Impact of Clinical Aspects and Pathophysiology Mechanisms of Acute Kidney Injury on Outcomes of Patients Affected by COVID-19—A Retrospective Cohort Study
Abstract
1. Introduction
2. Objectives
3. Methodology
3.1. Patients and Methods
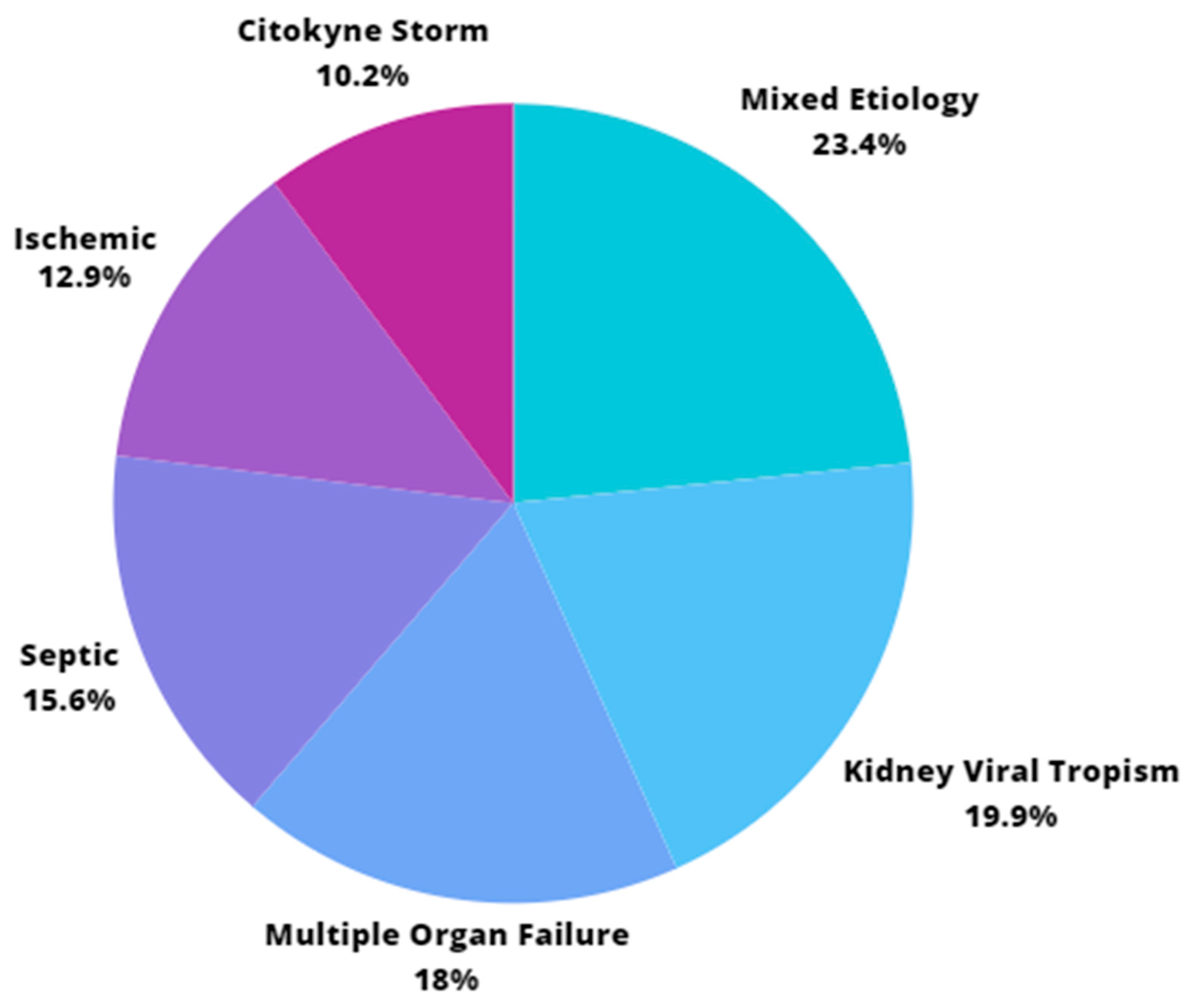
- Ischemic: when an ischemic insult (dehydration or hypotension) related to renal ischemia-reperfusion syndrome is identified, generating acute tubular necrosis [21]. It is associated with the presence of clinical signs of dehydration, hypotension, or requiring vasoactive drugs (with progression of the vasoactive drug weaning order after clinical measures) at the time of AKI diagnosis. They are related to conditions with no substantial changes in midstream (type 1) urine findings.
