Abstract
1. Background: Some reports have suggested that as many as one-half of all hospital inpatients identified as COVID-19-positive during the Omicron BA.1 variant-driven wave were incidental cases admitted primarily for reasons other than their viral infections. To date, however, there are no prospective longitudinal studies of a representative panel of hospitals based on pre-established criteria for determining whether a patient was, in fact, admitted as a result of the disease. 2. Materials and Methods: To fill this gap, we developed a formula to estimate the fraction of incidental COVID-19 hospitalizations that relies on measurable, population-based parameters. We applied our approach to a longitudinal panel of 164 counties throughout the United States, covering a 4-week interval ending in the first week of January 2022. 3. Results: Within this panel, we estimated that COVID-19 incidence was rising exponentially at a rate of 9.34% per day (95% CI, 8.93–9.87). Assuming that only one-quarter of all Omicron BA.1 infections had been reported by public authorities, we further estimated the aggregate prevalence of active SARS-CoV-2 infection during the first week of January to be 3.45%. During the same week, among 250 high-COVID-volume hospitals within our 164-county panel, an estimated one in four inpatients was COVID-positive. Based upon these estimates, we computed that 10.6% of such COVID-19-positive hospitalized patients were incidental infections. Across individual counties, the median fraction of incidental COVID-19 hospitalizations was 9.5%, with an interquartile range of 6.7 to 12.7%. 4. Conclusion: Incidental COVID-19 infections appear to have been a nontrivial fraction of all COVID-19-positive hospitalized patients during the Omicron BA.1 wave. In the aggregate, however, the burden of patients admitted for complications of their viral infections was far greater.
1. Introduction
During the massive wave of Omicron variant-driven SARS-CoV-2 infections in the United States during the winter of 2021–2022, total hospitalizations among infected persons reached 161,876 on 19 January 2022, a nationwide census substantially exceeding the peaks recorded during the previous winter of 2020–2021 and the Delta variant-driven wave of July–October 2021 [1]. Yet, some observers cautioned that more than half of all hospital inpatients identified as COVID-19-positive during the Omicron BA.1 surge were so-called incidental cases admitted primarily for reasons other than their viral infections [2,3,4]. Such estimates, if accurate, would imply that raw counts of COVID-19-associated hospitalizations substantially overstated not only the typical severity of Omicron BA.1 infection, but also the overall impact of the surge on healthcare resource utilization.
The notion that some patients hospitalized for entirely unrelated reasons happen to be SARS-CoV-2-positive appears entirely reasonable, especially in view of the substantial fraction of asymptomatic cases among Omicron BA.1 infections [5]. The real problem is putting an accurate bound on the proportion of such incidental hospitalizations. To that end, the ideal study design would be to prospectively follow a representative, longitudinal cohort of individuals, evaluating each hospitalization according to pre-established, objective criteria for determining whether each SARS-CoV-2-positive patient was in fact admitted as a result of their infection.
At issue here are more than technical matters of disease classification, but also fundamental questions of ultimate causation [6]. Consider a patient with hypertensive heart disease who is hospitalized for a tachyarrhythmia due to the mild hypoxemia he suffered from COVID-19. Or consider a young adult recently infected with SARS-CoV-2 who continues to drive while suffering from viremia-induced headache, fever, body aches, dizziness and fatigue and, as a result, gets into a major car crash. The former patient’s admission diagnoses might include such proximate causes as cardiomegaly and atrial fibrillation, while the latter patient’s admission diagnoses might list acute brain injury, spleen laceration and a pelvic fracture. However, the ultimate cause in both cases was COVID-19.
Unfortunately, the few documented research studies to date have not achieved this methodological ideal. A tabulation of 2688 COVID-19-positive hospitalized patients posted by the Massachusetts Department of Public Health during the Omicron BA.1 wave [7] designated only 1337 (48.8%) as hospitalized due to their SARS-CoV-2 infections. However, these non-incidental admissions were restricted to those patients who had been administered dexamethasone, a treatment principally indicated for severe cases requiring high-flow oxygen but contraindicated in some hospitalized COVID-19 patients [8,9].
A retrospective review of electronic health records of 1123 patients found to be polymerase chain reaction (PCR) positive from 7 days before to 14 days after admission to one of four U.S. hospital systems [10] did not cover the period of the Omicron BA.1 surge. Among the 292 cases (or 26%) retrospectively classified as incidental, about two-thirds involved such diagnoses as acute kidney injury, diabetes, anemia, thrombocytopenia and acute respiratory failure, which could well have been precipitated or exacerbated by SARS-CoV-2 infection [11,12,13].
A smaller study of PCR-positive admissions to a tertiary-care hospital in Rotterdam, the Netherlands, during the Omicron BA.1 wave [14] reported that 46 (30.5%) of 151 adults and 8 (38.1%) of 21 pediatric patients had no COVID-19 symptoms or only mild symptoms that did not require any treatment. Once again, there is a genuine question whether treatment of self-reported symptoms is the appropriate criterion for determining whether SARS-CoV-2 causally contributed to a patient’s hospital admission.
In another retrospective review of a large database of electronic health records from 960 U.S. hospitals, the estimated proportions of incidental COVID-19 admissions during the Omicron BA.1 surge in January 2022 were: 25% among elderly patients aged 65 years or more, 45% among adults aged 19–64 years and 65% among pediatric patients aged 18 years or younger [15]. Patients were classified as incidental if they tested positive on admission but had no record of treatment for COVID-19 or a related respiratory condition. The search criteria did not include nirmatrelvir-ritonavir (Paxlovid™), an oral antiviral combination that entered into widespread use upon its emergency approval by the U.S. Food and Drug Administration in December 2021 [16]. As with other retrospective searches of electronic health records, this study did squarely confront the fundamental issue of causation.
The aim of the present study is to inquire whether the gap in our understanding of the extent of incidental hospitalizations during the Omicron BA.1 wave in the United States can be filled with the tools of integrative epidemiology, an emerging discipline that combines diverse, often unconventional data sources to attack knotty problems of disease causation [17,18].
To that end, we developed a formula to estimate the fraction of incidental COVID-19 hospitalizations that relies upon objectively measurable, population-based parameters. We applied this approach to a longitudinal panel of 164 counties throughout the United States, which contained 250 high-COVID-volume hospitals. Our analysis covered the 4-week interval ending in the first week of January 2022, during which time the Omicron BA.1 variant of SARS-CoV-2 was far and away the dominant strain [19].
2. Materials and Methods
2.1. Defining the Fraction of Incidental COVID Hospitalizations,
We developed a model to formally define the fraction of incidental COVID-19 hospitalizations and to express this fraction as a function of observable quantities. To that end, consider a closed population at a particular point in time. We refer to any individual within this population who has a detectable SARS-CoV-2 infection at that time as a COVID-19 case. This definition does not require that the individual’s infection has in fact been detected, either via a positive test or any other means. Nor does it require that the individual has any symptoms of COVID-19.
Let (where 1 > > 0) denote the proportion of all COVID-19 cases that are hospitalized because of their illness. For shorthand, we refer to these as the severe cases. We refer to all other COVID-19 cases, whether they are hospitalized or not, as non-severe. While the severe cases are, by definition, hospitalized because of their COVID-19 illness, some of the remaining non-severe cases will also be hospitalized for non-COVID reasons. We refer to the latter group as incidental COVID-19 hospitalizations. Let denote the proportion of non-severe cases that are hospitalized, where 1 > > 0. Then, by Bayes’ rule, the proportion of all COVID-19 hospitalizations that are incidental is:
We refer to as the fraction of incidental COVID-19 hospitalizations. We see that is increasing in and decreasing in .
2.2. Rendering , the Fraction of Incidental Hospitalizations, as a Function of Observables
Ideally, one would estimate the quantity in Equation (1) by longitudinally following a closed cohort of individuals as they contracted COVID-19 and then observing who was hospitalized because of their illness. Within this longitudinal cohort, we could also estimate by tracking who was hospitalized for other reasons. In the absence of such a longitudinal study, we can still proceed provided we make a key simplifying assumption, namely, that those individuals without COVID-19 have the same probability of hospitalization as those with non-severe COVID-19. We examine the validity of this key assumption later in the Discussion.
