Biological Pollution of Indoor Air, Its Assessment and Control Methods
Definition
1. Introduction
2. Classification of Air Pollution
2.1. Chemical Contaminants
2.2. Physical Contaminants
2.3. Microbiological Contaminants
2.3.1. Bacterial Contamination of Indoor Air
2.3.2. Viral Indoor Air Pollution
2.3.3. Contamination of Indoor Air by Fungal Spores
3. Test Methods for Microbial Contamination of Indoor Air
3.1. Microscopic Methods for Bioaerosol Studies
3.2. Culture-Based Bioaerosol Test Methods
3.3. Molecular Methods for Bioaerosol Studies
3.4. Metabolic Bioaerosol Test Methods
4. Methods of Air Sampling for Bioaerosol Studies
4.1. Koch Sedimentation Method
4.2. Impactor Method
4.3. Filter Method (Aspiration)
4.4. Electroprecipitation Method
5. Microbiological Assessment of Air Pollution Control
6. Methods for Cleaning Indoor Air
6.1. Mechanical Air Purification Methods
6.2. Physical Methods of Air Purification
6.3. Chemical Air Purification Methods
6.4. Biological Methods of Air Purification
6.4.1. Purification Systems Based on Plants and Microalgae
6.4.2. Microbial-Based Treatment Systems
7. Analytical Techniques for the Analysis of Volatile Substances
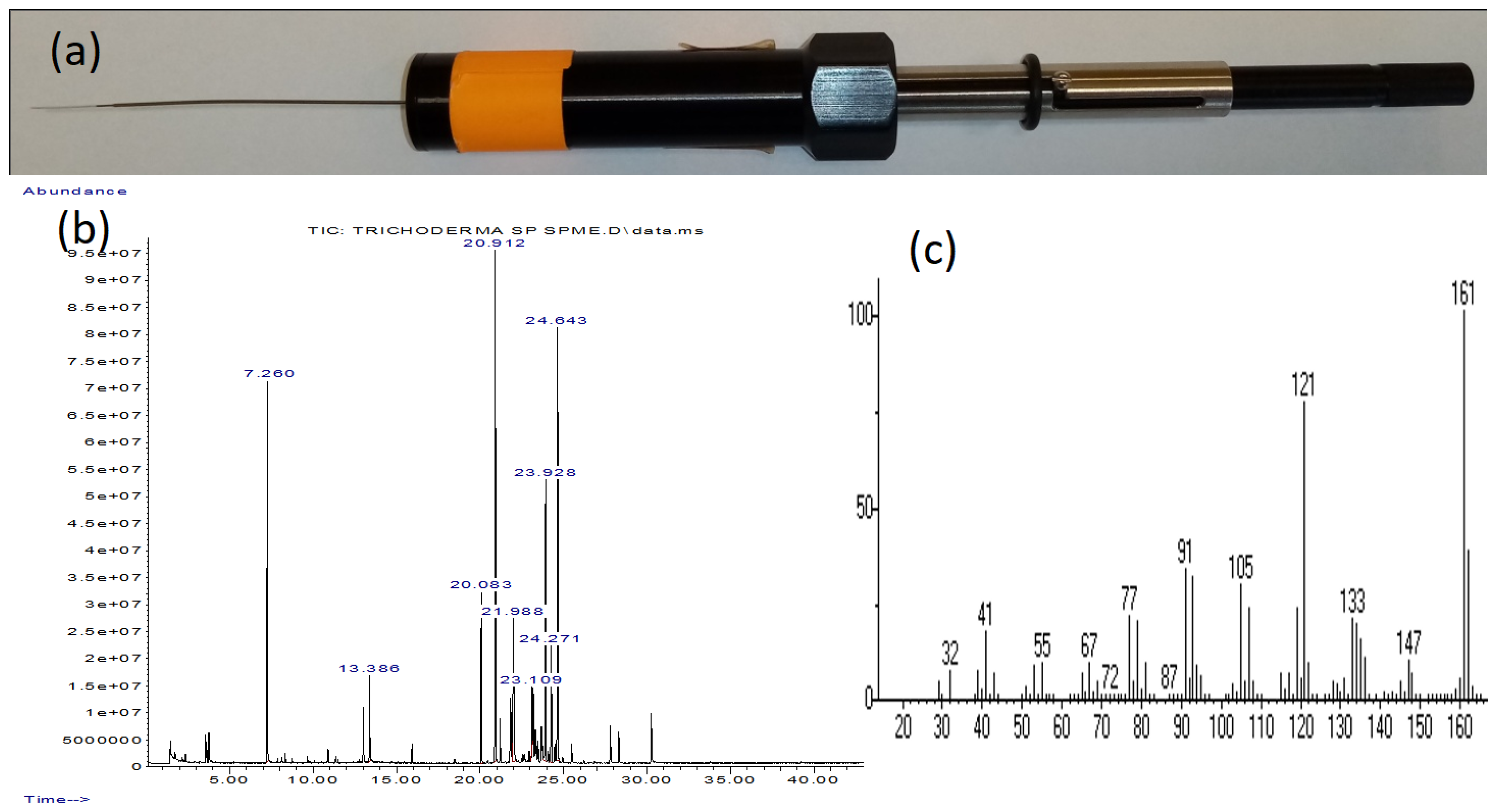
7.1. Stationary Phase Microextraction
7.2. Gas Chromatography
- (a)
- Wall-coated open-tebular (WCOT) column—the inner wall of the column is coated with a sorption layer;
- (b)
- Porous-layer open-tebular (PLOT) column—column with porous adsorbent;
- (c)
- Support-coated open-tebular (SCOT) column—column in which a liquid polymer layer, characterised by a high density, is applied to the surface of the adsorbent and which is the most commonly used column of this type today.
7.3. Mass Spectrometry
7.4. Analysis of Chromatograms and Mass Spectra Obtained by the Gas Chromatography-Mass Spectrometry Technique
8. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Khan, A.H.; Karuppayil, S.M. Fungal pollution of indoor environments and its management. Saudi J. Biol. Sci. 2012, 19, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lee, S.; Chan, L. Indoor air quality investigation at air-conditioned and non-air-conditioned markets in Hong Kong. Sci. Total Environ. 2004, 323, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Mannan, M.; Al-Ghamdi, S.G. Indoor air quality in buildings: A comprehensive review on the factors influencing air pollution in residential and commercial structure. Int. J. Environ. Res. Public Health 2021, 18, 3276. [Google Scholar] [CrossRef]
- Yang, W.; Sohn, J.; Kim, J.; Son, B.; Park, J. Indoor air quality investigation according to age of the school buildings in Korea. J. Environ. Manag. 2009, 90, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Butarewicz, A. Mikrobiologiczna jakosc powietrza w budynku Wydzialu Budownictwa i Inzynierii Srodowiska Politechniki Bialostockiej. Rocz. Państwowego Zakładu Hig. 2005, 2, 199–206. [Google Scholar]
- Baek, S. Assessing indoor air quality. In Encyclopedia of Environmental Health; Elsevier: Oxford, UK, 2019; pp. 191–198. [Google Scholar]
- Al-Rawi, M.; Lazonby, A.; Wai, A.A. Assessing Indoor Environmental Quality in a Crowded Low-Quality Built Environment: A Case Study. Atmosphere 2022, 13, 1703. [Google Scholar] [CrossRef]