- Septic: a septic condition of fever, impaired consciousness, and multiple organ failure, including kidneys, with positive blood cultures for pathogens compatible with this condition. It is usually accompanied by significant neutrophilia with a left shift [20].
- Nephrotoxic due to rhabdomyolysis: when musculoskeletal cells lysis occurs due to COVID-19, releasing nephrotoxic content [22]. It is diagnosed when creatine phosphokinase (CPK) exceeds 5000 IU at the time of the AKI diagnosis. Due to its manifestation elucidated throughout the study, this etiology of AKI is no longer considered in isolation and is now included in the Mixed etiology of AKI since patients who develop AKI due to rhabdomyolysis, as demonstrated by our preliminary analyses, suffer from this condition in synergism with other AKI COVID-19-associated etiologies.
- Associated with Cytokine Storm [14,15,16]: a subtype of multiple organ failure that occurs when there is a defective immune response, mainly with intense production of IL-6, IL-8, αTNF, type I, and III interferon. It is diagnosed in the presence of a fever higher than 39 degrees Celsius for more than 12 h throughout the day, for 48 h consecutive to the diagnosis of AKI.
- Associated with COVID-19 Multiple Organ Failure: a condition in which there is failure of two or more organ systems, including the kidneys, concomitantly or sequentially [12,13,14]. It is diagnosed when, concomitantly with AKI, there is a need for mechanical ventilation and vasoactive drug escalation with great difficulty in the weaning order. Furthermore, laboratory parameters compatible with MOF may occur concomitantly.
- Associated with Kidney Viral Tropism: when the renal tubules and podocytes are directly affected by the entry of the SARS-CoV-2 virus [15,16,17]. Identified with a midstream (type 1) urine test, the presence of hematuria, proteinuria, leukocyturia, epithelial cell sediment, and casts is assessed. It is usually identified when there is new hematuria or a large increase in previously mild hematuria at the time of AKI diagnosis, with no systemic factors indicating overlapping etiologies.
- Mixed: when there is more than one mechanism associated with COVID-19. Example: Viral Tropism and Cytokine Storm [23].
3.2. Data Management and Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. 2023 Data.Who.Int, WHO Coronavirus (COVID-19) Dashboard > Data [Dashboard]. Available online: https://data.who.int/dashboards/covid19/data (accessed on 15 February 2022).
- Ahmadian, E.; Hosseiniyan Khatibi, S.M.; Razi Soofiyani, S.; Abediazar, S.; Shoja, M.M.; Ardalan, M.; Zununi Vahed, S. COVID-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev. Med. Virol. 2021, 31, 2176. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/rmv.2176 (accessed on 23 December 2023). [CrossRef] [PubMed]
- Braun, F.; Lütgehetmann, M.; Pfefferle, S.; Wong, M.N.; Carsten, A.; Lindenmeyer, M.T.; Nörz, D.; Heinrich, F.; Meißner, K.; Wichmann, D.; et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 2020, 396, 597–598. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Chaudhary, K.; Saha, A.; Chauhan, K.; Vaid, A.; Zhao, S.; Paranjpe, I.; Somani, S.; Richter, F.; Miotto, R.; et al. AKI in Hospitalized Patients with COVID-19. J. Am. Soc. Nephrol. 2021, 32, 151–160. [Google Scholar] [CrossRef] [PubMed]
- National Center for Emerging and Zoonotic Infectious Diseases (U.S.). Division of Healthcare Quality Promotion. Hospital Toolkit for Adult Sepsis Surveillance. 1 May 2018. Available online: https://stacks.cdc.gov/view/cdc/132387 (accessed on 29 March 2024).
