Chemopreventive Effects of Selenium and Selenocompounds in the Treatment of Lymphoma
Abstract
:1. Introduction
2. Lymphomagenesis and Classification of Lymphomas
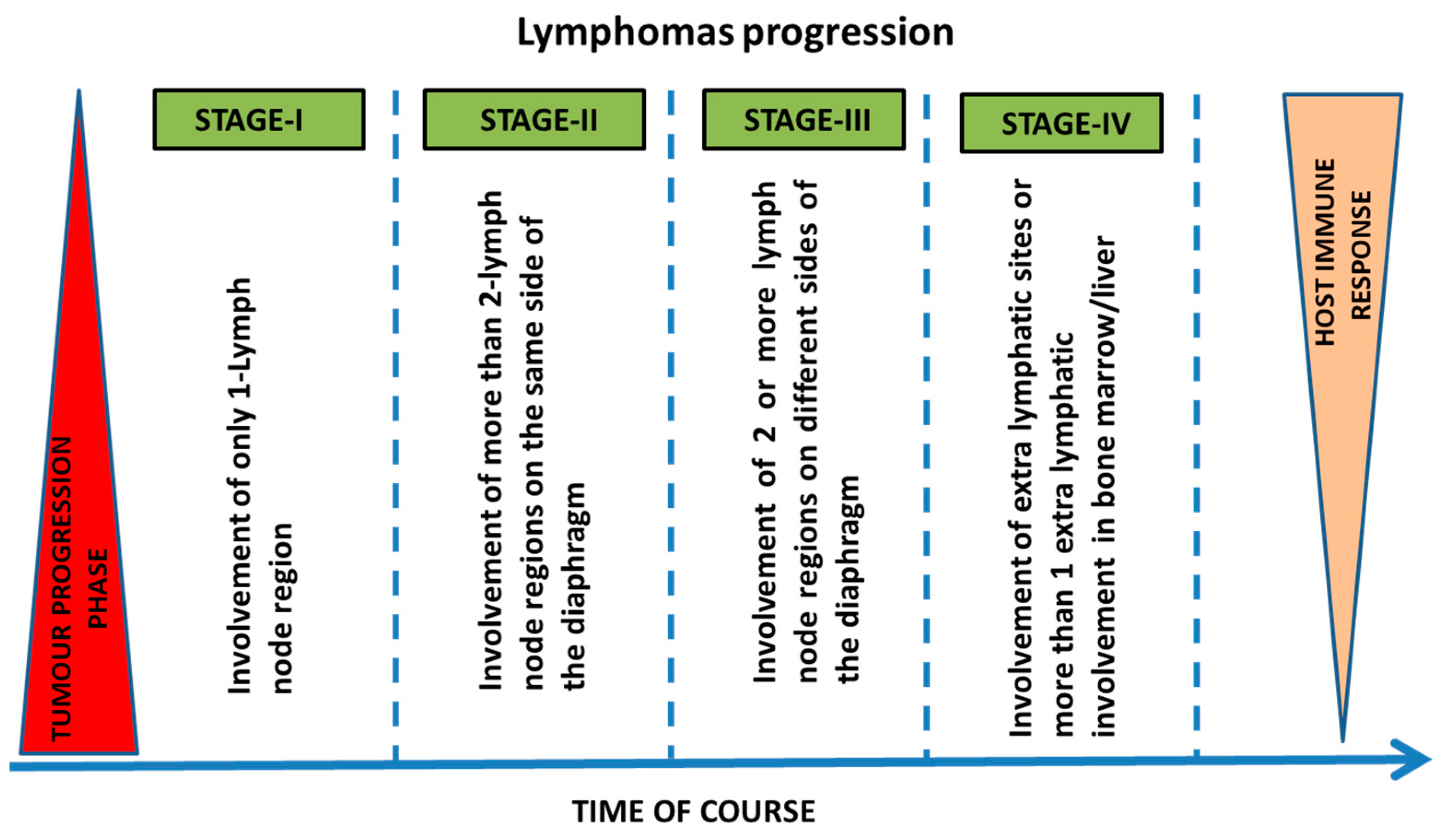
2.1. Stages and Progression of Lymphomas
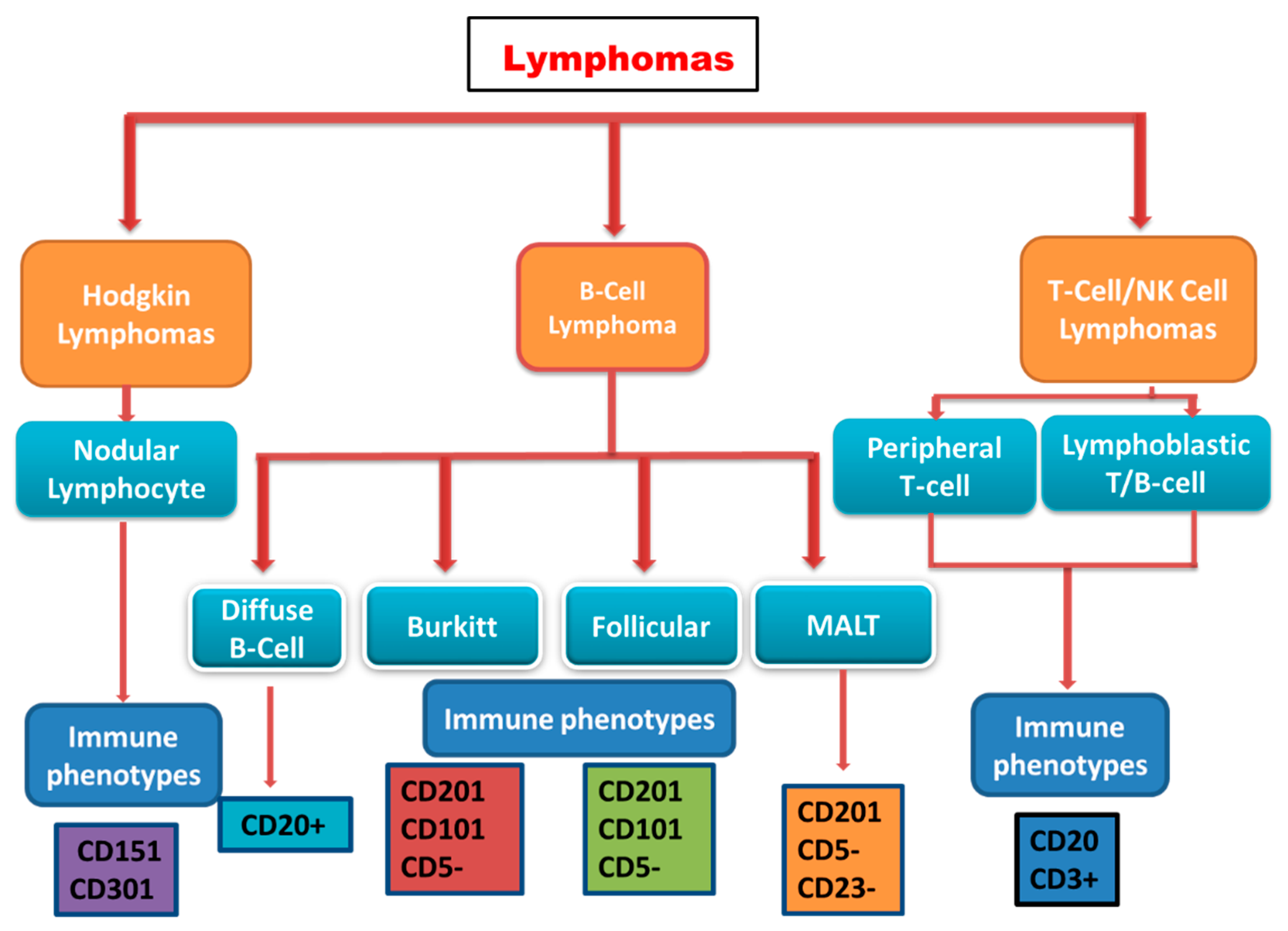
2.2. Classification of Lymphomas
2.2.1. Hodgkin Lymphoma
2.2.2. Non-Hodgkin Lymphoma
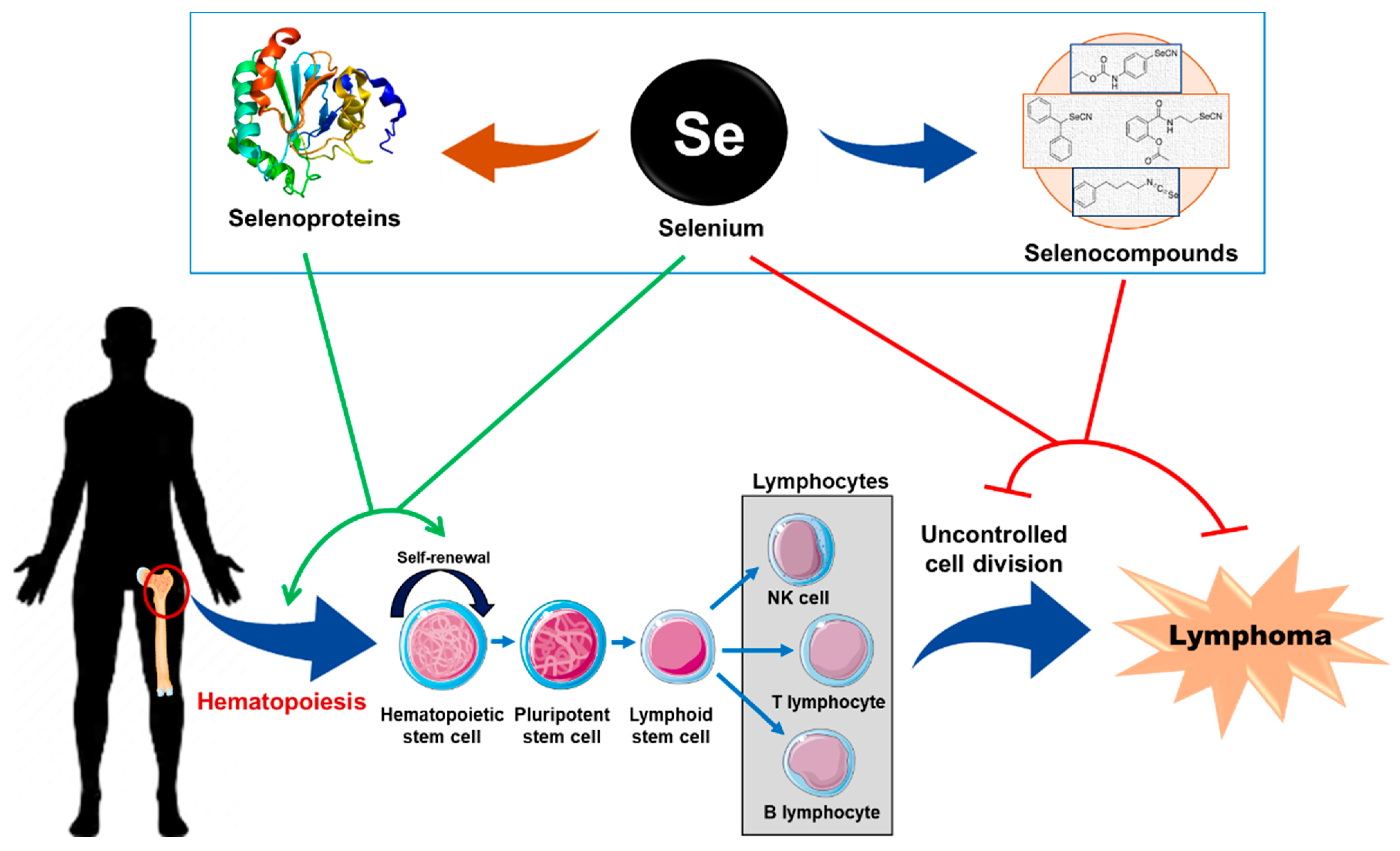
3. Biological Role of Se and SePs in Hematopoiesis and Lymphoma
3.1. Role of Se and SePs in Hematopoiesis
3.2. Role of Se and SePs in Lymphoma
3.3. Effect of Se Supplementation in Patients Undergoing Hematopoietic Stem Cell Transplantation
4. Physico-Chemical and Anticancer Properties of Se
4.1. The Historical Perspectives of Se
4.2. Physico-Chemical Properties of Se
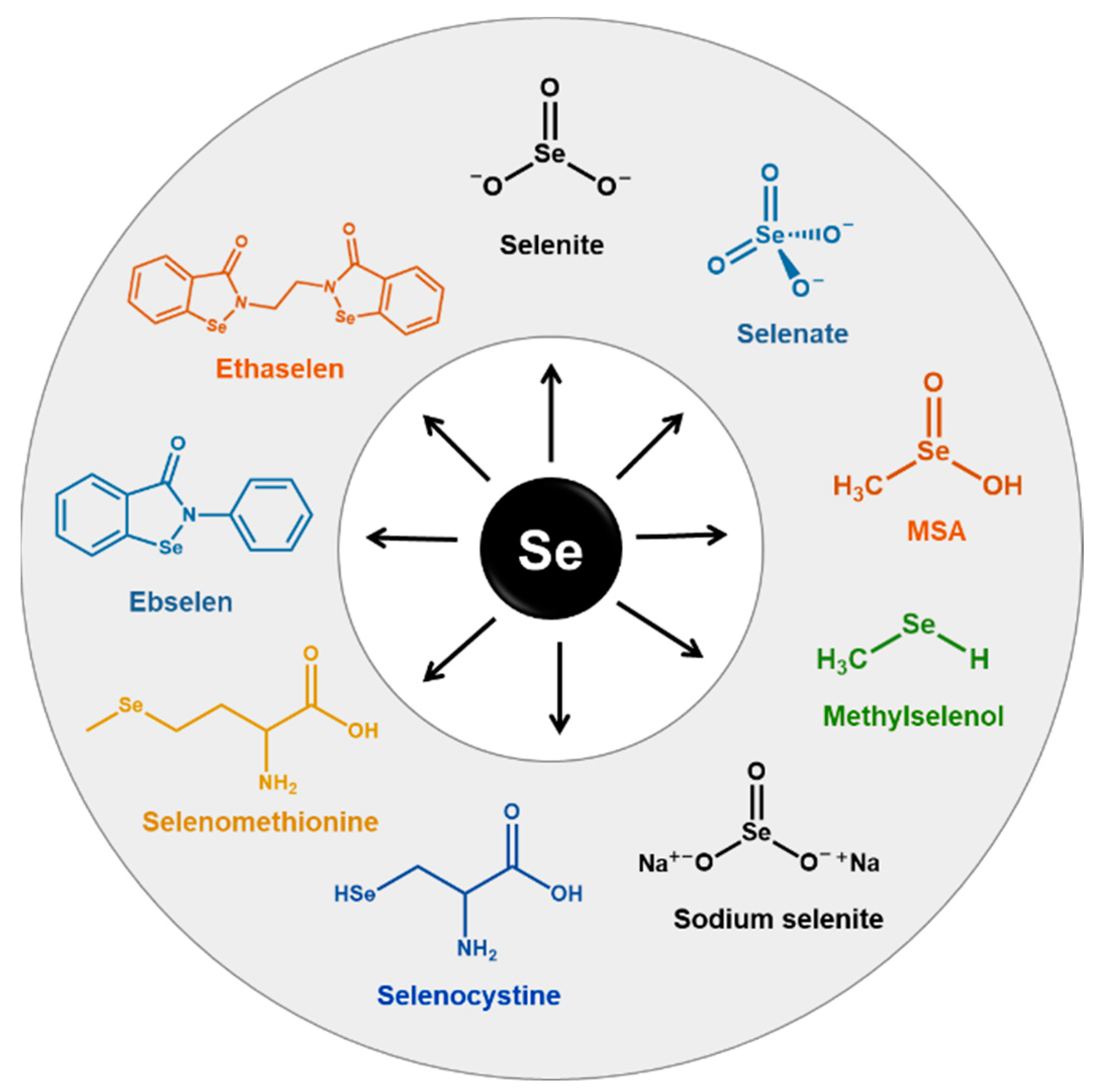
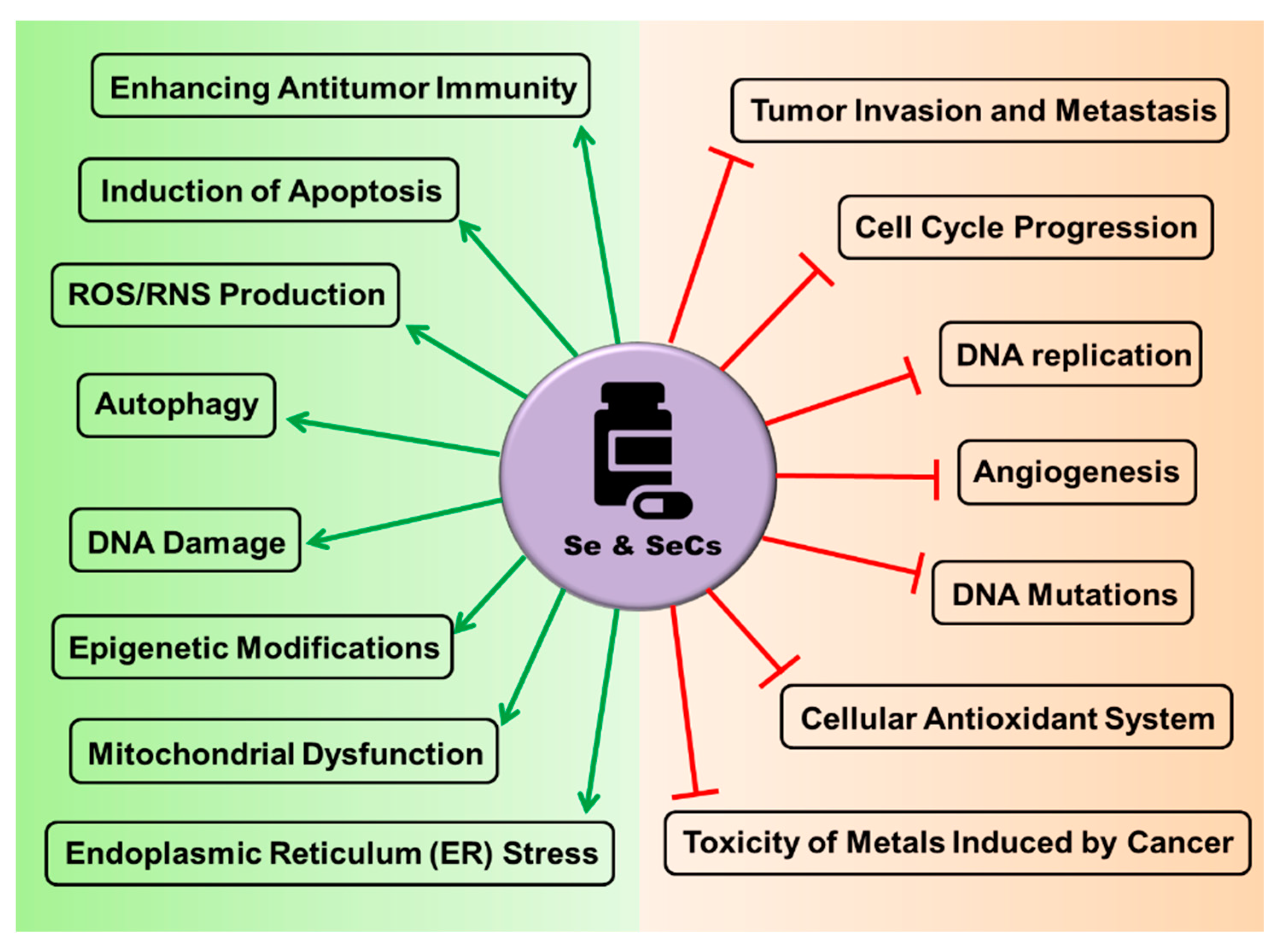
4.3. The Rationale behind the Use of Se and SeCs as Anticancer Agents
5. Implication of Se and SeCs for the Treatment of Lymphomas
5.1. Effect of Se and SeCs on Lymphoma Cultures In Vitro
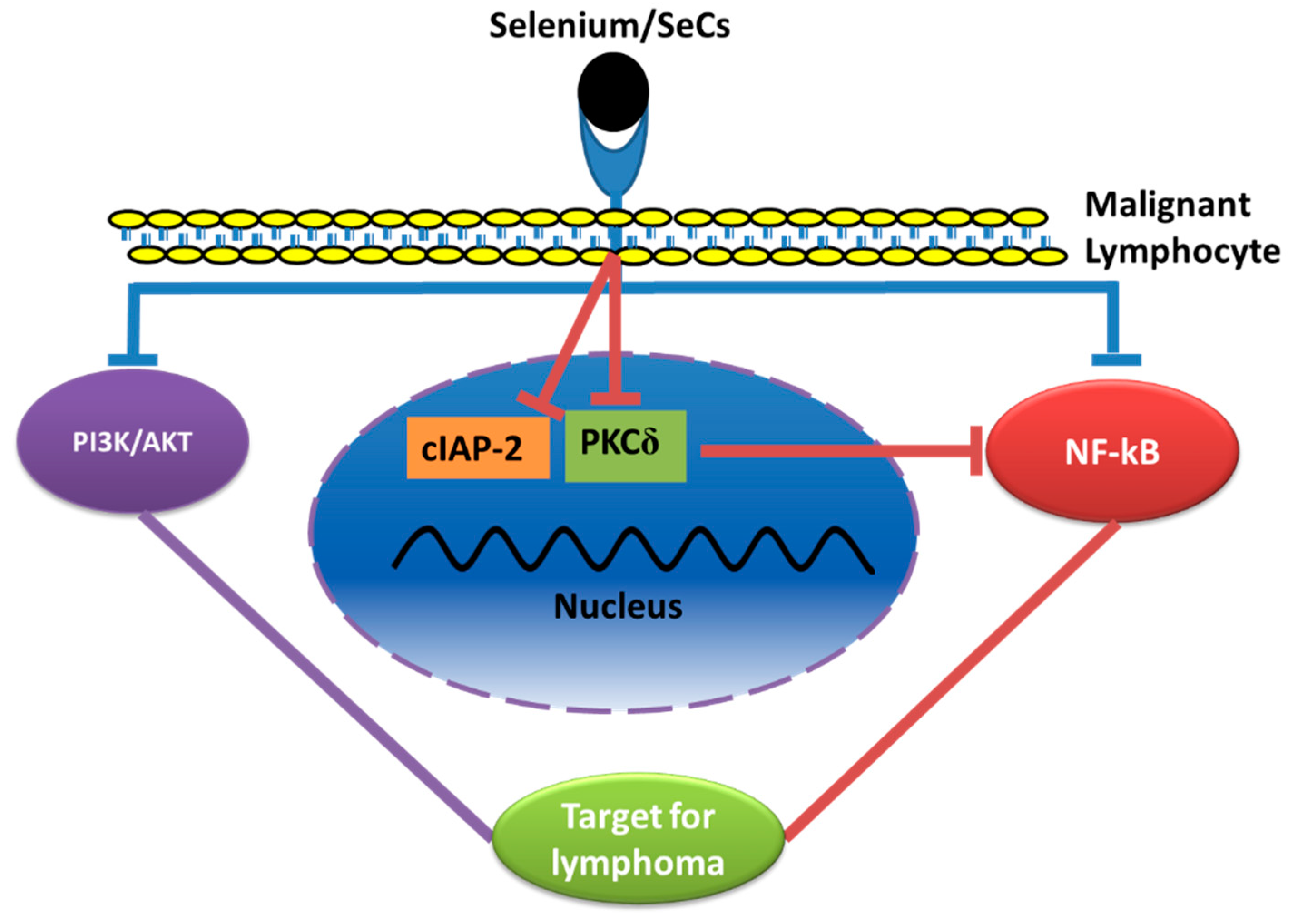
5.2. Effects of Se/SeCs on the PI3-Kinase/Akt, and MAP Kinase Pathways
5.3. Effects of Se/SeCs through the Nuclear Factor Kappa B (NF-κB) Pathway
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haque, A.; Brazeau, D.; Amin, A.R. Perspectives on natural compounds in chemoprevention and treatment of cancer: An update with new promising compounds. Eur. J. Cancer 2021, 149, 165–183. [Google Scholar] [CrossRef]
- Golla, U. Emergence of nutraceuticals as the alternative medications for pharmaceuticals. Int. J. Complement Alt. Med. 2018, 11, 155–158. [Google Scholar] [CrossRef] [Green Version]
- Amin, A.R.; Kucuk, O.; Khuri, F.R.; Shin, D.M. Perspectives for cancer prevention with natural compounds. J. Clin. Oncol 2009, 27, 2712–2725. [Google Scholar] [CrossRef] [Green Version]
- Harvie, M. Nutritional supplements and cancer: Potential benefits and proven harms. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, e478–e486. [Google Scholar] [CrossRef] [Green Version]