- Gradisnik, L.; Bunc, G.; Ravnik, J.; Velnar, T. Enhancing Surgical Safety: Microbiological Air Control in Operating Theatres at University Medical Centre Maribor. Diagnostics 2024, 14, 1054. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, M.; Ikutegbe, C.A.; Auckaili, A.; Farid, M.M. Sustainable technologies to improve indoor air quality in a residential house—A case study in Waikato, New Zealand. Energy Build. 2021, 250, 111283. [Google Scholar] [CrossRef]
- Zhang, J.; Smith, K.R. Indoor air pollution: A global health concern. Br. Med Bull. 2003, 68, 209–225. [Google Scholar] [CrossRef]
- Guo, H. Source apportionment of volatile organic compounds in Hong Kong homes. Build. Environ. 2011, 46, 2280–2286. [Google Scholar] [CrossRef]
- McDonnell, G.; Burke, P. Disinfection: Is it time to reconsider Spaulding? J. Hosp. Infect. 2011, 78, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Yang, C.S. Fungal contamination as a major contributor to sick building syndrome. Adv. Appl. Microbiol. 2004, 55, 31–112. [Google Scholar] [PubMed]
- Siemiński, M. Środowiskowe Zagrożenia Zdrowia; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2008. [Google Scholar]
- Harazin, B.; Zielińnski, G. Zawodowa Ekspozycja na Miejscowe Wibracje w Polsce. Med. Pr. 2004, 55, 465–472. (In Polish) [Google Scholar]
- An, H.; Mainelis, G.; Yao, M. Evaluation of a high-volume portable bioaerosol sampler in laboratory and field environments. Indoor Air 2004, 14, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Kołwzan, B.; Adamiak, W.; Grabas, K.; Pawełczyk, A. Podstawy Mikrobiologii w Ochronie Środowiska; Oficyna Wydawnicza Politechniki Wrocławskiej: Wrocław, Poland, 2005. (In Polish) [Google Scholar]
- Krzysztofik, B. Mikrobiologia Powietrza; Wydawnictwo Politechiki Warszawskiej: Warszawa, Poland, 1992. [Google Scholar]
- Górny, R. Biologiczne czynniki szkodliwe: Normy, zalecenia i propozycje wartości dopuszczalnych. Podstawy I Metod. Oceny Środowiska Pr. 2004, 3, 17–39. (In Polish) [Google Scholar]
- Law, A.K.; Chau, C.K.; Chan, G.Y. Characteristics of bioaerosol profile in office buildings in Hong Kong. Build. Environ. 2001, 36, 527–541. [Google Scholar] [CrossRef]
- Agranovski, I.E.; Agranovski, V.; Reponen, T.; Willeke, K.; Grinshpun, S.A. Development and evaluation of a new personal sampler for culturable airborne microorganisms. Atmos. Environ. 2002, 36, 889–898. [Google Scholar] [CrossRef]
- Kaiser, K.; Wolski, A. Kontrola czystości mikrobiologicznej powietrza. Tech. Chłodnicza I Klimatyzacyjna 2007, 4, 158–162. (In Polish) [Google Scholar]
- Cabral, J.P. Can we use indoor fungi as bioindicators of indoor air quality? Historical perspectives and open questions. Sci. Total Environ. 2010, 408, 4285–4295. [Google Scholar] [CrossRef]
- Krzyśko-upicka, T. Przegląd metod stosowanych wykrywania grzybów w pomieszczeniach. In Proceedings of the Materiały Konferencyjne, VI Międzynarodowa Konferencja Naukowa–Mikotoksyny w Środowisku Człowieka i Zwierząt, Bydgoszcz, Poland, 25–27 September 2002; pp. 203–205. (In Polish). [Google Scholar]
