Oxidative Stress in Relation to Aging and Exercise
Definition
:1. Introduction
2. Oxidative Stress
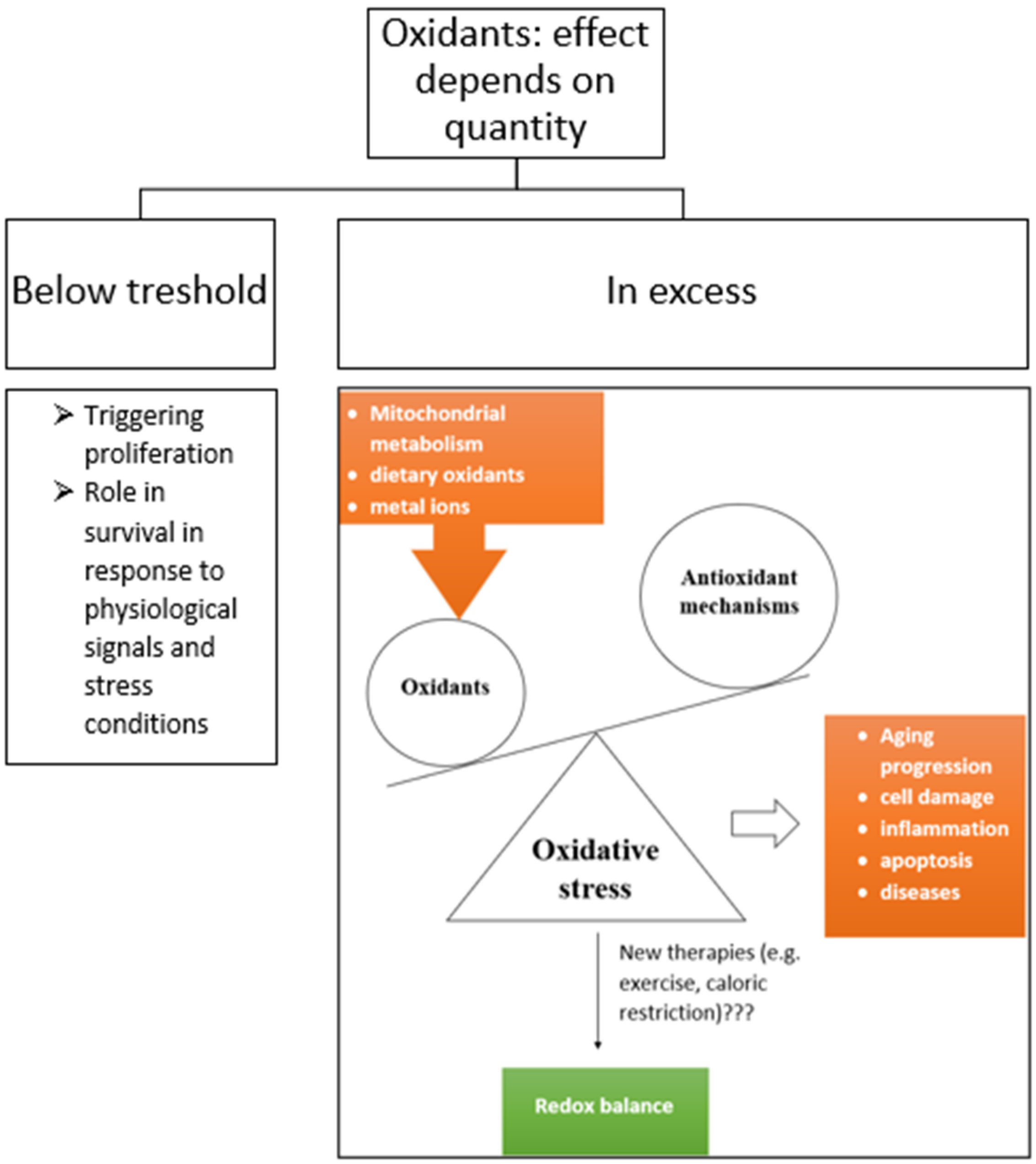
2.1. Reactive Oxygen Species
2.2. Antioxidants and Mechanisms of Their Action
2.2.1. Superoxide Dismutase
2.2.2. Glutathione Peroxidase, Glutathione Reductase, and Glutathione S-Transferase
2.2.3. Oxidized and Reduced Glutathione
2.2.4. Catalase
2.2.5. Others
2.3. Effects of Oxidative Stress
3. Oxidative Stress in Relation to Aging
3.1. Free Radical Theory of Aging
3.2. Telomere Shortening
3.3. Cellular Senescence
4. Oxidative Stress in Relation to Lifestyle
4.1. Exercise
4.2. Diet
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Longo, M.; Bellastella, G.; Maiorino, M.I.; Meier, J.J.; Esposito, K.; Giugliano, D. Diabetes and Aging: From Treatment Goals to Pharmacologic Therapy. Front. Endocrinol. 2019, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Caseiro, A.; Rocha, C.; Silva, A.M.; Ferreira, C.; Silva, I.; Clemente, M.; Cipriano, I.; Saraiva, M.; Barreira, R.; Azenha, J.; et al. Effects of A Personalized Intervention Program on the Biochemical and Hematological Profile in Community Dwelling Old Adults-The AGA@4life Intervention Model. Int. J. Env. Res Public Health 2020, 17, 718. [Google Scholar] [CrossRef] [PubMed]
- Ovadya, Y.; Krizhanovsky, V. Strategies targeting cellular senescence. J. Clin. Invest 2018, 128, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, Antioxidants, and the Degenerative Diseases of Aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef]
- Junqueira, V.B.C.; Barros, S.B.M.; Chan, S.S.; Rodrigues, L.; Giavarotti, L.; Abud, R.L.; Deucher, G.P. Aging and oxidative stress. Mol. Asp. Med. 2004, 25, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Polidori, M.C.; Troiano, L.; Cherubini, A.; Cecchetti, R.; Pini, G.; Straatman, M.; Monti, D.; Stahl, W.; Sies, H.; et al. Plasma antioxidants and longevity: A study on healthy centenarians. Free Radic. Biol. Med. 2000, 28, 1243–1248. [Google Scholar] [CrossRef]
- Kirkwood, T.B.L.; Kowald, A. The free-radical theory of ageing--older, wiser and still alive: Modelling positional effects of the primary targets of ROS reveals new support. Bioessays 2012, 34, 692–700. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Li, C.; Feng, F.; Xiong, X.; Li, R.; Chen, N. Exercise coupled with dietary restriction reduces oxidative stress in male adolescents with obesity. J. Sports Sci. 2017, 35, 663–668. [Google Scholar] [CrossRef]
