postpandemic Period. Science 2020, 368, 860–868. [Google Scholar] [CrossRef]
- Lavine, J.S.; Bjornstad, O.N.; Antia, R. Immunological Characteristics Govern the Transition of COVID-19 to Endemicity. Science 2021, 371, 741–745. [Google Scholar] [CrossRef]
- Saad-Roy, C.M.; Wagner, C.E.; Baker, R.E.; Morris, S.E.; Farrar, J.; Graham, A.L.; Levin, S.A.; Mina, M.J.; Metcalf, C.J.E.; Grenfell, B.T. Immune Life History, Vaccination, and the Dynamics of SARS-CoV-2 over the next 5 Years. Science 2020, 370, 811–818. [Google Scholar] [CrossRef]
- Giannitsarou, C.; Kissler, S.; Toxvaerd, F. Waning Immunity and the Second Wave: Some Projections for SARS-CoV-2. University of Cambridge, Faculty of Economics, Centre for Economic Policy Research Discussion Paper No. DP14852. 2020. Available online: https://ssrn.com/abstract=3628172 (accessed on 7 October 2021).
- Santé Publique France. Point Epidémiologique n°78 du 26 aout 2021. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-26-aout-2021 (accessed on 7 October 2021).
- Kiem, C.T.; Massonnaud, A.; Levy-Bruhl, D.; Poletto, C.; Colizza, V.; Bosetti, P.; Fontanet, A.; Gabet, A.; Olié, V.; Zanetti, L.; et al. modelling study investigating short and medium-term challenges for COVID-19 vaccination: From prioritisation to the relaxation of measures. EClinicalMedicine 2021, 38, 101001. [Google Scholar] [CrossRef] [PubMed]
- European Center for Disease Prevention and Controle. Implications for the EU-EEA on the Spread of SARS-CoV-2 Delta-VOC; ECDC: Stockholm, Sweden, 2021. [Google Scholar]
- Santé Publique France. Résultats de la vague 26 de l’enquête CoviPrev (15–21 juillet 2021). Available online: https://www.santepubliquefrance.fr/etudes-et-enquetes/coviprev-une-enquete-pour-suivre-l-evolution-des-comportements-et-de-la-sante-mentale-pendant-l-epidemie-de-covid-19 (accessed on 7 October 2021).
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.; Lim, E.; Touizer, E.; Meng, B.; Abdullahi, A.; CITIID-NIHR BioResource COVID-19 Collaboration; Elmer, A.; et al. Age-Related Immune Response Heterogeneity to SARS-CoV-2 Vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Avis Relatif au Contact Tracing et à L’application des Mesures Barrières Chez les Personnes Totalement Vaccinées Contre le Covid-19. Haut Conseil de Santé Publique, France. Available online: https://www.hcsp.fr/Explore.cgi/AvisRapportsDomaine?clefr=1069 (accessed on 7 October 2021).
- Childs, L.; Dick, D.W.; Feng, Z.; Heffernan, J.M.; Li, J.; Röst, G. Modeling Waning and Boosting of COVID-19 in Canada with Vaccination. medRxiv 2021. to be submitted. [Google Scholar] [CrossRef]
- Carlsson, R.-M.; Childs, L.M.; Feng, Z.; Glasser, J.W.; Heffernan, J.M.; Li, J.; Röst, G. Modeling the Waning and Boosting of Immunity from Infection or Vaccination. J. Theor. Biol. 2020, 497, 110265. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Jit, M.; Warren-Gash, C.; Guthrie, B.; Wang, H.H.; Mercer, S.W.; Sanderson, C.; McKee, M.; Troeger, C.; Ong, K.L.; et al. Global, Regional, and National Estimates of the Population at Increased Risk of Severe COVID-19 Due to Underlying Health Conditions in 2020: A Modelling Study. Lancet Glob. Health 2020, 8, e1003–e1017. [Google Scholar] [CrossRef]
- Crawford, K.H.D.; Dingens, A.S.; Eguia, R.; Wolf, C.R.; Wilcox, N.; Logue, J.K.; Shuey, K.; Casto, A.M.; Fiala, B.; Wrenn, S.; et al. Dynamics of Neutralizing Antibody Titers in the Months After Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Infect. Dis. 2021, 223, 197–205. [Google Scholar] [CrossRef]
