The Evolution of Serological Assays during Two Years of the COVID-19 Pandemic: From an Easy-to-Use Screening Tool for Identifying Current Infections to Laboratory Algorithms for Discovering Immune Protection and Optimizing Vaccine Administration
Abstract
1. Introduction
Search Strategy
2. Diagnosis of SARS-CoV-2: Immunoglobulins as Useful Markers of Infection
2.1. Serological Tests
2.1.1. Detection Methods
2.1.2. Immunochromatographic Enzyme Immunoassay
2.1.3. Electrochemiluminescence Immunoassay
2.1.4. Chemiluminescence Immunoassay
2.1.5. Enzyme-Linked Immunosorbent Assay
2.2. The Evaluation of Serological Tests
2.2.1. Anti-Nucleocapsid Antigen Tests
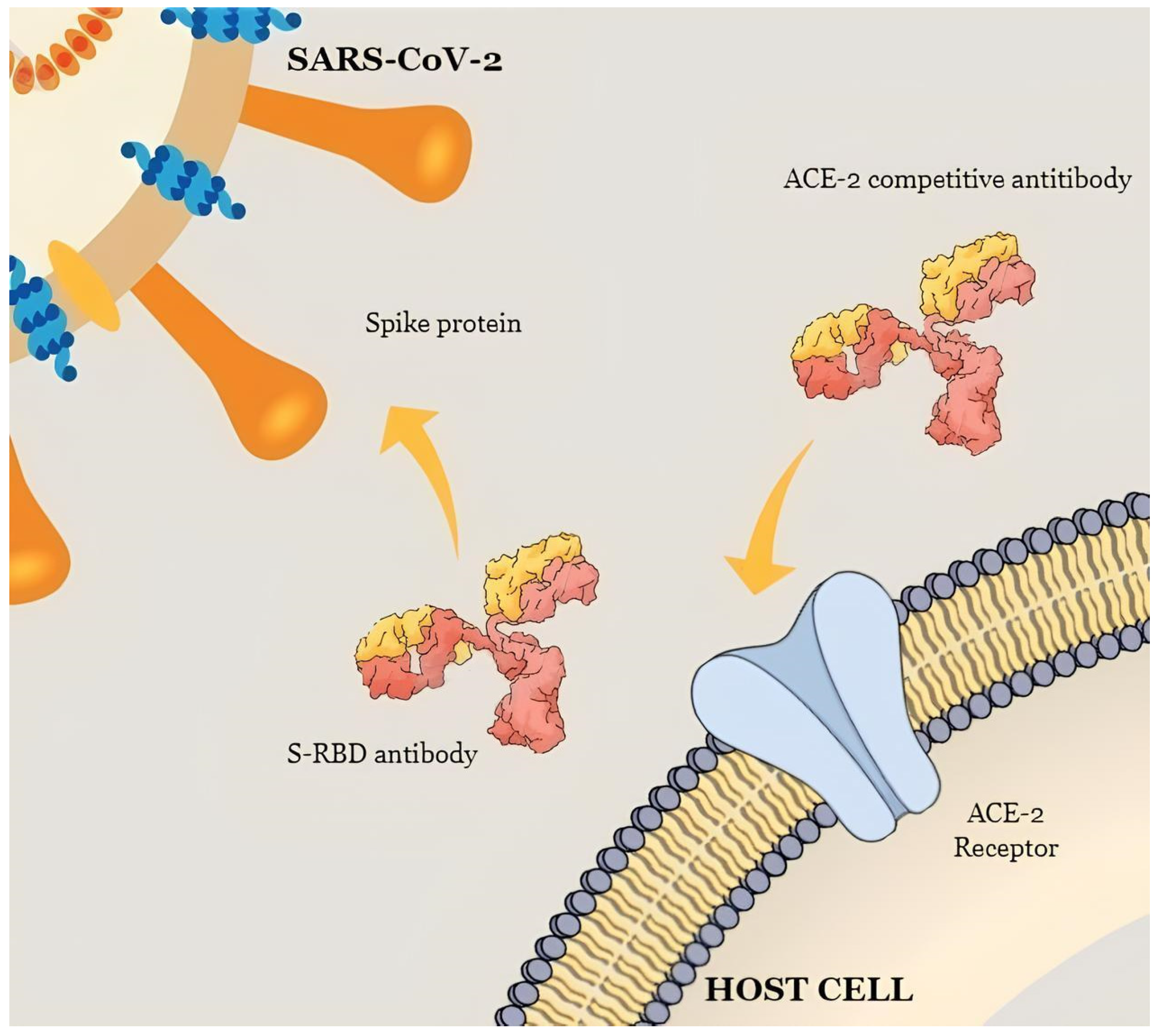
2.2.2. Anti-Spike and Anti-S-RBD Antigen Tests
2.2.3. Neutralizing Antibody Assays
2.3. International Guidelines for the Use of Serological Assays
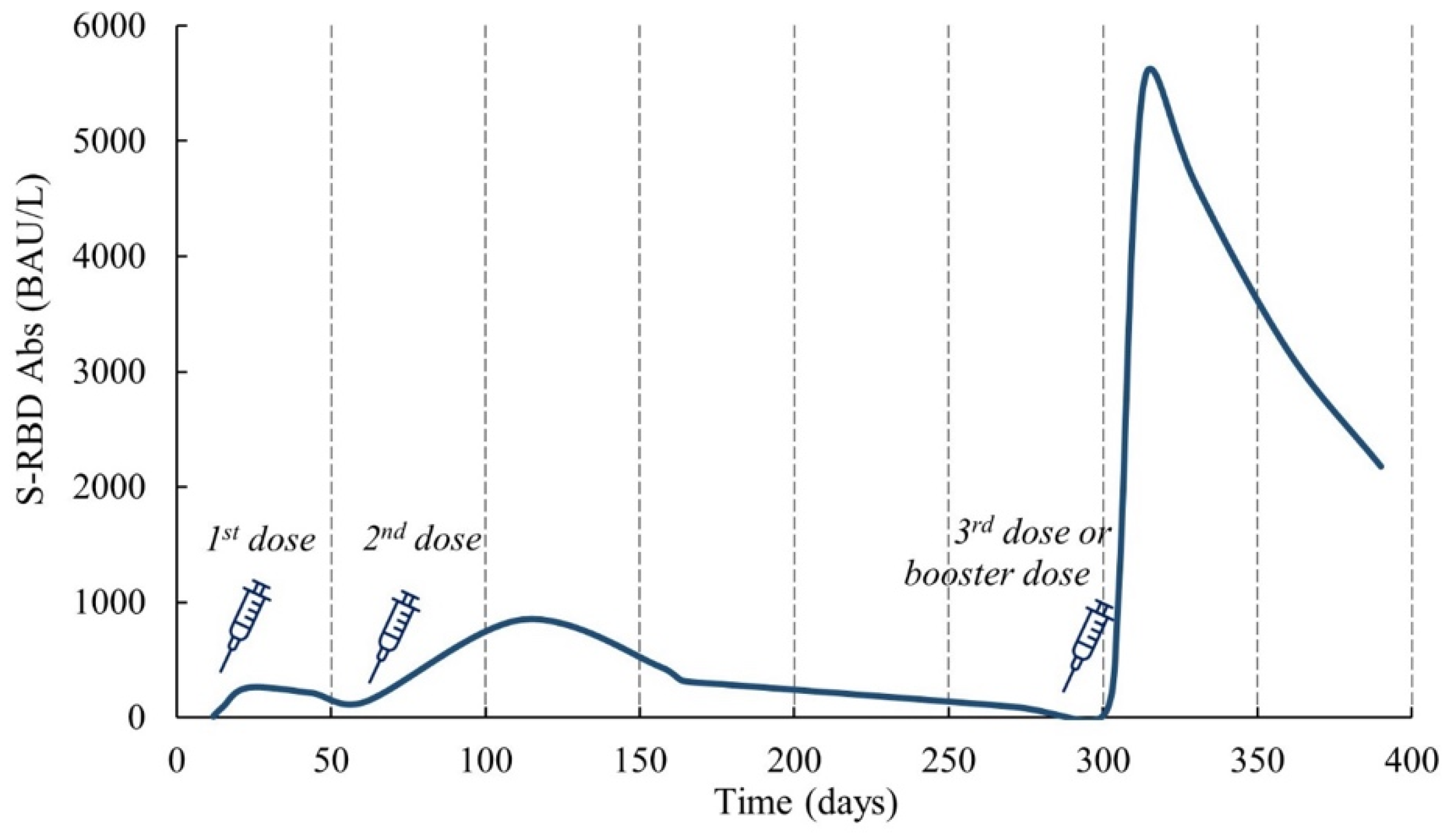
3. The Persistence and Duration of the Humoral Immune Response
4. The Use of the Interferon Gamma (IFN-γ) Release Assay to Assess the Cellular Response to SARS-CoV-2
5. Possible Laboratory Algorithm for COVID-19 Serological Tests
6. Understanding the Evolution and Implications of the Serological Test in COVID-19 Variants
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Brochot, E.; Demey, B.; Handala, L.; François, C.; Duverlie, G.; Castelain, S. Comparison of Different Serological Assays for SARS-CoV-2 in Real Life. J. Clin. Virol. 2020, 130, 104569. [Google Scholar] [CrossRef] [PubMed]
- Lambrou, A.S.; South, E.; Ballou, E.S.; Paden, C.R.; Fuller, J.A.; Bart, S.M.; Butryn, D.M.; Novak, R.T.; Browning, S.D.; Kirby, A.E.; et al. Early Detection and Surveillance of the SARS-CoV-2 Variant BA.2.86—Worldwide, July–October 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1162–1167. [Google Scholar]
- Liu, Y.; Rocklöv, J. The Reproductive Number of the Delta Variant of SARS-CoV-2 Is Far Higher Compared to the Ancestral SARS-CoV-2 Virus. J. Travel Med. 2021, 28, taab124. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, Y.; Jian, F.; Song, W.; Yisimayi, A.; Chen, X.; Xu, Y.; Wang, P.; Wang, J.; Yu, L.; et al. Antigenicity and Infectivity Characterisation of SARS-CoV-2 BA.2.86. Lancet Infect. Dis. 2023, 23, e457–e459. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Li, T.; Zhang, S.; Wang, L.; Wu, X.; Liu, J. The Genetic Sequence, Origin, and Diagnosis of SARS-CoV-2. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Drew, R.J.; O’Donnell, S.; LeBlanc, D.; McMahon, M.; Natin, D. The Importance of Cycle Threshold Values in Interpreting Molecular Tests for SARS-CoV-2. Diagn. Microbiol. Infect. Dis. 2020, 98, 115130. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Peng, Y.; Huang, B.; Ding, X.; Wang, X.; Niu, P.; Meng, J.; Zhu, Z.; Zhang, Z.; Wang, J.; et al. Genome Composition and Divergence of the Novel Coronavirus (2019-NCoV) Originating in China. Cell Host Microbe 2020, 27, 325–328. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral Targets for Vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Simple Statistical Measures for Diagnostic Accuracy Assessment. J. Thorac. Oncol. 2010, 5, 763–764. [Google Scholar] [CrossRef]
- Ghaffari, A.; Meurant, R.; Ardakani, A. COVID-19 Serological Tests: How Well Do They Actually Perform? Diagnostics 2020, 10, 453. [Google Scholar] [CrossRef]
