Arbuscular mycorrhizal Fungi as Inspiration for Sustainable Technology
Abstract
:1. Background
1.1. Arbuscular mycorrhizal Fungi
1.2. Phosphorus Is a Key Element
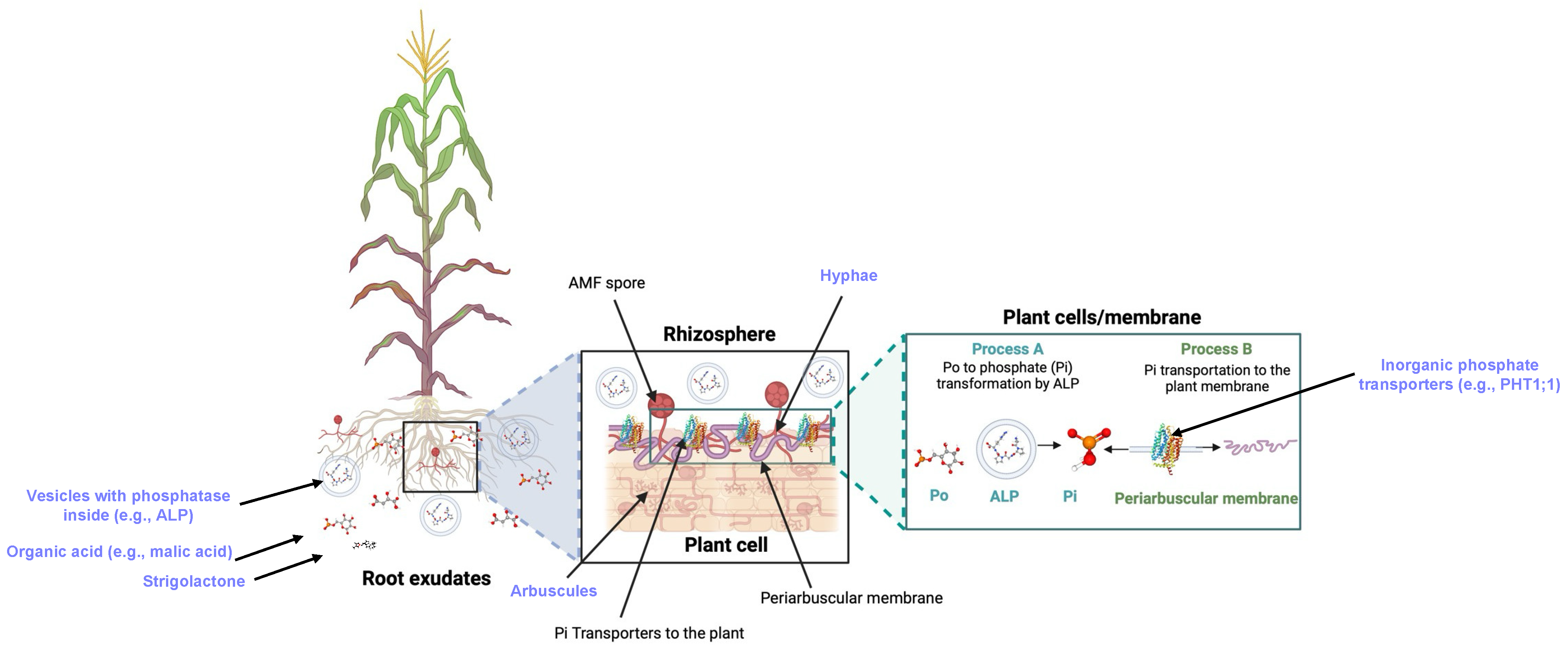
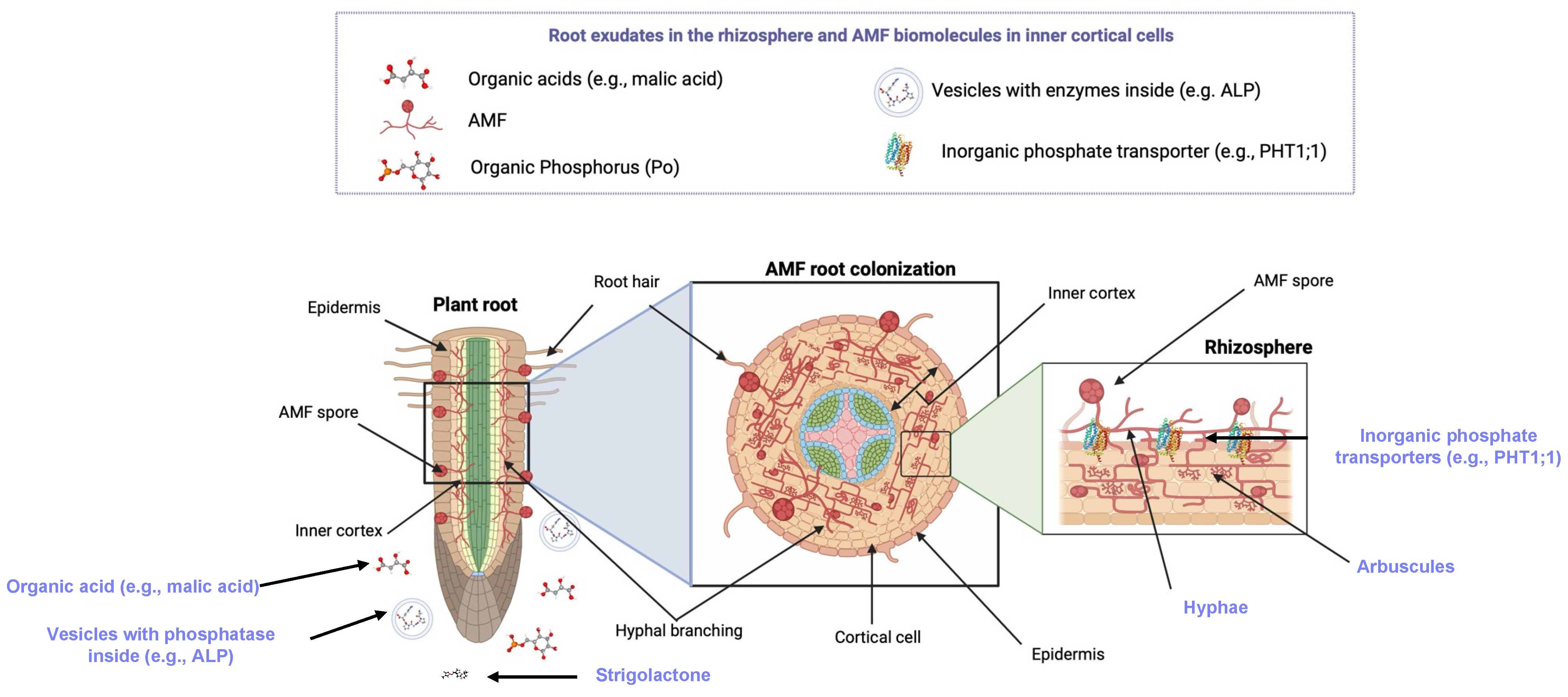
2. Mechanisms of P Uptake by AMF–Plant Systems
2.1. AMF
2.2. Root Exudates
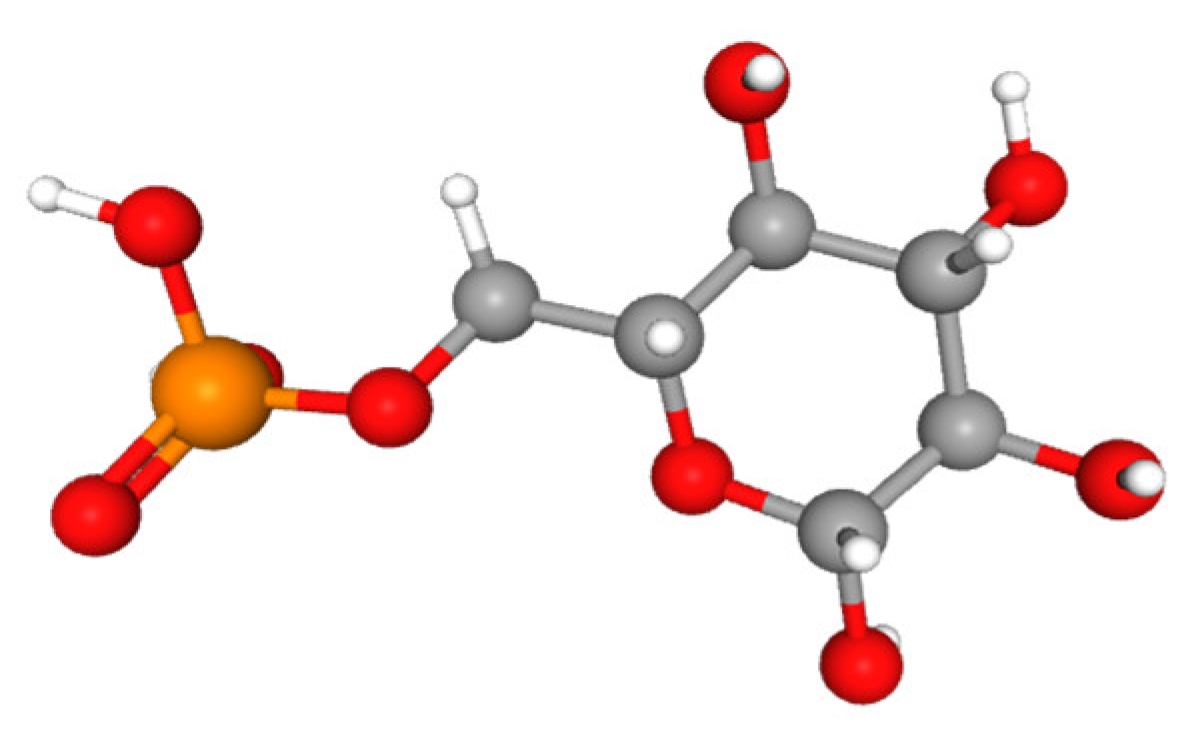
2.3. Organic Phosphorus
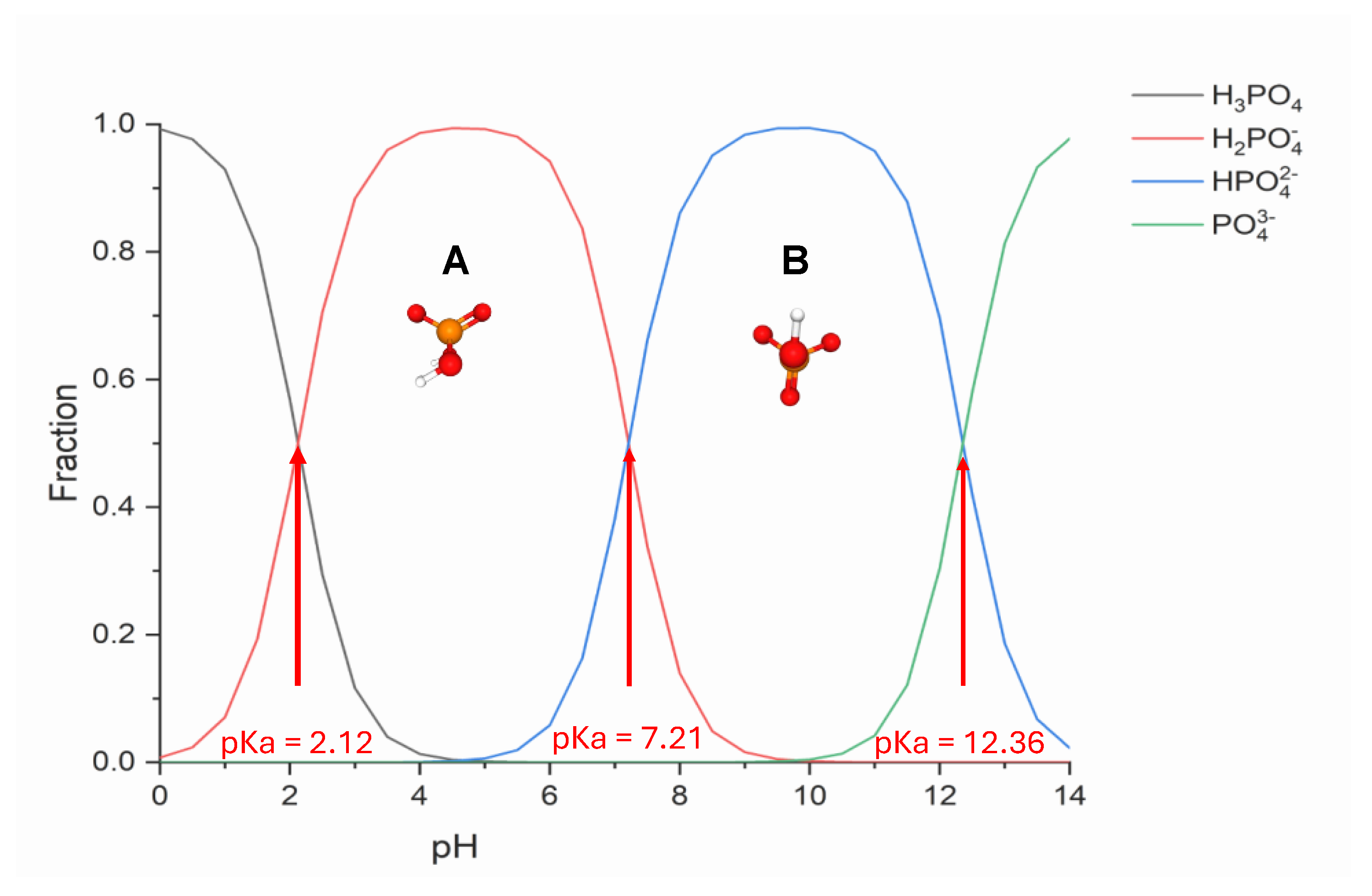
2.4. Inorganic Phosphorus
2.5. Pi Transporters
2.6. Enzymes
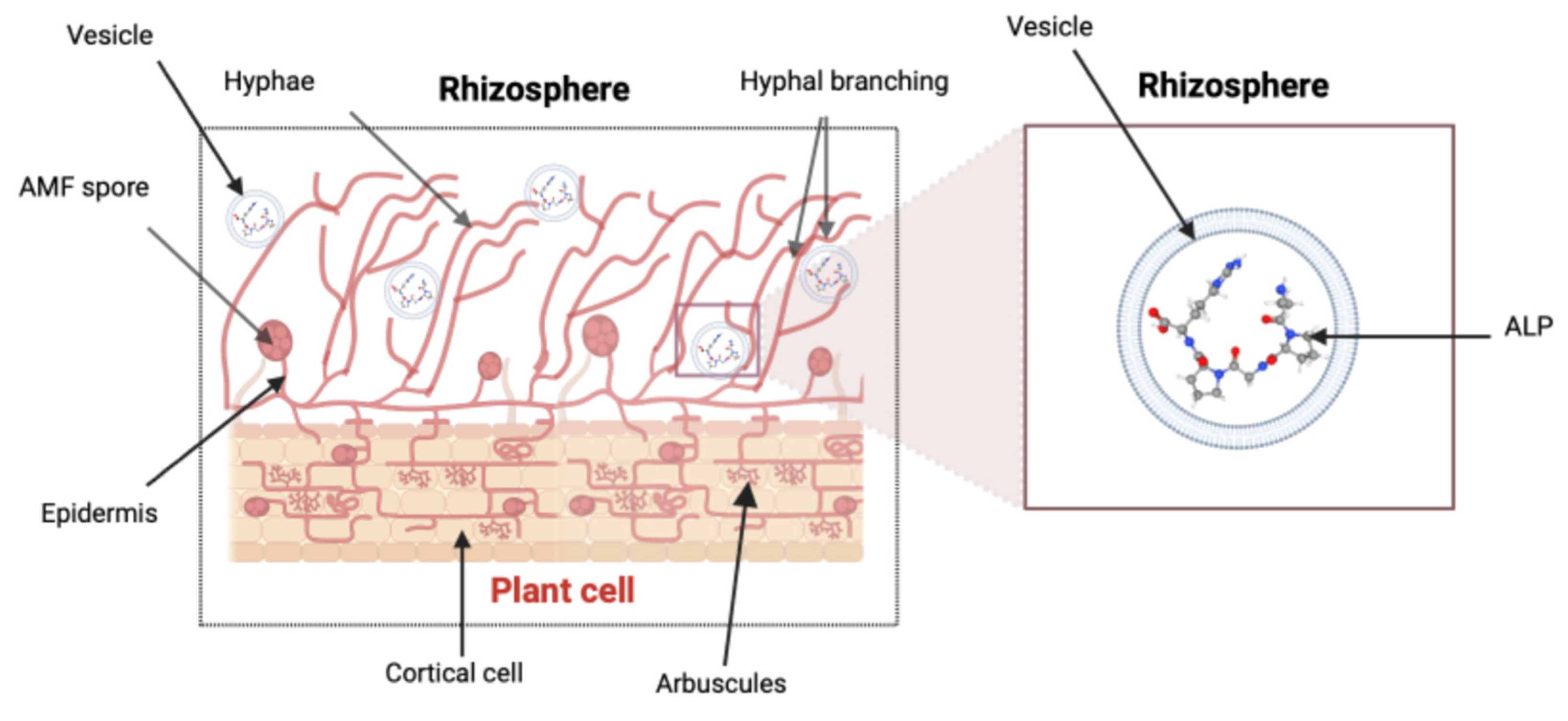
2.7. Arbuscules
2.8. Hyphae
2.9. Vesicles
2.10. Summary: Benefits of AMF in Soil
3. Future Research Opportunities
3.1. Monitoring P within Natural Systems
3.2. P Recovery
3.3. Enhancing P Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagyaraj, D.J.; Sharma, M.P.; Maiti, D. Phosphorus Nutrition of Crops through Arbuscular Mycorrhizal Fungi. Curr. Sci. 2015, 108, 1288–1293. [Google Scholar]
- Bhattacharya, A. Chapter 5—Changing Environmental Condition and Phosphorus-Use Efficiency in Plants. In Changing Climate and Resource Use Efficiency in Plants; Bhattacharya, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 241–305. ISBN 978-0-12-816209-5. [Google Scholar]