- Dufloo, J.; Grzelak, L. Asymptomatic and Symptomatic SARS-CoV-2 Infections Elicit Polyfunctional Antibodies. Cell Rep. Med. 2021, 2, 100275. [Google Scholar] [CrossRef]
- Piccoli, L.; Park, Y.-J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 2020, 183, 1024–1042.e21. [Google Scholar] [CrossRef]
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial Undocumented Infection Facilitates the Rapid Dissemination of Novel Coronavirus (SARS-CoV-2). Science 2020, 368, 489–493. [Google Scholar] [CrossRef] [Green Version]
- Prem, K.; Zandvoort, K.V.; Klepac, P.; Eggo, R.M.; Davies, N.G.; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group; Cook, A.R.; Jit, M. Projecting contact matrices in 177 geographical regions: An update and comparison with empirical data for the COVID-19 era. PLoS Comput. Biol. 2021, 17, e1009098. [Google Scholar] [CrossRef]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal Dynamics in Viral Shedding and Transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [Green Version]
- Bi, Q.; Wu, Y.; Mei, S.; Ye, C.; Zou, X.; Zhang, Z.; Liu, X.; Wei, L.; Truelove, S.A.; Zhang, T.; et al. Epidemiology and Transmission of COVID-19 in 391 Cases and 1286 of Their Close Contacts in Shenzhen, China: A Retrospective Cohort Study. Lancet Infect. Dis. 2020, 20, 911–919. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gilson, N.; Atti, A.; et al. Effectiveness of BNT162b2 MRNA Vaccine Against Infection and COVID-19 Vaccine Coverage in Healthcare Workers in England, Multicentre Prospective Cohort Study (the SIREN Study). SSRN Electron. J. 2021. Available online: https://ssrn.com/abstract=3790399 (accessed on 7 October 2021). [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 MRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Gethings, O.; Vihta, K.D.; Jones, J.; House, T.; VanSteenHouse, H.; Bell, I.; et al. Impact of Vaccination on New SARS-CoV-2 Infections in the United Kingdom. Nat. Med. 2021, 27, 1370–1378. [Google Scholar] [CrossRef]
- Charmet, T.; Schaeffer, L.; Grant, R.; Galmiche, S.; Chény, O.; Von Platen, C.; Maurizot, A.; Rogoff, A.; Omar, F.; David, C.; et al. Impact of Original, B.1.1.7, and B.1.351/P.1 SARS-CoV-2 Lineages on Vaccine Effectiveness of Two Doses of COVID-19 MRNA Vaccines: Results from a Nationwide Case-Control Study in France. Lancet Reg. Health-Eur. 2021, 8, 100171. [Google Scholar] [CrossRef]
- Diekmann, O.; Heesterbeek, J.A.P.; Roberts, M.G. The Construction of Next-Generation Matrices for Compartmental Epidemic Models. J. R. Soc. Interface 2010, 7, 873–885. [Google Scholar] [CrossRef] [Green Version]
- Van Vinh Chau, N.; Lam, V.T.; Dung, N.T.; Yen, L.M.; Minh, N.; Hung, L.M.; Ngoc, N.M.; Dung, N.T.; Man, D.N.H.; Nguyet, L.A.; et al. The Natural History and Transmission Potential of Asymptomatic Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Clin. Infect. Dis. 2020, 71, 2679–2687. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Jian, S.-W.; Liu, D.P.; Ng, T.C.; Huang, W.T.; Lin, H.H.; Taiwan COVID-19 Outbreak Investigation Team. Contact Tracing Assessment of COVID-19 Transmission Dynamics in Taiwan and Risk at Different Exposure Periods Before and After Symptom Onset. JAMA Intern. Med. 2020, 180, 1156. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, L.-M.; Wan, L.; Xiang, T.X.; Le, A.; Liu, J.M.; Peiris, M.; Poon, L.L.M.; Zhang, W. Viral Dynamics in Mild and Severe Cases of COVID-19. Lancet Infect. Dis. 2020, 20, 656–657. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Fan, J.; Yu, F.; Feng, B.; Lou, B.; Zou, Q.; Xie, G.; Lin, S.; Wang, R.; Yang, X.; et al. Viral Load Dynamics and Disease Severity in Patients Infected with SARS-CoV-2 in Zhejiang Province, China, January-March 2020: Retrospective Cohort Study. BMJ 2020, 369, m1443. [Google Scholar] [CrossRef] [Green Version]