- Shang, B.; Wang, X.-Y.; Yuan, J.-W.; Vabret, A.; Wu, X.-D.; Yang, R.-F.; Tian, L.; Ji, Y.-Y.; Deubel, V.; Sun, B. Characterization and Application of Monoclonal Antibodies against N Protein of SARS-Coronavirus. Biochem. Biophys. Res. Commun. 2005, 336, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Pieri, M.; Ciotti, M.; Carlozzi, N.; Frassanito, M.L.; Meloni, A.; Cistera, A.; Turchetti, G.; Niscola, S.; Labate, G.; Calugi, G.; et al. SARS-CoV-2 Infection Serology Validation of Different Methods: Usefulness of IgA in the Early Phase of Infection. Clin. Chim. Acta 2020, 511, 28–32. [Google Scholar] [CrossRef]
- Pieri, M.; Nuccetelli, M.; Nicolai, E.; Sarubbi, S.; Grelli, S.; Bernardini, S. Clinical Validation of a Second Generation Anti-SARS-CoV-2 IgG and IgM Automated Chemiluminescent Immunoassay. J. Med. Virol. 2021, 93, 2523–2528. [Google Scholar] [CrossRef]
- Dudley, D.J. The Immune System in Health and Disease. Baillieres Clin. Obstet. Gynaecol. 1992, 6, 393–416. [Google Scholar] [CrossRef]
- Nuccetelli, M.; Pieri, M.; Gisone, F.; Bernardini, S. Combined Anti-SARS-CoV-2 IgA, IgG, and IgM Detection as a Better Strategy to Prevent Second Infection Spreading Waves. Immunol. Investig. 2022, 51, 233–245. [Google Scholar] [CrossRef]
- Nuccetelli, M.; Pieri, M.; Gisone, F.; Sarubbi, S.; Ciotti, M.; Andreoni, M.; Bernardini, S. Evaluation of a New Simultaneous Anti-SARS-CoV-2 IgA, IgM and IgG Screening Automated Assay Based on Native Inactivated Virus. Int. Immunopharmacol. 2021, 92, 107330. [Google Scholar] [CrossRef]
- Napodano, C.; Callà, C.; Fiorita, A.; Marino, M.; Taddei, E.; di Cesare, T.; Passali, G.C.; di Santo, R.; Stefanile, A.; Fantoni, M.; et al. Salivary Biomarkers in Covid-19 Patients: Towards a Wide-Scale Test for Monitoring Disease Activity. J. Pers. Med. 2021, 11, 385. [Google Scholar] [CrossRef]
- Tomassetti, F.; Nuccetelli, M.; Sarubbi, S.; Gisone, F.; Ciotti, M.; Spinazzola, F.; Ricotta, C.; Cagnoli, M.; Borgatti, M.; Iannetta, M.; et al. Evaluation of S-RBD and High Specificity ACE-2-Binding Antibodies on SARS-CoV-2 Patients after Six Months from Infection. Int. Immunopharmacol. 2021, 99, 108013. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO International Standard First WHO International Standard for Anti-SARS-CoV-2 Immunoglobulin (Human); World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Infantino, M.; Pieri, M.; Nuccetelli, M.; Grossi, V.; Lari, B.; Tomassetti, F.; Calugi, G.; Pancani, S.; Benucci, M.; Casprini, P.; et al. The WHO International Standard for COVID-19 Serological Tests: Towards Harmonization of Anti-Spike Assays. Int. Immunopharmacol. 2021, 100, 108095. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Options for the Use of Rapid Antigen Detection Tests for COVID-19 in the EU/EEA—First Update, 26 October 2021; ECDC: Stockholm, Sweden, 2021. [Google Scholar]
- Chotpitayasunondh, T.; Fisher, D.A.; Hsueh, P.-R.; Lee, P.-I.; Nogales Crespo, K.; Ruxrungtham, K. Exploring the Role of Serology Testing to Strengthen Vaccination Initiatives and Policies for COVID-19 in Asia Pacific Countries and Territories: A Discussion Paper. Int. J. Transl. Med. 2022, 2, 275–308. [Google Scholar] [CrossRef]
- Lo Sasso, B.; Giglio, R.V.; Vidali, M.; Scazzone, C.; Bivona, G.; Gambino, C.M.; Ciaccio, A.M.; Agnello, L.; Ciaccio, M. Evaluation of Anti-SARS-Cov-2 S-RBD IgG Antibodies after COVID-19 MRNA BNT162b2 Vaccine. Diagnostics 2021, 11, 1135. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, L.; Park, Y.-J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 2020, 183, 1024–1042.e21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; He, P.; Liu, H.; Wei, H.; Yu, J. A Luciferase Based Automated Assay for Rapid and Sensitive Detection of SARS-CoV-2 Antibodies. Anal. Chim. Acta 2023, 1238, 340633. [Google Scholar] [CrossRef]