Let (where 1 > > 0) denote the proportion of individuals in the entire population who are COVID-19 cases. We refer to as the prevalence of COVID-19. Now focus more sharply on just those individuals who are hospitalized. We test every hospitalized patient to determine who is COVID-19-positive and who is COVID-19-negative. Let (where 1 > > 0) denote the fraction of all hospitalized individuals who are COVID-19-positive. In the Supplementary Materials, we derive the following expression for :
In Equation (2), π is now a function of the prevalence p of COVID-19 in the population, the fraction c of all hospitalized patients who are COVID-19-positive, and the proportion q of COVID-19-positive individuals requiring hospitalization because of their disease. Table 1 below summarizes these points.

Table 1.
Component Parameters in the Derivation of the Fraction of Incidental Hospitalizations, .
2.3. Rendering , the Prevalence of COVID-19, as a Function of Observables
To estimate the prevalence , we would ordinarily rely upon the classic formula in epidemiology, that is, prevalence equals the incidence of SARS-CoV-2 infection per unit time multiplied by the average duration of infection. The difficulty with this formula is that it applies only to a population with a stable incidence rate, and that is certainly not the case here [20].
Let denote the incidence rate of SARS-CoV-2 infection at time . We assume that incidence is growing exponentially, that is, , where . Let the duration of infection have an exponential distribution with mean , where > 0. In the Supplementary Materials, we show that the prevalence of infection can be approximated as:
When the incidence is stable (that is, ), this formula collapses to the classic result = . To estimate the prevalence during an exponentially growing epidemic, we therefore need data on the incidence , the growth rate of infection and the mean duration of infection.
It is widely acknowledged that COVID-19 cases have been and continue to be significantly underreported [21,22,23,24]. Accordingly, to estimate the incidence from data on reported cases, we follow the usual approach of incorporating an under-ascertainment factor into our analysis [25]. Let denote the reported incidence of COVID-19 and let > 0 denote the fraction of COVID cases that are reported at time . Then, we have:
Accordingly, to estimate , we need data on the parameters , , and , as indicated in Table 2. For clarity, we have dropped the time argument from the functions , and .

Table 2.
Component Parameters in the Derivation of COVID Prevalence, .
2.4. Data: Cohort of 250 High-COVID-Volume Hospitals
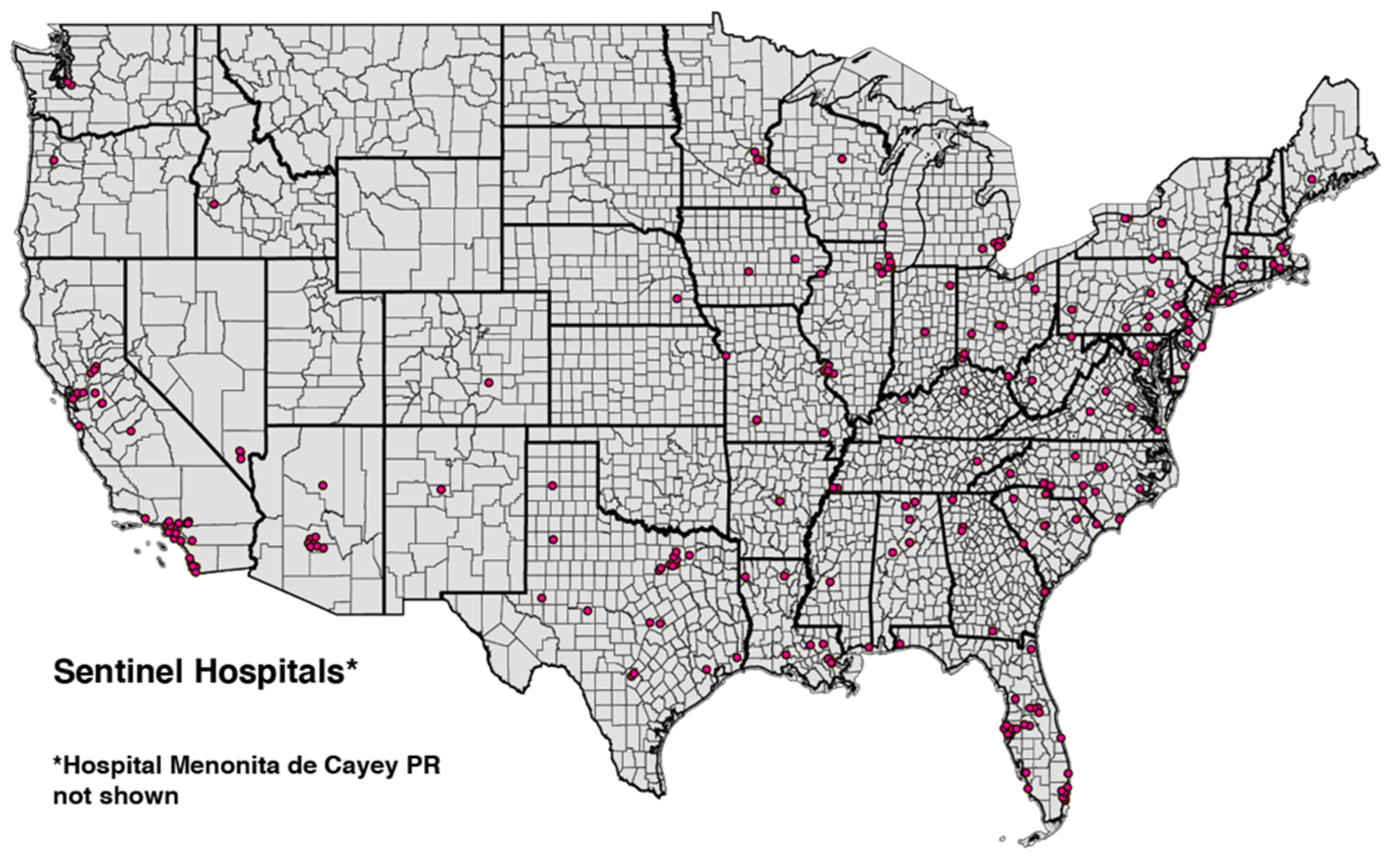
From a database of U.S. hospitals maintained by the Department of Health and Human services (HHS) [26], we identified the 250 hospitals with the highest cumulative volume of emergency department visits for COVID-19 from the week ending 25 June 2021 through the week ending 10 December 2021. These high-volume hospitals occupied 164 counties in 41 states and territories. Figure 1 displays a map of hospital locations within the continental United States.
Figure 1.
U.S. Continental Map Showing Locations of 249 of the 250 High-Volume Hospitals. Hospital Menonita de Cayey, Cayey, Puerto Rico, is not shown. State and county boundaries are indicated.
The hospital database maintained by HHS reported data on a weekly basis. For each of the 250 high-volume hospitals, we extracted the following three variables from the week ending 25 June 2021 through the week ending 7 January 2022.
- inpatient_beds_used_7_day_avg, defined as “Average of total number of staffed inpatient beds that are occupied reported during the 7-day period”.
- total_adult_patients_hospitalized_confirmed_covid_7_day_avg, defined as “Average number of patients currently hospitalized in an adult inpatient bed who have laboratory-confirmed COVID-19, including those in observation beds. This average includes patients who have both laboratory-confirmed COVID-19 and laboratory-confirmed influenza”.
- total_pediatric_patients_hospitalized_confirmed_and_suspected_covid_7_day_avg, defined as “Average number of patients currently hospitalized in a pediatric inpatient bed, including NICU, PICU, newborn, and nursery, who are suspected or laboratory-confirmed-positive for COVID-19. This average includes those in observation beds reported in the 7-day period”.
2.5. Estimating , the Fraction of Hospitalized Patients Who Are COVID-Positive
For each hospital and each week, we calculated the number of COVID-19-positive patients as the sum of variables (ii) and (iii) above, while variable (i) gave the total number of hospital patients. For each week, summing over all 250 hospitals, we computed cohort-wide numbers of COVID-19-positive inpatients and total inpatients, from which we then computed , the fraction of all inpatients who were COVID-19-positive. While we display the entire timeline of the fraction in Figure 2 below, we relied on the most recently available value of for the week ending 7 January 2022 in our calculations of the fraction of incidental hospitalizations .
2.6. Data: Confirmed COVID Incidence in 164 Counties
For each of the 164 counties covering our cohort of 250 high-volume hospitals, we downloaded weekly reported COVID-19 incidence from the Counties tab of Excel spreadsheets regularly issued by the White House COVID-19 Team as Community Profile Reports [27]. We extracted three variables from each report: Population, Cases—last 7 days and Cases per 100 k—last 7 days. These variables were extracted from the reports of December 20 (covering the week from 13 to 19 December), 27 December (covering 20–26 December), 3 January (covering 27 December–2 January) and January 10 (covering 3–9 January). The resulting database contained a balanced panel of 4 serial observations on each of 164 counties. We show a whiskers-on-box plot of the variable Cases per 100 k—last 7 days in Figure 3 below.
