Association of Oxidative Stress Markers with Vascular Stiffness Parameters in Patients with Diabetic Neuropathy
Abstract
:1. Introduction
2. Materials and Methods
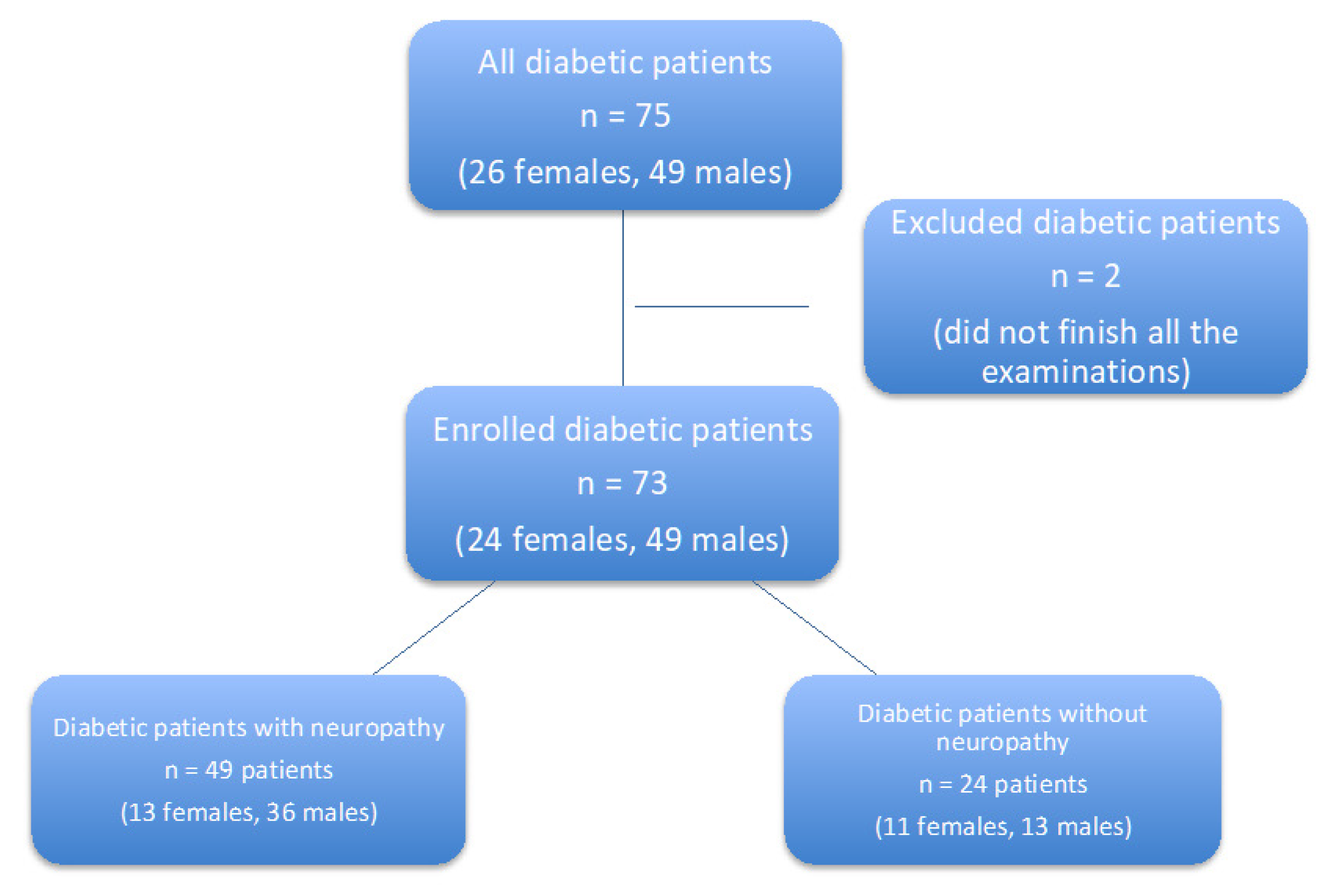
2.1. Study Design
2.2. Laboratory Methods
2.3. Measurement of Vascular Stiffness
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ao DBP | aortic diastolic pressure |
| AGE | advanced glycation end-products |
| AiX | augmentation index |
| ALA | alpha-lipoic acid, thioctic acid |
| AOPP | advanced oxidation protein products |
| Ao PBP | aortic pulse pressure |
| Ao SBP | aortic systolic pressure |
| AP | augmentation pressure |
| ApoA | apolipoprotein A |
| ApoB | apolipoprotein B |
| BMI | body mass index |
| BP | blood pressure |
| BPM | beats per minute |
| Chol | cholesterol |
| Chol/HDL | cholesterol and HDL ratio |
| CRP | C-reactive protein |
| DM | diabetes mellitus |
| ECG | electrocarography |
| EMG | electromyography |
| GPx | glutathione peroxidase |
| HbA1c | glycated haemoglobin |
| HDL | high density lipoprotein, |
| LDL | low density lipoprotein |
| LOX-1 | lecithin-like oxLDL receptor |
| MACE | major cardiovascular events |
| MDA | malondialdehyde |
| NADPH | nicotinamid adenin dinucleotide phosphate |
| NOS | nithic oxide synthase |
| DNA | deoxyribonucleic acid |
| nonHDL | cholesterol contained in potentially atherogenic lipoprotein particles (LDL, IDL, VLDL, residual chylomicrons) |
| OHA | oral hypoglycemic agents |
| OxLDL | oxidized low density lipoprotein |
| PAI-1 | plasminogen activator inhibitor 1 |
| PWV | pulse wave velocity |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| sRAGE | soluble AGE receptor |
| TAG | triacylglycerols |
| TAS | total antioxidant status |
References
- American Diabetes Association. Introduction: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. 1), S1–S2. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Salway, J.G.; Whitehead, L.; Finnegan, J.A.; Karunanayaka, A.; Barnett, D.; Payne, R.B. Effect of myo-inositol on peripheral-nerve function in diabetes. Lancet 1978, 2, 1282–1284. [Google Scholar] [CrossRef]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.M.; Hayes, J.M.; McLean, L.L.; Vivekanandan-Giri, A.; Pennathur, S.; Feldman, E.L. Dyslipidemia-induced neuropathy in mice: The role of oxLDL/LOX-1. Diabetes 2009, 58, 2376–2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, K.; Elzinga, S.; Eid, S.; Figueroa-Romero, C.; Hinder, L.M.; Pacut, C.; Feldman, E.L.; Hur, J. Genome-wide DNA methylation profiling of human diabetic peripheral neuropathy in subjects with type 2 diabetes mellitus. Epigenetics 2019, 14, 766–779. [Google Scholar] [CrossRef] [PubMed]
