Main Carotenoids Produced by Microorganisms
Definition
:1. Introduction
1.1. Brief History of Carotenoids Discovery and Production
1.2. What Are Carotenoids?
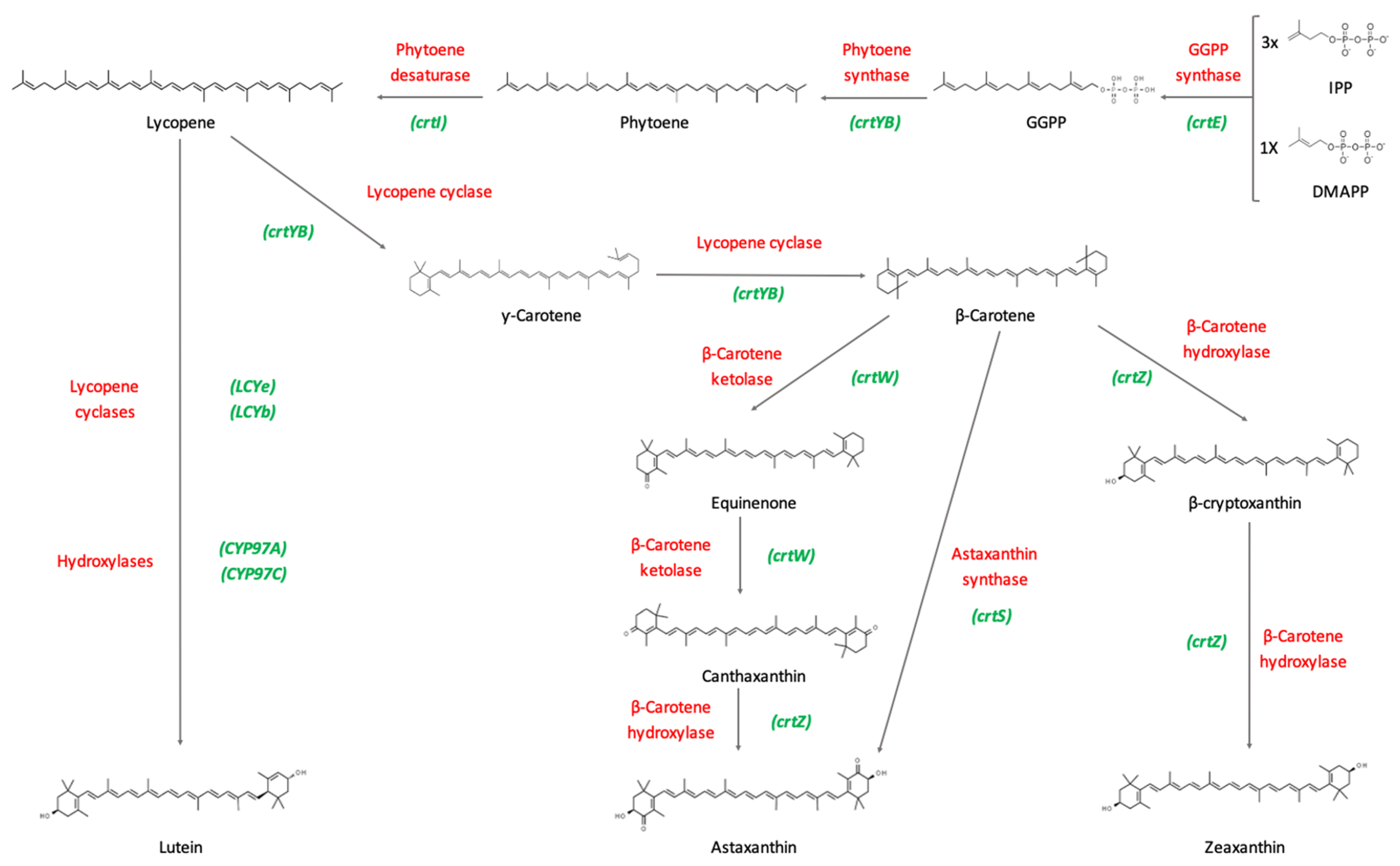
1.3. Biosynthetic Pathways of Carotenoids
2. Carotenoids Market
3. Relevance of Carotenoids
3.1. Astaxanthin
3.2. β-Carotene
3.3. Canthaxanthin
3.4. Lutein
3.5. Lycopene
3.6. Zeaxanthin
4. Biotechnological Production of Carotenoids
4.1. Astaxanthin
4.2. β-Carotene
4.3. Canthaxanthin
4.4. Lutein
4.5. Lycopene
4.6. Zeaxanthin
| Carotenoid | Strain | Titer/Yield | Reference |
|---|---|---|---|
| Astaxanthin | Xanthophyllomyces dendrorhous (Phaffia rhodozyma) | 350.0 mg/L (4.1 mg/g DCW) | [94] |
| Yarrowia lipolytica | 285.0 mg/L (6 mg/g DCW) | [96] | |
| Saccharomyces cerevisiae | 47.0 mg/L (8 mg/g DCW) | [95] | |
| Haematococcus pluvialis | 29.3 mg/g DCW | [97] | |
| Chlorella zofingiensis | 3.9 mg/g DCW | [98] | |
| Coelastrella striolata | 1.5 mg/g DCW | [99] | |
| Escherichia coli | 6.17 mg/g DCW | [91] | |
| β-carotene | Yarrowia lipolytica | 90 mg/g DCW | [131] |
| Blakeslea trispora | 78.0 mg/g DCW | [110] | |
| Sporobolomyces roseus | 2.6 mg/g DCW | [132] | |
| Rhodotorula glutinis | 1.3 mg/g DCW | [132] | |
| Saccharomyces cerevisiae | 5.9 mg/g DCW | [133] | |
| Dunaliella salina | 25.2 mg/g DCW | [134] | |
| Coelastrella striolata | 7.0 mg/g DCW | [99] | |
| Chlorella zofingiensis | 7.2 mg/g DCW | [108] | |
| Canthaxanthin | Aspergillus carbonarius | 206.0 mg/g DCW | [63] |
| Chlorella zofingiensis | 0.26 mg/g DCW | [135] | |
| Dietzia natronolimnaea | 0.9 mg/g DCW | [136] | |
| Rhodococcus maris | 13.4 µg/g DCW | [137] | |
| Coelastrella striolata | 47.5 mg/g DCW | [99] | |
| Escherichia coli | 10.6 mg/g DCW | [90] | |
| Lutein | Scenedesmus bijugus | 2.9 mg/g DCW | [138] |
| Chlorella sorokiniana | 5.9 mg/g DCW | ||
| Chlorella sp. | 2.3 mg/g DCW | ||
| Coelastrella sp. | 6.5 mg/g DCW | ||
| Vischeria stellata | 1.5 mg/g DCW | ||
| Chlorella zofingiensis | 13.8 mg/g DCW | [108] | |
| Lycopene | Blakeslea trispora | 24.0 mg/g DCW | [139] |
| Rhodotorula glutinis | 340.0 mg/L | [140] | |
| Yarrowia lipolytica | 21.1 mg/g DCW | [141] | |
| Dietzia natronolimnaea | 0.9 mg/g DCW | [142] | |
| Dunaliella salina | 0.7 mg/L | [143] | |
| Saccharomyces cerevisiae | 3.3 g/L | [125] | |
| Pichia pastoris | 9.3 mg/g DCW | [144] | |
| Zeaxanthin | Mucor circinelloides | 10.0 µg/g DCW | [145] |
| Mesoflavibacter zeaxanthinifaciens | 0.9 mg/g DCW | [146] | |
| Muricauda lutaonensis | 2.7 mg/g DCW | [127] | |
| Siansivirga zeaxanthinifaciens | 6.5 mg/g DCW | [147] | |
| Sphingomonas natatoria | 4.9 mg/g DCW | [148] | |
| Escherichia coli | 12.0 mg/g DCW | [89] | |
| Algibacter sp. | 11.4 mg/g DCW | [149] | |
| Paracoccus zeaxanthinifaciens | 1.3 mg/g DCW | [150] | |
| Pseudomonas putida | 7.0 mg/g DCW | [130] | |
| Chlorella zofingiensis | 7.0 mg/g DCW | [108] | |
| Arthorbacter gardavensis | 1.5 mg/g DCW | [151] | |
| Chlamydomonas reinhardtii | 7.3 mg/g DCW | [129] |
5. Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Entry Link on the Encyclopedia Platform
References
- Saini, R.K.; Keum, Y.S. Progress in Microbial Carotenoids Production. Indian J. Microbiol. 2017, 57, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Pfander, H.; Lanz, M.; Traber, B. Synthesis of carotenoids. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1997; Volume 20, pp. 561–612. [Google Scholar]
- Barreiro, C.; Barredo, J. (Eds.) Carotenoids production: A healthy and profitable industry. In Microbial Carotenoids: Methods and Protocols; Springer Science+Business Media: New York, NY, USA, 2018; pp. 45–55. ISBN 9781493987429. [Google Scholar]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From plants to food and feed industries. In Microbial Carotenoids: Methods and Protocols; Barreiro, C., Barredo, J., Eds.; Springer Science: Amsterdam, The Netherlands, 2018; pp. 57–71. [Google Scholar]
- Bhosale, P.; Bernstein, P.S. Microbial xanthophylls. Appl. Microbiol. Biotechnol. 2005, 68, 445–455. [Google Scholar] [CrossRef]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Do Nascimento, T.C.; Jacob-Lopes, E.; De Rosso, V.V.; Zepka, L.Q. Carotenoids—A brief overview on its structure, biosynthesis, synthesis, and applications. Prog. Carotenoid Res. 2018, 1–16. [Google Scholar] [CrossRef]