2.7. Estimating the Exponential Rate of Increase in COVID Incidence, , and Reported COVID Incidence,
We relied upon the county-specific data derived from the Community Profile Reports [27] to estimate the exponential rate of increase in COVID-19 incidence, . To that end, we ran the following log-linear fixed-effects regression model on our panel of 4 serial weekly observations (indexed = 1,…,4) on 164 counties (indexed = 1,…,164):
In Equation (5), the observations correspond to the data variable Cases—last 7 days, while the observations represent the ending date of each of the four weeks. The parameter is an overall constant term, the parameter is a county-specific fixed effect and the term is a spherical error. The parameter is estimated by ordinary least squares.
To test whether the data adequately fit an exponential growth model, we plotted the reported incidence for all 164 counties combined against to check for serial correlation of residuals. The reported incidence was computed as = , where denotes Cases per 100 k—last 7 days and denotes Population. We show a plot of versus in Figure 4 below. While we used a continuous-time model to develop our formula for prevalence in Equation (3), here we use the discrete time notation to refer to computations made from the panel of 164 observations in each of 4 successive weeks.
2.8. Estimating the Fraction of COVID-19 Cases Reported, , and Actual COVID-19 Incidence,
For our estimate of the fraction of COVID-19 cases reported, , we relied upon estimates issued by the Institute for Health Metrics and Evaluation (IHME) [28]. The authors estimated that by late December 2021 and early January 2022, , what they termed the “infection-detection rate”, had fallen to 0.25. (See Figure 8-1 in [28]). By relying on reported COVID-19 incidence rather than actual COVID-19 incidence to estimate above, we effectively assumed to be constant during the 4 weeks covered by our panel, and we do so here as well. Thus, for each week = 1, …,4, we compute actual incidence as = = .
2.9. Estimating q, the Proportion Hospitalized
A contemporaneous technical report from the UK Health Security Agency [29] noted that 3019 Omicron cases were hospitalized among 528,176 Omicron cases total, which gives 0.006. While hospitalization practices may differ in the UK’s National Health Service, this source has the advantage that the denominator closely approximated all Omicron infections, and not just symptomatic cases.
2.10. Aggregate and County-Specific Estimates of the Fraction of Incidental Hospitalizations,
We estimated the fraction of incidental hospitalizations not only for all 164 counties combined, but also for each county individually. We focused on the first week in January, that is, for the final week = 4 in our four-week panel. For the aggregate calculation, we estimated the reported incidence and the proportion of hospitalized patients who were COVID-19-positive for all counties combined. (Since we are focusing on a specific week, we have dropped the subscripts ). Given the estimated parameters , , and , we then computed an aggregate value of . For the county-specific calculations, we estimated the reported incidence and the proportion of hospitalized patients who were COVID-positive for each county (again dropping the subscript ). Assuming the parameters , , θ and to be constant across counties, we then computed county-specific values for each .
3. Results
3.1. Aggregate Fraction of Hospitalized Patients Who Are COVID-19-Positive,
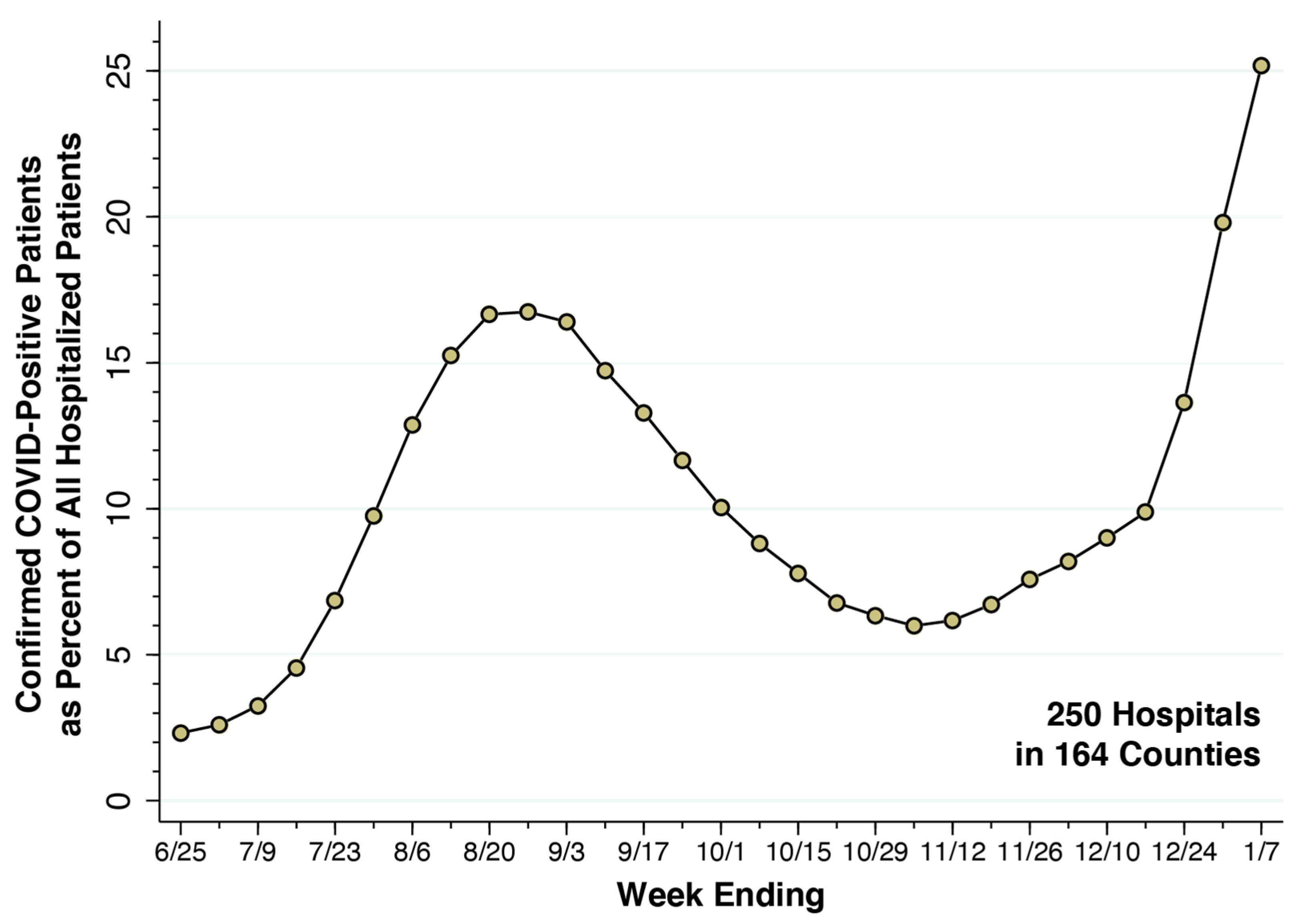
From the week ending 25 June 2021 through the week ending 7 January 2022, Figure 2 plots the fraction of all hospital inpatients who were COVID-19-positive in our cohort of 250 high-volume hospitals. During the summer’s Delta wave, this fraction rose to 16.74 percent for the week ending 27 August 2021. During the Omicron wave, the fraction rose rapidly, rising to 25.17 percent for the week ending 7 January 2022. We took the latter datum as our estimate of the parameter . Accordingly, about one in four inpatients of our cohort of 250 high-COVID-volume hospitals was hospitalized.
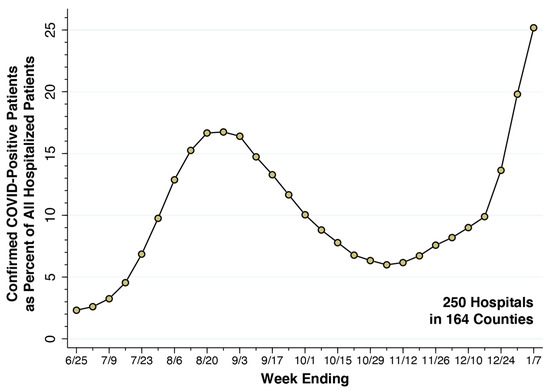
Figure 2.