- Lee, E.-H.; Eo, J.-K.; Ka, K.-H.; Eom, A.-H. Diversity of Arbuscular Mycorrhizal Fungi and Their Roles in Ecosystems. Mycobiology 2013, 41, 121. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Mendoza, I.E.; Dewbre, G.R.; Harrison, M.J. A Phosphate Transporter Gene from the Extra-Radical Mycelium of an Arbuscular Mycorrhizal Fungus Glomus Intraradices Is Regulated in Response to Phosphate in the Environment. Mol. Plant-Microbe Interact. 2001, 14, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Doydora, S.; Gatiboni, L.; Grieger, K.; Hesterberg, D.; Jones, J.L.; McLamore, E.S.; Peters, R.; Sozzani, R.; Van den Broeck, L.; Duckworth, O.W. Accessing Legacy Phosphorus in Soils. Soil Syst. 2020, 4, 74. [Google Scholar] [CrossRef]
- Qin, Z.; Peng, Y.; Yang, G.; Feng, G.; Christie, P.; Zhou, J.; Zhang, J.; Li, X.; Gai, J. Relationship between Phosphorus Uptake via Indigenous Arbuscular Mycorrhizal Fungi and Crop Response: A 32P-Labeling Study. Appl. Soil Ecol. 2022, 180, 104624. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Hajiboland, R. Introduction to Arbuscular Mycorrhizal Fungi and Higher Plant Symbiosis: Characteristic Features, Functions, and Applications. In Arbuscular Mycorrhizal Fungi and Higher Plants: Fundamentals and Applications; Ahammed, G.J., Hajiboland, R., Eds.; Springer Nature: Singapore, 2024; pp. 1–17. ISBN 978-981-9982-20-2. [Google Scholar]
- National Center for Biotechnology Information PubChem Compound Summary for CID 15102684, 5-Deoxystrigol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/15102684 (accessed on 25 May 2024).
- National Center for Biotechnology Information PubChem Compound Summary for CID 5958, Glucose 6-Phosphate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5958 (accessed on 25 May 2024).
- Gupta, M.; Finer-Moore, J.; Stroud, R.M. PDB Entry - 8FV- PiPT Y150A. Available online: https://www.wwpdb.org/pdb?id=pdb_00008fvz (accessed on 1 June 2024).
- Aggarwal, A.; Kadian, N.; Tanwar, A.; Yadav, A.; Gupta, K.K. Role of Arbuscular Mycorrhizal Fungi (AMF) in Global Sustainable Development. J. Appl. Nat. Sci. 2011, 3, 340–351. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus Dynamics: From Soil to Plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of Arbuscular Mycorrhizal Fungi, Phosphate–Solubilizing Bacteria, and Silicon to P Uptake by Plant. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Huang, T.-K.; Kuo, H.-F.; Chiou, T.-J. Role of Vacuoles in Phosphorus Storage and Remobilization. J. Exp. Bot. 2017, 68, 3045–3055. [Google Scholar] [CrossRef]
- Qi, S.; Wang, J.; Wan, L.; Dai, Z.; da Silva Matos, D.M.; Du, D.; Egan, S.; Bonser, S.P.; Thomas, T.; Moles, A.T. Arbuscular Mycorrhizal Fungi Contribute to Phosphorous Uptake and Allocation Strategies of Solidago Canadensis in a Phosphorous-Deficient Environment. Front. Plant Sci. 2022, 13, 831654. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y. The Function of Root Exudates in the Root Colonization by Beneficial Soil Rhizobacteria. Biology 2024, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Paszkowski, U. Mechanisms and Impact of Symbiotic Phosphate Acquisition. Cold Spring Harb. Perspect. Biol. 2019, 11, a034603. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information PubChem Compound Summary for CID 1003, Dihydrogenphosphate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1003 (accessed on 25 May 2024).