- Barredo, J.; García-Estrada, C.; Kosalkova, K.; Barreiro, C. Biosynthesis of astaxanthin as a main carotenoid in the eterobasidiomycetous yeast Xanthophyllomyces dendrorhous. J. Fungi 2017, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Gómez-García, M.R.; Ochoa-Alejo, N. Biochemistry and molecular biology of carotenoid biosynthesis in chili peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053. [Google Scholar] [CrossRef]
- Moran, N.A.; Jarvik, T. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 2010, 328, 624–627. [Google Scholar] [CrossRef]
- Barreiro, C.; Gutiérrez, S.; Olivera, E.R. Fungal Horizontal Gene Transfer: A History Beyond the Phylogenetic Kingdoms. In Horizontal Gene Transfer; Villa, T., Viñas, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 315–336. ISBN 9783030218621. [Google Scholar]
- Klassen, J.L. Phylogenetic and evolutionary patterns in microbial carotenoid biosynthesis are revealed by comparative genomics. PLoS ONE 2010, 5, e11257. [Google Scholar] [CrossRef]
- Hammond, B.R.; Renzi, L.M. Carotenoids. Adv. Nutr. 2013, 4, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Isler, O. Introduction. In Carotenoids; Isler, O., Gutmann, H., Ulrich, S., Eds.; Springer Basel AG: Basel, Switzerland, 1971; pp. 12–25. ISBN 978-3-0348-5832-8. [Google Scholar]
- Isler, O. History and Industrial Application of Carotenoids and Vitamin A (1). Pure Appl. Chem. 1979, 51, 447–462. [Google Scholar] [CrossRef]
- José Bagur, M.; Alonso Salinas, G.; Jiménez-Monreal, A.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G. Saffron: An old medicinal plant and a potential novel functional food. Molecules 2017, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.H.; Strack, D. Carotenoids and their cleavage products: Biosynthesis and functions. Nat. Prod. Rep. 2011, 28, 663–692. [Google Scholar] [CrossRef]
- Pandita, D. Saffron (Crocus sativus L.): Phytochemistry, therapeutic significance and omics-based biology. In Medicinal and Aromatic Plants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 325–396. ISBN 978-0-12-819590-1. [Google Scholar]
- Singla, R.K.; Bhat, V.G. Crocin: An overview. Indo Glob. J. Pharm. Sci. 2014, 1, 281–286. [Google Scholar]
- Sourkes, T.L. The discovery and early history of carotene. Bull. Hist. Chem. 2009, 34, 33. [Google Scholar]
- Vogele, A.C. Effect of environmental factors upon the color of the tomato and the watermelon. Plant Physiol. 1937, 12, 929–955. [Google Scholar] [CrossRef]
- Meroni, E.; Raikos, V. Lycopene in beverage emulsions: Optimizing formulation design and processing effects for enhanced delivery. Beverages 2018, 4, 14. [Google Scholar] [CrossRef]
- The xanthophyll group of yellow colouring matters. Proc. R. Soc. Lond. 1904, 72, 165–176. [CrossRef]
- Willstätter, R.; Mieg, W. Untersuchungen über Chlorophyll; IV. Ueber die gelben Begleiter des Chlorophylls. Justus Liebig’s Ann. Chem. 1907, 355, 1–28. [Google Scholar] [CrossRef]
- Buttriss, J.L.; Welch, A.A.; Kearney, J.M.; Lanham-New, S.A. (Eds.) Public Health Nutrition, 2nd ed.; Wiley-Blackwell: Hoboken, FJ, USA, 2017; ISBN 978-1-118-66097-3. [Google Scholar]
- Isler, O.; Huber, W.; Ronco, A.; Kofler, M. Synthese des Vitamin A. Helv. Chim. Acta 1947, 30, 1911–1927. [Google Scholar] [CrossRef] [PubMed]
- Ernst, H. Recent advances in industrial carotenoid synthesis. Pure Appl. Chem. 2002, 74, 2213–2226. [Google Scholar] [CrossRef]
- Bogacz-Radomska, L.; Harasym, J. β-Carotene—Properties and production methods. Food Qual. Saf. 2018, 2, 69–74. [Google Scholar] [CrossRef]
- Khachik, F. Distribution and metabolism of dietary carotenoids in humans as a criterion for development of nutritional supplements. Pure Appl. Chem. 2006, 78, 1551–1557. [Google Scholar] [CrossRef]
- Demain, A.L.; Sánchez, S. Advancement of biotechnology by genetic modifications. In Microbial Carotenoids: Methods and Protocols; Barreiro, C., Barredo, J.L., Eds.; Springer Nature: New York, NY, USA, 2018; Volume 1852, pp. 1–43. ISBN 9781493987429. [Google Scholar]