Fraction of All Inpatients Who Were COVID-Positive in a Cohort of 250 High-Volume Hospitals, Weeks Ending 25 June 2021 through 7 January 2022. Source: U.S. Department of Health and Human Services [26]. For the most recent week ending 7 January 2022, the fraction of inpatients who were COVID-positive was 0.2517, which we took as the value of the parameter . The corresponding odds ratio was = 0.336. The underlying data are given in Supplementary Table S1.
Figure 2.
Fraction of All Inpatients Who Were COVID-Positive in a Cohort of 250 High-Volume Hospitals, Weeks Ending 25 June 2021 through 7 January 2022. Source: U.S. Department of Health and Human Services [26]. For the most recent week ending 7 January 2022, the fraction of inpatients who were COVID-positive was 0.2517, which we took as the value of the parameter . The corresponding odds ratio was = 0.336. The underlying data are given in Supplementary Table S1.
3.2. Growth Rate of COVID-19 Incidence, 164 Counties
Figure 3 displays a whiskers-on-box plot of reported weekly COVID-19 incidence in the 164 counties containing our 250-hospital cohort. The plot covers the four weeks of data through the week ending 9 January 2022. During the latter week, Potter County, TX, which includes Amarillo, was at the 25th percentile of reported incidence (1069 cases per 100,000 population). Baltimore County, MD was at the median (1466 cases per 100,000 population), and Hartford County, CT was at the 75th percentile (1858 cases per 100,000 population). The two counties with the highest reported incidence were Miami-Dade County, FL (4065 per 100,000 population) and Bronx County, NY (3865 per 100,000 population).
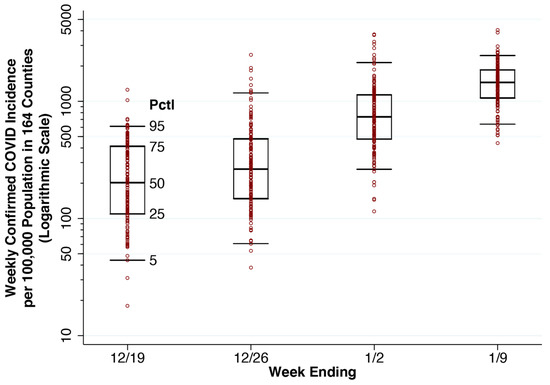
Figure 3.
Whiskers-on-Box Plots of Weekly Confirmed COVID-19 Incidence per 100,000 Population in the 164 Counties Containing the 250 Study Hospitals, Weeks Ending 19 December 2021 Through 9 January 2022. For each week, the 5th, 25th, 50th, 75th and 95th percentiles are superimposed upon the individual county-specific datapoints. A total of 11 datapoints with zero incident cases are omitted from the first two weeks.
Figure 3.
Whiskers-on-Box Plots of Weekly Confirmed COVID-19 Incidence per 100,000 Population in the 164 Counties Containing the 250 Study Hospitals, Weeks Ending 19 December 2021 Through 9 January 2022. For each week, the 5th, 25th, 50th, 75th and 95th percentiles are superimposed upon the individual county-specific datapoints. A total of 11 datapoints with zero incident cases are omitted from the first two weeks.
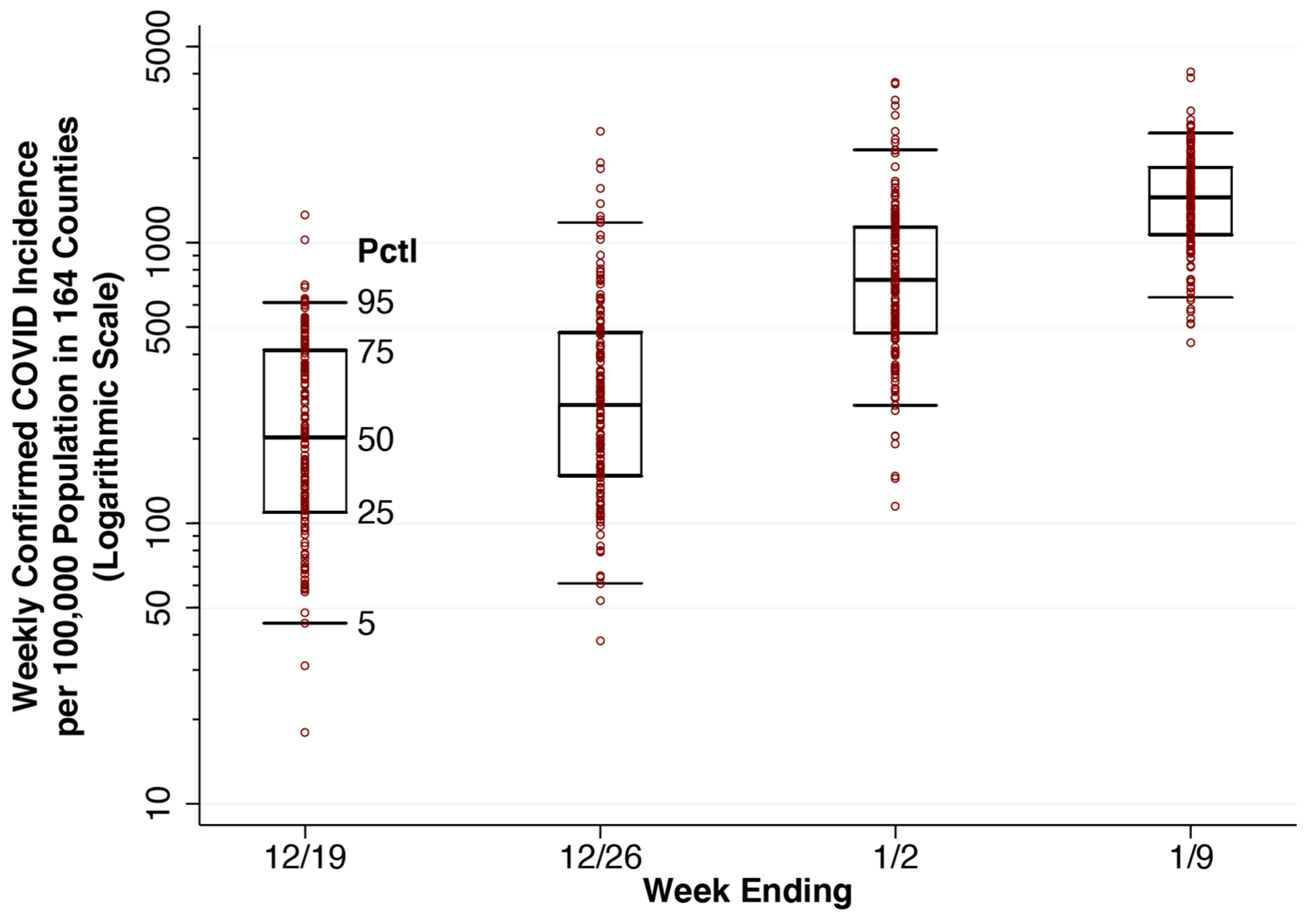
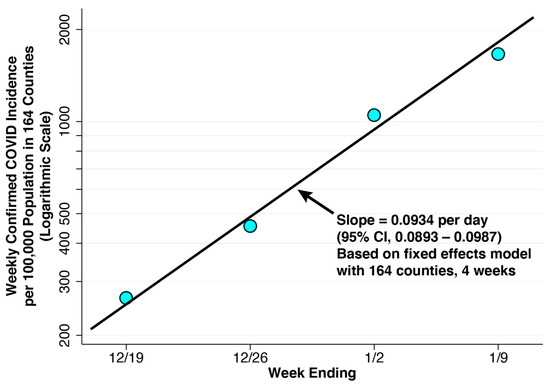
Figure 4.
Weekly Confirmed COVID-19 Incidence per 100,000 Population in the 164 Counties Containing the 250 Study Hospitals, Weeks Ending 19 December 2021 Through 9 January 2022. The superimposed line has a slope of 0.0934 per day, derived from a linear fixed effects regression model of the logarithm of weekly COVID-19 cases versus the date on which each week ended (Equation (5)). The balanced panel had 4 observations for each of 164 counties. No serial correlation of residuals is evident.
Figure 4.
Weekly Confirmed COVID-19 Incidence per 100,000 Population in the 164 Counties Containing the 250 Study Hospitals, Weeks Ending 19 December 2021 Through 9 January 2022. The superimposed line has a slope of 0.0934 per day, derived from a linear fixed effects regression model of the logarithm of weekly COVID-19 cases versus the date on which each week ended (Equation (5)). The balanced panel had 4 observations for each of 164 counties. No serial correlation of residuals is evident.
