Impact of Metabolic States on SARS-CoV-2 Vaccine Responses in Mouse Models of Obesity and Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Experiments
2.1.1. Ethical Statement
2.1.2. Animals and STZ Treatment
2.2. Recombinant Protein Expression and Purification
2.3. mRNA-LNP Vaccine Production
2.4. Immunization (Dosage)
2.5. Immunization (Diabetic Mouse Model Experiment)
2.6. Glucose Tolerance Test
2.7. Analysis of Antibodies by Multiplex Microsphere Immunoassay (MIA)
2.8. Splenocyte Preparation and ELISpot Assays
2.9. Microneutralization Assay
2.10. Avidity Assay
2.11. Statistical Methods
3. Results
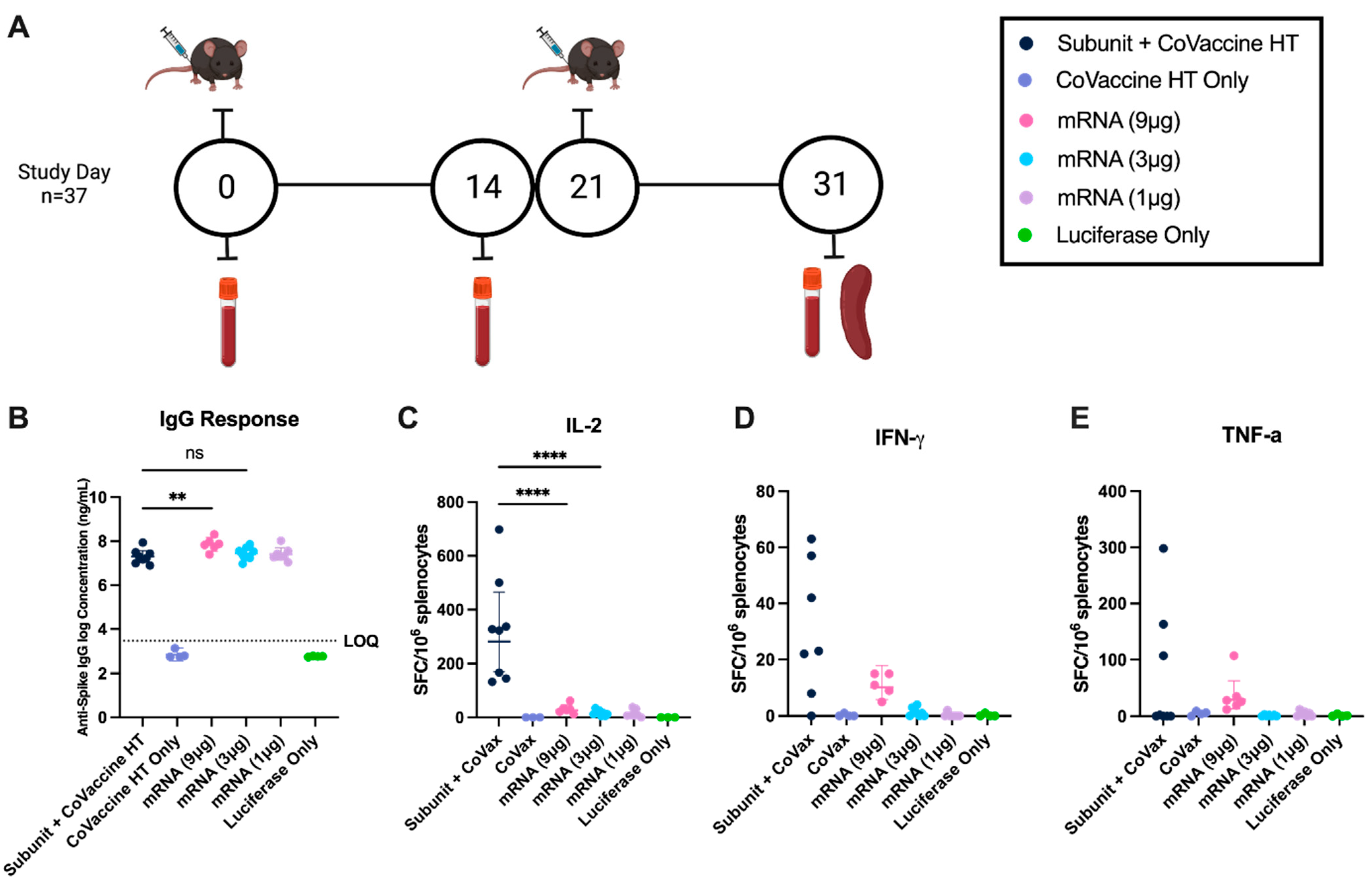
3.1. Comparative Humoral Responses to mRNA LNP Vaccines and Adjuvanted Subunit Vaccines at Equivalent Concentrations
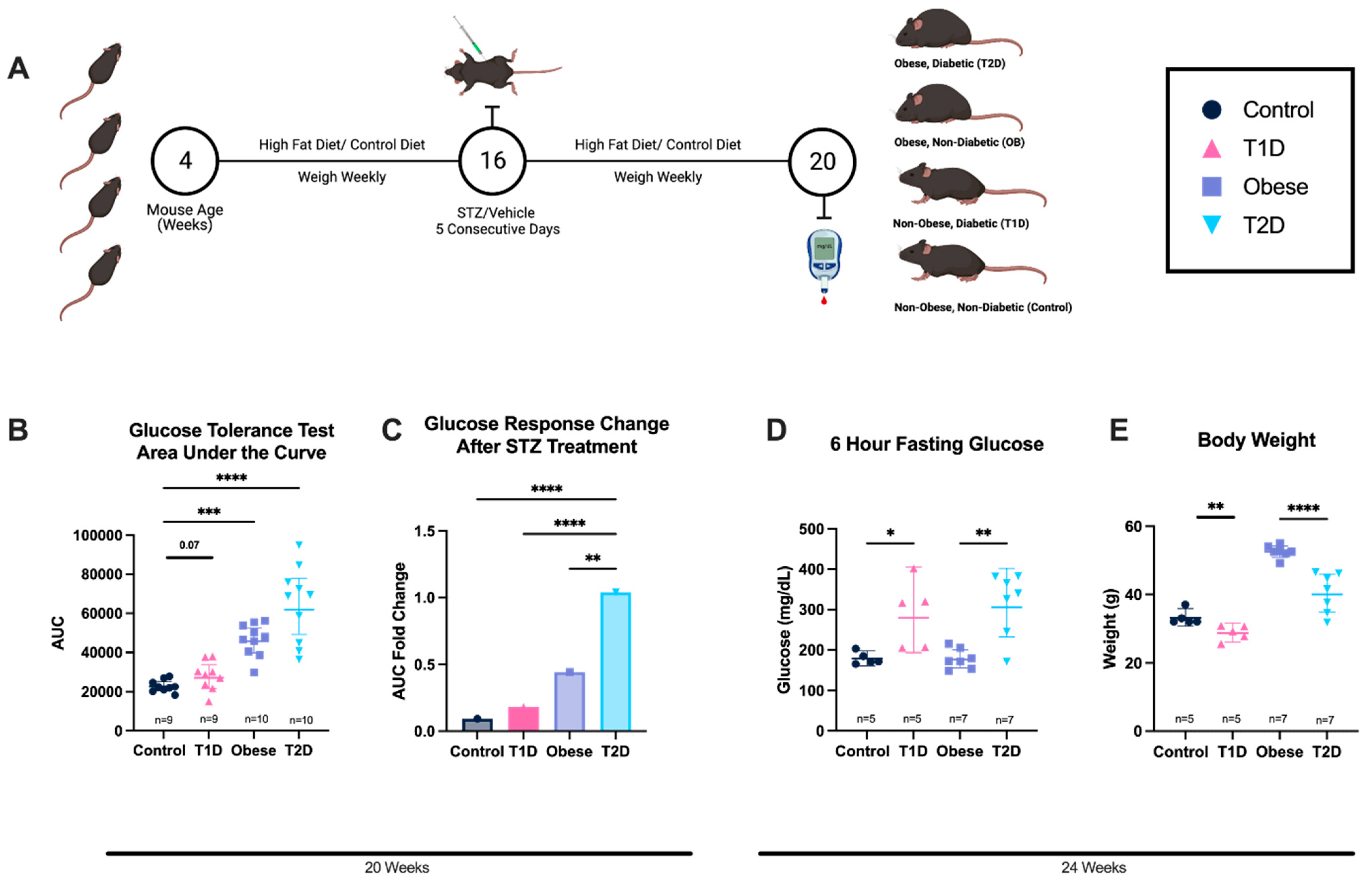
3.2. Diabetic States Are Induced via STZ Treatment and/or Diet-Induced Obesity