- Magalhães, L.E.; de Oliveira, P.G.S.; Favarin, A.J.; Yuasa, B.K.; Cardoso, P.A.; Zamoner, W.; Ponce, D. Acute kidney injury in coronavirus infectious disease: A study of incidence, risk factors, and prognosis during the first wave of the disease in Brazil. Int. Urol. Nephrol. 2023, 55, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Delorey, T.M.; Ziegler, C.G.K.; Heimberg, G.; Normand, R.; Yang, Y.; Segerstolpe, Å.; Abbondanza, D.; Fleming, S.J.; Subramanian, A.; Montoro, D.T.; et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 2021, 595, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Kanneganti, T.D. Innate immunity: The first line of defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef]
- Menez, S.; Parikh, C.R. Overview of acute kidney manifestations and management of patients with COVID-19. Am. J. Physiol.-Ren. Physiol. 2021, 321, 403–410. [Google Scholar] [CrossRef]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef]
- Su, H.; Yang, M.; Wan, C.; Yi, L.X.; Tang, F.; Zhu, H.Y.; Yi, F.; Yang, H.C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Rubin, L.; Peng, T.; Liu, L.; Xing, X.; Lazarovici, P.; Zheng, W. Cytokine storm in COVID-19: From viral infection to immune responses, diagnosis and therapy. Int. J. Biol. Sci. 2022, 18, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Rovito, R.; Augello, M.; Ben-Haim, A.; Bono, V.; d’Arminio Monforte, A.; Marchetti, G. Hallmarks of severe COVID-19 pathogenesis: A pas de deux between viral and host factors. Front. Immunol. 2022, 13, 912336. [Google Scholar] [CrossRef] [PubMed]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Kleymenov, D.A.; Bykonia, E.N.; Popova, L.I.; Mazunina, E.P.; Gushchin, V.A.; Kolobukhina, L.V.; Burgasova, O.A.; Kruzhkova, I.S.; Kuznetsova, N.A.; Shidlovskaya, E.V.; et al. A Deep Look Into COVID-19 Severity through Dynamic Changes in Blood Cytokine Levels. Front. Immunol. 2021, 12, 771609. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.E.; Endre, Z.H. Definitions, phenotypes, and subphenotypes in acute kidney injury-Moving towards precision medicine. Nephrology 2023, 28, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Endre, Z.H.; Mehta, R.L. Identification of acute kidney injury subphenotypes. Curr. Opin. Crit. Care 2020, 26, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Acute Kidney Injury. KDIGO. 2012. Available online: https://kdigo.org/guidelines/acute-kidney-injury/ (accessed on 15 February 2022).