- Román-Pintos, L.M.; Villegas-Rivera, G.; Rodríguez-Carrizalez, A.D.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. Diabetic Polyneuropathy in Type 2 Diabetes Mellitus: Inflammation, Oxidative Stress, and Mitochondrial Function. J. Diabetes Res. 2016, 2016, 3425617. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, D.; Ametov, A.; Barinov, A.; Dyck, P.J.; Gurieva, I.; Low, P.A.; Munzel, U.; Yakhno, N.; Raz, I.; Novosadova, M.; et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: The SYDNEY 2 trial. Diabetes Care 2006, 29, 2365–2370. [Google Scholar] [CrossRef] [Green Version]
- Ametov, A.S.; Barinov, A.; Dyck, P.J.; Hermann, R.; Kozlova, N.; Litchy, W.J.; Low, P.A.; Nehrdich, D.; Novosadova, M.; O’Brien, P.C.; et al. The sensory symptoms of diabetic polyneuropathy are improved with alpha-lipoic acid: The SYDNEY trial. Diabetes Care 2003, 26, 770–776, Erratum in Diabetes Care 2003, 26, 2227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanas, N.; Ziegler, D. Efficacy of α-lipoic acid in diabetic neuropathy. Expert Opin. Pharmacother. 2014, 15, 2721–2731. [Google Scholar] [CrossRef]
- Vallianou, N.; Evangelopoulos, A.; Koutalas, P. Alpha-lipoic Acid and diabetic neuropathy. Rev. Diabet. Stud. 2009, 6, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Prenner, S.B.; Chirinos, J.A. Arterial stiffness in diabetes mellitus. Atherosclerosis 2015, 238, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Dohi, Y.; Takase, H.; Yamashita, S.; Fujii, S.; Ohte, N. Oxidative Stress is Closely Associated with Increased Arterial Stiffness, Especially in Aged Male Smokers without Previous Cardiovascular Events: A Cross-Sectional Study. J. Atheroscler. Thromb. 2017, 24, 1186–1198. [Google Scholar] [CrossRef] [Green Version]
- Kawamoto, R.; Ninomiyax, D.; Kusunoki, T.; Kasai, Y.; Ohtsuka, N.; Kumagi, T. Oxidative stress is associated with increased arterial stiffness in middle-aged and elderly community-dwelling persons. J. Clin. Gerontol. 2016, 7, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Villa, M.; Parravano, M.; Micheli, A.; Gaddini, L.; Matteucci, A.; Mallozzi, C.; Facchiano, F.; Malchiodi-Albedi, F.; Pricci, F. A quick, simple method for detecting circulating fluorescent advanced glycation end-products: Correlation with in vitro and in vivo non-enzymatic glycation. Metabolism 2017, 71, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajdova, J.; Karasek, D.; Goldmannova, D.; Krystynik, O.; Schovanek, J.; Vaverkova, H.; Zadrazil, J. Pulse wave analysis and diabetes mellitus. A systematic review. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2017, 161, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.Y.; Kim, Y.S. The Role of Advanced Glycation End Products in Diabetic Vascular Complications. Diabetes Metab. J. 2018, 42, 188–195. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Oxidative stress in type 2 diabetes mellitus. In Oxidative Stress in Aging; Miwa, S., Beckman, K., Muller, F., Eds.; Humana Press: Totowa, NJ, USA, 2008; pp. 191–211. [Google Scholar]
- Spahis, S.; Borys, J.M.; Levy, E. Metabolic Syndrome as a Multifaceted Risk Factor for Oxidative Stress. Antioxid. Redox Signal. 2017, 26, 445–461. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [Green Version]
- Alessi, M.C.; Poggi, M.; Juhan-Vague, I. Plasminogen activator inhibitor-1, adipose tissue and insulin resistance. Curr. Opin. Lipidol. 2007, 18, 240–245. [Google Scholar] [CrossRef]
- Jung, R.G.; Motazedian, P.; Ramirez, F.D.; Simard, T.; Di Santo, P.; Visintini, S.; Faraz, M.A.; Labinaz, A.; Jung, Y.; Hibbert, B. Association between plasminogen activator inhibitor-1 and cardiovascular events: A systematic review and meta-analysis. Thromb. J. 2018, 16, 12. [Google Scholar] [CrossRef] [Green Version]
- Juhan-Vague, I.; Alessi, M.C.; Mavri, A.; Morange, P.E. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J. Thromb. Haemost. 2003, 1, 1575–1579. [Google Scholar] [CrossRef]
- Bilgili, S.; Celebiler, A.C.; Dogan, A.; Karaca, B. Inverse relationship between adiponectin and plasminogen activator inhibitor-1 in metabolic syndrome patients. Endocr. Regul. 2008, 42, 63–68. [Google Scholar]
- Edwards, J.L.; Vincent, A.M.; Cheng, H.T.; Feldman, E.L. Diabetic neuropathy: Mechanisms to management. Pharmacol. Ther. 2008, 120, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, C.R.; Salles, G.F. Aortic Stiffness as a Surrogate Endpoint to Micro- and Macrovascular Complications in Patients with Type 2 Diabetes. Int. J. Mol. Sci. 2016, 17, 2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Otsuki, T.; Maeda, S.; Mukai, J.; Ohki, M.; Nakanishi, M.; Yoshikawa, T. Association between plasma sLOX-1 concentration and arterial stiffness in middle-aged and older individuals. J. Clin. Biochem. Nutr. 2015, 57, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Brinkley, T.E.; Nicklas, B.J.; Kanaya, A.M.; Satterfield, S.; Lakatta, E.G.; Simonsick, E.M.; Sutton-Tyrrell, K.; Kritchevsky, S.B. Plasma oxidized low-density lipoprotein levels and arterial stiffness in older adults: The health, aging, and body composition study. Hypertension 2009, 53, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.Y.; Kim, J.Y.; Paik, J.K.; Kim, O.Y.; Paik, Y.H.; Lee, E.J.; Lee, J.H. The association of specific metabolites of lipid metabolism with markers of oxidative stress, inflammation and arterial stiffness in men with newly diagnosed type 2 diabetes. Clin. Endocrinol. 2012, 76, 674–682. [Google Scholar] [CrossRef]