3.3. Cell-Mediated Immune Responses After Immunization with Adjuvanted Subunit and mRNA Vaccines in Mice with Altered Metabolic States
3.4. Anti-Spike IgG Concentration After Immunization with Adjuvanted Subunit and mRNA Vaccines Is Reduced in Mice with Altered Metabolic States
3.5. Subunit Adjuvanted with CoVaccine HT and mRNA Vaccines Elicit a More Balanced Immune Response Compared to the Subunit Vaccine Adjuvanted with Alum
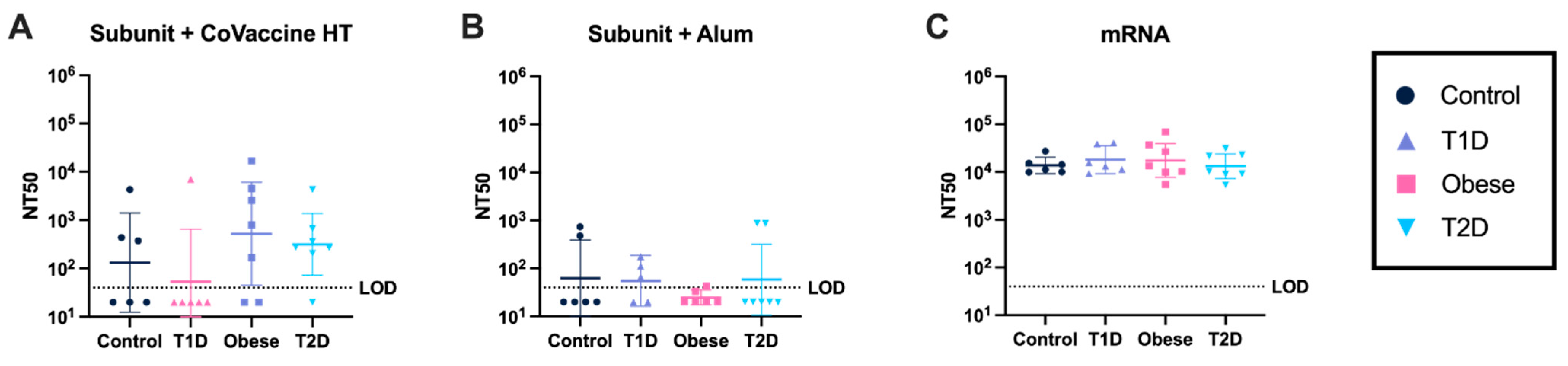
3.6. mRNA Vaccines Elicit Consistently High Virus-Neutralizing Antibody Responses Under All Metabolic Conditions
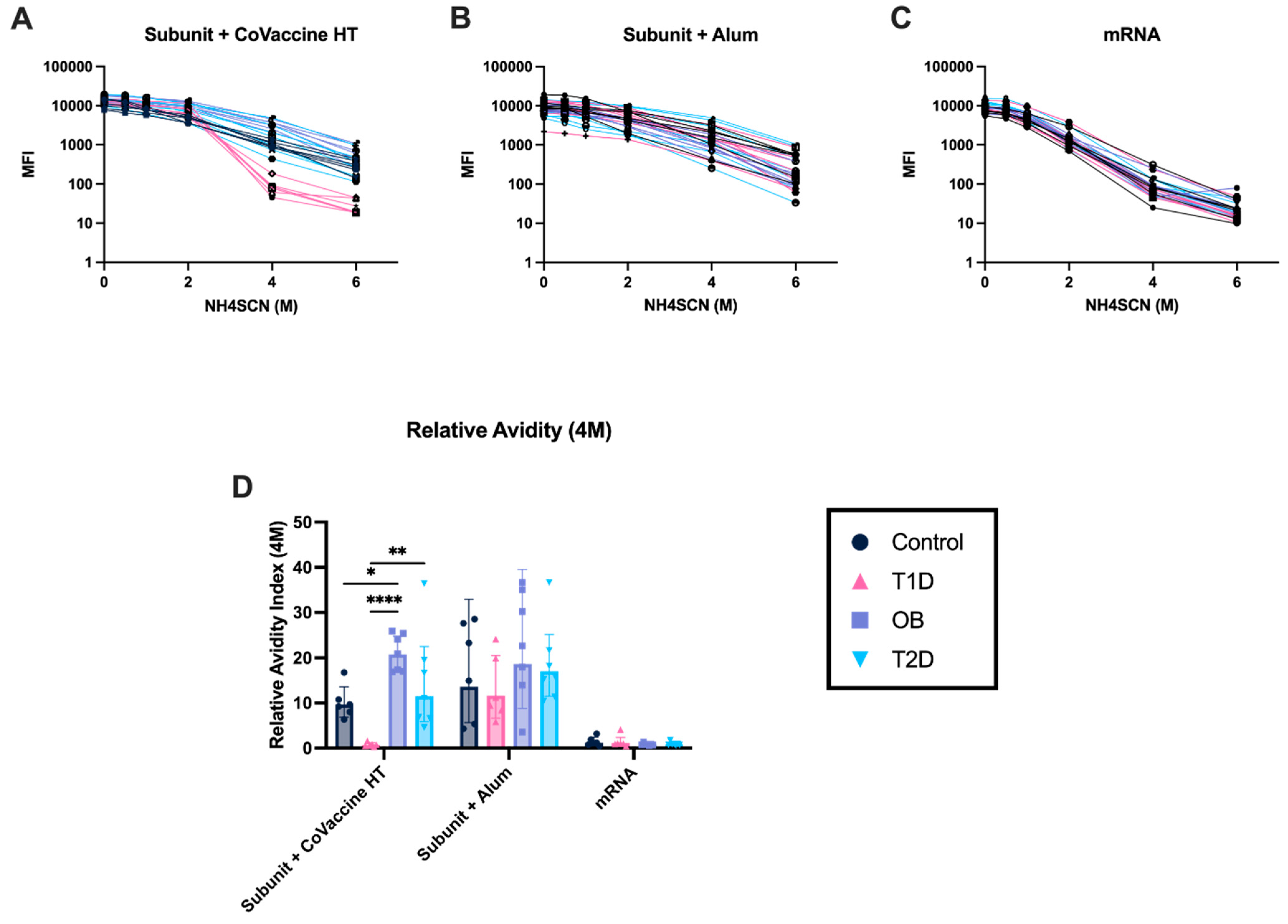
3.7. Adjuvanted Subunit Vaccines Elicit Antibodies with Stronger Avidity After Ten Days Post-Second Immunization Compared to mRNA Vaccines
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Javed, B.; Sarwer, A.; Soto, E.B.; Mashwani, Z.U.R. The coronavirus (COVID-19) pandemic’s impact on mental health. Int. J. Health Plan. Manag. 