- Meira, C.; Silva, D.; Santos, I.; Barreto, B.; Rocha, V.; Santos, E.; Dos Reis, B.; Evangelista, A.; Ribeiro Dos Santos, R.; Machado, B.; et al. Diagnostic Performance of Three ELISAs for Detection of Antibodies against SARS-CoV-2 in Human Samples. Sci. World J. 2022, 2022, 7754329. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, I.; Dahma, H.; Wolff, F.; Dauby, N.; Delaunoy, S.; Wuyts, M.; Detemmerman, C.; Duterme, C.; Vandenberg, O.; Martin, C.; et al. Neutralizing Antibody Responses Following Natural SARS-CoV-2 Infection: Dynamics and Correlation with Commercial Serologic Tests. J. Clin. Virol. 2021, 144, 104988. [Google Scholar] [CrossRef]
- Nuccetelli, M.; Pieri, M.; Grelli, S.; Ciotti, M.; Miano, R.; Andreoni, M.; Bernardini, S. SARS-CoV-2 Infection Serology: A Useful Tool to Overcome Lockdown? Cell Death Discov. 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-NCoV and Naming It SARS-CoV-2. Nat. Microbiol 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Chia, W.N.; Tan, C.W.; Foo, R.; Kang, A.E.Z.; Peng, Y.; Sivalingam, V.; Tiu, C.; Ong, X.M.; Zhu, F.; Young, B.E.; et al. Serological Differentiation between COVID-19 and SARS Infections. Emerg. Microbes Infect. 2020, 9, 1497–1505. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and Immunogenicity of the ChAdOx1 NCoV-19 Vaccine against SARS-CoV-2: A Preliminary Report of a Phase 1/2, Single-Blind, Randomised Controlled Trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An MRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Pinto, D.; Park, Y.-J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; de Marco, A.; et al. Cross-Neutralization of SARS-CoV-2 by a Human Monoclonal SARS-CoV Antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef]
- Röltgen, K.; Powell, A.E.; Wirz, O.F.; Stevens, B.A.; Hogan, C.A.; Najeeb, J.; Hunter, M.; Wang, H.; Sahoo, M.K.; Huang, C.; et al. Defining the Features and Duration of Antibody Responses to SARS-CoV-2 Infection Associated with Disease Severity and Outcome. Sci. Immunol. 2020, 5, eabe0240. [Google Scholar] [CrossRef]
- Zhao, F.; Yuan, M.; Keating, C.; Shaabani, N.; Limbo, O.; Joyce, C.; Woehl, J.; Barman, S.; Burns, A.; Tran, Q.; et al. Broadening a SARS-CoV-1–Neutralizing Antibody for Potent SARS-CoV-2 Neutralization through Directed Evolution. Sci. Signal. 2023, 16, eabk3516. [Google Scholar] [CrossRef]
- Zedan, H.T.; Yassine, H.M.; Al-Sadeq, D.W.; Liu, N.; Qotba, H.; Nicolai, E.; Pieri, M.; Bernardini, S.; Abu-Raddad, L.J.; Nasrallah, G.K. Evaluation of Commercially Available Fully Automated and ELISA-Based Assays for Detecting Anti-SARS-CoV-2 Neutralizing Antibodies. Sci. Rep. 2022, 12, 19020. [Google Scholar] [CrossRef] [PubMed]
- Santos da Silva, E.; Servais, J.-Y.; Kohnen, M.; Arendt, V.; Staub, T.; The Con-Vince Consortium; The CoVaLux Consortium; Krüger, R.; Fagherazzi, G.; Wilmes, P.; et al. Validation of a SARS-CoV-2 Surrogate Neutralization Test Detecting Neutralizing Antibodies against the Major Variants of Concern. Int. J. Mol. Sci. 2023, 24, 14965. [Google Scholar] [CrossRef]