- Xue, D.; Abdallah, I.I.; de Haan, I.E.M.; Sibbald, M.J.J.B.; Quax, W.J. Enhanced C30 carotenoid production in Bacillus subtilis by systematic overexpression of MEP pathway genes. Appl. Microbiol. Biotechnol. 2015, 99, 5907–5915. [Google Scholar] [CrossRef] [PubMed]
- Furubayashi, M.; Ikezumi, M.; Takaichi, S.; Maoka, T.; Hemmi, H.; Ogawa, T.; Saito, K.; Tobias, A.V.; Umeno, D. A highly selective biosynthetic pathway to non-natural C50 carotenoids assembled from moderately selective enzymes. Nat. Commun. 2015, 6, 7534. [Google Scholar] [CrossRef]
- Gong, G.; Liu, L.; Zhang, X.; Tan, T. Multi-omics metabolism analysis on irradiation-induced oxidative stress to Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2019, 103, 361–374. [Google Scholar] [CrossRef]
- Lehmann, M.; Vamvaka, E.; Torrado, A.; Jahns, P.; Dann, M.; Rosenhammer, L.; Aziba, A.; Leister, D.; Rühle, T. Introduction of the carotenoid biosynthesis α-branch into Synechocystis sp. PCC 6803 for lutein production. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Visser, H.; van Ooyen, A.J.J.; Verdoes, J.C. Metabolic engineering of the astaxanthin-biosynthetic pathway of Xanthophyllomyces dendrorhous. FEMS Yeast Res. 2003, 4, 221–231. [Google Scholar] [CrossRef]
- Wang, E.; Dong, C.; Zhang, P.; Roberts, T.H.; Park, R.F. Carotenoid biosynthesis and the evolution of carotenogenesis genes in rust fungi. Fungal Biol. 2021, 125, 400–411. [Google Scholar] [CrossRef]
- Rebelo, B.A.; Farrona, S.; Ventura, M.R.; Abranches, R. Canthaxanthin, a red-hot carotenoid: Applications, synthesis, and biosynthetic evolution. Plants 2020, 9, 1039. [Google Scholar] [CrossRef]
- Misawa, N.; Kajiwara, S.; Kondo, K.; Yokoyama, A.; Satomi, Y.; Saito, T.; Miki, W.; Ohtani, T. Canthaxanthin biosynthesis by the conversion of methylene to keto groups in a hydrocarbon β-carotene by a single gene. Biochem. Biophys. Res. Commun. 1995, 209, 867–876. [Google Scholar] [CrossRef]
- Rodríguez-Sáiz, M.; de la Fuente, J.L.; Barredo, J.L. Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Appl. Microbiol. Biotechnol. 2010, 88, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N.; Satomi, Y.; Kondo, K.; Yokoyama, A.; Kajiwara, S.; Saito, T.; Ohtani, T.; Miki, W. Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J. Bacteriol. 1995, 177, 6575–6584. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine carotenoids: Biological functions and commercial applications. Mar. Drugs 2011, 9, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S.R.; Mishra, S. Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. J. Funct. Foods 2020, 67, 103867. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Sathasivam, R.; Ki, J.-S. A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef]
- Granado, F.; Olmedilla, B.; Blanco, I. Nutritional and clinical relevance of lutein in human health. Br. J. Nutr. 2003, 90, 487. [Google Scholar] [CrossRef]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Nabi, F.; Arain, M.A.; Rajput, N.; Alagawany, M.; Soomro, J.; Umer, M.; Soomro, F.; Wang, Z.; Ye, R.; Liu, J. Health benefits of carotenoids and potential application in poultry industry: A review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1809–1818. [Google Scholar] [CrossRef]
- Bjerkeng, B. Carotenoids in Aquaculture: Fish and Crustaceans. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Basel: Basel, Switzerland, 2008; Volume 4, pp. 237–254. ISBN 978-3-7643-7498-3. [Google Scholar]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A potential mitochondrial-targeted antioxidant treatment in diseases and with aging. Oxid. Med. Cell. Longev. 2019, 2019, 3849692. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Duan, C.; Yi, S.; Gao, Z.; Xiao, C.; Agathos, S.N.; Wang, G.; Li, J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.; Biesalski, H.K. β-Carotene is an important vitamin A source for humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef]
- Weber, D.; Grune, T. The contribution of β-carotene to vitamin A supply of humans. Mol. Nutr. Food Res. 2012, 56, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-W.; Sun, Z.-H.; Tong, W.-W.; Yang, K.; Guo, K.-Q.; Liu, G.; Pan, A. Dietary intake and circulating concentrations of carotenoids and risk of type 2 diabetes: A dose-response meta-analysis of prospective observational studies. Adv. Nutr. 2021, 12, 1723–1733. [Google Scholar] [CrossRef]