- Ogórek, R.; Pląskowska, E. Analiza mikologiczna powietrza wybranych pomieszczeń użytku publicznego. Doniesienie wstępne. Med Mycol. 2011, 18, 24. (In Polish) [Google Scholar]
- Chmiel, M.; Frączek, K.; Grzyb, J. Problemy monitoringu zanieczyszczeń mikrobiologicznych powietrza. Woda-Środowisko Wiej. 2015, 15, 17–27. (In Polish) [Google Scholar]
- Sadigh, A.; Fataei, E.; Arzanloo, M.; Imani, A.A. Bacteria bioaerosol in the indoor air of educational microenvironments: Measuring exposures and assessing health effects. J. Environ. Health Sci. Eng. 2021, 19, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Franck, U.; Herbarth, O.; Röder, S.; Schlink, U.; Borte, M.; Diez, U.; Krämer, U.; Lehmann, I. Respiratory effects of indoor particles in young children are size dependent. Sci. Total Environ. 2011, 409, 1621–1631. [Google Scholar] [CrossRef]
- Brickus, L.S.; Siqueira, L.F.; de Aquino Neto, F.R.; Cardoso, J.N. Occurrence of airborne bacteria and fungi in bayside offices in Rio de Janeiro, Brazil. Indoor Built Environ. 1998, 7, 270–275. [Google Scholar] [CrossRef]
- Gołofit-Szymczak, M.; Skowroń, J. Zagrożenia mikrobiologiczne w pomieszczeniach biurowych. Bezpieczeństwo Pr. Nauka Prakt. 2005, 3, 29–31. (In Polish) [Google Scholar]
- Stojek, N. Zagrożenie bakteriami z rodzaju Legionella w środowisku pracy. Podstawy I Metod. Oceny Środowiska Pr. 2004, 3, 61–67. (In Polish) [Google Scholar]
- Abouelela, M.E.; Assaf, H.K.; Abdelhamid, R.A.; Elkhyat, E.S.; Sayed, A.M.; Oszako, T.; Belbahri, L.; El Zowalaty, A.E.; Abdelkader, M.S.A. Identification of potential SARS-CoV-2 main protease and spike protein inhibitors from the genus aloe: An in silico study for drug development. Molecules 2021, 26, 1767. [Google Scholar] [CrossRef] [PubMed]
- Vlaskin, M.S. Review of air disinfection approaches and proposal for thermal inactivation of airborne viruses as a life-style and an instrument to fight pandemics. Appl. Therm. Eng. 2022, 202, 117855. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Elias, B.; Bar-Yam, Y. Could air filtration reduce COVID-19 severity and spread. N. Engl. Complex Syst. Inst. 2020, 9, 1–4. [Google Scholar]
- Ryńska, J. Filtracja i Oczyszczanie Powietrza z Pyłów Zawieszonych i Wirusów. Rynek Instal. 2021, 11, 45–48. (In Polish) [Google Scholar]
- Fan, M.; Fu, Z.; Wang, J.; Wang, Z.; Suo, H.; Kong, X.; Li, H. A review of different ventilation modes on thermal comfort, air quality and virus spread control. Build. Environ. 2022, 212, 108831. [Google Scholar] [CrossRef]
- Libudzisz, Z.; Kowal, K.; Żakowska, Z. Mikrobiologia Techniczna. T. 1, Mikroorganizmy i Środowiska ich Występowania; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2007. (In Polish) [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Meklin, T.; Reponen, T.; Toivola, M.; Koponen, V.; Husman, T.; Hyvärinen, A.; Nevalainen, A. Size distributions of airborne microbes in moisture-damaged and reference school buildings of two construction types. Atmos. Environ. 2002, 36, 6031–6039. [Google Scholar] [CrossRef]
- Jo, W.K.; Kang, J.H. Workplace exposure to bioaerosols in pet shops, pet clinics, and flower gardens. Chemosphere 2006, 65, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Piontek, M. Występowanie Grzybów Pleśniowych w Budownictwie Mieszkaniowym; Oficyna Wydawnicza Uniwersytetu Zielonogórskiego: Zielona Góra, Poland, 1998. (In Polish) [Google Scholar]