- National Center for Biotechnology Information PubChem Compound Summary for CID 18985873, Alkaline Phosphatase. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/18985873 (accessed on 25 May 2024).
- Parniske, M. Arbuscular Mycorrhiza: The Mother of Plant Root Endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Tisserant, E.; Malbreil, M.; Kuo, A.; Kohler, A.; Symeonidi, A.; Balestrini, R.; Charron, P.; Duensing, N.; Frei dit Frey, N.; Gianinazzi-Pearson, V.; et al. Genome of an Arbuscular Mycorrhizal Fungus Provides Insight into the Oldest Plant Symbiosis. Proc. Natl. Acad. Sci. USA 2013, 110, 20117–20122. [Google Scholar] [CrossRef]
- Kapulnik, Y.; Koltai, H. Strigolactone Involvement in Root Development, Response to Abiotic Stress, and Interactions with the Biotic Soil Environment. Plant Physiol. 2014, 166, 560–569. [Google Scholar] [CrossRef]
- Marro, N.; Lidoy, J.; Chico, M.Á.; Rial, C.; García, J.; Varela, R.M.; Macías, F.A.; Pozo, M.J.; Janoušková, M.; López-Ráez, J.A. Strigolactones: New Players in the Nitrogen–Phosphorus Signalling Interplay. Plant Cell Environ. 2022, 45, 512–527. [Google Scholar] [CrossRef]
- Feng, W.; Wang, T.; Zhu, Y.; Sun, F.; Giesy, J.P.; Wu, F. Chemical Composition, Sources, and Ecological Effect of Organic Phosphorus in Water Ecosystems: A Review. Carbon Res. 2023, 2, 12. [Google Scholar] [CrossRef]
- George, T.S.; Giles, C.D.; Menezes-Blackburn, D.; Condron, L.M.; Gama-Rodrigues, A.C.; Jaisi, D.; Lang, F.; Neal, A.L.; Stutter, M.I.; Almeida, D.S.; et al. Organic Phosphorus in the Terrestrial Environment: A Perspective on the State of the Art and Future Priorities. Plant Soil 2018, 427, 191–208. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, F.; He, Z.; Guo, J.; Qu, X.; Xie, F.; Giesy, J.P.; Liao, H.; Guo, F. Characterization of Organic Phosphorus in Lake Sediments by Sequential Fractionation and Enzymatic Hydrolysis. Environ. Sci. Technol. 2013, 47, 7679–7687. [Google Scholar] [CrossRef]
- Wan, J.; Yuan, X.; Han, L.; Ye, H.; Yang, X. Characteristics and Distribution of Organic Phosphorus Fractions in the Surface Sediments of the Inflow Rivers around Hongze Lake, China. Int. J. Environ. Res. Public. Health 2020, 17, 648. [Google Scholar] [CrossRef]
- Thomas Sims, J.; Pierzynski, G.M. Chemistry of Phosphorus in Soils. In Chemical Processes in Soils; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 151–192. ISBN 978-0-89118-892-6. [Google Scholar]
- Hinsinger, P. Bioavailability of Soil Inorganic P in the Rhizosphere as Affected by Root-Induced Chemical Changes: A Review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Compound Summary for CID 3681305, Hydrogen Phosphate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3681305 (accessed on 25 May 2024).
- Role of Soil Enzymes in Sustainable Crop Production. Available online: https://www.sciencedirect.com/science/article/pii/B9780128132807000335 (accessed on 29 April 2024).
- Eivazi, F.; Tabatabai, M.A. Phosphatases in Soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Compound Summary for CID 12951370, Acid Phosphatase. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/12951370 (accessed on 25 May 2024).
- Devi, S.H.; Bhupenchandra, I.; Sinyorita, S.; Chongtham, S.K.; Devi, E.L.; Devi, S.H.; Bhupenchandra, I.; Sinyorita, S.; Chongtham, S.K.; Devi, E.L. Mycorrhizal Fungi and Sustainable Agriculture. In Nitrogen in Agriculture—Physiological, Agricultural and Ecological Aspects; IntechOpen: London, UK, 2021; ISBN 978-1-83968-492-0. [Google Scholar]
- Dhanker, R.; Chaudhary, S.; Kumari, A.; Kumar, R.; Goyal, S. Symbiotic Signaling: Insights from Arbuscular Mycorrhizal Symbiosis. In Plant Microbe Symbiosis; Varma, A., Tripathi, S., Prasad, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 75–103. ISBN 978-3-030-36248-5. [Google Scholar]
- Wang, Y.-J.; Wu, Q.-S. Influence of Sugar Metabolism on the Dialogue between Arbuscular Mycorrhizal Fungi and Plants. Hortic. Adv. 2023, 1, 2. [Google Scholar] [CrossRef]
- SJones, S. The Underground Network: Arbuscular Mycorrhizal Fungi—Sebastian Jones. Available online: https://microbewiki.kenyon.edu/index.php/The_Underground_Network:_Arbuscular_Mycorrhizal_Fungi_-_Sebastian_Jones (accessed on 27 May 2024).