- Mary, A.E.P.; Artavia Mora, J.I.; Ronda Borzone, P.A.; Richards, S.E.; Kies, A.K. Vitamin E and beta-carotene status of dairy cows: A survey of plasma levels and supplementation practices. Animal 2021, 15, 100303. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.J.; Herdt, T.H.; Seymour, W.M.; Duffield, T.F.; Leslie, K.E. Peripartum serum vitamin E, retinol, and beta-carotene in dairy cattle and their associations with disease. J. Dairy Sci. 2004, 87, 609–619. [Google Scholar] [CrossRef]
- Haxo, F. Carotenoids of the mushroom Cantharellus cinnabarinus. Bot. Gaz. 1950, 112, 228–232. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Rimbach, G. Canthaxanthin: From molecule to function. Mol. Nutr. Food Res. 2017, 61, 1–49. [Google Scholar] [CrossRef]
- Krupa, D.; Nakkeeran, E.; Kumaresan, N.; Vijayalakshmi, G.; Subramanian, R. Extraction, purification and concentration of partially saturated canthaxanthin from Aspergillus carbonarius. Bioresour. Technol. 2010, 101, 7598–7604. [Google Scholar] [CrossRef] [PubMed]
- Nasri Nasrabadi, M.R.; Razavi, S.H. Enhancement of canthaxanthin production from Dietzia natronolimnaea HS-1 in a fed-batch process using trace elements and statistical methods. Braz. J. Chem. Eng. 2010, 27, 517–529. [Google Scholar] [CrossRef]
- Hojjati, M.; Razavi, S.H.; Rezaei, K.; Gilani, K. Stabilization of canthaxanthin produced by Dietzia natronolimnaea HS-1 with spray drying microencapsulation. J. Food Sci. Technol. 2014, 51, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, R.A.; Warwar, R.E.; Buerk, B.M. Canthaxanthin retinopathy with visual loss: A case report and review. Case Rep. Ophthalmol. Med. 2013, 2013, 1600469. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on the Re-Evaluation of Canthaxanthin (E 161 g) as a Food Additive. EFSA J. 2010, 8, 1852. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Cao, Y.; Howard, A.N.; Alvarez-Calderon, F. Macular pigment response to a supplement containing meso-zeaxanthin, lutein and zeaxanthin. Nutr. Metab. 2007, 4, 12. [Google Scholar] [CrossRef]
- Ma, L.; Yan, S.-F.; Huang, Y.-M.; Lu, X.-R.; Qian, F.; Pang, H.-L.; Xu, X.-R.; Zou, Z.-Y.; Dong, P.-C.; Xiao, X.; et al. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology 2012, 119, 2290–2297. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A mechanistic review of β-carotene, lutein, and zeaxanthin in eye health and disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Udenigwe, C.C.; Aluko, R.E. Lutein and zeaxanthin: Production technology, bioavailability, mechanisms of action, visual function, and health claim status. Trends Food Sci. Technol. 2016, 49, 74–84. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: Results from a double-blinded, placebo-controlled, crossover study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Kavalappa, Y.P.; Gopal, S.S.; Ponesakki, G. Lutein inhibits breast cancer cell growth by suppressing antioxidant and cell survival signals and induces apoptosis. J. Cell. Physiol. 2021, 236, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Mcdonald, K.; Caldarella, S.M.; Chung, H.; Troen, A.M.; Snodderly, D.M. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr. Neurosci. 2008, 11, 75–83. [Google Scholar] [CrossRef]
- Breithaupt, D.R. Xanthophylls in Poultry Feeding. In Carotenoids: Volume 4: Natural Functions; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Basel: Basel, Switzerland, 2008; pp. 255–264. ISBN 978-3-7643-7499-0. [Google Scholar]
- Sirri, F.; Iaffaldano, N.; Minelli, G.; Meluzzi, A.; Rosato, M.P.; Franchini, A. Comparative pigmentation efficiency of high dietary levels of apo-ester and marigold extract on quality traits of whole liquid egg of two strains of laying hens. J. Appl. Poult. Res. 2007, 16, 429–437. [Google Scholar] [CrossRef]
- Hadden, W.L.; Watkins, R.H.; Levy, L.W.; Regalado, E.; Rivadeneira, D.M.; van Breemen, R.B.; Schwartz, S.J. Carotenoid composition of Marigold (Tagetes erecta) flower extract used as nutritional supplement. J. Agric. Food Chem. 1999, 47, 4189–4194. [Google Scholar] [CrossRef]