- Samet, J.M.; Spengler, J.D. Indoor environments and health: Moving into the 21st century. Am. J. Public Health 2003, 93, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Maus, R.; Goppelsröder, A.; Umhauer, H. Survival of bacterial and mold spores in air filter media. Atmos. Environ. 2001, 35, 105–113. [Google Scholar] [CrossRef]
- Sivasubramani, S.K.; Niemeier, R.T.; Reponen, T.; Grinshpun, S.A. Fungal spore source strength tester: Laboratory evaluation of a new concept. Sci. Total Environ. 2004, 329, 75–86. [Google Scholar] [CrossRef]
- Doherty, W.O.; Mousavioun, P.; Fellows, C.M. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crop. Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- Bonetta, S.; Bonetta, S.; Mosso, S.; Sampò, S.; Carraro, E. Assessment of microbiological indoor air quality in an Italian office building equipped with an HVAC system. Environ. Monit. Assess. 2010, 161, 473–483. [Google Scholar] [CrossRef]
- Li, A.; Liu, Z.; Zhu, X.; Liu, Y.; Wang, Q. The effect of air-conditioning parameters and deposition dust on microbial growth in supply air ducts. Energy Build. 2010, 42, 449–454. [Google Scholar] [CrossRef]
- Noris, F.; Siegel, J.A.; Kinney, K.A. Evaluation of HVAC filters as a sampling mechanism for indoor microbial communities. Atmos. Environ. 2011, 45, 338–346. [Google Scholar] [CrossRef]
- Fan, G.; Xie, J.; Yoshino, H.; Yanagi, U.; Zhang, H.; Li, Z.; Li, N.; Lv, Y.; Liu, J.; Zhu, S.; et al. Investigation of fungal contamination in urban houses with children in six major Chinese cities: Genus and concentration characteristics. Build. Environ. 2021, 205, 108229. [Google Scholar] [CrossRef]
- Nabrdalik, M.; Latala, A. Wystepowanie grzybów strzepkowych w obiektach budowlanych. Rocz. Państwowego Zakładu Hig. 2003, 1, 119–127. (In Polish) [Google Scholar]
- Gonzalez-Martin, J.; Kraakman, N.J.R.; Perez, C.; Lebrero, R.; Munoz, R. A state–of–the-art review on indoor air pollution and strategies for indoor air pollution control. Chemosphere 2021, 262, 128376. [Google Scholar] [CrossRef] [PubMed]
- Michałkiewicz, M. Metody Badań Mikrobiologicznego Zanieczyszczenia Powietrza na Terenach Oczyszczalni ścieków - Przegląd Literaturowy. Kosmos 2019, 68, 475–487. (In Polish) [Google Scholar] [CrossRef]
- Patentalakis, N.; Pantidou, A.; Kalogerakis, N. Determination of enterobacteria in air and wastewater samples from a wastewater treatment plant by epi-fluorescence microscopy. Water Air Soil Pollut. Focus 2008, 8, 107–115. [Google Scholar] [CrossRef]
- Gołofit-Szymczak, M.; awniczek-Wałczyk, A.; Górny, R.L. Ilościowa i jakościowa kontrola szkodliwych czynników biologicznych w środowisku pracy. Podstawy Metod. Oceny Środowiska Pr. 2013, 2, 5–17. (In Polish) [Google Scholar] [CrossRef]
- Dutkiewicz, J. Bacteria and fungi in organic dust as potential health hazard. Ann. Agric. Environ. Med. 1997, 4, 11–16. [Google Scholar]
- Shampo, M.A.; Kyle, R.A. Kary B. Mullis—Nobel Laureate for procedure to replicate DNA. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2002; Volume 77, p. 606. [Google Scholar]