- Evans, S.O.; Khairuddin, P.F.; Jameson, M.B. Optimising Selenium for Modulation of Cancer Treatments. Anticancer Res. 2017, 37, 6497–6509. [Google Scholar] [CrossRef] [Green Version]
- Foster, L.; Sumar, S. Selenium in health and disease: A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 211–228. [Google Scholar] [CrossRef] [PubMed]
- El-Bayoumy, K.; Sinha, R. Molecular chemoprevention by selenium: A genomic approach. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2005, 591, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid Redox Signal 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Brown, K.M.; Arthur, J.R. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Kieliszek, M.; Blazejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.; Lee, J.; Wu, C.; Guo, X.; Lee, B.J.; Chun, J.S.; Kim, J.H. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Chambers, I.; Frampton, J.; Goldfarb, P.; Affara, N.; McBain, W.; Harrison, P.R. The structure of the mouse glutathione peroxidase gene: The selenocysteine in the active site is encoded by the ‘termination’ codon, TGA. EMBO J. 1986, 5, 1221–1227. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Li, H.; Ying, Z.; Liu, X. Research progress on separation of selenoproteins/Se-enriched peptides and their physiological activities. Food Funct. 2021, 12, 1390–1401. [Google Scholar] [CrossRef]
- Ganther, H.E. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: Complexities with thioredoxin reductase. Carcinogenesis 1999, 20, 1657–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellinger, F.P.; Raman, A.V.; Reeves, M.A.; Berry, M.J. Regulation and function of selenoproteins in human disease. Biochem. J. 2009, 422, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Li, D.; Feng, X.; Zhao, F.; Li, C.; Zheng, S.; Lyu, J. A pan-cancer study of selenoprotein genes as promising targets for cancer therapy. BMC Med. Genomics 2021, 14, 78. [Google Scholar] [CrossRef]
- Kaweme, N.M.; Zhou, S.; Changwe, G.J.; Zhou, F. The significant role of redox system in myeloid leukemia: From pathogenesis to therapeutic applications. Biomark Res. 2020, 8, 63. [Google Scholar] [CrossRef]