- Andrino, A.; Guggenberger, G.; Kernchen, S.; Mikutta, R.; Sauheitl, L.; Boy, J. Production of Organic Acids by Arbuscular Mycorrhizal Fungi and Their Contribution in the Mobilization of Phosphorus Bound to Iron Oxides. Front. Plant Sci. 2021, 12, 661842. [Google Scholar] [CrossRef]
- Wang, D.; Lv, S.; Jiang, P.; Li, Y. Roles, Regulation, and Agricultural Application of Plant Phosphate Transporters. Front. Plant Sci. 2017, 8, 817. [Google Scholar] [CrossRef]
- Walder, F.; Boller, T.; Wiemken, A.; Courty, P.-E. Regulation of Plants’ Phosphate Uptake in Common Mycorrhizal Networks: Role of Intraradical Fungal Phosphate Transporters. Plant Signal. Behav. 2016, 11, e1131372. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Hasan, M.M.; Teixeira da Silva, J.A.; Li, X. Regulation of Phosphorus Uptake and Utilization: Transitioning from Current Knowledge to Practical Strategies. Cell. Mol. Biol. Lett. 2016, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Aini, N.; Dwi Yamika, W.S.; Wahidah Pahlevi, R. The Effect of Nutrient Concentration and Inoculation of PGPR and AMF on the Yield and Fruit Quality of Hydroponic Cherry Tomatoes (Lycopersicum Esculentum Mill. Var. Cerasiforme). J. Appl. Hortic. 2019, 21, 116–122. [Google Scholar] [CrossRef]
- Lanfranco, L.; Fiorilli, V.; Venice, F.; Bonfante, P. Strigolactones Cross the Kingdoms: Plants, Fungi, and Bacteria in the Arbuscular Mycorrhizal Symbiosis. J. Exp. Bot. 2018, 69, 2175–2188. [Google Scholar] [CrossRef]
- Czarnecki, O.; Yang, J.; Weston, D.J.; Tuskan, G.A.; Chen, J.-G. A Dual Role of Strigolactones in Phosphate Acquisition and Utilization in Plants. Int. J. Mol. Sci. 2013, 14, 7681–7701. [Google Scholar] [CrossRef]
- Boyno, G.; Rezaee Danesh, Y.; Demir, S.; Teniz, N.; Mulet, J.M.; Porcel, R. The Complex Interplay between Arbuscular Mycorrhizal Fungi and Strigolactone: Mechanisms, Sinergies, Applications and Future Directions. Int. J. Mol. Sci. 2023, 24, 16774. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Czarnecki, O.; Chourey, K.; Yang, J.; Tuskan, G.A.; Hurst, G.B.; Pan, C.; Chen, J.-G. Strigolactone-Regulated Proteins Revealed by iTRAQ-Based Quantitative Proteomics in Arabidopsis. J. Proteome Res. 2014, 13, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of Arbuscular Mycorrhizal Fungi in Regulating Growth, Enhancing Productivity, and Potentially Influencing Ecosystems under Abiotic and Biotic Stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Cela, F.; Avio, L.; Giordani, T.; Vangelisti, A.; Cavallini, A.; Turrini, A.; Sbrana, C.; Pardossi, A.; Incrocci, L. Arbuscular Mycorrhizal Fungi Increase Nutritional Quality of Soilless Grown Lettuce While Overcoming Low Phosphorus Supply. Foods 2022, 11, 3612. [Google Scholar] [CrossRef] [PubMed]
- Hage-Ahmed, K.; Rosner, K.; Steinkellner, S. Arbuscular Mycorrhizal Fungi and Their Response to Pesticides. Pest Manag. Sci. 2019, 75, 583–590. [Google Scholar] [CrossRef]
- Piliarová, M.; Ondreickova, K.; Hudcovicova, M.; Mihálik, D.; Kraic, J. Arbuscular Mycorrhizal Fungi—Their Life and Function in Ecosystem. Agric. Polnohospodárstvo 2019, 65, 3–15. [Google Scholar] [CrossRef]
- Garagounis, C.; Delkis, N.; Papadopoulou, K.K. Unraveling the Roles of Plant Specialized Metabolites: Using Synthetic Biology to Design Molecular Biosensors. New Phytol. 2021, 231, 1338–1352. [Google Scholar] [CrossRef]
- Alam, M.M.; Srinivasan, V.; Mueller, A.V.; Gu, A.Z. Status and Advances in Technologies for Phosphorus Species Detection and Characterization in Natural Environment- A Comprehensive Review. Talanta 2021, 233, 122458. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, S.; Wang, S.; Yan, X.; Qian, J.; Pan, B. Phosphorus in Water: A Review on the Speciation Analysis and Species Specific Removal Strategies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 435–456. [Google Scholar] [CrossRef]
- McLamore, E.; Duckworth, O.; Boyer, T.H.; Marshall, A.-M.; Call, D.F.; Bhadha, J.H.; Guzmán, S. Perspective: Phosphorus Monitoring Must Be Rooted in Sustainability Frameworks Spanning Material Scale to Human Scale. Water Res. X 2023, 19, 100168. [Google Scholar] [CrossRef]
- Evaluating Sustainable Development Pathways for Protein- and Peptide-Based Bioadsorbents for Phosphorus Recovery from Wastewater|Environmental Science & Technology. Available online: https://pubs.acs.org/doi/full/10.1021/acs.est.3c04016 (accessed on 24 May 2024).