- Mullis, K.B.; Faloona, F.A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 155, pp. 335–350. [Google Scholar]
- Bakal, A.; Górny, R.; Ławniczek-Wałczyk, A.; Cyprowski, M. Zastosowanie metod biologii molekularnej w ocenie narażenia zawodowego na szkodliwe czynniki biologiczne. Podstawy Metod. Oceny Środowiska Pr. 2017, 3, 5–16. (In Polish) [Google Scholar] [CrossRef]
- Stärk, K.D.; Nicolet, J.; Frey, J. Detection of Mycoplasma hyopneumoniae by air sampling with a nested PCR assay. Appl. Environ. Microbiol. 1998, 64, 543–548. [Google Scholar] [CrossRef]
- Gutarowska, B. Przegląd metod oceny zanieczyszczenia mikrobiologicznego powietrza. In Problemy jakości powietrza wewnętrznego w Polsce; Wyd. Inst. Ogrzew. Went. Politech. Warsz.: Warszawa, Poland, 2002; pp. 93–102. (In Polish) [Google Scholar]
- Frac, M. Ocena Mikologiczna osadu z Oczyszczalni ścieków Mleczarskich oraz jego wpływ na różnorodność funkcjonalną Mikroorganizmów Glebowych; Instytut Agrofizyki im. Bohdana Dobrzańskiego, Polska Akademia Nauk: Lublin, Poland, 2012; Volume 1. (In Polish) [Google Scholar]
- Krajewska-Kułak, E.; Gniadek, A.; Łukaszuk, C.R.; Macura, A.B.; Kułak, W. Wstępne porównanie wyników badań zanieczyszczenia powietrza grzybami z wykorzystaniem aparatu SAS SUPER 100 i MAS 100. Doniesienie wstępne. Med. Mycol./Mikol. 2009, 16, 221–227. (In Polish) [Google Scholar]
- Luengas, A.; Barona, A.; Hort, C.; Gallastegui, G.; Platel, V.; Elias, A. A review of indoor air treatment technologies. Rev. Environ. Sci. Bio/Technol. 2015, 14, 499–522. [Google Scholar] [CrossRef]
- Chenari, B.; Carrilho, J.D.; da Silva, M.G. Towards sustainable, energy-efficient and healthy ventilation strategies in buildings: A review. Renew. Sustain. Energy Rev. 2016, 59, 1426–1447. [Google Scholar] [CrossRef]
- Lewandowski, R.; Jóźwik, P. Aktualne techniki oczyszczania powietrza z czynników biologicznych. Hygeia Public Health 2017, 52, 103–110. (In Polish) [Google Scholar]
- Guieysse, B.; Hort, C.; Platel, V.; Munoz, R.; Ondarts, M.; Revah, S. Biological treatment of indoor air for VOC removal: Potential and challenges. Biotechnol. Adv. 2008, 26, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Park, D.; Kim, K.; Lim, S.M.; Yu, N.H.; Kim, S.; Kim, H.Y.; Jung, K.S.; Jang, J.Y.; Park, J.C.; et al. Characterization of Bacillus amyloliquefaciens DA12 showing potent antifungal activity against mycotoxigenic Fusarium species. Plant Pathol. J. 2017, 33, 499. [Google Scholar] [CrossRef] [PubMed]
- Prussin, A.J.; Marr, L.C. Sources of airborne microorganisms in the built environment. Microbiome 2015, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lv, X.; Tong, S.; Zhang, T.; Song, Y.; Wang, Y.; Hao, Z.; Huang, C.; Xia, D. Modification of zeolite by metal and adsorption desulfurization of organic sulfide in natural gas. J. Nat. Gas Sci. Eng. 2019, 69, 102941. [Google Scholar] [CrossRef]
- Tang, X.; Wei, J.; Kong, Z.; Zhang, H.; Tian, J. Introduction of amino and rGO into PP nonwoven surface for removal of gaseous aromatic pollutants and particulate matter from air. Appl. Surf. Sci. 2020, 511, 145631. [Google Scholar] [CrossRef]