- Sack, M.N.; Fyhrquist, F.Y.; Saijonmaa, O.J.; Fuster, V.; Kovacic, J.C. Basic Biology of Oxidative Stress and the Cardiovascular System: Part 1 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 196–211. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Robb, E.L.; Christoff, C.A.; Maddalena, L.A.; Stuart, J.A. Mitochondrial reactive oxygen species (ROS) in animal cells: Relevance to aging and normal physiology. Can. J. Zool. 2014, 92, 603–613. [Google Scholar] [CrossRef]
- Ahmad, M.; Wolberg, A.; Kahwaji, C.I. Biochemistry, Electron Transport Chain; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Childs, S.; Haroune, N.; Williams, L.; Gronow, M. Determination of cellular glutathione:glutathione disulfide ratio in prostate cancer cells by high performance liquid chromatography with electrochemical detection. J. Chromatogr. A 2016, 1437, 67–73. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Close, D.C.; Hagerman, A.E. Oxidative Stress, Exercise, and Aging; Imperial College Press: London, UK; Distributed by World Scientific Pub: Hackensack, NJ, USA, 2006. [Google Scholar]
- Ignarro, L.J. Nitric Oxide: Biology and Pathobiology, 1st ed.; Academic Press: San Diego, MA, USA, 2000. [Google Scholar]
- Blaise, G.A.; Gauvin, D.; Gangal, M.; Authier, S. Nitric oxide, cell signaling and cell death. Toxicology 2005, 208, 177–192. [Google Scholar] [CrossRef]
- Wendehenne, D.; Pugin, A.; Klessig, D.F.; Durner, J. Nitric oxide: Comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 2001, 6, 177–183. [Google Scholar] [CrossRef]
- Simpkins, J.W.; Wang, J.; Wang, X.; Perez, E.; Prokai, L.; Dykens, J.A. Mitochondria play a central role in estrogen-induced neuroprotection. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 69–83. [Google Scholar] [CrossRef]
- Wu, W.; Chen, M.; Luo, T.; Fan, Y.; Zhang, J.; Zhang, Y.; Zhang, Q.; Sapin-Minet, A.; Gaucher, C.; Xia, X. ROS and GSH-responsive S-nitrosoglutathione functionalized polymeric nanoparticles to overcome multidrug resistance in cancer. Acta Biomater 2020, 103, 259–271. [Google Scholar] [CrossRef]
- Gupta, I.; Shetti, A.; Keluskar, V.; Bagewadi, A. Assessment of Serum Enzymatic Antioxidant Levels in Patients with Recurrent Aphthous Stomatitis: A Case Control Study. Enzym. Res. 2014, 2014, 340814–340819. [Google Scholar] [CrossRef]
- Aztatzi-Aguilar, O.G.; Sierra-Vargas, M.P.; Ortega-Romero, M.; Jiménez-Corona, A.E. Osteopontin’s relationship with malnutrition and oxidative stress in adolescents. A pilot study. PLoS ONE 2021, 16, e0249057. [Google Scholar] [CrossRef]
- Phillips, N.H. Superoxide Dismutase (SOD): Sources, Therapeutic Uses and Health Benefits; Nova Biomedical: New York, NY, USA, 2016. [Google Scholar]
- Żwierełło, W.; Styburski, D.; Maruszewska, A.; Piorun, K.; Skórka-Majewicz, M.; Czerwińska, M.; Maciejewska, D.; Baranowska-Bosiacka, I.; Krajewski, A.; Gutowska, I. Bioelements in the treatment of burn injuries—The complex review of metabolism and supplementation (copper, selenium, zinc, iron, manganese, chromium and magnesium). J. Trace Elem. Med. Biol. 2020, 62, 126616. [Google Scholar] [CrossRef] [PubMed]