- Nicolai, E.; Tomassetti, F.; Pelagalli, M.; Sarubbi, S.; Minieri, M.; Nisini, A.; Nuccetelli, M.; Ciotti, M.; Pieri, M.; Bernardini, S. The Antibodies’ Response to SARS-CoV-2 Vaccination: 1-Year Follow Up. Biomedicines 2023, 11, 2661. [Google Scholar] [CrossRef] [PubMed]
- Pieri, M.; Infantino, M.; Manfredi, M.; Nuccetelli, M.; Grossi, V.; Lari, B.; Tomassetti, F.; Sarubbi, S.; Russo, E.; Amedei, A.; et al. Performance Evaluation of Four Surrogate Virus Neutralization Tests (SVNTs) in Comparison to the in Vivo Gold Standard Test. Front. Biosci. 2022, 27, 74. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sempowski, G.D.; Saunders, K.O.; Acharya, P.; Haynes, B.F. SARS-CoV-2 Neutralizing Antibodies for COVID-19 Prevention and Treatment. Annu. Rev. Med. 2022, 73, 1–16. [Google Scholar] [CrossRef]
- Lau, E.H.Y.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.W.; Chan, W.; Chiu, S.S.; Ko, R.L.W.; Chan, K.H.; Cheng, S.M.S.; Perera, R.A.P.M.; et al. Neutralizing Antibody Titres in SARS-CoV-2 Infections. Nat. Commun. 2021, 12, 63. [Google Scholar] [CrossRef]
- Cristiano, A.; Pieri, M.; Sarubbi, S.; Pelagalli, M.; Calugi, G.; Tomassetti, F.; Bernardini, S.; Nuccetelli, M. Evaluation of Serological Anti-SARS-CoV-2 Chemiluminescent Immunoassays Correlated to Live Virus Neutralization Test, for the Detection of Anti-RBD Antibodies as a Relevant Alternative in COVID-19 Large-Scale Neutralizing Activity Monitoring. Clin. Immunol. 2022, 234, 108918. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, A.; Nuccetelli, M.; Pieri, M.; Sarubbi, S.; Pelagalli, M.; Calugi, G.; Tomassetti, F.; Bernardini, S. Serological Anti-SARS-CoV-2 Neutralizing Antibodies Association to Live Virus Neutralizing Test Titers in COVID-19 Paucisymptomatic/Symptomatic Patients and Vaccinated Subjects. Int. Immunopharmacol. 2021, 101, 108215. [Google Scholar] [CrossRef] [PubMed]
- Maciola, A.K.; La Raja, M.; Pacenti, M.; Salata, C.; De Silvestro, G.; Rosato, A.; Pasqual, G. Neutralizing Antibody Responses to SARS-CoV-2 in Recovered COVID-19 Patients Are Variable and Correlate With Disease Severity and Receptor-Binding Domain Recognition. Front. Immunol. 2022, 13, 830710. [Google Scholar] [CrossRef] [PubMed]
- Morales-Núñez, J.J.; Muñoz-Valle, J.F.; Torres-Hernández, P.C.; Hernández-Bello, J. Overview of Neutralizing Antibodies and Their Potential in COVID-19. Vaccines 2021, 9, 1376. [Google Scholar] [CrossRef] [PubMed]
- Pezzati, L.; Milazzo, L.; Carrozzo, G.; Kullmann, C.; Oreni, L.; Beltrami, M.; Caronni, S.; Lai, A.; Caberlotto, L.; Ottomano, C.; et al. Evaluation of Residual Humoral Immune Response against SARS-CoV-2 by a Surrogate Virus Neutralization Test (SVNT) 9 Months after BNT162b2 Primary Vaccination. J. Infect. Chemother. 2023, 29, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Abebe, E.C.; Dejenie, T.A. Protective Roles and Protective Mechanisms of Neutralizing Antibodies against SARS-CoV-2 Infection and Their Potential Clinical Implications. Front. Immunol. 2023, 14, 1055457. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, K.; Cheng, S.; Binder, R.A.; Mantis, N.J.; Crawford, J.M.; Okoye, N.; Braun, J.G.; Joung, S.; Wang, M.; Lozanski, G.; et al. Clinical Utility of SARS-CoV-2 Serological Testing and Defining a Correlate of Protection. Vaccines 2023, 11, 1644. [Google Scholar] [CrossRef] [PubMed]