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated Transmissibility and Impact of SARS-CoV-2 Lineage B.1.1.7 in England. Science 2021, 372, eabg3055. [Google Scholar] [CrossRef]
- Gaymard, A.; Bosetti, P.; Feri, A.; Destras, G.; Enouf, V.; Andronico, A.; Burrel, S.; Behillil, S.; Sauvage, C.; Bal, A.; et al. Early Assessment of Diffusion and Possible Expansion of SARS-CoV-2 Lineage 20I/501Y.V1 (B.1.1.7, Variant of Concern 202012/01) in France, January to March 2021. Eurosurveillance 2021, 26, 2100133. [Google Scholar] [CrossRef]
- Campbell, F.; Archer, B.; Laurenson-Schafer, H.; Jinnai, Y.; Konings, F.; Batra, N.; Pavlin, B.; Vandemaele, K.; Van Kerkhove, M.D.; Jombart, T.; et al. Increased Transmissibility and Global Spread of SARS-CoV-2 Variants of Concern as at June 2021. Eurosurveillance 2021, 26, 2100509. [Google Scholar] [CrossRef]
- Les Actions du Gouvernement. Available online: https://www.gouvernement.fr/info-coronavirus/les-actions-du-gouvernement (accessed on 7 October 2021).
- Liu, X.; Huang, J.; Li, C.; Zhao, Y.; Wang, D.; Huang, Z.; Yang, K. The Role of Seasonality in the Spread of COVID-19 Pandemic. Environ. Res. 2021, 195, 110874. [Google Scholar] [CrossRef]
- Nombre de Lits de Réanimation, de Soins Intensifs et de Soins Continus En France, Fin 2013 et 2019. DRESS. 2021. Available online: https://drees.solidarites-sante.gouv.fr/article/nombre-de-lits-de-reanimation-de-soins-intensifs-et-de-soins-continus-en-france-fin-2013-et (accessed on 6 August 2021).
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Kiem, C.T.; Bosetti, P.; Hozé, N.; Paireau, J.; Cauchemez, S. Impact de L’accélération de la Vaccination sur L’épidémie du Variant Delta en France Métropolitaine; Institut Pasteur: Paris, France, 2021; Available online: https://modelisation-covid19.pasteur.fr/variant/Institut_Pasteur_Acceleration_vaccination_et_Delta_20210726.pdf (accessed on 7 October 2021).
- Bosetti, P.; Kiem, C.T.; Andronico, A.; Colizza, V.; Yazdanpanah, Y.; Fontanet, A.; Benamouzig, D.; Cauchemez, S. Epidemiology and Control of SARS-CoV-2 Epidemics in Partially Vaccinated Populations: A Modeling Study Applied to France; Institut Pasteur: Paris, France, 2021; to be submitted; Available online: https://hal-pasteur.archives-ouvertes.fr/pasteur-03272638 (accessed on 7 October 2021).
- Mesa, D.O.; Hogan, A.B.; Watson, O.J.; Charles, G.D.; Hauck, K.; Ghani, A.C.; Winskill, P. Report 43: Quantifying the Impact of Vaccine Hesitancy in Prolonging the Need for Non-Pharmaceutical Interventions to Control the COVID-19 Pandemic. Imp. Coll. Lond. 2021. to be submitted. [Google Scholar] [CrossRef]
- Hozé, N.; Paireau, J.; Lapidus, N.; Kiem, C.T.; Salje, H.; Severi, G.; Touvier, M.; Zins, M.; de Lamballerie, X.; Lévy-Bruhl, D.; et al. Monitoring the Proportion of the Population Infected by SARS-CoV-2 Using Age-Stratified Hospitalisation and Serological Data: A Modelling Study. Lancet Public Health 2021, 6, e408–e415. [Google Scholar] [CrossRef]
- Les Données de la Vaccination Contre la COVID, Assurance Maladie. Available online: https://datavaccin-covid.ameli.fr/pages/type-vaccins/ (accessed on 7 October 2021).