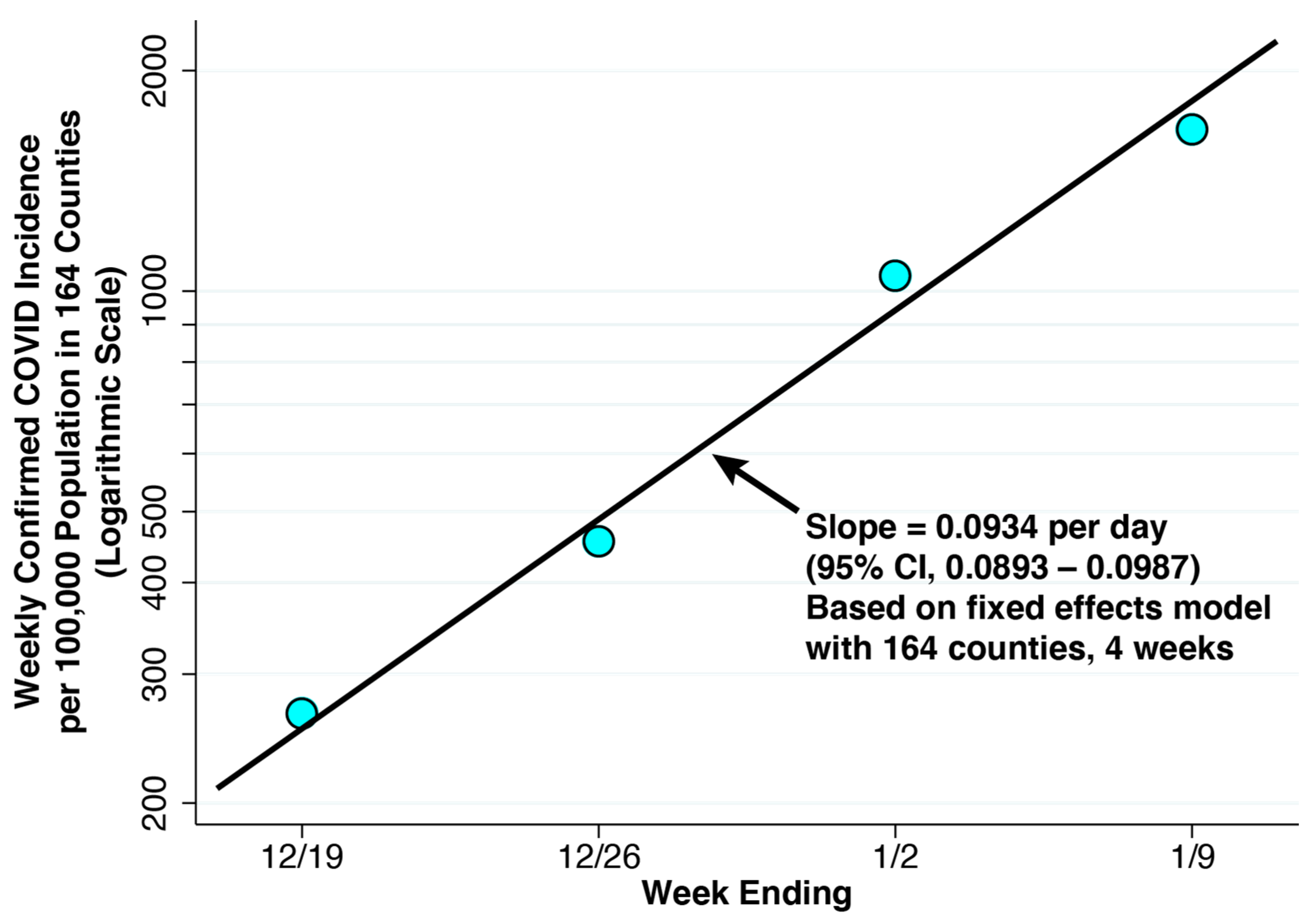
For the same four weeks, Figure 4 plots the population-weighted mean values of confirmed COVID-19 incidence among all 164 counties combined. These values represent our estimates of reported incidence . The fixed-effect log linear regression described on our panel of 164 counties over 4 weeks, described in Equation (5), gave an estimate of = 0.0934 per day (95% confidence interval: 0.0893–0.0987). The fitted line in the figure with the same slope shows no evidence of the serial correlation of residuals, thus supporting a model of recent exponential growth.
3.3. Actual COVID-19 Incidence and Prevalence, 164 Counties
For the most recent week ending 9 January 2022, the mean weekly confirmed COVID-19 incidence for the 164 counties in our panel was 1663 per 100,000 population (Figure 3), which comes to a reported incidence of r = 237.6 per 100,000 per day. Given our estimate of the fraction of cases reported at = 0.25, we obtain an estimate of actual incidence of = = 950.4 per 100,000 per day.
The U.S. Centers for Disease Control and Prevention (CDC) has estimated that the mean duration of infectiousness for Omicron is 5–6 days [30]. That gives us = = 0.182. From Equation (3), we thus estimate actual COVID prevalence in our panel of 164 counties during the most recent week ending January 9 to be , which comes to 3.45 percent of the population.
3.4. Estimated Fraction of Incidental Hospitalizations
Given estimates of the parameters = 0.006, = 0.2517 and = 0.0345, we relied upon Equation (2) to compute = 0.106. That is, we estimated 10.6 percent incidental COVID hospitalizations for the 164 counties containing our high-volume hospital cohort during the first week of January 2022. Our calculations are summarized in Table 3.

Table 3.
Estimates of Component Parameters in the Derivation of the Fraction of Incidental Hospitalizations, .
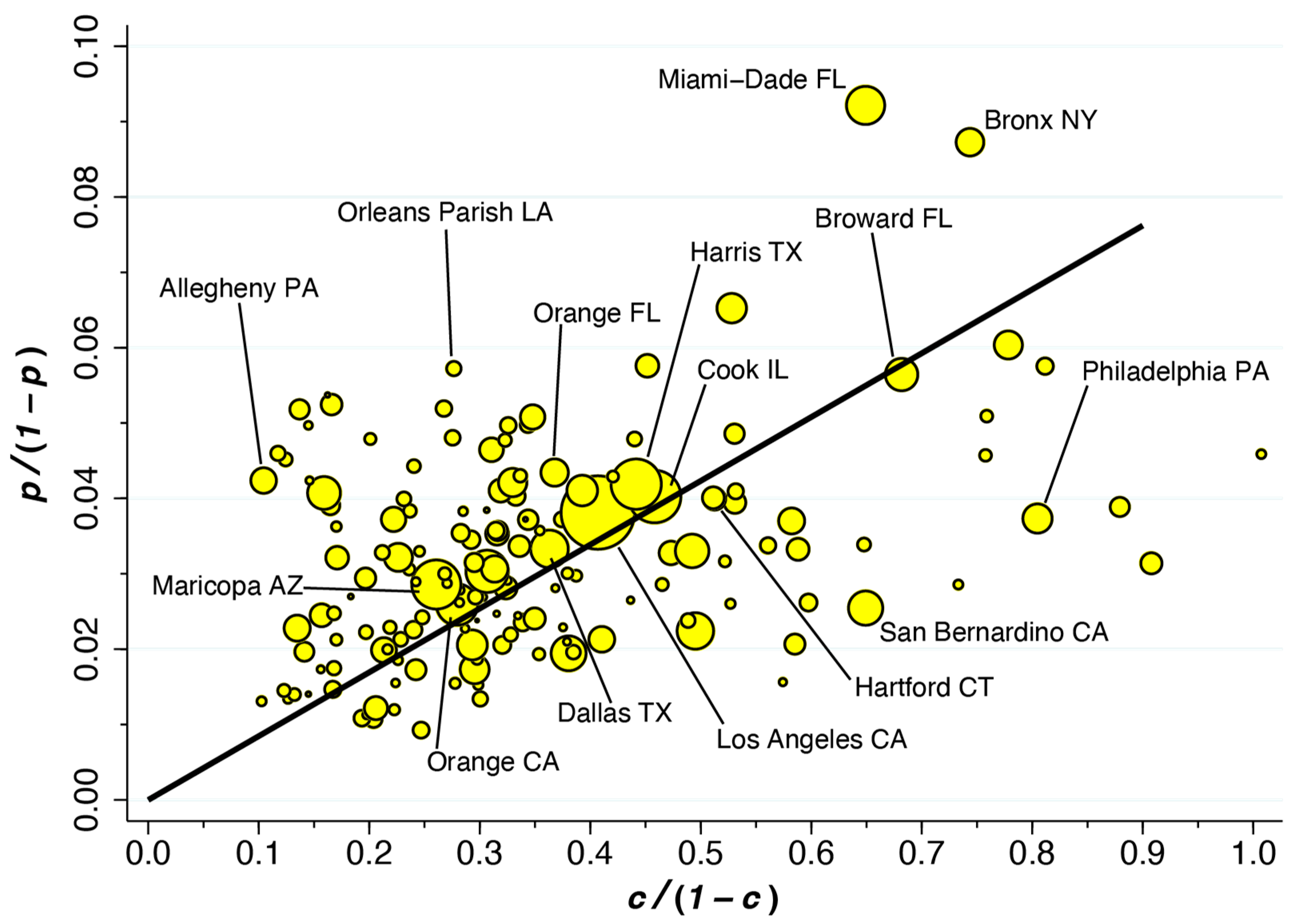
3.5. Variability of the Fraction of Incidental Hospitalizations, , across Counties
Our overall estimate of = 10.6 percent is an average that does not capture its variability across the 164 counties under study. When we applied the formula of Equation (2) individually to each county, once again restricting the computations to the first week of January 2022, the median value of was 9.5 percent, with an interquartile range of 6.7 to 12.7 percent.