- Grinshpun, S.; Adhikari, A.; Li, C.; Reponen, T.; Yermakov, M.; Schoenitz, M.; Dreizin, E.; Trunov, M.; Mohan, S. Thermal inactivation of airborne viable Bacillus subtilis spores by short-term exposure in axially heated air flow. J. Aerosol Sci. 2010, 41, 352–363. [Google Scholar] [CrossRef]
- Coleman, W.H.; Chen, D.; Li, Y.q.; Cowan, A.E.; Setlow, P. How moist heat kills spores of Bacillus subtilis. J. Bacteriol. 2007, 189, 8458–8466. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Lee, J.E.; Kim, S.S. Thermal effects on bacterial bioaerosols in continuous air flow. Sci. Total Environ. 2009, 407, 4723–4730. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Yoon, K.Y.; Park, J.H.; Hwang, J. Application of air ions for bacterial de-colonization in air filters contaminated by aerosolized bacteria. Sci. Total Environ. 2011, 409, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Kałużny, J.; Muszyński, Z.; Kałużny, B. Ophthalmic operating theatre–chance of limiting contamination and infection risk. Part I. Decontamination of the microbial air pollution. Klin. Oczna/Acta Ophthalmol. Pol. 2008, 110, 102–107. [Google Scholar]
- Huang, R.; Agranovski, I.; Pyankov, O.; Grinshpun, S. Removal of viable bioaerosol particles with a low-efficiency HVAC filter enhanced by continuous emission of unipolar air ions. Indoor Air 2008, 18, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Reed, N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, J.; Schepers, D.; Bottiger, J.; Edmonds, J. UV-C decontamination of aerosolized and surface-bound single spores and bioclusters. Aerosol Sci. Technol. 2014, 48, 450–457. [Google Scholar] [CrossRef]
- Małecka, I.; Borowski, G. Dezynfekcja powietrza promieniami UV i promieniową jonizacją katalityczną w instalacjach wentylacyjnych. Zesz. Nauk. Inżynieria Lądowa Wodna Kształtowaniu Środowiska 2011, 3, 25–30. (In Polish) [Google Scholar]
- Vaze, N.D.; Gallagher, M.J.; Park, S.; Fridman, G.; Vasilets, V.N.; Gutsol, A.F.; Anandan, S.; Friedman, G.; Fridman, A.A. Inactivation of bacteria in flight by direct exposure to nonthermal plasma. IEEE Trans. Plasma Sci. 2010, 38, 3234–3240. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, Y.; Sun, K.; Chen, Q.; Shen, F.; Zhang, J.; Yao, M.; Zhu, T.; Fang, J. Rapid inactivation of biological species in the air using atmospheric pressure nonthermal plasma. Environ. Sci. Technol. 2012, 46, 3360–3368. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.S.; Zhang, Z. Performance of air cleaners for removing multiple volatile organic compounds in indoor air. ASHRAE Trans. 2005, 111, 1101–1114. [Google Scholar]
- Iordache, D.; Niculae, D. Microwave thermal oxidation of gas emissions resulting from the risky medical waste incineration. UPB Sci. Bull. Ser. C 2012, 74, 169–180. [Google Scholar]
- Hildebrandt, R.; Zgórska, A. Fizyczne i chemiczne metody oczyszczania powietrza w pomieszczeniach i obiektach zamkniętych w aspekcie zapobiegania zakażeniu COVID-19. Przemysł Chem. 2021, 1, 55–62. (In Polish) [Google Scholar] [CrossRef]