- Stevens, J.; Waters, R.; Sieniawska, C.; Kassam, S.; Montoto, S.; Fitzgibbon, J.; Rohatiner, A.; Lister, A.; Joel, S. Serum selenium concentration at diagnosis and outcome in patients with haematological malignancies. Br. J. Haematol. 2011, 154, 448–456. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Shanbhag, S.; Ambinder, R.F. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J. Clin. 2018, 68, 116–132. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; IARC Press: Lyon, France, 2008; Volume 2. [Google Scholar]
- Skarin, A.T.; Dorfman, D.M. Non-Hodgkin’s lymphomas: Current classification and management. CA Cancer J. Clin. 1997, 47, 351–372. [Google Scholar] [CrossRef] [Green Version]
- Endrizzi, L.; Fiorentino, M.V.; Salvagno, L.; Segati, R.; Pappagallo, G.L.; Fosser, V. Serum lactate dehydrogenase (LDH) as a prognostic index for non-Hodgkin’s lymphoma. Eur. J. Cancer Clin. Oncol. 1982, 18, 945–949. [Google Scholar] [CrossRef]
- Elstrom, R.; Guan, L.; Baker, G.; Nakhoda, K.; Vergilio, J.-A.; Zhuang, H.; Pitsilos, S.; Bagg, A.; Downs, L.; Mehrotra, A. Utility of FDG-PET scanning in lymphoma by WHO classification. Blood J. Am. Soc. Hematol. 2003, 101, 3875–3876. [Google Scholar] [CrossRef]
- Dahmus, J.; Rosario, M.; Clarke, K. Risk of Lymphoma Associated with Anti-TNF Therapy in Patients with Inflammatory Bowel Disease: Implications for Therapy. Clin. Exp. Gastroenterol. 2020, 13, 339–350. [Google Scholar] [CrossRef]
- Mercer, L.K.; Galloway, J.B.; Lunt, M.; Davies, R.; Low, A.L.; Dixon, W.G.; Watson, K.D.; Consortium, B.C.C.; Symmons, D.P.; Hyrich, K.L. Risk of lymphoma in patients exposed to antitumour necrosis factor therapy: Results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann. Rheum. Dis. 2017, 76, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Clinical Practice Guidelines in Oncology: B-Cell Lymphomas. 2018. Available online: www.nccn.org. (accessed on 12 March 2021).