Figure 5 displays this variability in the form of a scatterplot of versus for 162 of 164 counties. If all counties had the same value of , they would line up along the superimposed ray from the origin. Those datapoints above the ray have higher values of , while those below the ray have lower values. Geometrically speaking, the value of at each datapoint depends on the slope of the ray drawn from that point back to the origin. Thus, Allegheny County, PA, which houses Pittsburgh, has the highest estimated value of 40.4 percent. By contrast, while Miami-Dade has the highest estimated prevalence of = 8.4 percent, the estimated fraction of incidental COVID hospitalizations is 14.1 percent. As Equation (2) shows, when the overall prevalence of COVID-19 in the county is higher, the fraction of incidental COVID-19 hospitalizations tends to be higher. However, as hospitals in the county fill up with COVID-19 patients, thus pushing higher, the fraction of incidental COVID hospitalizations tends to go lower.
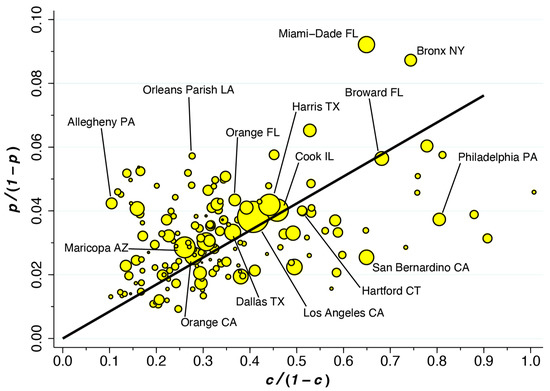
Figure 5.
Plot of Versus Among 162 of 164 Counties During the First Week of January 2022. The ray from the origin has slope 0.085 (95% CI: 0.079–0.090) based on a population-weighted regression. = prevalence of COVID; = fraction of hospitalized patients who are COVID-positive.
4. Discussion
Assuming only one-quarter (that is, = 0.25) of all Omicron infections were reported by public authorities, we estimated the aggregate prevalence of active SARS-CoV-2 infection during the first week of January to be 3.45% (that is, = 0.0345). During the same week, among 250 high-COVID-volume hospitals within our 164-county panel, an estimated one in four inpatients were found to be COVID-19-positive (more precisely, = 0.2517). Applying these estimates to our population-based formula, we found that among all COVID-19-positive hospitalized patients in all 164 counties combined, an estimated 10.6% were incidental infections (that is, = 0.106). Across individual counties, the median fraction of incidental COVID-19 hospitalizations was 9.5%, with an interquartile range of 6.7 to 12.7%.
4.1. Limitations
The fundamental drawback of the current study is that it is a modeling exercise. It does not adhere to the standard of a prospective study of hospitalized COVID-19 patients set forth in the Introduction. As a modeling exercise, its principal limitations include uncertainty in the fraction of reported cases; its critical but non-testable assumption about the equality of hospitalization rates; its reliance on non-U.S. data sources for assessing the probability that a SARS-CoV-2 infected individual would have a severe case requiring hospitalization; and the significant variability in the estimated fraction of incidental cases across counties. Each of these limitations is addressed in turn.
4.2. Fraction of Reported COVID-19 Cases, , as a Principal Source of Uncertainty
The principal source of uncertainty in our estimate of incidental COVID-19 hospitalization is the fraction of reported COVID cases. During the Omicron wave, as noted above, this fraction declined to 25 percent of actual cases [28], in great part as a result of a surge in asymptomatic and mildly symptomatic infections, as well as an increasing proportion of rapid test results not tabulated by public health authorities. Our formula for the prevalence of infection (Equation (3)) is a linear function of the actual incidence , which is in turn directly proportional to the inverse of (Equation (4)). Thus, if only 20 percent of cases were reported (that is, = 0.20), our estimate of COVID-19 prevalence would increase to 4.3 percent and the resulting estimate of the fraction of incidental hospitalizations would rise to 13.3 percent. At the other extreme, if as many as 50 percent of cases were reported (that is, = 0.50), our estimate of COVID-19 prevalence would drop to 1.39 percent and the resulting estimate of would fall to 4.1 percent.
4.3. Equality of Hospitalization Rates as a Critical Assumption
In the derivation of our population-based formula, we made the key assumption that those individuals without COVID-19 have the same probability of hospitalization as those with non-severe COVID-19. Strict adherence to the notion of causality would seem to require this assumption. If a concurrent COVID-19 infection increases the probability of hospitalization, then the infection has a causal role in the hospitalization and is thus not incidental. If a hospitalization is purely incidental, then the probability of hospitalization would be the same with or without COVID-19.
Consider again our patient with hypertensive heart disease who, as a result of high fevers and dehydration from an Omicron infection, develops atrial fibrillation, a cardiac arrhythmia. He needs to be hospitalized to get his heart rate down and convert his heart rhythm back to normal. His would not be an incidental COVID-19 hospitalization. Consider instead a patient with a history of extreme myopia since childhood who suffers a spontaneous retinal detachment and is hospitalized for eye surgery. During her admission workup, she is found to be COVID-19-positive. Since the infection did not apparently affect her probability of hospitalization, hers would be an incidental COVID-19 hospitalization. (This is not to deny that the COVID-19 epidemic has delayed the time when patients have sought treatment and thus affected the severity of vision loss upon initial presentation [31]).
The problem with the foregoing logic is that individuals in the two groups—those without COVID-19 and those with non-severe COVID-19—are different people. Thus, one individual with a white-collar occupation may be able to work remotely and does not contract COVID-19. Another individual with a blue-collar occupation cannot work remotely and comes down with a non-severe infection at work. The latter individual may also have a higher risk of hospitalization from an onsite workplace accident.
Generalizing our formula for , we suppose that those without a COVID-19 infection have a hospitalization probability that is not necessarily equal to the hospitalization probability of those with non-severe COVID-19. Then, the fraction of incidental COVID-19 hospitalizations becomes , where is the relative risk of hospitalization between the two groups and is as already defined in Equation (2). This does not mean that our strong assumption that = 1 necessarily understates . In the foregoing example comparing white-collar and blue-collar workers, < 1 and, thus, would be overstated. More generally, when those individuals who take precautions to reduce the risk of infection, such as getting vaccinated [32], also tend to adopt other preventive measures to reduce the risk of hospitalization generally, such as not smoking, our strong assumption that = 1 will tend to overstate .
4.4. Reliability of the Estimate of from UK Data
Relying on data reported by the UK Health Security Agency [29], we took , the probability that an infected individual would have a severe case requiring hospitalization, as 0.006. It is worth inquiring whether there are any U.S.-based sources that might provide a more reliable estimate. The difficulty is that the computation of needs to be based upon a population denominator that includes all cases of COVID-19, even asymptomatic and unreported cases.
A study of symptomatic patients infected with the Omicron variant in the Houston Methodist hospital system [33] revealed that out of 2232 symptomatic patients, 313 were admitted to the hospital. This source, it would seem, yields an estimate of = 0.14, which is an order of magnitude greater than the estimate derived from the UK data. The problem with relying upon this alternative data source is that the denominator reflects symptomatic patients primarily presenting to the emergency department, rather than all COVID-19 cases. In fact, the percentage reported by Houston Methodist is quite close to the rate of 15.1 hospital admissions per 100 emergency department visits for COVID that we computed from our 250-hospital cohort, as shown in Supplementary Figure S1.