2020, 35, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Giuntella, O.; Hyde, K.; Saccardo, S.; Sadoff, S. Lifestyle and mental health disruptions during COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2016632118. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; Mcginn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052. [Google Scholar] [CrossRef]
- Cowie, C.C.; Casagrande, S.S.; Geiss, L.S. Prevalence and Incidence of Type 2 Diabetes and Prediabetes. In Diabetes in America; Cowie, C.C., Casagrande, S.S., Menke, A., Cissell, M.A., Eberhardt, M.S., Meigs, J.B., Gregg, E.W., Knowler, W.C., Barrett-Connor, E., Becker, D.J., et al., Eds.; National Institute of Diabetes and Digestive and Kidney Diseases (US): Bethesda, MD, USA, 2018. [Google Scholar]
- Rowley, W.R.; Bezold, C. Creating Public Awareness: State 2025 Diabetes Forecasts. Popul. Health Manag. 2012, 15, 194–200. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- McElhaney, J.E.; Pinkoski, M.J.; Au, D.; Lechelt, K.E.; Bleackley, R.C.; Meneilly, G.S. Helper and cytotoxic T lymphocyte responses to influenza vaccination in healthy compared to diabetic elderly. Vaccine 1996, 14, 539–544. [Google Scholar] [CrossRef]
- Mcelhaney, J.E.; Garneau, H.; Camous, X.; Dupuis, G.; Pawelec, G.; Baehl, S.; Tessier, D.; Frost, E.H.; Frasca, D.; Larbi, A.; et al. Predictors of the antibody response to influenza vaccination in older adults with type 2 diabetes. BMJ Open Diabetes Res. Care 2015, 3, e000140. [Google Scholar] [CrossRef]
- Vasireddy, D.; Atluri, P.; Malayala, S.V.; Vanaparthy, R.; Mohan, G. Review of COVID-19 Vaccines Approved in the United States of America for Emergency Use. J. Clin. Med. Res. 2021, 13, 204–213. [Google Scholar] [CrossRef]
- COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 7 February 2023).