- Wilks, S. Cases of enlargement of the lymphatic glands and spleen (or, Hodgkin’s disease) with remarks. Guy’s Hosp. Rep. 1856, 11, 56–67. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA A Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Morton, L.M.; Wang, S.S.; Devesa, S.S.; Hartge, P.; Weisenburger, D.D.; Linet, M.S. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006, 107, 265–276. [Google Scholar] [CrossRef]
- Harty, L.C.; Lin, A.Y.; Goldstein, A.M.; Jaffe, E.S.; Carrington, M.; Tucker, M.A.; Modi, W.S. HLA-DR, HLA-DQ, and TAP genes in familial Hodgkin disease. Blood J. Am. Soc. Hematol. 2002, 99, 690–693. [Google Scholar] [CrossRef] [Green Version]
- Hooper, W.C.; Holman, R.C.; Clarke, M.; Chorba, T.L. Trends in non-hodgkin lymphoma (NHL) and HIV-associated NHL deaths in the United States. Am. J. Hematol. 2001, 66, 159–166. [Google Scholar] [CrossRef]
- Tirelli, U.; Spina, M.; Gaidano, G.; Vaccher, E.; Franceschi, S.; Carbone, A. Epidemiological, biological and clinical features of HIV-related lymphomas in the era of highly active antiretroviral therapy. Aids 2000, 14, 1675–1688. [Google Scholar] [CrossRef] [Green Version]
- International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J. Natl. Cancer Inst. 2000, 92, 1823–1830. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Yoo, M.H.; Carlson, B.A.; Gladyshev, V.N. Selenoproteins that function in cancer prevention and promotion. Biochim. Biophys. Acta 2009, 1790, 1541–1545. [Google Scholar] [CrossRef] [Green Version]
- Schweizer, U.; Fradejas-Villar, N. Why 21? The significance of selenoproteins for human health revealed by inborn errors of metabolism. FASEB J. 2016, 30, 3669–3681. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, K.L. Evaluating selenium poisoning. Ann. Clin. Lab. Sci. 2006, 36, 409–420. [Google Scholar]
- Elsaid, R.; Soares-da-Silva, F.; Peixoto, M.; Amiri, D.; Mackowski, N.; Pereira, P.; Bandeira, A.; Cumano, A. Hematopoiesis: A Layered Organization Across Chordate Species. Front. Cell Dev. Biol. 2020, 8, 606642. [Google Scholar] [CrossRef]