- Authority, E.F.S. Safety of synthetic lycopene—Scientific opinion of the panel on scientific panel on dietetic products, nutrition and allergies. EFSA J. 2008, 6, 676. [Google Scholar] [CrossRef]
- Aghajanpour, M.; Nazer, M.R.; Obeidavi, Z.; Akbari, M.; Ezati, P.; Kor, N.M. Functional foods and their role in cancer prevention and health promotion: A comprehensive review. Am. J. Cancer Res. 2017, 7, 740–769. [Google Scholar]
- Marzocco, S.; Singla, R.K.; Capasso, A. Multifaceted effects of lycopene: A boulevard to the multitarget-based treatment for cancer. Molecules 2021, 26, 5333. [Google Scholar] [CrossRef]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases—A critical review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar] [CrossRef]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov, A.G. Lycopene and vascular health. Front. Pharmacol. 2018, 9, 521. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on the Substantiation of Health Claims Related to Lycopene and Protection of DNA, Proteins and Lipids from Oxidative Damage (ID 1608, 1609, 1611, 1662, 1663, 1664, 1899, 1942, 2081, 2082, 2142, 2374), Protection of the Skin from UV-Indu. EFSA J. 2011, 9, 2031. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kamat, M.Y. The carotenoid pigment zeaxanthin—A review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 29–49. [Google Scholar] [CrossRef]
- Khodaiyan, F.; Razavi, S.H.; Emam-Djomeh, Z.; Mousavi, S.M.A.; Hejazi, M.A. Effect of culture conditions on canthaxanthin production by Dietzia natronolimnaea HS-1. J. Microbiol. Biotechnol. 2007, 17, 195–201. [Google Scholar]
- Jing, Y.; Guo, F.; Zhang, S.; Dong, W.; Zhou, J.; Xin, F.; Zhang, W.; Jiang, M. Recent Advances on Biological Synthesis of Lycopene by Using Industrial Yeast. Ind. Eng. Chem. Res. 2021, 60, 3485–3494. [Google Scholar] [CrossRef]
- Sanchez, S.; Ruiz, B.; Rodríguez-Sanoja, R.; Flores-Cotera, L.B. Microbial production of carotenoids. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 194–233. ISBN 978-0-85709-354-7. [Google Scholar]
- Misawa, N. Carotenoids. In Comprehensive Natural Products II; Liu, H.W.B., Mander, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 733–753. ISBN 978-0-08-045382-8. [Google Scholar]
- Li, X.-R.; Tian, G.-Q.; Shen, H.-J.; Liu, J.-Z. Metabolic engineering of Escherichia coli to produce zeaxanthin. J. Ind. Microbiol. Biotechnol. 2015, 42, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Scaife, M.A.; Prince, C.A.; Norman, A.; Armenta, R.E. Progress toward an Escherichia coli canthaxanthin bioprocess. Process Biochem. 2012, 47, 2500–2509. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, H.; Tang, J.; Bi, C.; Li, Q.; Zhang, X. Coordinated Expression of Astaxanthin Biosynthesis Genes for Improved Astaxanthin Production in Escherichia coli. J. Agric. Food Chem. 2020, 68, 14917–14927. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Feng, X.; Rasool, A.; Sun, W.; Li, C. Production of plant natural products through engineered Yarrowia lipolytica. Biotechnol. Adv. 2020, 43, 107555. [Google Scholar] [CrossRef]
- de la Fuente, J.L.; Rodríguez-Sáiz, M.; Schleissner, C.; Díez, B.; Peiro, E.; Barredo, J.L. High-titer production of astaxanthin by the semi-industrial fermentation of Xanthophyllomyces dendrorhous. J. Biotechnol. 2010, 148, 144–146. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, W.; Li, A.; Wang, F.; Yao, Z.; Bian, Q.; Zhu, Y.; Yu, H.; Ye, L. Alleviation of metabolic bottleneck by combinatorial engineering enhanced astaxanthin synthesis in Saccharomyces cerevisiae. Enzym. Microb. Technol. 2017, 100, 28–36. [Google Scholar] [CrossRef]
- Tramontin, L.R.R.; Kildegaard, K.R.; Sudarsan, S.; Borodina, I. Enhancement of Astaxanthin Biosynthesis in Oleaginous Yeast Yarrowia lipolytica via Microalgal Pathway. Microorganisms 2019, 7, 472. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Ding, W.; Zhao, Y.; Xu, J.-W.; Zhao, P.; Li, T.; Ma, H.; Yu, X. Enhanced astaxanthin production from Haematococcus pluvialis using butylated hydroxyanisole. J. Biotechnol. 2016, 236, 199–207. [Google Scholar] [CrossRef]