- Davies, A.; Pottage, T.; Bennett, A.; Walker, J. Gaseous and air decontamination technologies for Clostridium difficile in the healthcare environment. J. Hosp. Infect. 2011, 77, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.; Sabourin, C.; Choi, Y.; Richter, W.; Rudnicki, D.; Riggs, K.; Taylor, M.; Chang, J. Decontamination assessment of Bacillus anthracis, Bacillus subtilis, and Geobacillus stearothermophilus spores on indoor surfaces using a hydrogen peroxide gas generator. J. Appl. Microbiol. 2005, 99, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Kraakman, N.J.R.; González-Martín, J.; Pérez, C.; Lebrero, R.; Muñoz, R. Recent advances in biological systems for improving indoor air quality. Rev. Environ. Sci. Bio/Technol. 2021, 20, 363–387. [Google Scholar] [CrossRef]
- Skowron, K.; Grudlewska, K.; Krawczyk, A.; Gospodarek-Komkowska, E. The effectiveness of radiant catalytic ionization in inactivation of Listeria monocytogenes planktonic and biofilm cells from food and food contact surfaces as a method of food preservation. J. Appl. Microbiol. 2018, 124, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Soreanu, G.; Dumont, É. From Biofiltration to Promising Options in Gaseous Fluxes Biotreatment: Recent Developments, New Trends, Advances, and Opportunities; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Klepacka, K. Biologiczne oczyszczanie gazów z ksylenu, styrenu oraz ich mieszanin w bioreaktorach strużkowych-aktualny stan wiedzy i kierunki rozwoju. Pr. Nauk. Inst. Inż. Chem. Pol. Akad. Nauk. 2010, 14, 41–58. (In Polish) [Google Scholar]
- Irga, P.; Torpy, F.; Burchett, M. Can hydroculture be used to enhance the performance of indoor plants for the removal of air pollutants? Atmos. Environ. 2013, 77, 267–271. [Google Scholar] [CrossRef]
- Cervera Sardá, R.; Gómez Pioz, J. Architectural bio-photo reactors: Harvesting microalgae on the surface of architecture. In Biotechnologies and Biomimetics for Civil Engineering; Springer: Berlin/Heidelberg, Germany, 2014; pp. 163–179. [Google Scholar]
- Moya, T.A.; van den Dobbelsteen, A.; Ottele, M.; Bluyssen, P.M. A review of green systems within the indoor environment. Indoor Built Environ. 2019, 28, 298–309. [Google Scholar] [CrossRef]
- Anbalagan, A.; Toledo-Cervantes, A.; Posadas, E.; Rojo, E.M.; Lebrero, R.; González-Sánchez, A.; Nehrenheim, E.; Muñoz, R. Continuous photosynthetic abatement of CO2 and volatile organic compounds from exhaust gas coupled to wastewater treatment: Evaluation of tubular algal-bacterial photobioreactor. J. CO2 Util. 2017, 21, 353–359. [Google Scholar] [CrossRef]
- Żarczyński, A.; Rosiak, K.; Anielak, P.; Ziemiński, K.; Wolf, W. Praktyczne metody usuwania siarkowodoru z biogazu. II, Zastosowanie roztworów sorpcyjnych i metod biologicznych. Acta Innov. 2015, 15, 57–71. (In Polish) [Google Scholar]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Pawliszyn, J. Solid Phase Microextraction: Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Sparkman, O.D.; Penton, Z.; Kitson, F.G. Gas Chromatography and Mass Spectrometry: A Practical Guide; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Spietelun, A.; Kloskowski, A.; Chrzanowski, W.; Namieśnik, J. Understanding solid-phase microextraction: Key factors influencing the extraction process and trends in improving the technique. Chem. Rev. 2013, 113, 1667–1685. [Google Scholar] [CrossRef]