- Conrad, M.; Jakupoglu, C.; Moreno, S.G.; Lippl, S.; Banjac, A.; Schneider, M.; Beck, H.; Hatzopoulos, A.K.; Just, U.; Sinowatz, F.; et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol. Cell Biol. 2004, 24, 9414–9423. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Carlson, B.A.; Paulson, R.F.; Prabhu, K.S. The intricate role of selenium and selenoproteins in erythropoiesis. Free Radic. Biol. Med. 2018, 127, 165–171. [Google Scholar] [CrossRef]
- Liao, C.; Hardison, R.C.; Kennett, M.J.; Carlson, B.A.; Paulson, R.F.; Prabhu, K.S. Selenoproteins regulate stress erythroid progenitors and spleen microenvironment during stress erythropoiesis. Blood 2018, 131, 2568–2580. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Selenium in Human Health and Disease: An Overview. In Selenium; Michalke, B., Ed.; Molecular and Integrative Toxicology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ehudin, M.A.; Golla, U.; Trivedi, D.; Potlakayala, S.D.; Rudrabhatla, S.V.; Desai, D.; Dovat, S.; Claxton, D.; Sharma, A. Therapeutic Benefits of Selenium in Hematological Malignancies. Int. J. Mol. Sci. 2022, 23, 7972. [Google Scholar] [CrossRef]
- Muecke, R.; Schomburg, L.; Buentzel, J.; Kisters, K.; Micke, O.; German Working Group Trace Elements and Electrolytes in Oncology-AKTE. Selenium or no selenium—That is the question in tumor patients: A new controversy. Integr. Cancer Ther. 2010, 9, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Last, K.W.; Cornelius, V.; Delves, T.; Sieniawska, C.; Fitzgibbon, J.; Norton, A.; Amess, J.; Wilson, A.; Rohatiner, A.Z.; Lister, T.A. Presentation serum selenium predicts for overall survival, dose delivery, and first treatment response in aggressive non-Hodgkin’s lymphoma. J. Clin. Oncol. 2003, 21, 2335–2341. [Google Scholar] [CrossRef]
- Deffuant, C.; Celerier, P.; Boiteau, H.; Litoux, P.; Dreno, B. Serum selenium in melanoma and epidermotropic cutaneous T-cell lymphoma. Acta Derm. Venereol. 1994, 74, 90–92. [Google Scholar]
- Ozgen, I.T.; Dagdemir, A.; Elli, M.; Saraymen, R.; Pinarli, F.G.; Fisgin, T.; Albayrak, D.; Acar, S. Hair selenium status in children with leukemia and lymphoma. J Pediatr Hematol Oncol 2007, 29, 519–522. [Google Scholar] [CrossRef]