- Nagy, A.; Alhatlani, B. An overview of current COVID-19 vaccine platforms. Comput. Struct. Biotechnol. J. 2021, 19, 2508–2517. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the Cold Reality of mRNA Vaccine Stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zheng, B.-J.; Lu, L.; Zhou, Y.; Jiang, S.; Du, L. Advancements in the development of subunit influenza vaccines. Microbes Infect. 2015, 17, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Goldblatt, D.; Alter, G.; Crotty, S.; Plotkin, S.A. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol. Rev. 2022, 310, 6–26. [Google Scholar] [CrossRef]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef]
- Like, A.A.; Rossini, A.A. Streptozotocin-Induced Pancreatic Insulitis: New Model of Diabetes Mellitus. Science 1976, 193, 415–417. [Google Scholar] [CrossRef]
- Surwit, R.S.; Kuhn, C.M.; Cochrane, C.; McCubbin, J.A.; Feinglos, M.N. Diet-Induced Type II Diabetes in C57BL/6J Mice. Diabetes 1988, 37, 1163–1167. [Google Scholar] [CrossRef]
- Yin, R.; Xue, Y.; Hu, J.; Hu, X.; Shen, Q. The effects of diet and streptozotocin on metabolism and gut microbiota in a type 2 diabetes mellitus mouse model. Food Agric. Immunol. 2020, 31, 723–739. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, Z.; Yang, S.; Sun, L.; Yu, Z.; Wang, G. Role of Adaptive and Innate Immunity in Type 2 Diabetes Mellitus. J. Diabetes Res. 2018, 2018, 7457269. [Google Scholar] [CrossRef]
- Ignacio, R.M.C.; Kim, C.-S.; Kim, S.-K. Immunological Profiling of Obesity. J. Lifestyle Med. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Gorter, K.; Hak, E.; Goudzwaard, W.; Schellevis, F.; Hoepelman, A.; Rutten, G. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin. Infect. Dis. 2005, 41, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, S.E.; Hoepelman, A.I. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Weerarathna, T.; McCarthy, J.S.; Davis, T.M. Common infections in diabetes: Pathogenesis, management and relationship to glycaemic control. Diabetes/Metab. Res. Rev. 2007, 23, 3–13. [Google Scholar] [CrossRef]
- Sarkar, I.; Garg, R.; van Drunen Littel-van den Hurk, S. Selection of adjuvants for vaccines targeting specific pathogens. Expert. Rev. Vaccines 2019, 18, 505–521. [Google Scholar] [CrossRef]
- Haun, B.K.; Lai, C.-Y.; Williams, C.A.; Wong, T.A.S.; Lieberman, M.M.; Pessaint, L.; Andersen, H.; Lehrer, A.T. CoVaccine HT™ Adjuvant Potentiates Robust Immune Responses to Recombinant SARS-CoV-2 Spike S1 Immunization. Front. Immunol. 2020, 11, 599587. [Google Scholar] [CrossRef]
- Lai, C.-Y.; To, A.; Wong, T.A.S.; Lieberman, M.M.; Clements, D.E.; Senda, J.T.; Ball, A.H.; Pessaint, L.; Andersen, H.; Furuyama, W.; et al. Recombinant protein subunit SARS-CoV-2 vaccines formulated with CoVaccine HT™ adjuvant induce broad, Th1 biased, humoral and cellular immune responses in mice. Vaccine X 2021, 9, 100126. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Adipose Tissue Inflammation Induces B Cell Inflammation and Decreases B Cell Function in Aging. Front. Immunol. 2017, 8, 1003. [Google Scholar] [CrossRef]
- De Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, V.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes/Metab. Res. Rev. 2022, 38, e3465. [Google Scholar] [CrossRef] [PubMed]