4.5. Variability of across Counties
While our aggregate measure of the incidental COVID-19 fraction for all 164 counties is on the order of 11 percent, Figure 5 shows significant variability across individual counties. Some of this variability is no doubt due to sampling errors in the proportions of inpatients who were COVID-19-positive, especially in counties with only one high-volume hospital. Our assumption that the fraction of COVID-19 cases reported was uniform across counties may have introduced errors in the county-specific incidence estimates = . Likewise, our assumption that the exponential rate of increase in COVID-19 incidence was uniform across counties may have introduced errors in the county-specific prevalence estimates = .
Still, some of the variability of the variability may be due to systemic differences between hospitals in their range of services and patient populations. For example, a hospital may have a large, specialized transplant service with many immunosuppressed patients who are persistently COVID-19-positive.
4.6. Extensions
We chose to analyze the time period from December 2021 to January 2022, when COVID-19 incidence was exponentially rising (Figure 3 and Figure 4). This choice allowed us to apply a simplified formula (Equation (3)) to estimate the prevalence of the disease. We focused on the Omicron BA.1 wave, when the reported incidence of infection was markedly higher than that in other waves thus far observed in the United States. During such a period of maximum disease prevalence, we would expect the fraction of incidental COVID-19 hospitalizations to likewise have approached its peak (Equation (2)). Still, our methodology can be extended to other time periods during the SARS-CoV-2 epidemic. Application to extended intervals when incidence is neither uniformly increasing nor decreasing would require a more complex model of disease prevalence, as derived in the Supplementary Materials.
We chose to analyze a longitudinal panel of 164 counties throughout the United States. This choice was intended to cover a representative, high-density, mostly urban population. Still, our methodology can be extended to other countries and to population subgroups based on demographic characteristics, occupation and vaccination status. Such extensions would require group-specific data on the prevalence of infection, which would in turn require group-specific estimates of the fraction underreported. We would also need group-specific data on the fraction of all hospitalized patients who are COVID-19 positive, as well as the proportion of infected individuals who are hospitalized because of their COVID-19 illness.
4.7. Policy Implications
Our findings imply that, in the aggregate, the burden of patients admitted for complications of their COVID-19 infections is far greater than the number of incidental cases. Consequently, real-time surveillance data on COVID-19 hospitalization rates can still be relied upon to gauge the clinical severity of the disease, as well as its impact on limited healthcare manpower [34,35], other limited healthcare resources [36,37,38], healthcare costs [39] and excess mortality [40].
Moreover, COVID-19 hospitalization rates can still be used as a reliable endpoint to evaluate public policies to reduce morbidity and mortality from the disease. In particular, the repeated observation [32,41] that populations with higher rates of vaccination against SARS-CoV-2 have lower risks of hospitalization similarly retains its public policy significance. Hospitalization rates can likewise continue to be used as an endpoint to assess the impact on non-pharmacological public policies [42].
5. Conclusions
Our population-based estimates suggest that incidental COVID-19 infections have indeed been a nontrivial fraction of all COVID-positive hospitalized patients, but they fall far short of the proportions suggested by some sources [2,3,4,7,10,14,15]. Even under our conservative assumption that there were four times as many COVID-19 infections as cases reported by public authorities, there were not nearly enough prevalent COVID-19 cases to push the fraction of incidental hospitalizations even close to the one-half mark suggested by those sources. Our examples of the patient with hypertensive heart disease who developed a cardiac arrythmia and the young man who got into a car crash as a result of their viral infections suggest that the indirect methodologies employed in prior studies have been overly restrictive in their assessment of COVID-19-caused admissions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/covid3050054/s1, Figure S1: Hospital Admissions for COVID-19 per 100 Emergency Department Visits in a Cohort of 250 High-Volume Hospitals, Weeks Ending 25 June 2021, through 7 January 2022. Table S1: Data Underlying Figure 2.
Funding
This research received no external funding.
Institutional Review Board Statement
This study relies exclusively on publicly available data that contain no individual identifiers. No human studies approval was required.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data and materials have been posted at Open Science Framework: https://osf.io/7umje/.
Acknowledgments
This article represents the sole opinion of its author and does not necessarily represent the opinions of the Massachusetts Institute of Technology, Eisner Health or any other organization.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
| IHME | Institute for Health Metrics and Evaluation |
| CDC | U.S. Centers for Disease Control and Prevention |
| HHS | U.S. Department of Health and Human Services |
| PCR | Polymerase Chain Reaction |
References
- Johns Hopkins University. United States. Data Timeline: Daily COVID-19 Hospitalizations; Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/region/united-states (accessed on 15 June 2022).
- Murray, S.G.; Croci, R.; Wachter, R.M. Is a Patient Hospitalized ‘with’ COVID or ‘for’ COVID? It Can Be Hard to Tell; Washington Post. Available online: https://www.washingtonpost.com/outlook/2022/01/07/hospitalization-covid-statistics-incidental/ (accessed on 7 January 2022).
- Jackson Health System. Jackson Health System Hospitals Currently Have 564 Patients Who Have Tested Positive for COVID-19 (Tweet). Twitter.com. Available online: https://twitter.com/JacksonHealth/status/1481268904970358790 (accessed on 12 January 2022).
- New York Governor. Governor Hochul Updates New Yorkers on State’s Progress Combating COVID-19 Press Release. Available online: https://www.governor.ny.gov/news/governor-hochul-updates-new-yorkers-states-progress-combating-covid-19-131 (accessed on 7 January 2022).
- Garrett, N.; Tapley, A.; Andriesen, J.; Seocharan, I.; Fisher, L.H.; Bunts, L.; Espy, N.; Wallis, C.L.; Randhawa, A.K.; Miner, M.D.; et al. High Asymptomatic Carriage With the Omicron Variant in South Africa. Clin. Infect. Dis. 2022, 75, e289–e292. [Google Scholar] [CrossRef]
- Mayr, E. Cause and effect in biology. Science 1961, 134, 1501–1506. [Google Scholar] [CrossRef]
- Massachusetts Department of Public Health. COVID-19 Hospitalizations (Powerpoint Presentation); Commonwealth of Massachusetts, Executive Office of Health and Human Services. Available online: https://www.mass.gov/doc/incidental-covid-19-report-january-25-2022/download (accessed on 25 January 2022).
- U.S. National Institutes of Health. Therapeutic Management of Hospitalized Adults with COVID-19; NIH COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/ (accessed on 24 February 2022).
- Chen, F.; Hao, L.; Zhu, S.; Yang, X.; Shi, W.; Zheng, K.; Wang, T.; Chen, H. Potential Adverse Effects of Dexamethasone Therapy on COVID-19 Patients: Review and Recommendations. Infect. Dis. Ther. 2021, 10, 1907–1931. [Google Scholar] [CrossRef]
- Klann, J.G.; Strasser, Z.H.; Hutch, M.R.; Kennedy, C.J.; Marwaha, J.S.; Morris, M.; Samayamuthu, M.J.; Pfaff, A.C.; Estiri, H.; South, A.M.; et al. Distinguishing Admissions Specifically for COVID-19 from Incidental SARS-CoV-2 Admissions: National Retrospective Electronic Health Record Study. J. Med. Internet Res. 2022, 24, e37931. [Google Scholar] [CrossRef]
- Legrand, M.; Bell, S.; Forni, L.; Joannidis, M.; Koyner, J.L.; Liu, K.; Cantaluppi, V. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol. 2021, 17, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Landstra, C.P.; de Koning, E.J.P. COVID-19 and Diabetes: Understanding the Interrelationship and Risks for a Severe Course. Front. Endocrinol. 2021, 12, 649525. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Khakwani, M.; Venkatadasari, I.; Horgan, C.; Giles, H.; Jobanputra, S.; Lokare, A.; Ewing, J.; Paneesha, S.; Murthy, V. Thrombocytopenia as an initial manifestation of COVID-19; case series and literature review. Br. J. Haematol. 2020, 189, 1057–1058. [Google Scholar] [CrossRef] [PubMed]
- Voor In ‘t Holt, A.F.; Haanappel, C.P.; Rahamat-Langendoen, J.; Molenkamp, R.; van Nood, E.; Van den Toorn, L.M.; Peeters, R.P.; van Rossum, A.M.C.; Severin, J.A. Admissions to a large tertiary care hospital and Omicron BA.1 and BA.2 SARS-CoV-2 PCR positivity: Primary, contributing, or incidental COVID-19. Int. J. Infect. Dis. 2022, 122, 665–668. [Google Scholar] [CrossRef]
- Thayer, J.; Hirz, K.W.; Sandberg, N.; Berg, D.V.; Miller, A.R.; Posner, X.; Dalecki, A. A Bright Side: Hospitalizations for COVID-19 Might Be Overcounted, Especially Among Kids; Epic Research. Available online: https://epicresearch.org/articles/a-bright-side-hospitalizations-for-covid-19-might-be-overcounted-especially-among-kids (accessed on 4 May 2022).