- Bergeri, I.; Lewis, H.C.; Subissi, L.; Nardone, A.; Valenciano, M.; Cheng, B.; Glonti, K.; Williams, B.; Abejirinde, I.O.; Simniceanu, A.; et al. Early Epidemiological Investigations: World Health Organization UNITY Protocols Provide a Standardized and Timely International Investigation Framework during the COVID-19 Pandemic. Influenza Other Respir. Viruses 2022, 16, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Interim Guidelines for COVID-19 Antibody Testing—Interim Guidelines for COVID-19 Antibody Testing in Clinical and Public Health Settings; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2022. [Google Scholar]
- Schipani, M.C.; Tomassetti, F.; Polidori, I.; Ricci, P.; Frassanito, M.L.; Seraceni, S.; Morello, M.; Nicolai, E.; Aquaro, S.; Bernardini, S.; et al. Evaluation of Natural and Vaccine-Induced Anti-SARS-CoV-2 Immunity: A Comparative Study between Different Groups of Volunteers. Diseases 2022, 10, 25. [Google Scholar] [CrossRef]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II Study of COVID-19 RNA Vaccine BNT162b1 in Adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Ismail, A.A. Serological Tests for COVID-19 Antibodies: Limitations Must Be Recognized. Ann. Clin. Biochem. 2020, 57, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Pozzetto, B.; Legros, V.; Djebali, S.; Barateau, V.; Guibert, N.; Villard, M.; Peyrot, L.; Allatif, O.; Fassier, J.-B.; Massardier-Pilonchéry, A.; et al. Immunogenicity and Efficacy of Heterologous ChAdOx1-BNT162b2 Vaccination. Nature 2021, 600, 701–706. [Google Scholar] [CrossRef]
- Alfego, D.; Sullivan, A.; Poirier, B.; Williams, J.; Grover, A.; Gillim, L.; Adcock, D.; Letovsky, S. A Population-Based Analysis of the Longevity of SARS-CoV-2 Antibody Seropositivity in the United States. EClinicalMedicine 2021, 36, 100902. [Google Scholar] [CrossRef]
- Ferrari, D.; di Resta, C.; Tomaiuolo, R.; Sabetta, E.; Pontillo, M.; Motta, A.; Locatelli, M. Long-Term Antibody Persistence and Exceptional Vaccination Response on Previously SARS-CoV-2 Infected Subjects. Vaccine 2021, 39, 4256–4260. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive Immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, E.; Diotti, R.A.; Strollo, M.; Rolla, S.; Ambrosi, A.; Locatelli, M.; Burioni, R.; Mancini, N.; Clementi, M.; Clementi, N. Weak Correlation between Antibody Titers and Neutralizing Activity in Sera from SARS-CoV-2 Infected Subjects. J. Med. Virol. 2021, 93, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.H.D.; Dingens, A.S.; Eguia, R.; Wolf, C.R.; Wilcox, N.; Logue, J.K.; Shuey, K.; Casto, A.M.; Fiala, B.; Wrenn, S.; et al. Dynamics of Neutralizing Antibody Titers in the Months After Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Infect. Dis. 2021, 223, 197–205. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Y.; Xiao, M.; Chen, L.; Zhao, Y.; Zhang, H.; Long, P.; Zhou, Y.; Xu, X.; Lei, Y.; et al. Dynamics of the SARS-CoV-2 Antibody Response up to 10 Months after Infection. Cell Mol. Immunol. 2021, 18, 1832–1834. [Google Scholar] [CrossRef]
- Addetia, A.; Crawford, K.H.D.; Dingens, A.; Zhu, H.; Roychoudhury, P.; Huang, M.-L.; Jerome, K.R.; Bloom, J.D.; Greninger, A.L. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J. Clin. Microbiol. 2020, 58, e02107-20. [Google Scholar] [CrossRef]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and Immunological Assessment of Asymptomatic SARS-CoV-2 Infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M.; Cosma, C.; Padoan, A. SARS-CoV-2 Antibody Assay after Vaccination: One Size Does Not Fit All. Clin. Chem. Lab. Med. 2021, 59, e380–e381. [Google Scholar] [CrossRef]