- Soetedjo, N.N.M.; Iryaningrum, M.R.; Lawrensia, S.; Permana, H. Antibody response following SARS-CoV-2 vaccination among patients with type 2 diabetes mellitus: A systematic review. Diabetes Metab. Syndr. 2022, 16, 102406. [Google Scholar] [CrossRef] [PubMed]
- Van Hoang, D.; Yamamoto, S.; Fukunaga, A.; Inoue, Y.; Mizoue, T.; Ohmagari, N. Metabolic syndrome and the immunogenicity of Pfizer–BioNTech vaccine: A cross-sectional study in Japanese healthcare workers. Diabetol. Metab. Syndr. 2022, 14, 149. [Google Scholar] [CrossRef]
- Marfella, R.; D’Onofrio, N.; Sardu, C.; Scisciola, L.; Maggi, P.; Coppola, N.; Romano, C.; Messina, V.; Turriziani, F.; Siniscalchi, M.; et al. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: The CAVEAT study. Diabetes Obes. Metab. 2022, 24, 160–165. [Google Scholar] [CrossRef]
- Laczkó, D.; Hogan, M.J.; Toulmin, S.A.; Hicks, P.; Lederer, K.; Gaudette, B.T.; Castaño, D.; Amanat, F.; Muramatsu, H.; Oguin, T.H.; et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity 2020, 53, 724–732.e727. [Google Scholar] [CrossRef]
- Vadovics, M.; Muramatsu, H.; Sárközy, A.; Pardi, N. Production and Evaluation of Nucleoside-Modified mRNA Vaccines for Infectious Diseases. Methods Mol. Biol. 2024, 2786, 167–181. [Google Scholar]
- Baiersdörfer, M.; Boros, G.; Muramatsu, H.; Mahiny, A.; Vlatkovic, I.; Sahin, U.; Karikó, K. A Facile Method for the Removal of dsRNA Contaminant from In Vitro-Transcribed mRNA. Mol. Ther. Nucleic Acids 2019, 15, 26–35. [Google Scholar] [CrossRef]
- Blom, A.G.; Luuk, A. Sucrose fatty acid sulphate esters as novel vaccine adjuvants: Effect of the chemical composition. Vaccine 2004, 23, 743–754. [Google Scholar] [CrossRef]
- Stevens, N.E.; Fraser, C.K.; Alsharifi, M.; Brown, M.P.; Diener, K.R.; Hayball, J.D. An empirical approach towards the efficient and optimal production of influenza-neutralizing ovine polyclonal antibodies demonstrates that the novel adjuvant CoVaccine HT™ is functionally superior to Freund’s adjuvant. PLoS ONE 2013, 8, e68895. [Google Scholar] [CrossRef]
- Harris, J.R.; Soliakov, A.; Lewis, R.J.; Depoix, F.; Watkinson, A.; Lakey, J.H. Alhydrogel® adjuvant, ultrasonic dispersion and protein binding: A TEM and analytical study. Micron 2012, 43, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Marzi, A.; Fletcher, P.; Feldmann, F.; Saturday, G.; Hanley, P.W.; Feldmann, H. Species-specific immunogenicity and protective efficacy of a vesicular stomatitis virus-based Sudan virus vaccine: A challenge study in macaques. Lancet Microbe 2023, 4, e171–e178. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.L.; Clancy, C.S.; Griffin, A.J.; Shifflett, K.; Gourdine, T.; Thomas, T.; Long, C.M.; Furuyama, W.; Marzi, A. Optimization of Single-Dose VSV-Based COVID-19 Vaccination in Hamsters. Front. Immunol. 2021, 12, 788235. [Google Scholar] [CrossRef] [PubMed]
- To, A.; Wong, T.A.S.; Lieberman, M.M.; Thompson, K.; Ball, A.H.; Pessaint, L.; Greenhouse, J.; Daham, N.; Cook, A.; Narvaez, B.; et al. A Recombinant Subunit Vaccine Induces a Potent, Broadly Neutralizing, and Durable Antibody Response in Macaques against the SARS-CoV-2 P.1 (Gamma) Variant. ACS Infect. Dis. 2022, 8, 825–840. [Google Scholar] [CrossRef]
- Martin, R.M.; Brady, J.L.; Lew, A.M. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 1998, 212, 187–192. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Ferreira, M.U.; Katzin, A.M. The assessment of antibody affinity distribution by thiocyanate elution: A simple dose-response approach. J. Immunol. Methods 1995, 187, 297–305. [Google Scholar] [CrossRef]