- Beguin, Y.; Weber, G.; Delbrouck, J.M.; Roelandts, I.; Robaye, G.; Bury, J.; Fillet, G. Serum trace elements during chemotherapy for acute myelogenous leukemia. Acta Pharmacol Toxicol 1986, 59 (Suppl. 7), 270–273. [Google Scholar] [CrossRef]
- Beguin, Y.; Brasseur, F.; Weber, G.; Bury, J.; Delbrouck, J.M.; Roelandts, I.; Robaye, G.; Fillet, G. Observations of serum trace elements in chronic lymphocytic leukemia. Cancer 1987, 60, 1842–1846. [Google Scholar] [CrossRef]
- Asfour, I.A.; El-kholy, N.M.; Ayoub, M.S.; Ahmed, M.B.; Bakarman, A.A. Selenium and glutathione peroxidase status in adult Egyptian patients with acute myeloid leukemia. Biol. Trace Elem. Res. 2009, 132, 85–92. [Google Scholar] [CrossRef]
- Shacter, E.; Williams, J.A.; Hinson, R.M.; Senturker, S.; Lee, Y.J. Oxidative stress interferes with cancer chemotherapy: Inhibition of lymphoma cell apoptosis and phagocytosis. Blood 2000, 96, 307–313. [Google Scholar] [CrossRef]
- Taguchi, T.; Kurata, M.; Onishi, I.; Kinowaki, Y.; Sato, Y.; Shiono, S.; Ishibashi, S.; Ikeda, M.; Yamamoto, M.; Kitagawa, M.; et al. SECISBP2 is a novel prognostic predictor that regulates selenoproteins in diffuse large B-cell lymphoma. Lab. Investig. 2021, 101, 218–227. [Google Scholar] [CrossRef]
- Tabbara, I.A.; Zimmerman, K.; Morgan, C.; Nahleh, Z. Allogeneic hematopoietic stem cell transplantation: Complications and results. Arch. Intern. Med. 2002, 162, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Schots, R.; Kaufman, L.; Van Riet, I.; Ben Othman, T.; De Waele, M.; Van Camp, B.; Demanet, C. Proinflammatory cytokines and their role in the development of major transplant-related complications in the early phase after allogeneic bone marrow transplantation. Leukemia 2003, 17, 1150–1156. [Google Scholar] [CrossRef]