- Mackay, J.E.; Cavagnaro, T.R.; Müller Stöver, D.S.; Macdonald, L.M.; Grønlund, M.; Jakobsen, I. A Key Role for Arbuscular Mycorrhiza in Plant Acquisition of P from Sewage Sludge Recycled to Soil. Soil Biol. Biochem. 2017, 115, 11–20. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Ma, F.; Zhang, X.; Fu, D. Arbuscular Mycorrhiza Improved Phosphorus Efficiency in Paddy Fields. Ecol. Eng. 2016, 95, 64–72. [Google Scholar] [CrossRef]
- Nussaume, L.; Kanno, S.; Javot, H.; Marin, E.; Nakanishi, T.M.; Thibaud, M.-C. Phosphate Import in Plants: Focus on the PHT1 Transporters. Front. Plant Sci. 2011, 2, 83. [Google Scholar] [CrossRef] [PubMed]




| Biomolecule | Role in Soil Processes | Role in AMF | Plant Root Processes | References |
|---|---|---|---|---|
| Phosphatases | Hydrolyze organic P (Po) to release inorganic phosphate (Pi, Ortho-P) as reaction products for plant absorption. | Secreted by fungi (e.g., AMF) to transform (mineralization of) Po into Pi. | Root-associated phosphatases hydrolyze Po in the rhizosphere, making it available for the plant. | [2,11,12,13] |
| Phosphate transporters | Pi is released from the arbuscular into the peri arbuscular membrane by the PHT1 transporter. | PHT (e.g., PHT1) family transport mycorrhiza-specific nutrients, inducible through AMF symbiosis with plant hosts, from the soil to the shoot and mycorrhizal symbiotic interface. | Phosphate transporters, such as the PHT1 family, are present in plant roots’ root epidermis and are expressed for the uptake of phosphate. | [39,40,41] |
| Organic acids | The process involves the solubilization of P from mineral surfaces through ligand ex-change or the ligand-promoted dissolution of Fe oxides. | AMF microbiota promotes plant growth by the production of organic acids. | Plant root responses to P starvation result in the exudation of organic acids like citric and malic acid in the rhizosphere. | [6,38,42] |
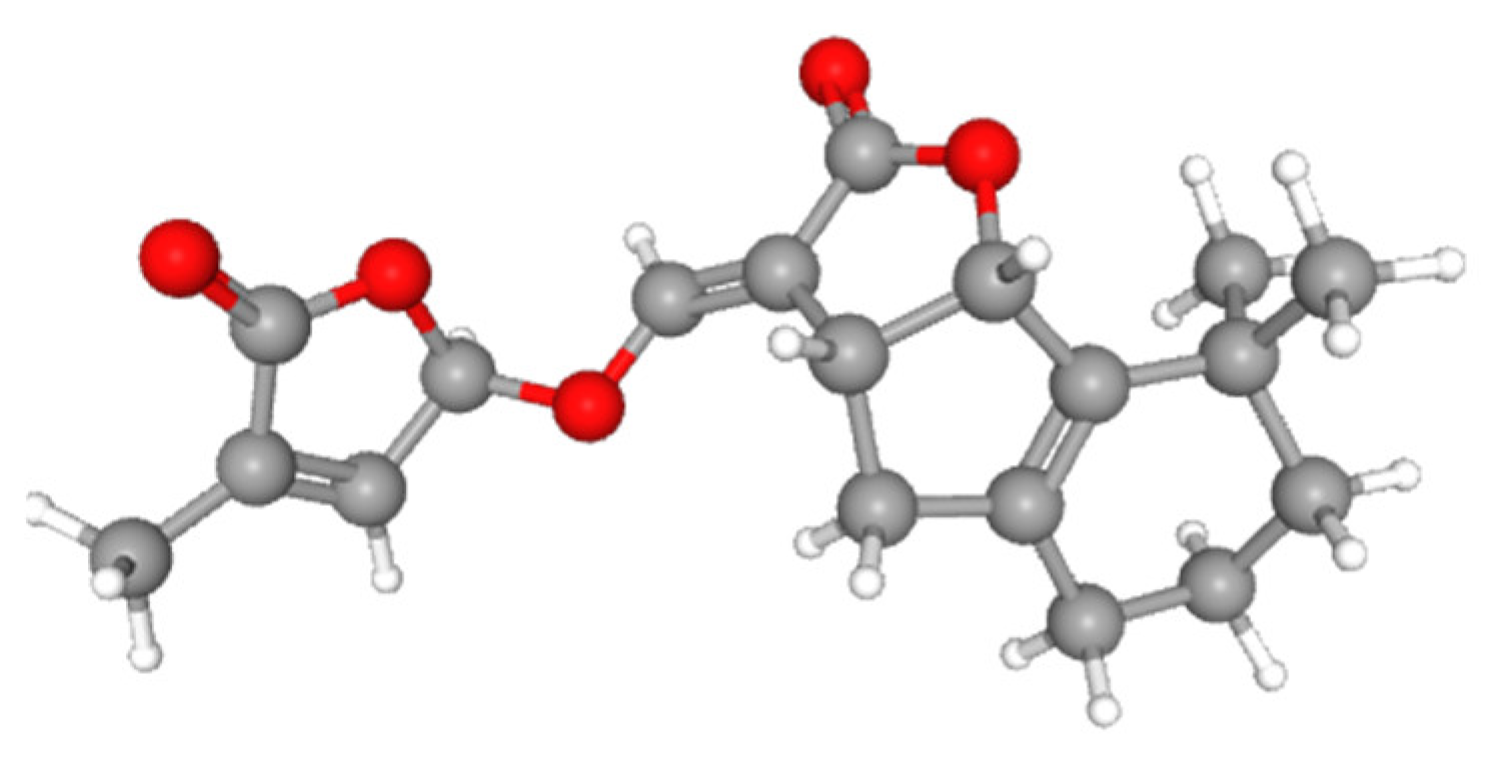
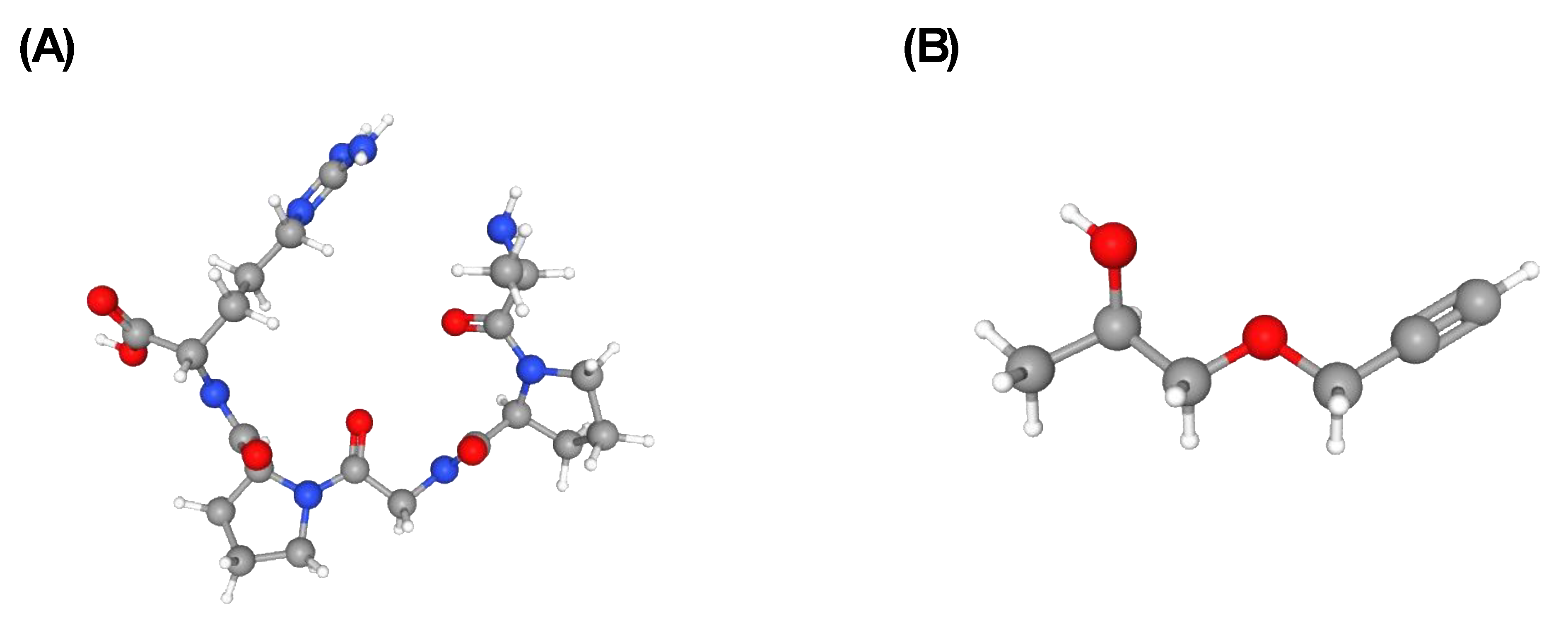