- Mao, X.; Wu, T.; Sun, D.; Zhang, Z.; Chen, F. Differential responses of the green microalga Chlorella zofingiensis to the starvation of various nutrients for oil and astaxanthin production. Bioresour. Technol. 2018, 249, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Hattori, H.; Hirano, M. Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata. Food Chem. 2007, 100, 656–661. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. J. Mar. Sci. Eng. 2020, 8, 789. [Google Scholar] [CrossRef]
- Pan, X.; Wang, B.; Duan, R.; Jia, J.; Li, J.; Xiong, W.; Ling, X.; Chen, C.; Huang, X.; Zhang, G.; et al. Enhancing astaxanthin accumulation in Xanthophyllomyces dendrorhous by a phytohormone: Metabolomic and gene expression profiles. Microb. Biotechnol. 2020, 13, 1446–1460. [Google Scholar] [CrossRef]
- Milas, N.A.; Davis, P.; Belič, I.; Fleš, D.A. Synthesis of β-Carotene. J. Am. Chem. Soc. 1950, 72, 4844. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Barreto, D.W.; Coelho, M.A.Z. Technological Aspects of β-Carotene Production. Food Bioprocess Technol. 2011, 4, 693–701. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Hu, I.-C. Production of potential coproducts from microalgae. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 345–358. [Google Scholar]
- Huang, W.; Lin, Y.; He, M.; Gong, Y.; Huang, J. Induced High-Yield Production of Zeaxanthin, Lutein, and β-Carotene by a Mutant of Chlorella zofingiensis. J. Agric. Food Chem. 2018, 66, 891–897. [Google Scholar] [CrossRef]
- Massoud, R.; Khosravi-Darani, K. A Review on the Impacts of Process Variables on Microbial Production of Carotenoid Pigments. In Food Biosynthesis; Elsevier: Amsterdam, The Netherlands, 2017; pp. 183–211. ISBN 978-0-12-811372-1. [Google Scholar]
- Nanou, K.; Roukas, T.; Papadakis, E. Improved production of carotenes from synthetic medium by Blakeslea trispora in a bubble column reactor. Biochem. Eng. J. 2012, 67, 203–207. [Google Scholar] [CrossRef]
- Martínez-Cámara, S.; Rubio, S.; del Río, H.; Rodríguez-Sáiz, M.; Barredo, J.-L. Lycopene production by mated fermentation of Blakeslea trispora. In Microbial Carotenoids; Barreiro, C., Barredo, J., Eds.; Springer Protocols: New York, NY, USA, 2018; pp. 257–268. [Google Scholar] [CrossRef]
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A.M. Carotenoids and some other pigments from fungi and yeasts. Metabolites 2021, 11, 92. [Google Scholar] [CrossRef]
- Barreiro, C.; Barredo, J.-L. (Eds.) Microbial Carotenoids. Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1852, ISBN 978-1-4939-8741-2. [Google Scholar]
- Fernández-Sevilla, J.M.; Acién Fernández, F.G.; Molina Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef]
- Saha, S.K.; Ermis, H.; Murray, P. Marine Microalgae for Potential Lutein Production. Appl. Sci. 2020, 10, 6457. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef]
- Liu, J.; Gerken, H.; Huang, J.; Chen, F. Engineering of an endogenous phytoene desaturase gene as a dominant selectable marker for Chlamydomonas reinhardtii transformation and enhanced biosynthesis of carotenoids. Process Biochem. 2013, 48, 788–795. [Google Scholar] [CrossRef]
- Rathod, J.P.; Vira, C.; Lali, A.M.; Prakash, G. Metabolic Engineering of Chlamydomonas reinhardtii for Enhanced β-Carotene and Lutein Production. Appl. Biochem. Biotechnol. 2020, 190, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Chen, C.-Y.; Hasunuma, T.; Kondo, A.; Chang, C.-H.; Ng, I.-S.; Chang, J.-S. Enhancing lutein production with mixotrophic cultivation of Chlorella sorokiniana MB-1-M12 using different bioprocess operation strategies. Bioresour. Technol. 2019, 278, 17–25. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Karatza, D.; Chianese, S.; Iovine, A.; Casella, P.; Marino, T.; Musmarra, D. Bench-Scale Cultivation of Microalgae Scenedesmus almeriensis for CO2 Capture and Lutein Production. Energies 2019, 12, 2806. [Google Scholar] [CrossRef]
- Chen, W.-C.; Hsu, Y.-C.; Chang, J.-S.; Ho, S.-H.; Wang, L.-F.; Wei, Y.-H. Enhancing production of lutein by a mixotrophic cultivation system using microalga Scenedesmus obliquus CWL-1. Bioresour. Technol. 2019, 291, 121891. [Google Scholar] [CrossRef]