- Takatsuka, H.; Takemoto, Y.; Yamada, S.; Wada, H.; Tamura, S.; Fujimori, Y.; Okamoto, T.; Suehiro, A.; Kanamaru, A.; Kakishita, E. Complications after bone marrow transplantation are manifestations of systemic inflammatory response syndrome. Bone Marrow Transpl. 2000, 26, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Spencer, A.; Horvath, N.; Gibson, J.; Prince, H.M.; Herrmann, R.; Bashford, J.; Joske, D.; Grigg, A.; McKendrick, J.; Prosser, I.; et al. Prospective randomised trial of amifostine cytoprotection in myeloma patients undergoing high-dose melphalan conditioned autologous stem cell transplantation. Bone Marrow Transpl. 2005, 35, 971–977. [Google Scholar] [CrossRef] [Green Version]
- Levine, J.E.; Paczesny, S.; Mineishi, S.; Braun, T.; Choi, S.W.; Hutchinson, R.J.; Jones, D.; Khaled, Y.; Kitko, C.L.; Bickley, D.; et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood 2008, 111, 2470–2475. [Google Scholar] [CrossRef]
- Duntas, L.H. Selenium and inflammation: Underlying anti-inflammatory mechanisms. Horm. Metab. Res. 2009, 41, 443–447. [Google Scholar] [CrossRef]
- Daeian, N.; Radfar, M.; Jahangard-Rafsanjani, Z.; Hadjibabaie, M.; Ghavamzadeh, A. Selenium supplementation in patients undergoing hematopoietic stem cell transplantation: Effects on pro-inflammatory cytokines levels. Daru 2014, 22, 51. [Google Scholar] [CrossRef] [Green Version]
- Dickerson, O.; Smith, T. Selenium, tellurium, and osmium. In Occupational Medicine, 3rd ed.; Mosby: St. Louis, MO, USA, 1994; Volume 9. [Google Scholar]
- Mihajlovic, M. Selenium toxicity in domestic animals. Glas. Srp. Akad. Nauka Med. 1992, 42, 131–144. [Google Scholar]
- Draize, J.; Beath, O. Observations on the pathology of blind staggers and alkali disease. J. Am. Vet. Med. Assoc. 1935, 86, 753–763. [Google Scholar]
- Frastke, K.; Painter, E. Selenium in proteins from toxic foodstuffs. 1. Remarks on the occurrence and nature of the selenium present in a number of foodstuffs or their derived products. Cereal Chem. 1936, 13, 67–70. [Google Scholar]
- Rosenfeld, I.; Beath, O.A. Pathology of Selenium Poisoning; University of Wyoming, Agricultural Experiment Station: Laramie, WY, USA, 1946. [Google Scholar]