- RANDOX. Superoxide Dismutase (Ransod). Available online: https://www.randox.com/superoxide-dismutase-ransod/?highlight=Ransod# (accessed on 11 February 2022).
- Simões, S.I.; Delgado, T.C.; Lopes, R.M.; Jesus, S.; Ferreira, A.A.; Morais, J.A.; Cruz, M.E.M.; Corvo, M.L.; Martins, M.B.F. Developments in the rat adjuvant arthritis model and its use in therapeutic evaluation of novel non-invasive treatment by SOD in Transfersomes. J. Control Release 2005, 103, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Strange, R.C. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology 2000, 61, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Strange, R.C.; Spiteri, M.A.; Ramachandran, S.; Fryer, A.A. Glutathione-S-transferase family of enzymes. Mutat. Res. 2001, 482, 21–26. [Google Scholar] [CrossRef]
- Kanwal, R.; Pandey, M.; Bhaskaran, N.; Maclennan, G.T.; Fu, P.; Ponsky, L.E.; Gupta, S. Protection against oxidative DNA damage and stress in human prostate by glutathione S-transferase P1. Mol. Carcinog. 2014, 53, 8–18. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Fitri, L.E.; Iskandar, A.; Sardjono, T.W.; Erliana, U.D.; Rahmawati, W.; Candradikusuma, D.; Saputra, U.B.; Suhartono, E.; Setiawan, B.; Sulistyaningsih, E. Plasma glutathione and oxidized glutathione level, glutathione/oxidized glutathione ratio, and albumin concentration in complicated and uncomplicated falciparum malaria. Asian Pac. J. Trop. Biomed. 2016, 6, 646–650. [Google Scholar] [CrossRef]
- Lai, Y.; Li, M.; Liao, X.; Zou, L. Smartphone-Assisted Colorimetric Detection of Glutathione and Glutathione Reductase Activity in Human Serum and Mouse Liver Using Hemin/G-Quadruplex DNAzyme. Molecules 2021, 26, 5016. [Google Scholar] [CrossRef] [PubMed]
- Nannenga, B.L.; Shi, D.; Hattne, J.; Reyes, F.E.; Gonen, T. Structure of catalase determined by MicroED. Elife 2014, 3, e03600. [Google Scholar] [CrossRef]
- Iskusnykh, I.Y.; Zakharova, A.A.; Pathak, D. Glutathione in Brain Disorders and Aging. Molecules 2022, 27, 324. [Google Scholar] [CrossRef]
- Yamawaki, H.; Haendeler, J.; Berk, B.C. Thioredoxin: A Key Regulator of Cardiovascular Homeostasis. Circ. Res. 2003, 93, 1029–1033. [Google Scholar] [CrossRef]
- Dworzański, J.; Strycharz-Dudziak, M.; Kliszczewska, E.; Kiełczykowska, M.; Dworzańska, A.; Drop, B.; Polz-Dacewicz, M. Glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in patients with diabetes mellitus type 2 infected with Epstein-Barr virus. PLoS ONE 2020, 15, e0230374. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I. Functional Foods; IntechOpen: London, UK, 2018. [Google Scholar]