- Dimitrov, J.D.; Lacroix-Desmazes, S.; Kaveri, S.V. Important parameters for evaluation of antibody avidity by immunosorbent assay. Anal. Biochem. 2011, 418, 149–151. [Google Scholar] [CrossRef]
- Willems, S.J.; Castells, M.C.; Baptist, A.P. The Magnification of Health Disparities During the COVID-19 Pandemic. J. Allergy Clin. Immunol. Pract. 2022, 10, 903–908. [Google Scholar] [CrossRef]
- Mude, W.; Oguoma, V.M.; Nyanhanda, T.; Mwanri, L.; Njue, C. Racial disparities in COVID-19 pandemic cases, hospitalisations, and deaths: A systematic review and meta-analysis. J. Glob. Health 2021, 11, 05015. [Google Scholar] [CrossRef]
- Russell, C.D.; Lone, N.I.; Baillie, J.K. Comorbidities, multimorbidity and COVID-19. Nat. Med. 2023, 29, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Neidich, S.D.; Green, W.D.; Rebeles, J.; Karlsson, E.A.; Schultz-Cherry, S.; Noah, T.L.; Chakladar, S.; Hudgens, M.G.; Weir, S.S.; Beck, M.A. Increased risk of influenza among vaccinated adults who are obese. Int. J. Obes. 2017, 41, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Alterki, A.; Sindhu, S.; Alahmad, B.; Hammad, M.; Al-Sabah, S.; Alghounaim, M.; Jamal, M.H.; Aldei, A.; Mairza, M.J.; et al. Robust Antibody Levels in Both Diabetic and Non-Diabetic Individuals After BNT162b2 mRNA COVID-19 Vaccination. Front. Immunol. 2021, 12, 752233. [Google Scholar] [CrossRef] [PubMed]
- Medina, L.O.; To, A.; Lieberman, M.M.; Wong, T.A.S.; Namekar, M.; Nakano, E.; Andersen, H.; Yalley-Ogunro, J.; Greenhouse, J.; Higgs, S.; et al. A Recombinant Subunit Based Zika Virus Vaccine Is Efficacious in Non-human Primates. Front. Immunol. 2018, 9, 2464. [Google Scholar] [CrossRef]
- Lehrer, A.T.; Wong, T.S.; Lieberman, M.M.; Humphreys, T.; Clements, D.E.; Bakken, R.R.; Hart, M.K.; Pratt, W.D.; Dye, J.M. Recombinant proteins of Zaire ebolavirus induce potent humoral and cellular immune responses and protect against live virus infection in mice. Vaccine 2018, 36, 3090–3100. [Google Scholar] [CrossRef]
- Mahdi Abdel Hamid, M.; Remarque, E.J.; Van Duivenvoorde, L.M.; Van Der Werff, N.; Walraven, V.; Faber, B.W.; Kocken, C.H.M.; Thomas, A.W. Vaccination with Plasmodium knowlesi AMA1 Formulated in the Novel Adjuvant Co-Vaccine HT™ Protects against Blood-Stage Challenge in Rhesus Macaques. PLoS ONE 2011, 6, e20547. [Google Scholar] [CrossRef]
- Kahn, R.; Schrag, S.J.; Verani, J.R.; Lipsitch, M. Identifying and Alleviating Bias Due to Differential Depletion of Susceptible People in Postmarketing Evaluations of COVID-19 Vaccines. Am. J. Epidemiol. 2022, 191, 800–811. [Google Scholar] [CrossRef]
- Arunachalam, A.B.; Vile, S.; Rosas, A. A Mouse Immunogenicity Model for the Evaluation of Meningococcal Conjugate Vaccines. Front. Immunol. 2022, 13, 814088. [Google Scholar] [CrossRef]
- Hosokawa, M.; Dolci, W.; Thorens, B. Differential sensitivity of GLUT1- and GLUT2-expressing beta cells to streptozotocin. Biochem. Biophys. Res. Commun. 2001, 289, 1114–1117. [Google Scholar] [CrossRef]