- Rashedi, R.; Samieefar, N.; Masoumi, N.; Mohseni, S.; Rezaei, N. COVID-19 Vaccines Mix-and-Match: The Concept, the Efficacy and the Doubts. J. Med. Virol. 2022, 94, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Sood, N.; Pernet, O.; Lam, C.N.; Klipp, A.; Kotha, R.; Kovacs, A.; Hu, H. Seroprevalence of Antibodies Specific to Receptor Binding Domain of SARS-CoV-2 and Vaccination Coverage Among Adults in Los Angeles County, April 2021: The LA Pandemic Surveillance Cohort Study. JAMA Netw. Open 2022, 5, e2144258. [Google Scholar] [CrossRef] [PubMed]
- Morales-Núñez, J.J.; Muñoz-Valle, J.F.; Meza-López, C.; Wang, L.-F.; Machado Sulbarán, A.C.; Torres-Hernández, P.C.; Bedolla-Barajas, M.; de la O-Gómez, B.; Balcázar-Félix, P.; Hernández-Bello, J. Neutralizing Antibodies Titers and Side Effects in Response to BNT162b2 Vaccine in Healthcare Workers with and without Prior SARS-CoV-2 Infection. Vaccines 2021, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 Variants on the Total CD4+ and CD8+ T Cell Reactivity in Infected or Vaccinated Individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Brand, I.; Gilberg, L.; Bruger, J.; Garí, M.; Wieser, A.; Eser, T.M.; Frese, J.; Ahmed, M.I.M.; Rubio-Acero, R.; Guggenbuehl Noller, J.M.; et al. Broad T Cell Targeting of Structural Proteins After SARS-CoV-2 Infection: High Throughput Assessment of T Cell Reactivity Using an Automated Interferon Gamma Release Assay. Front. Immunol. 2021, 12, 688436. [Google Scholar] [CrossRef]
- Lalvani, A.; Pareek, M. Interferon Gamma Release Assays: Principles and Practice. Enferm. Infecc. Microbiol. Clin. 2010, 28, 245–252. [Google Scholar] [CrossRef]
- Kruse, M.; Dark, C.; Aspden, M.; Cochrane, D.; Competiello, R.; Peltz, M.; Torres, L.; Wrighton-Smith, P.; Dudek, M. Performance of the T-SPOTⓇ.COVID Test for Detecting SARS-CoV-2-Responsive T Cells. Int. J. Infect. Dis. 2021, 113, 155–161. [Google Scholar] [CrossRef]
- Renaudineau, Y.; Abravanel, F.; Izopet, J.; Bost, C.; Treiner, E.; Congy, N.; Blancher, A. Novel T Cell Interferon Gamma Release Assay (IGRA) Using Spike Recombinant Protein for COVID19 Vaccine Response and Nucleocapsid for SARS-Cov2 Response. Clin. Immunol. 2022, 237, 108979. [Google Scholar] [CrossRef]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological Mechanisms of Vaccine-Induced Protection against COVID-19 in Humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Ibarrondo, F.J.; Fulcher, J.A.; Goodman-Meza, D.; Elliott, J.; Hofmann, C.; Hausner, M.A.; Ferbas, K.G.; Tobin, N.H.; Aldrovandi, G.M.; Yang, O.O. Rapid Decay of Anti–SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N. Engl. J. Med. 2020, 383, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Barmania, F.; Mellet, J.; Peta, K.; Strydom, A.; Viljoen, I.M.; James, W.; Gordon, S.; Pepper, M.S. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front. Immunol. 2022, 12, 809244. [Google Scholar] [CrossRef]
- Broccolo, F. Optimizing Effectiveness of COVID-19 Vaccination: Will Laboratory Stewardship Play a Role? Clin. Chem. Lab. Med. 2022, 60, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T Cell Immune Response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Cromer, D.; Juno, J.A.; Khoury, D.; Reynaldi, A.; Wheatley, A.K.; Kent, S.J.; Davenport, M.P. Prospects for Durable Immune Control of SARS-CoV-2 and Prevention of Reinfection. Nat. Rev. Immunol. 2021, 21, 395–404. [Google Scholar] [CrossRef] [PubMed]