- Zheng, Q.Y.; Li, Y.; Liang, S.J.; Chen, X.M.; Tang, M.; Rao, Z.S.; Li, G.Q.; Feng, J.L.; Zhong, Y.; Chen, J.; et al. Light deficiency attenuates acute kidney disease development in an in vivo experimental renal ischemia and reperfusion injury model. Cell Death Discov. 2022, 8, 399. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Trinidad, R. Rhabdomyolysis: A syndrome to be considered. Med. Clin. 2022, 158, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, H.; Li, J.; Xu, B.; Xu, J. The mechanism of multiple organ dysfunction syndrome in patients with COVID-19. J. Med. Virol. 2022, 94, 1886–1892. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- Zamoner, W.; Santos, C.A.S.; Magalhães, L.E.; de Oliveira, P.G.S.; Balbi, A.L.; Ponce, D. Acute Kidney Injury in COVID-19: 90 Days of the Pandemic in a Brazilian Public Hospital. Front. Med. 2021, 8, 622577. [Google Scholar] [CrossRef]
- Yuasa, B.K.; Magalhães, L.E.; de Oliveira, P.G.S.; Yokota, L.G.; Cardoso, P.A.; Zamoner, W.; Balbi, A.L.; Ponce, D. Acute Kidney Injury in Elderly Patients with Coronavirus Infectious Disease: A Study of Incidence, Risk Factors, and Prognosis in Brazil. Front. Nephrol. 2022, 2, 896891. [Google Scholar] [CrossRef]
- Médicos são Recomendados a não Realizarem Autópsia em Casos de COVID-19. Jornal da USP no Ar. 2020. p. 1. Available online: https://jornal.usp.br/?p=311199 (accessed on 15 February 2022).
- Mumtaz, H.; Ejaz, M.K.; Tayyab, M.; Vohra, L.I.; Sapkota, S.; Hasan, M.; Saqib, M. APACHE scoring as an indicator of mortality rate in ICU patients: A cohort study. Ann. Med. Surg 2023, 85, 416–421. [Google Scholar] [CrossRef]
- Kumar, S.; Gattani, S.C.; Baheti, A.H.; Dubey, A. Comparison of the Performance of APACHE II, SOFA, and mNUTRIC Scoring Systems in Critically Ill Patients: A 2-year Cross-sectional Study. Indian J. Crit Care Med. 2020, 24, 1057–1061. [Google Scholar] [CrossRef]
- Liaño, F. Severity of acute renal failure: The need of measurement. Nephrol Dial Transplant. 1994, 9, 229–238. [Google Scholar] [PubMed]