- Blanco, A.M.; Moreno, J.; Del Campo, J.A.; Rivas, J.; Guerrero, M.G. Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl. Microbiol. Biotechnol. 2007, 73, 1259–1266. [Google Scholar] [CrossRef]
- Cámara, M.; de Cortes Sánchez-Mata, M.; Fernández-Ruiz, V.; Cámara, R.M.; Manzoor, S.; Caceres, J.O. Lycopene: A Review of Chemical and Biological Activity Related to Beneficial Health Effects. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 40, pp. 383–426. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Meneguzzo, F.; Ilharco, L.M.; Pagliaro, M. Lycopene: Emerging Production Methods and Applications of a Valued Carotenoid. ACS Sustain. Chem. Eng. 2016, 4, 643–650. [Google Scholar] [CrossRef]
- Shi, B.; Ma, T.; Ye, Z.; Li, X.; Huang, Y.; Zhou, Z.; Ding, Y.; Deng, Z.; Liu, T. Systematic Metabolic Engineering of Saccharomyces cerevisiae for Lycopene Overproduction. J. Agric. Food Chem. 2019, 67, 11148–11157. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, N.; Lazar, Z.; Chatzivasileiou, A.; Ward, V.; Chen, J.; Zhou, J.; Stephanopoulos, G. Enhancing isoprenoid synthesis in Yarrowia lipolytica by expressing the isopentenol utilization pathway and modulating intracellular hydrophobicity. Metab. Eng. 2020, 61, 344–351. [Google Scholar] [CrossRef]
- Hameed, A.; Arun, A.B.; Ho, H.-P.; Chang, C.-M.J.; Rekha, P.D.; Lee, M.-R.; Singh, S.; Young, C.-C. Supercritical Carbon Dioxide Micronization of Zeaxanthin from Moderately Thermophilic Bacteria Muricauda lutaonensis CC-HSB-11 T. J. Agric. Food Chem. 2011, 59, 4119–4124. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Sun, J.; Xue, C.; Mao, X. Biotechnological production of zeaxanthin by microorganisms. Trends Food Sci. Technol. 2017, 71, 225–234. [Google Scholar] [CrossRef]
- Song, I.; Kim, J.; Baek, K.; Choi, Y.; Shin, B.; Jin, E. The generation of metabolic changes for the production of high-purity zeaxanthin mediated by CRISPR-Cas9 in Chlamydomonas reinhardtii. Microb. Cell Fact. 2020, 19, 220. [Google Scholar] [CrossRef]
- Beuttler, H.; Hoffmann, J.; Jeske, M.; Hauer, B.; Schmid, R.D.; Altenbuchner, J.; Urlacher, V.B. Biosynthesis of zeaxanthin in recombinant Pseudomonas putida. Appl. Microbiol. Biotechnol. 2011, 89, 1137–1147. [Google Scholar] [CrossRef]
- Larroude, M.; Celinska, E.; Back, A.; Thomas, S.; Nicaud, J.-M.; Ledesma-Amaro, R. A synthetic biology approach to transform Yarrowia lipolytica into a competitive biotechnological producer of β-carotene. Biotechnol. Bioeng. 2018, 115, 464–472. [Google Scholar] [CrossRef]
- Petrik, S.; Marova, I.; Haronikova, A.; Kostovova, I.; Breierova, E. Production of biomass, carotenoid and other lipid metabolites by several red yeast strains cultivated on waste glycerol from biofuel production—A comparative screening study. Ann. Microbiol. 2013, 63, 1537–1551. [Google Scholar] [CrossRef]
- Verwaal, R.; Wang, J.; Meijnen, J.-P.; Visser, H.; Sandmann, G.; van den Berg, J.A.; van Ooyen, A.J.J. High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl. Environ. Microbiol. 2007, 73, 4342–4350. [Google Scholar] [CrossRef] [PubMed]
- Gonabadi, E.; Samadlouie, H.R.; Shafafi Zenoozian, M. Optimization of culture conditions for enhanced Dunaliella salina productions in mixotrophic culture. Prep. Biochem. Biotechnol. 2021, 1–9. [Google Scholar] [CrossRef]
- Li, H.-B.; Fan, K.-W.; Chen, F. Isolation and purification of canthaxanthin from the microalga Chlorella zofingiensis by high-speed counter-current chromatography. J. Sep. Sci. 2006, 29, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Nasri Nasrabadi, M.R.; Razavi, S.H. Use of response surface methodology in a fed-batch process for optimization of tricarboxylic acid cycle intermediates to achieve high levels of canthaxanthin from Dietzia natronolimnaea HS-1. J. Biosci. Bioeng. 2010, 109, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, S.M. Microbial canthaxanthin: Perspectives on biochemistry and biotechnological production. Eng. Life Sci. 2013, 13, 408–417. [Google Scholar] [CrossRef]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Sashidhar, B.; Adholeya, A. The isolation and identification of new microalgal strains producing oil and carotenoid simultaneously with biofuel potential. Bioresour. Technol. 2016, 211, 556–565. [Google Scholar] [CrossRef]