- Vinereanu, I.V.; Peride, I.; Niculae, A.; Tiron, A.T.; Caragheorgheopol, A.; Manda, D.; Checherita, I.A. The Relationship between Advanced Oxidation Protein Products, Vascular Calcifications and Arterial Stiffness in Predialysis Chronic Kidney Disease Patients. Medicina 2021, 57, 452. [Google Scholar] [CrossRef] [PubMed]
- Sri-Amad, R.; Huipao, N.; Prasertsri, P.; Roengrit, T. Aortic Pulse Wave Velocity, Ankle-Brachial Index, and Malondialdehyde in Older Adults with or without Metabolic Syndrome. Pulse 2020, 8, 31–39. [Google Scholar] [CrossRef]
- Villegas-Rodríguez, M.E.; Uribarri, J.; Solorio-Meza, S.E.; Fajardo-Araujo, M.E.; Cai, W.; Torres-Graciano, S.; Rangel-Salazar, R.; Wrobel, K.; Garay-Sevilla, M.E. The AGE-RAGE Axis and Its Relationship to Markers of Cardiovascular Disease in Newly Diagnosed Diabetic Patients. PLoS ONE 2016, 11, e0159175. [Google Scholar] [CrossRef] [Green Version]
- Birukov, A.; Cuadrat, R.; Polemiti, E.; Eichelmann, F.; Schulze, M.B. Advanced glycation end-products, measured as skin autofluorescence, associate with vascular stiffness in diabetic, pre-diabetic and normoglycemic individuals: A cross-sectional study. Cardiovasc. Diabetol. 2021, 20, 110. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Huang, Q.-F.; Cheng, Y.-B.; Guo, Q.-H.; Chen, Q.; Li, Y.; Wang, J.-G. A Comparative Study on Skin and Plasma Advanced Glycation End Products and Their Associations with Arterial Stiffness. Pulse 2017, 4, 208–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wannamethee, S.G.; Welsh, P.; Papacosta, O.; Ellins, E.A.; Halcox, J.P.J.; Whincup, P.H.; Sattar, N. Circulating soluble receptor for advanced glycation end product: Cross-sectional associations with cardiac markers and subclinical vascular disease in older men with and without diabetes. Atherosclerosis 2017, 264, 36–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porasuphatana, S.; Suddee, S.; Nartnampong, A.; Konsil, J.; Harnwong, B.; Santaweesuk, A. Glycemic and oxidative status of patients with type 2 diabetes mellitus following oral administration of alpha-lipoic acid: A randomized double-blinded placebo-controlled study. Asia Pac. J. Clin. Nutr. 2012, 21, 12–21. [Google Scholar]