- Balbi, A.L.; Gabriel, D.P.; Barsante, R.C.; Caramori, J.T.; Martin, L.C.; Barreti, P. Mortalidade e prognóstico específico em pacientes com insuficiência renal aguda. Rev. Assoc. Med. Bras. 2005, 51, 318–322. [Google Scholar] [CrossRef]
- Fernandes, N.M.; Pinto, P.d.o.s.S.; Lacet, T.B.; Rodrigues, D.F.; Bastos, M.G.; Stella, S.R.; Cendoroglo Neto, M. APACHE II and ATN-ISS in acute renal failure (ARF) in intensive care unit (ICU) and non-ICU. Rev. Assoc. Med. Bras. 1992, 55, 434–441. [Google Scholar] [CrossRef][Green Version]
| General n = 372 | Death n = 272 (73.1) | Hospital Discharge n = 100 (26.95) | p-Value | |
|---|---|---|---|---|
| ICU [n, (%)] | 328 (88.2) | 261 (79.6) | 67 (67) | 0.0001 |
| VAD [n, (%] | 316 (84.9) | 258 (81.6) | 58 (58) | 0.0001 |
| MV [n, (%)] | 321 (86.3) | 262 (81.6) | 59 (59) | 0.0001 |
| Dialysis [n, (%)] | 242 (65.1) | 204 (84.3) | 38 (38) | 0.0001 |
| HTU [n, (%)] | 186 (50) | 142 (76.3) | 44 (44) | 0.005 |
| PTU [n, (%)] | 176 (47.3) | 133 (75.6) | 43 (43) | 0.047 |
| KDIGO 3 [n, (%)] | 241 (64.8) | 199 (82.6) | 42 (42) | 0.0001 |
| DLP [n, (%)] | 96 (25.9) | 59 (61.5) | 37 (37) | 0.002 |
| CKD [n, (%)] | 55 (14.8) | 35 (63.6) | 20 (20) | 0.086 |
| APACHE | 18.6 (12.2–25.1) | 19.7 (5.3–34.1) | 14.4 (8.3–20.5) | 0.0001 |
| SOFA | 7.9 (4.2–11.7) | 8.58 (5.08–12.08) | 5.41 (1.91–8.91) | 0.0001 |
| AGE | 61.4 (46.6–76.2) | 62.72 (48.7–76.7) | 57.8 (41.15–74.45) | 0.004 |
| length of stay (days) | 21.4 (4.8–37.9) | 18.1 (4.5–31.6) | 30.3 (10.05–50.55) | 0.0001 |
| ATN-ISS | 0.74 (0.54–0.94) | 0.786 (0.64–0.93) | 0.595 (0.453–0.737) | 0.0001 |
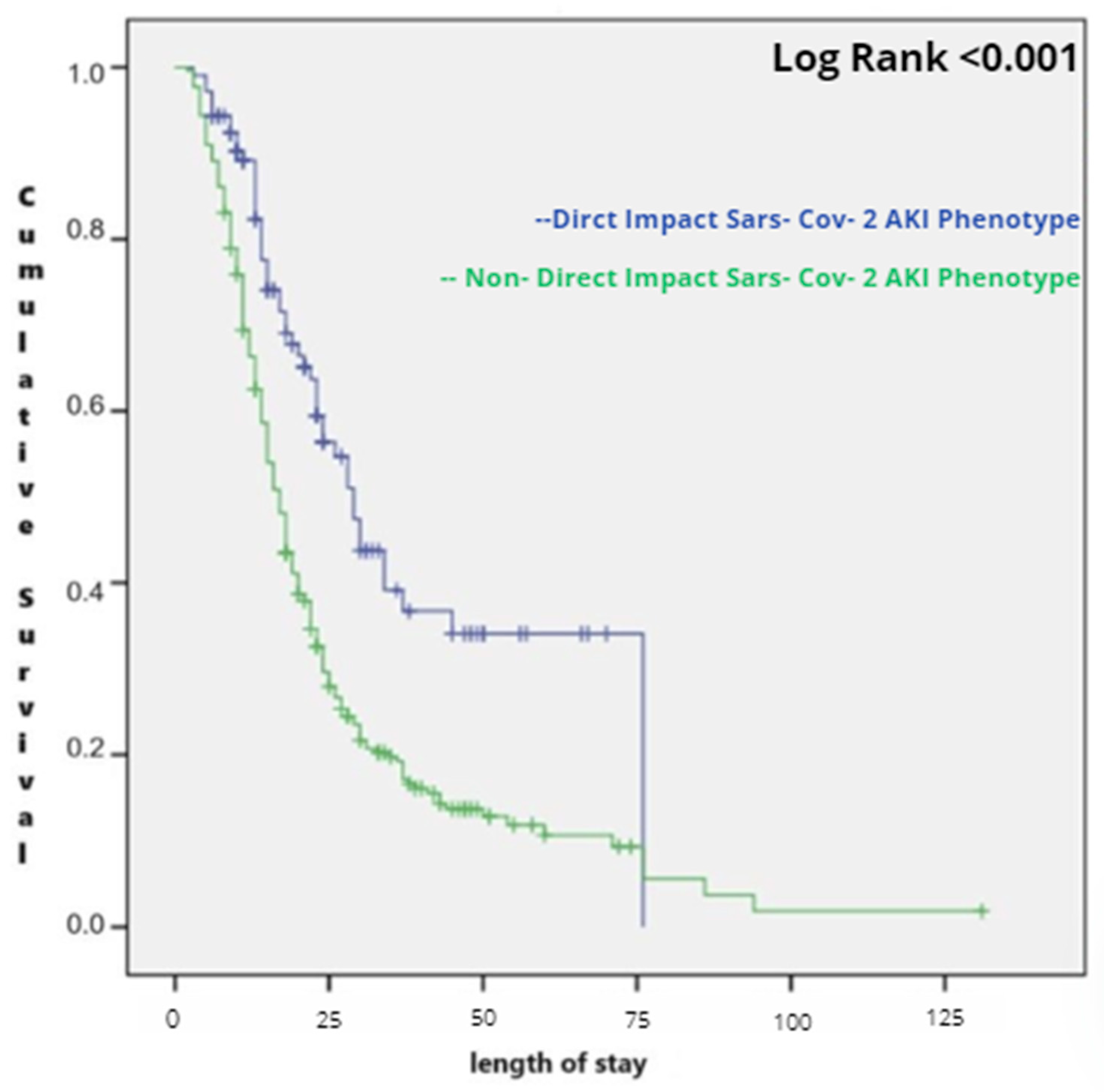
| phenotype direct impact of SARS-CoV-2 on kidney | 266 (71.5) | 223 (83.8) | 43 (16.2) | <0.001 |
| indirect impact of SARS-CoV-2 on kidney | 106 (28.5) | 49 (46.3) | 57 (53.7) |
| Cytokine Storm n = 38 | Viral Tropism n = 74 | MOF n = 67 | Septic n = 58 | Ischemic n = 48 | Mixed COVID n = 87 | p-Value | |