- Wang, X.; Yuen, T.T.-T.; Dou, Y.; Hu, J.; Li, R.; Zeng, Z.; Lin, X.; Gong, H.; Chan, C.H.-C.; Yoon, C.; et al. Vaccine-induced protection against SARS-CoV-2 requires IFN-γ-driven cellular immune response. Nat. Commun. 2023, 14, 3440. [Google Scholar] [CrossRef]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.W. The immune cells in adipose tissue. Diabetes Obes. Metab. 2013, 15, 34–38. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Xu, D. The roles of T cells in obese adipose tissue inflammation. Adipocyte 2021, 10, 435–445. [Google Scholar] [CrossRef]
- Somi Sankaran, P. High-fat-diet induced obesity and diabetes mellitus in Th1 and Th2 biased mice strains: A brief overview and hypothesis. Chronic Dis. Transl. Med. 2023, 9, 14–19. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Xiong, E.; Hong, R.; Lu, Q.; Ohno, H.; Wang, J.-Y. Role of the IgM Fc Receptor in Immunity and Tolerance. Front. Immunol. 2019, 10, 529. [Google Scholar] [CrossRef]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e2877. [Google Scholar] [CrossRef]
- Lederer, K.; Castaño, D.; Gómez Atria, D.; Oguin, T.H., 3rd; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 2020, 53, 1281–1295.e1285. [Google Scholar] [CrossRef] [PubMed]
- Lederer, K.; Bettini, E.; Parvathaneni, K.; Painter, M.M.; Agarwal, D.; Lundgreen, K.A.; Weirick, M.; Muralidharan, K.; Castaño, D.; Goel, R.R.; et al. Germinal center responses to SARS-CoV-2 mRNA vaccines in healthy and immunocompromised individuals. Cell 2022, 185, 1008–1024.e1015. [Google Scholar] [CrossRef] [PubMed]
- Budroni, S.; Buricchi, F.; Cavallone, A.; Bourguignon, P.; Caubet, M.; Dewar, V.; D’Oro, U.; Finco, O.; Garçon, N.; El Idrissi, M.; et al. Antibody avidity, persistence, and response to antigen recall: Comparison of vaccine adjuvants. Npj Vaccines 2021, 6, 78. [Google Scholar] [CrossRef]
- Tang, J.; Grubbs, G.; Lee, Y.; Huang, C.; Ravichandran, S.; Forgacs, D.; Golding, H.; Ross, T.M.; Khurana, S. Antibody affinity maturation and cross-variant activity following SARS-CoV-2 mRNA vaccination: Impact of prior exposure and sex. EBioMedicine 2021, 74, 103748. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Kool, M.; Willart, M.A.; Hammad, H. Mechanism of action of clinically approved adjuvants. Curr. Opin. Immunol. 2009, 21, 23–29. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, O.A.; Fujimoto, B.; Wong, T.A.S.; To, A.; Odo, T.; Ball, A.; Haun, B.K.; Muramatsu, H.; Tam, Y.K.; Pardi, N.; et al. Impact of Metabolic States on SARS-CoV-2 Vaccine Responses in Mouse Models of Obesity and Diabetes. COVID 2025, 5, 2. https://doi.org/10.3390/covid5010002
Smith OA, Fujimoto B, Wong TAS, To A, Odo T, Ball A, Haun BK, Muramatsu H, Tam YK, Pardi N, et al. Impact of Metabolic States on SARS-CoV-2 Vaccine Responses in Mouse Models of Obesity and Diabetes. COVID. 2025; 5(1):2. https://doi.org/10.3390/covid5010002
Chicago/Turabian StyleSmith, Olivia A., Brent Fujimoto, Teri Ann S. Wong, Albert To, Troy Odo, Aquena Ball, Brien K. Haun, Hiromi Muramatsu, Ying K Tam, Norbert Pardi, and et al. 2025. "Impact of Metabolic States on SARS-CoV-2 Vaccine Responses in Mouse Models of Obesity and Diabetes" COVID 5, no. 1: 2. https://doi.org/10.3390/covid5010002
APA StyleSmith, O. A., Fujimoto, B., Wong, T. A. S., To, A., Odo, T., Ball, A., Haun, B. K., Muramatsu, H., Tam, Y. K., Pardi, N., & Lehrer, A. T. (2025). Impact of Metabolic States on SARS-CoV-2 Vaccine Responses in Mouse Models of Obesity and Diabetes. COVID, 5(1), 2. https://doi.org/10.3390/covid5010002