- Derosa, G.; D’Angelo, A.; Romano, D.; Maffioli, P. A Clinical Trial about a Food Supplement Containing α-Lipoic Acid on Oxidative Stress Markers in Type 2 Diabetic Patients. Int. J. Mol. Sci. 2016, 17, 1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Sharman, J.E.; Gunaruwan, P.; Knez, W.L.; Schmitt, M.; Marsh, S.A.; Wilson, G.R.; Cockcroft, J.R.; Coombes, J.S. Alpha-lipoic acid does not acutely affect resistance and conduit artery function or oxidative stress in healthy men. Br. J. Clin. Pharmacol. 2004, 58, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Núñez, V.M.; García-Martínez, B.I.; Rosado-Pérez, J.; Santiago-Osorio, E.; Pedraza-Chaverri, J.; Hernández-Abad, V.J. The Effect of 600 mg Alpha-lipoic Acid Supplementation on Oxidative Stress, Inflammation, and RAGE in Older Adults with Type 2 Diabetes Mellitus. Oxid. Med. Cell. Longev. 2019, 2019, 3276958. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Han, P.; Wu, N.; He, B.; Lu, Y.; Li, S.; Liu, Y.; Zhao, S.; Liu, L.; Li, Y. Amelioration of lipid abnormalities by α-lipoic acid through antioxidative and anti-inflammatory effects. Obesity 2011, 19, 1647–1653. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Hwang, S.J.; Vasan, R.S.; Larson, M.G.; Pencina, M.J.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J. Arterial stiffness and cardiovascular events: The Framingham Heart Study. Circulation 2010, 121, 505–511. [Google Scholar] [CrossRef] [Green Version]
| Patients (n = 49) | Controls (n = 24) | p | |
|---|---|---|---|
| BMI (kg/m2) | 30.6 (27.9–34.8) | 34.3 (31.3–39.5) | <0.05 |
| HbA1c (mmol/mol) | 66 (48–77) | 51 (43–75.2) | n.s. |
| Cholesterol (mmol/L) | 4.06 ± 0.86 | 4.35 ± 0.97 | n.s. |
| TAG (mmol/L) | 2.05 (1.61–3.63) | 1.55 (1.19–2.4) | n.s. |
| HDL (mmol/L) | 1.12 ± 0.29 | 1.18 ± 0.33 | n.s. |
| LDL (mmol/L) | 1.78 ± 0.63 | 2.33 ± 0.87 | <0.01 |
| Chol/HDL | 3.85 ± 1.34 | 3.93 ± 1.27 | n.s. |
| nonHDL | 2.94 ± 0.87 | 3.2 ± 1.01 | n.s. |
| ApoA (g/L) | 1.44 (1.23–1.55) | 1.46 (1.39–1.67) | n.s. |
| ApoB (g/L) | 0.9 (0.77–1.02) | 0.94 (0.81–1.1) | n.s. |
| Patients | Controls | p | |
|---|---|---|---|
| Ao SBP (mmHg) | 119.7 ± 13.1 | 117.2 ± 12.2 | n.s. |
| Ao DBP (mmHg) | 79.3 ± 9.9 | 81.9 ± 11.8 | n.s. |
| Ao PBP (mmHg) | 39.4 ± 10.7 | 33.5 ± 7.9 | <0.05 |
| AP (mmHg) | 9.9 ± 5.9 | 8.2 ± 4.2 | n.s. |
| AiX (%/75 bpm) | 21.7 ± 12.7 | 20.1 ± 8.2 | n.s. |
| PWV (m/s) | 11.6 ± 4.6 | 8.8 ± 1.9 | <0.001 |
| PAI-1 (ng/mL) | 45.8 (19.6–85.1) | 21 (13.5–36.4) | <0.05 |
| Oxidation Markers | Patients before | Patients after | p (Patients before and after Therapy) | Controls | p (Patients before vs. Controls |
|---|---|---|---|---|---|
| AOPP (µmol/L) | 211.2 | 142.5 | 2.54 × 10−6 | 193.8 | 0.39 |