- le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-Specific T Cell Immunity in Cases of COVID-19 and SARS, and Uninfected Controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Fernández-González, M.; Agulló, V.; Padilla, S.; García, J.A.; García-Abellán, J.; Botella, Á.; Mascarell, P.; Ruiz-García, M.; Gutiérrez, F. Clinical Performance of a Standardized SARS-CoV-2 Interferon-γ Release Assay for Simple Detection of T-Cell Responses after Infection or Vaccination. Clin. Infect. Dis. 2021, ciab1021. [Google Scholar] [CrossRef]
- Murugesan, K.; Jagannathan, P.; Pham, T.D.; Pandey, S.; Bonilla, H.F.; Jacobson, K.; Parsonnet, J.; Andrews, J.R.; Weiskopf, D.; Sette, A.; et al. Interferon-γ Release Assay for Accurate Detection of Severe Acute Respiratory Syndrome Coronavirus 2 T-Cell Response. Clin. Infect. Dis. 2021, 73, e3130–e3132. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, N.; Lee, S.K.; Cho, E.-J.; Hyun, J.; Park, M.-J.; Song, W.; Kim, H.S. Humoral and Cellular Responses to BNT162b2 as a Booster Following Two Doses of ChAdOx1 NCov-19 Determined Using Three SARS-CoV-2 Antibody Assays and an Interferon-Gamma Release Assay: A Prospective Longitudinal Study in Healthcare Workers. Front. Immunol. 2022, 13, 859019. [Google Scholar] [CrossRef]
- Seraceni, S.; Zocca, E.; Cervone, T.E.; Tomassetti, F.; Polidori, I.; Valisi, M.; Broccolo, F.; Calugi, G.; Bernardini, S.; Pieri, M. T-Cell Assay after COVID-19 Vaccination Could Be a Useful Tool? A Pilot Study on Interferon-Gamma Release Assay in Healthcare Workers. Diseases 2022, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. SARS-CoV-2 Variant Classifications and Definitions; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2023. [Google Scholar]
- Smirnova, A.; Baroonian, M. Reconstruction of Incidence Reporting Rate for SARS-CoV-2 Delta Variant of COVID-19 Pandemic in the US. Infect. Dis. Model. 2024, 9, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.S.; Farias, J.P.; Andreata-Santos, R.; Silva, M.P.; Brito, R.D.d.S.; Duarte Barbosa da Silva, M.; Peter, C.M.; Cirilo, M.V.d.F.; Luiz, W.B.; Birbrair, A.; et al. Neutralizing Antibody Response after Immunization with a COVID-19 Bivalent Vaccine: Insights to the Future. J. Med. Virol. 2024, 96, e29416. [Google Scholar] [CrossRef] [PubMed]
- Chittrakarn, S.; Siripaitoon, P.; Chusri, S.; Kanchanasuwan, S.; Charoenmak, B.; Hortiwakul, T.; Kantikit, P.; Kositpantawong, N. Comparative Immunogenicity and Neutralizing Antibody Responses Post Heterologous Vaccination with CoronaVac (Sinovac) and Vaxzevria (AstraZeneca) in HIV-Infected Patients with Varying CD4+ T Lymphocyte Counts. Hum. Vaccines Immunother. 2024, 20, 2309734. [Google Scholar] [CrossRef] [PubMed]
- Awwad, S.; Al-Hamdani, M.; Abdallah, A.M.; Abu-Madi, M. Laboratory Testing Efficiency during the COVID Pandemic: Findings from the Primary Health Care Corporation Laboratories in the State of Qatar. J. Infect. Public Health 2024, 17, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Pieri, M.; Nicolai, E.; Ciotti, M.; Nuccetelli, M.; Sarubbi, S.; Pelagalli, M.; Bernardini, S. Antibody Response to COVID-19 Vaccine: A Point of View That Can Help to Optimize Dose Distribution. Int. Immunopharmacol. 2022, 102, 108406. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, A.; Vizcarra, P.; Quereda, C.; Moreno, A.; Casado, J.L. IFN-Γ+ Cell Response and IFN-γ Release Concordance after in Vitro SARS-CoV-2 Stimulation. Eur. J. Clin. Investig. 2021, 51, e13636. [Google Scholar] [CrossRef]