| Strigolactone | Plant hormones that play a crucial role in promoting plant AMF associations during the release of P starvation in the rhizosphere. | Hypha branching and root colonization of symbiotic AMF are stimulated by these substances, which also encourage the production and release of SLs by plants, attracting beneficial microbes. | Transported from roots to shoots, these substances control shoot branching (primary root growth, lateral and adventitious root formation, and root hair development) and promote plant growth. | [23,43,44,45,46] |
| Arbuscules | Enhance nutrient uptake by forming associations in the rhizosphere | Major site or resource exchange within the soil regulates the colonization process between AMF plants. | Formed arbuscules within the inner root cortical cells of the host plant allowing plant–AMF nutrient exchange | [7,17,47] |
| Hyphae | Threadlike structures that extend into the soil matrix, increasing the surface area available for Pi uptake. | Serves as a conduct for P uptake and C exchange to AMF in return. Hyphal branches form the association with plant roots. | Increase surface area for nutrient exploration, enhancing the Pi acquisition in places where plant roots alone do not have access to it. | [7,41,48] |
| Vesicle | Serve as storage reservoirs for phosphatases, lipids, and carbohydrates. | Store and transport Pi acquired by AMF from soil to the plant host. | Enhance Pi transfer from AMF to the plant root cells within vesicle structures. Allows access to Pi more efficiently. | [7,49,50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, M.J.; Moreira, G.; Bhadha, J.H.; McLamore, E.S. Arbuscular mycorrhizal Fungi as Inspiration for Sustainable Technology. Encyclopedia 2024, 4, 1188-1200. https://doi.org/10.3390/encyclopedia4030077
Torres MJ, Moreira G, Bhadha JH, McLamore ES. Arbuscular mycorrhizal Fungi as Inspiration for Sustainable Technology. Encyclopedia. 2024; 4(3):1188-1200. https://doi.org/10.3390/encyclopedia4030077
Chicago/Turabian StyleTorres, Maria J., Geisianny Moreira, Jehangir H. Bhadha, and Eric S. McLamore. 2024. "Arbuscular mycorrhizal Fungi as Inspiration for Sustainable Technology" Encyclopedia 4, no. 3: 1188-1200. https://doi.org/10.3390/encyclopedia4030077