- Asarian, L.; Gloy, V.; Geary, N. Homeostasis. In Encyclopedia of Human Behavior, 2nd ed.; Ramachandran, V.S., Ed.; Academic Press: San Diego, MA, USA, 2012; pp. 324–333. [Google Scholar] [CrossRef]
- Vukovic, D.; Kiyan, V. Myocardial Ischemia: Causes, Symptoms and Treatment; Nova Science Publishers: Hauppauge, NY, USA, 2010. [Google Scholar]
- Sanchez-Roman, I.; Barja, G. Regulation of longevity and oxidative stress by nutritional interventions: Role of methionine restriction. Exp. Gerontol. 2013, 48, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Weindruch, R. Oxidative Stress, Caloric Restriction, and Aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Saretzki, G.; von Zglinicki, T. Replicative senescence as a model of aging: The role of oxidative stress and telomere shortening--an overview. Z. Für Gerontol. Und Geriatr. 1999, 32, 69–75. [Google Scholar] [CrossRef]
- Joseph, A.M.; Adhihetty, P.J.; Leeuwenburgh, C. Beneficial effects of exercise on age-related mitochondrial dysfunction and oxidative stress in skeletal muscle. J. Physiol. 2016, 594, 5105–5123. [Google Scholar] [CrossRef]
- Konopka, A.R.; Sreekumaran Nair, K. Mitochondrial and skeletal muscle health with advancing age. Mol. Cell Endocrinol. 2013, 379, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Hogan, M.C. Exercise and oxidative stress. J. Physiol. 2016, 594, 5079–5080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thirupathi, A.; Wang, M.; Lin, J.K.; Fekete, G.; István, B.; Baker, J.S.; Gu, Y. Effect of Different Exercise Modalities on Oxidative Stress: A Systematic Review. Bio. Med. Res. Int. 2021, 2021, 1947928. [Google Scholar] [CrossRef]
- Galli, D.; Carubbi, C.; Masselli, E.; Vaccarezza, M.; Presta, V.; Pozzi, G.; Ambrosini, L.; Gobbi, G.; Vitale, M.; Mirandola, P. Physical Activity and Redox Balance in the Elderly: Signal Transduction Mechanisms. Appl. Sci. 2021, 11, 2228. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Rowiński, R.; Kornatowski, M.; Dąbrowski, A.; Kędziora-Kornatowska, K.; Strachecka, A. Relation of Moderate Physical Activity to Blood Markers of Oxidative Stress and Antioxidant Defense in the Elderly. Oxidative Med. Cell. Longev. 2019, 2019, 5123628. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Hamadeh, M.J.; Kaczor, J.J.; Raha, S.; Debeer, J.; Tarnopolsky, M.A. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS ONE 2010, 5, e10778. [Google Scholar] [CrossRef]
- Aerobic Training. Available online: https://www.healthychildren.org/English/healthy-living/fitness/Pages/Aerobic-Training.aspx#:~:text=Aerobic%20training%20exercises%20are%20any,Jogging%20or%20running (accessed on 20 June 2022).