- Varshney, R.K. State of the Globe: Navigating the Impact of SARS-CoV-2 Mutations on COVID-19 Testing. J. Glob. Infect. Dis. 2023, 15, 41–42. [Google Scholar] [CrossRef]
| Method | Range of TAT | What It Tells Us | Antigen Target | Matrix |
|---|---|---|---|---|
| Rapid serological test (immunochromatographic enzyme immunoassay) | 10–30 min | The presence or absence (qualitative) of Abs against the virus | N protein | Capillary blood, serum |
| S protein or RBD region | ||||
| Electrochemiluminescent immunoassay (ECLIA) | 20/30 min | The presence or absence (quantitative) of total Abs units against the virus | N protein | Serum |
| S protein or RBD region | ||||
| Chemiluminescent immunoassay | 20/45 min | The presence or absence of IgG or IgM based on RLUs (semiquantitative) against the virus | S protein | Serum |
| Enzyme-linked immunosorbent assay (ELISA) | 2–5 h | The number of units of IgG, IgM, and IgA (quantitative) against the virus | N protein | Serum |
| S protein or RBD region |
| Vaccination Status | Anti-S Antibody | Anti-N Antibody | Interpretation |
|---|---|---|---|
| Vaccinated | + | + | Vaccinated and previously infected |
| Vaccinated | + | − | Vaccinated and not previously infected |
| Unvaccinated | + | + | Not vaccinated and previously infected |
| Unvaccinated | − | − | Not previously vaccinated nor infected |
| Vaccination status unknown | + | + | Previously infected, but may or may not have been vaccinated |
| Vaccination status unknown | + | − | Vaccinated with no previous infection |
| Vaccination status unknown | − | − | Not previously vaccinated nor infected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolai, E.; Tomassetti, F.; Pignalosa, S.; Redi, S.; Marino, M.; Basile, U.; Ciotti, M. The Evolution of Serological Assays during Two Years of the COVID-19 Pandemic: From an Easy-to-Use Screening Tool for Identifying Current Infections to Laboratory Algorithms for Discovering Immune Protection and Optimizing Vaccine Administration. COVID 2024, 4, 1272-1290. https://doi.org/10.3390/covid4080091
Nicolai E, Tomassetti F, Pignalosa S, Redi S, Marino M, Basile U, Ciotti M. The Evolution of Serological Assays during Two Years of the COVID-19 Pandemic: From an Easy-to-Use Screening Tool for Identifying Current Infections to Laboratory Algorithms for Discovering Immune Protection and Optimizing Vaccine Administration. COVID. 2024; 4(8):1272-1290. https://doi.org/10.3390/covid4080091
Chicago/Turabian StyleNicolai, Eleonora, Flaminia Tomassetti, Stefano Pignalosa, Serena Redi, Mariapaola Marino, Umberto Basile, and Marco Ciotti. 2024. "The Evolution of Serological Assays during Two Years of the COVID-19 Pandemic: From an Easy-to-Use Screening Tool for Identifying Current Infections to Laboratory Algorithms for Discovering Immune Protection and Optimizing Vaccine Administration" COVID 4, no. 8: 1272-1290. https://doi.org/10.3390/covid4080091
APA StyleNicolai, E., Tomassetti, F., Pignalosa, S., Redi, S., Marino, M., Basile, U., & Ciotti, M. (2024). The Evolution of Serological Assays during Two Years of the COVID-19 Pandemic: From an Easy-to-Use Screening Tool for Identifying Current Infections to Laboratory Algorithms for Discovering Immune Protection and Optimizing Vaccine Administration. COVID, 4(8), 1272-1290. https://doi.org/10.3390/covid4080091