|---|---|---|---|---|---|---|---|
| Death (%) | 34 (89.5) a | 51 (68.9) b | 59 (88.1) a | 32 (55.2) b | 17 (35.41) b | 79 (90.8) a | 0.0001 |
| VARIABLES | ODDS RATIO | CI-95% | p Value |
|---|---|---|---|
| APACHE II | 1.777 | 1.08–1.28 | <0.001 |
| HTU | 2.117 | 0.65–6.86 | 0.211 |
| DLP | 0.442 | 0.17–1.16 | 0.098 |
| ATN-ISS | 17.972 | 1.09–295.7 | 0.043 |
| DIALYSIS | 0.929 | 0.31–2.75 | 0.895 |
| AKI ETIOLOGY INVOLVING DIRECT IMPACT OF SARS-CoV-2 ON KIDNEY | 1.780 | 1.7–13.44 | 0.003 |
| ISCHEMIC PATHOPHYSIOLOGICAL MECHANISM OF AKI | 0.876 | 0.81–0.98 | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, P.A.; Yuasa, B.K.; Magalhães, L.E.; de Oliveira, P.G.S.; Favarin, A.J.; Zamoner, W.; Ponce, D. Impact of Clinical Aspects and Pathophysiology Mechanisms of Acute Kidney Injury on Outcomes of Patients Affected by COVID-19—A Retrospective Cohort Study. COVID 2024, 4, 1147-1156. https://doi.org/10.3390/covid4080080
Cardoso PA, Yuasa BK, Magalhães LE, de Oliveira PGS, Favarin AJ, Zamoner W, Ponce D. Impact of Clinical Aspects and Pathophysiology Mechanisms of Acute Kidney Injury on Outcomes of Patients Affected by COVID-19—A Retrospective Cohort Study. COVID. 2024; 4(8):1147-1156. https://doi.org/10.3390/covid4080080
Chicago/Turabian StyleCardoso, Pedro Andriolo, Bruna Kaori Yuasa, Luis Eduardo Magalhães, Paula Gabriela Sousa de Oliveira, Ana Julia Favarin, Welder Zamoner, and Daniela Ponce. 2024. "Impact of Clinical Aspects and Pathophysiology Mechanisms of Acute Kidney Injury on Outcomes of Patients Affected by COVID-19—A Retrospective Cohort Study" COVID 4, no. 8: 1147-1156. https://doi.org/10.3390/covid4080080
APA StyleCardoso, P. A., Yuasa, B. K., Magalhães, L. E., de Oliveira, P. G. S., Favarin, A. J., Zamoner, W., & Ponce, D. (2024). Impact of Clinical Aspects and Pathophysiology Mechanisms of Acute Kidney Injury on Outcomes of Patients Affected by COVID-19—A Retrospective Cohort Study. COVID, 4(8), 1147-1156. https://doi.org/10.3390/covid4080080