| AGE (AU/g) | 13,331.55 | 10,984.38 | 1.32 × 10−7 | 10,334.89 | 0.0031 |
| MDA (µmol/L) | 68.37 | 71.52 | 0.83 | 62.23 | 0.34 |
| OxLDL (U/L) | 40.42 | 38.25 | 0.03 | 45.63 | 0.94 |
| oxLDL | AOPP | AGE | MDA | |
|---|---|---|---|---|
| cholestrol | r = 0.76 p < 0.001 | r = 0.24 p < 0.001 | n.s. | n.s. |
| TAG | r = 0.5 p < 0.001 | r = 0.82 p < 0.001 | r = 0.35 p = 0.049 | n.s. |
| HDL | r = −0.18 p = 0.009 | r = −0.3 p = 0.005 | n.s. | n.s. |
| LDL | r = 0.46 p < 0.001 | n.s. | r = 0.22 p = 0.034 | n.s. |
| nonHDL | r = 0.84 p < 0.001 | r = 0.34 p < 0.001 | r = 0.06 p = 0.05 | n.s. |
| Chol/HDL | r = 0.71 p < 0.001 | r = 0.55 p < 0.001 | n.s. | n.s. |
| ApoB | r = 0.77 p = 0.008 | r = 0.1 p = 0.033 | n.s. | n.s. |
| ApoA | n.s. | n.s. | n.s. | r = −0.11 p = 0.001 |
| HbA1c | r = 0.32 p = 0.039 | r = 0.31 p = 0.048 | n.s. | n.s. |
| PAI-1 | r = 0.43 p = 0.003 | r = 0.55 p < 0.001 | n.s. | n.s. |
| BMI | r = 0.29 p = 0.036 | r = 0.44 p = 0.013 | n.s. | n.s. |
| CRP | n.s. | n.s. | r = 0.036 p < 0.001 | n.s. |
| oxLDL | AOPP | AGE | MDA | |
|---|---|---|---|---|
| SBP ao | r = 0.2 p < 0.05 | r = 0.098 p < 0.05 | n.s. | n.s. |
| DBP ao | r = 0.45 p < 0.01 | n.s. | n.s. | n.s. |
| AP | n.s. | n.s. | n.s. | n.s. |
| AiX/75 bpm | r = 0.46 p < 0.01 | n.s. | n.s. | n.s. |
| PVW | n.s. | n.s. | n.s. | n.s. |
| TAG | nonHDL | ApoB | HbA1c | SBP ao | DBP ao | AiX/75 bpm | |
|---|---|---|---|---|---|---|---|
| oxLDL | 2.712 ** | −2.343 * | 3.071 ** | 2.729 ** | 2.776 ** | ||
| AOPP | 7.094 *** | 2.293 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mačáková, D.; Plchová, M.; Cibičková, L.; Krystyník, O.; Karásek, D.; Zadrazil, J. Association of Oxidative Stress Markers with Vascular Stiffness Parameters in Patients with Diabetic Neuropathy. BioMed 2022, 2, 1-12. https://doi.org/10.3390/biomed2010001
Mačáková D, Plchová M, Cibičková L, Krystyník O, Karásek D, Zadrazil J. Association of Oxidative Stress Markers with Vascular Stiffness Parameters in Patients with Diabetic Neuropathy. BioMed. 2022; 2(1):1-12. https://doi.org/10.3390/biomed2010001
Chicago/Turabian StyleMačáková, Dominika, Markéta Plchová, Lubica Cibičková, Ondřej Krystyník, David Karásek, and Josef Zadrazil. 2022. "Association of Oxidative Stress Markers with Vascular Stiffness Parameters in Patients with Diabetic Neuropathy" BioMed 2, no. 1: 1-12. https://doi.org/10.3390/biomed2010001
APA StyleMačáková, D., Plchová, M., Cibičková, L., Krystyník, O., Karásek, D., & Zadrazil, J. (2022). Association of Oxidative Stress Markers with Vascular Stiffness Parameters in Patients with Diabetic Neuropathy. BioMed, 2(1), 1-12. https://doi.org/10.3390/biomed2010001