- AUSactive. Resistance Training–Health Benefits. Available online: https://www.betterhealth.vic.gov.au/health/healthyliving/resistance-training-health-benefits (accessed on 23 June 2022).
- Harper, C.; Gopalan, V.; Goh, J. Exercise rescues mitochondrial coupling in aged skeletal muscle: A comparison of different modalities in preventing sarcopenia. J. Transl. Med. 2021, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Valado, A.; Fortes, S.; Morais, M.; Rosado, J.; Figueiredo, J.P.; Barreira, R.; Caseiro, A. Hydrotherapy in the evaluation of enzymatic antioxidants in an elderly population. Eur. J. Public Health 2020, 30 (Suppl. 2), ckaa040-056. [Google Scholar] [CrossRef]
- Kliszczewicz, B.; John, Q.C.; Daniel, B.L.; Gretchen, O.D.; Michael, E.R.; Kyle, T.J. Acute Exercise and Oxidative Stress: CrossFit™ vs. Treadmill Bout. J. Hum. Kinet 2015, 47, 81–90. [Google Scholar] [CrossRef]
- Lanza, I.R.; Zabielski, P.; Klaus, K.A.; Morse, D.M.; Heppelmann, C.J.; Bergen, H.R.; Dasari, S.; Walrand, S.; Short, K.R.; Johnson, M.L.; et al. Chronic Caloric Restriction Preserves Mitochondrial Function in Senescence without Increasing Mitochondrial Biogenesis. Cell Metab. 2012, 16, 777–788. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.-M.; Dayem, A.A.; Cho, S.-G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci 2017, 18, 1544. [Google Scholar] [CrossRef]
- Everitt, A.V.; Roth, G.S.; Le Couteur, D.G.; Hilmer, S.N. Caloric restriction versus drug therapy to delay the onset of aging diseases and extend life. Age 2005, 27, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Rapisarda, P.; Fanella, F.; Maccarone, E. Reliability of Analytical Methods for Determining Anthocyanins in Blood Orange Juices. J. Agric. Food Chem. 2000, 48, 2249–2252. [Google Scholar] [CrossRef]
- Afanas’ev, I. Signaling and Damaging Functions of Free Radicals in Aging-Free Radical Theory, Hormesis, and TOR. Aging Dis. 2010, 1, 75–88. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verhaegen, D.; Smits, K.; Osório, N.; Caseiro, A. Oxidative Stress in Relation to Aging and Exercise. Encyclopedia 2022, 2, 1545-1558. https://doi.org/10.3390/encyclopedia2030105
Verhaegen D, Smits K, Osório N, Caseiro A. Oxidative Stress in Relation to Aging and Exercise. Encyclopedia. 2022; 2(3):1545-1558. https://doi.org/10.3390/encyclopedia2030105
Chicago/Turabian StyleVerhaegen, Dimphna, Kelly Smits, Nádia Osório, and Armando Caseiro. 2022. "Oxidative Stress in Relation to Aging and Exercise" Encyclopedia 2, no. 3: 1545-1558. https://doi.org/10.3390/encyclopedia2030105