- Pegklidou, K.; Mantzouridou, F.; Tsimidou, M.Z. Lycopene Production Using Blakeslea trispora in the Presence of 2-Methyl Imidazole: Yield, Selectivity, and Safety Aspects. J. Agric. Food Chem. 2008, 56, 4482–4490. [Google Scholar] [CrossRef]
- Hernández-Almanza, A.; Montañez-Sáenz, J.; Martínez-Ávila, C.; Rodríguez-Herrera, R.; Aguilar, C.N. Carotenoid production by Rhodotorula glutinis YB-252 in solid-state fermentation. Food Biosci. 2014, 7, 31–36. [Google Scholar] [CrossRef]
- Schwartz, C.; Frogue, K.; Misa, J.; Wheeldon, I. Host and Pathway Engineering for Enhanced Lycopene Biosynthesis in Yarrowia lipolytica. Front. Microbiol. 2017, 8, 2233. [Google Scholar] [CrossRef] [PubMed]
- Nasri Nasrabadi, M.R.; Razavi, S.H. High levels lycopene accumulation by Dietzia natronolimnaea HS-1 using lycopene cyclase inhibitors in a fed-batch process. Food Sci. Biotechnol. 2010, 19, 899–906. [Google Scholar] [CrossRef]
- Fazeli, M.R.; Tofighi, H.; Madadkar-Sobhani, A.; Shahverdi, A.R.; Nejad-Sattari, T.; Mirzaie, S.; Jamalifar, H. Nicotine inhibition of lycopene cyclase enhances accumulation of carotenoid intermediates by Dunaliella salina CCAP 19/18. Eur. J. Phycol. 2009, 44, 215–220. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Duan, Y.; Zheng, X.; Lin, Y.; Liang, S. Production of lycopene by metabolically engineered Pichia pastoris. Biosci. Biotechnol. Biochem. 2020, 84, 463–470. [Google Scholar] [CrossRef]
- Papp, T.; Velayos, A.; Bartók, T.; Eslava, A.P.; Vágvölgyi, C.; Iturriaga, E.A. Heterologous expression of astaxanthin biosynthesis genes in Mucor circinelloides. Appl. Microbiol. Biotechnol. 2006, 69, 526–531. [Google Scholar] [CrossRef]
- Asker, D.; Beppu, T.; Ueda, K. Mesoflavibacter zeaxanthinifaciens gen. nov., sp. nov., a novel zeaxanthin-producing marine bacterium of the family Flavobacteriaceae. Syst. Appl. Microbiol. 2007, 30, 291–296. [Google Scholar] [CrossRef]
- Hameed, A.; Shahina, M.; Lin, S.-Y.; Sridhar, K.R.; Young, L.-S.; Lee, M.-R.; Chen, W.-M.; Chou, J.-H.; Young, C.-C. Siansivirga zeaxanthinifaciens gen. nov., sp. nov., a novel zeaxanthin-producing member of the family Flavobacteriaceae isolated from coastal seawater of Taiwan. FEMS Microbiol. Lett. 2012, 333, 37–45. [Google Scholar] [CrossRef]
- Thawornwiriyanun, P.; Tanasupawat, S.; Dechsakulwatana, C.; Techkarnjanaruk, S.; Suntornsuk, W. Identification of Newly Zeaxanthin-Producing Bacteria Isolated from Sponges in the Gulf of Thailand and their Zeaxanthin Production. Appl. Biochem. Biotechnol. 2012, 167, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Issouf, M.; Mearns, S.A.; Fraser, K.A.; Hodgson, R. Biological production of Zeaxanthin. European Patent Application No. EP1893769B1, 2012. [Google Scholar]
- Joshi, C.; Singhal, R.S. Modelling and optimization of zeaxanthin production by Paracoccus zeaxanthinifaciens ATCC 21588 using hybrid genetic algorithm techniques. Biocatal. Agric. Biotechnol. 2016, 8, 228–235. [Google Scholar] [CrossRef]
- Ram, S.; Tirkey, S.R.; Kumar, M.A.; Mishra, S. Ameliorating process parameters for zeaxanthin yield in Arthrobacter gandavensis MTCC 25325. AMB Express 2020, 10, 69. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Cámara, S.; Ibañez, A.; Rubio, S.; Barreiro, C.; Barredo, J.-L. Main Carotenoids Produced by Microorganisms. Encyclopedia 2021, 1, 1223-1245. https://doi.org/10.3390/encyclopedia1040093
Martínez-Cámara S, Ibañez A, Rubio S, Barreiro C, Barredo J-L. Main Carotenoids Produced by Microorganisms. Encyclopedia. 2021; 1(4):1223-1245. https://doi.org/10.3390/encyclopedia1040093
Chicago/Turabian StyleMartínez-Cámara, Sonia, Ana Ibañez, Sara Rubio, Carlos Barreiro, and José-Luis Barredo. 2021. "Main Carotenoids Produced by Microorganisms" Encyclopedia 1, no. 4: 1223-1245. https://doi.org/10.3390/encyclopedia1040093
APA StyleMartínez-Cámara, S., Ibañez, A., Rubio, S., Barreiro, C., & Barredo, J. -L. (2021). Main Carotenoids Produced by Microorganisms. Encyclopedia, 1(4), 1223-1245. https://doi.org/10.3390/encyclopedia1040093