- U.S. Food & Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19 (accessed on 22 December 2021).
- Spitz, M.R.; Wu, X.; Mills, G. Integrative epidemiology: From risk assessment to outcome prediction. J. Clin. Oncol. 2005, 23, 267–275. [Google Scholar] [CrossRef]
- Spitz, M.R.; Caporaso, N.E.; Sellers, T.A. Integrative cancer epidemiology—The next generation. Cancer Discov. 2012, 2, 1087–1090. [Google Scholar] [CrossRef]
- U.S. Centers for Disease Control and Prevenetion. COVID Data Tracker: Variant Proportions. Available online: https://covid.cdc.gov/covid-data-tracker/#variant-proportions (accessed on 20 January 2022).
- Alho, J.M. On prevalence, incidence, and duration in general stable populations. Biometrics 1992, 48, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Danuser, G. Estimation of the fraction of COVID-19 infected people in U.S. states and countries worldwide. PLoS ONE 2021, 16, e0246772. [Google Scholar] [CrossRef] [PubMed]
- Havers, F.P.; Reed, C.; Lim, T.; Montgomery, J.M.; Klena, J.D.; Hall, A.J.; Thornburg, N.J. Seroprevalence of Antibodies to SARS-CoV-2 in Six Sites in the United States, March 23-May 3, 2020; MedRxiv. Available online: https://www.medrxiv.org/content/10.1101/2020.06.25.20140384v1 (accessed on 26 June 2020).
- Moghadas, S.M.; Fitzpatrick, M.C.; Sah, P.; Pandey, A.; Shoukat, A.; Singer, B.H.; Galvani, A.P. The implications of silent transmission for the control of COVID-19 outbreaks. Proc. Natl. Acad. Sci. USA 2020, 117, 17513–17515. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E. Critical Role of the Subways in the Initial Spread of SARS-CoV-2 in New York City. Front. Public Health 2021, 9, 754767. [Google Scholar] [CrossRef]
- Harris, J.E. Los Angeles County SARS-CoV-2 Epidemic: Critical Role of Multi-generational Intra-household Transmission. J. Bioecon. 2021, 23, 55–83. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. COVID-19 Reported Patient Impact and Hospital Capacity by Facility; HealthData.gov. Available online: https://healthdata.gov/Hospital/COVID-19-Reported-Patient-Impact-and-Hospital-Capa/anag-cw7u (accessed on 17 January 2022).
- White House COVID-19 Team. COVID-19 Community Profile Report; HealthData.gov, Excel Files for 12/20/2021, 12/27/2021, 1/3/2022, 1/10/2022. Available online: https://healthdata.gov/Health/COVID-19-Community-Profile-Report/gqxm-d9w9, (accessed on 1 October 2022).
- Institute for Health Metrics and Evaluation (IHME). COVID-19 Results Briefing: United States of America, January 8. Available online: https://www.healthdata.org/sites/default/files/files/102_briefing_United_States_of_America_3.pdf (accessed on 17 January 2022).
- UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England—Technical briefing: Update on Hospitalisation and Vaccine Effectiveness for Omicron VOC-21NOV-01 (B.1.1.529). Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1045619/Technical-Briefing-31-Dec-2021-Omicron_severity_update.pdf (accessed on 31 December 2021).
- U.S. Centers for Disease Control and Prevenetion. Ending Isolation and Precautions for People with COVID-19: Interim Guidance. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html (accessed on 14 January 2022).
- Patel, L.G.; Peck, T.; Starr, M.R.; Ammar, M.J.; Khan, M.A.; Yonekawa, Y.; Klufas, M.A.; Regillo, C.D.; Ho, A.C.; Xu, D. Clinical Presentation of Rhegmatogenous Retinal Detachment during the COVID-19 Pandemic: A Historical Cohort Study. Ophthalmology 2021, 128, 686–692. [Google Scholar] [CrossRef]
- Harris, J.E. COVID-19 Incidence and Hospitalization During the Delta Surge Were Inversely Related to Vaccination Coverage Among the Most Populous U.S. Counties. Health Policy Technol. 2021, 11, 100583. [Google Scholar] [CrossRef]
- Christensen, P.A.; Olsen, R.J.; Long, W.; Snehal, R.; Davis, J.J.; Saavedra, M.O.; Reppond, K.; Shyer, M.N.; Cambric, J.; Gadd, R.; et al. Signals of Significantly Increased Vaccine Breakthrough, Decreased Hospitalization Rates, and Less Severe Disease in Patients with COVID-19 Caused by the Omicron Variant of SARS-CoV-2 in Houston, Texas; medRxiv. Available online: https://www.medrxiv.org/content/10.1101/2021.12.30.21268560v3 (accessed on 12 January 2022).
- Grimm, C.A.; Hospitals Reported That the COVID-19 Pandemic Has Significantly Strained Health Care Delivery (Report in Brief OEI-09-21-00140) U.S. Department of Health and Human Services, Office of the Inspector General. Available online: https://oig.hhs.gov/oei/reports/OEI-09-21-00140.pdf (accessed on 12 April 2023).
- Office of the Assistant Secretary for Policy and Evaluation. Mpact of the COVID-19 Pandemic on the Hospital and Outpatient Clinician Workforce: Challenges and Policy Responses (Issue Brief No. HP-2022-13); U.S. Department of Health and Human Services. Available online: https://aspe.hhs.gov/sites/default/files/documents/9cc72124abd9ea25d58a22c7692dccb6/aspe-covid-workforce-report.pdf (accessed on 12 April 2023).
- Janke, A.T.; Mei, H.; Rothenberg, C.; Becher, R.D.; Lin, Z.; Venkatesh, A.K. Analysis of Hospital Resource Availability and COVID-19 Mortality Across the United States. J. Hosp. Med. 2021, 16, 211–214. [Google Scholar] [CrossRef]
- Fox, S.J.; Lachmann, M.; Tec, M.; Pasco, R.; Woody, S.; Du, Z.; Wang, X.; Ingle, T.A.; Javan, E.; Dahan, M.; et al. Real-time pandemic surveillance using hospital admissions and mobility data. Proc. Natl. Acad. Sci. USA 2022, 119. [Google Scholar] [CrossRef]
- Anesi, G.L.; Kerlin, M.P. The impact of resource limitations on care delivery and outcomes: Routine variation, the coronavirus disease 2019 pandemic, and persistent shortage. Curr. Opin. Crit. Care 2021, 27, 513–519. [Google Scholar] [CrossRef]
- DeMartino, J.K.; Swallow, E.; Goldschmidt, D.; Yang, K.; Viola, M.; Radtke, T.; Kirson, N. Direct health care costs associated with COVID-19 in the United States. J. Manag. Care Spec. Pharm. 2022, 28, 936–947. [Google Scholar] [CrossRef] [PubMed]
- French, G.; Hulse, M.; Nguyen, D.; Sobotka, K.; Webster, K.; Corman, J.; Aboagye-Nyame, B.; Dion, M.; Johnson, M.; Zalinger, B.; et al. Impact of Hospital Strain on Excess Deaths during the COVID-19 Pandemic—United States, July 2020–July 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Havers, F.P.; Pham, H.; Taylor, C.A.; Whitaker, M.; Patel, K.; Anglin, O.; Kambhampati, A.K.; Milucky, J.; Zell, E.; Moline, H.L.; et al. COVID-19-Associated Hospitalizations Among Vaccinated and Unvaccinated Adults 18 Years or Older in 13 US States, January 2021 to April 2022. JAMA Intern. Med. 2022, 182, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ren, R.; Kattan, M.W.; Jehi, L.; Cheng, Z.; Fang, K. Public Health Interventions’ Effect on Hospital Use in Patients With COVID-19: Comparative Study. JMIR Public Health Surveill. 2020, 6, e25174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).