- Nyberg, T.; Twohig, K.A.; Harris, R.J.; Seaman, S.R.; Flannagan, J.; Allen, H.; Charlett, A.; De Angelis, D.; Dabrera, G.; Presanis, A.M. Risk of Hospital Admission for Patients with SARS-CoV-2 Variant B.1.1.7: Cohort Analysis. BMJ 2021, 373, n1412. [Google Scholar] [CrossRef] [PubMed]
- Viguerie, A.; Lorenzo, G.; Auricchio, F.; Baroli, D.; Hughes, T.J.; Patton, A.; Reali, A.; Yankeelov, T.E.; Veneziani, A. Simulating the spread of COVID-19 via a spatially-resolved susceptible–exposed–infected–recovered–deceased (SEIRD) model with heterogeneous diffusion. Appl. Math. Lett. 2021, 111, 106617. [Google Scholar] [CrossRef]



| Parameter | Definition | Value | References | ||
|---|---|---|---|---|---|
| Fixed Parameters | |||||
| αi | Susceptibility by immune status i | α1 | α2 | α3 | Hypothesis, taken from Childs et al. [33] |
| 1 | 0.66 | 0.33 | |||
| βj | Infectivity by infection severity j | β2 | β3 | β4 | [39] |
| 0.045 | 0.089 | 0.009 | |||
| λim | Force of infection by immune status i and age m | Appendix A | Estimated | ||
| cma | Contact-rates between individuals in age group m and age group a | Described in Section 2.3 | [40] | ||
| R0 | Basic reproductive number | 2.9 | [4] | ||
| pjim | Proportion of S going to Ij by age m and by immune status i | Table S2 | [35] | ||
| Am | Population size by age m | Table S1 | INSEE * | ||
| γj | Recovery rate by infection severity j | γ2 | γ3 | γ4 | Hypothesis |
| 1/5 | 1/10 | 1/15 | |||
| κ | Rate of progress through the exposed compartments | 1/1.5 | [41,42,43] | ||
| Variable Parameters | |||||
| σ1im | Vaccination rate by age m and by immune status i for first dose | Described in Section 2.2 paragraph 2 | VAC-SI ** | ||
| σ2im | Vaccination rate by age m and by immune status i for second dose | 1/28 | |||
| ω | Waning rate of immunity | Main analysis | Sensitivity analysis | [21,22] | |
| 1/1095 | 1/365 | 0 | |||
| ρ | Vaccine efficacy against infections after 2 doses | Main analysis | Sensitivity analysis | [44,45,46,47] | |
| 0.8 | 0.9 | ||||
| 1 − ε | Vaccine efficacy against infections after 1 dose | Main analysis | Sensitivity analysis | [44,45,46,47] | |
| 0.5 | 0.7 | ||||
| 1 − q | Vaccine efficacy against severe cases after 1 dose | Main analysis | Sensitivity analysis | [44,45,46,47] | |
| 0.7 | 0.7 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vignals, C.; Dick, D.W.; Thiébaut, R.; Wittkop, L.; Prague, M.; Heffernan, J.M. Barrier Gesture Relaxation during Vaccination Campaign in France: Modelling Impact of Waning Immunity. COVID 2021, 1, 472-488. https://doi.org/10.3390/covid1020041
Vignals C, Dick DW, Thiébaut R, Wittkop L, Prague M, Heffernan JM. Barrier Gesture Relaxation during Vaccination Campaign in France: Modelling Impact of Waning Immunity. COVID. 2021; 1(2):472-488. https://doi.org/10.3390/covid1020041
Chicago/Turabian StyleVignals, Carole, David W. Dick, Rodolphe Thiébaut, Linda Wittkop, Mélanie Prague, and Jane M. Heffernan. 2021. "Barrier Gesture Relaxation during Vaccination Campaign in France: Modelling Impact of Waning Immunity" COVID 1, no. 2: 472-488. https://doi.org/10.3390/covid1020041
APA StyleVignals, C., Dick, D. W., Thiébaut, R., Wittkop, L., Prague, M., & Heffernan, J. M. (2021). Barrier Gesture Relaxation during Vaccination Campaign in France: Modelling Impact of Waning Immunity. COVID, 1(2), 472-488. https://doi.org/10.3390/covid1020041