- James, A.T.; Martin, A.J. Gas-liquid partition chromatography: The separation and micro-estimation of volatile fatty acids from formic acid to dodecanoic acid. Biochem. J. 1952, 50, 679. [Google Scholar] [CrossRef]
- Kolb, B.; Ettre, L.S. Static Headspace-Gas Chromatography: Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Scott, R.P. Introduction to Analytical Gas Chromatography, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Herbert, C.G.; Johnstone, R.A. Mass Spectrometry Basics; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- De Hoffmann, E.; Stroobant, V. Mass Spectrometry: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Watson, J.T.; Sparkman, O.D. Introduction to Mass Spectrometry: Instrumentation, Applications, and Strategies for Data Interpretation; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Bouchonnet, S. Introduction to GC-MS Coupling; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Hübschmann, H.J. Handbook of GC-MS: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
| Type of Environment | Permissible No. of Microorg. in 1 m3 | |
|---|---|---|
| Total No. of Microorg. on MPA Medium | Total No. of Fungi | |
| Operating rooms | 100 | 0 |
| Residential houses | 1000–2000 | 200–300 |
| College lecture hall | 1500 | 200 |
| Food industry production room | 600 | 0 |
| Production halls of the pharmaceutical industry | 100 | 0 |
| Cowsheds | 150,000 | 5000 |
| Piggeries | 200,000 | 10,000 |
| Chicken coops | 100,000 | 2000 |
| Total No. [m−3] | Number [m−3] | Degree | |||
|---|---|---|---|---|---|
| Actinomycotes | Hemolytic Type Straphylococci | Pseudomonas fluorescens | |||
| <1000 | <10 | none | none | none | not polluted |
| 1000–3000 | 10–100 | 1–25 | 1–50 | 1–50 | moderately polluted |
| >3000 | >100 | >25 | >50 | >50 | heavily polluted |
| Total No. [m−3] | Degree of Atmospheric Air Pollution |
|---|---|
| <3000 | air not polluted |
| 3000–5000 | averagely clean atmospheric air, especially in early autumn and late autumn |
| 5000–10,000 | pollution that may have a negative impact on human natural environments |
| >10,000 | pollution that threatens the human environment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stocka, N.; Butarewicz, A.; Stocki, M.; Borowik, P.; Oszako, T. Biological Pollution of Indoor Air, Its Assessment and Control Methods. Encyclopedia 2024, 4, 1217-1235. https://doi.org/10.3390/encyclopedia4030079
Stocka N, Butarewicz A, Stocki M, Borowik P, Oszako T. Biological Pollution of Indoor Air, Its Assessment and Control Methods. Encyclopedia. 2024; 4(3):1217-1235. https://doi.org/10.3390/encyclopedia4030079
Chicago/Turabian StyleStocka, Natalia, Andrzej Butarewicz, Marcin Stocki, Piotr Borowik, and Tomasz Oszako. 2024. "Biological Pollution of Indoor Air, Its Assessment and Control Methods" Encyclopedia 4, no. 3: 1217-1235. https://doi.org/10.3390/encyclopedia4030079
APA StyleStocka, N., Butarewicz, A., Stocki, M., Borowik, P., & Oszako, T. (2024). Biological Pollution of Indoor Air, Its Assessment and Control Methods. Encyclopedia, 4(3), 1217-1235. https://doi.org/10.3390/encyclopedia4030079