- O’Toole, D.; Raisbeck, M.; Case, J.; Whitson, T. Selenium-induced “blind staggers” and related myths. A commentary on the extent of historical livestock losses attributed to selenosis on western US rangelands. Vet. Pathol. 1996, 33, 104–116. [Google Scholar] [CrossRef]
- Combs, G., Jr. Growing interest in selenium. West. J. Med. 1990, 153, 192. [Google Scholar] [PubMed]
- Walker, C.; Klein, F. Selenium—Its therapeutic value, especially in cancer, American Med. 628, as cited by Schrauzer, CH, 1979, Trace elements in carcinogenesis. Adv. Nutr. Res. 1915, 2, 219–244. [Google Scholar]
- Bartolini, D.; Sancineto, L.; Fabro de Bem, A.; Tew, K.D.; Santi, C.; Radi, R.; Toquato, P.; Galli, F. Selenocompounds in Cancer Therapy: An Overview. Adv. Cancer Res. 2017, 136, 259–302. [Google Scholar] [CrossRef] [PubMed]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, G.; Srivastava, D.K.; Mishra, V. Positive and Negative Impacts of Selenium on Human Health and Phytotoxicity. In Selenium Contamination in Water, 1st ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 73–90. [Google Scholar] [CrossRef]
- Roychowdhury, M. A review of safety and health hazards of metalorganic compounds. Am. Ind. Hyg. Assoc. J. 1993, 54, 607–614. [Google Scholar] [CrossRef]
- Betz, A.L.; Firth, J.A.; Goldstein, G.W. Polarity of the blood-brain barrier: Distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res. 1980, 192, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Montero, A.J.; Jassem, J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs 2011, 71, 1385–1396. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Abdulah, R.; Miyazaki, K.; Nakazawa, M.; Koyama, H. Chemical forms of selenium for cancer prevention. J. Trace Elem. Med. Biol. 2005, 19, 141–150. [Google Scholar] [CrossRef]
- Dwivedi, C.; Shah, C.P.; Singh, K.; Kumar, M.; Bajaj, P.N. An organic acid-induced synthesis and characterization of selenium nanoparticles. J. Nanotechnol. 2011, 2011, 651971. [Google Scholar] [CrossRef] [Green Version]
- Ingole, A.R.; Thakare, S.R.; Khati, N.; Wankhade, A.V.; Burghate, D. Green synthesis of selenium nanoparticles under ambient condition. Chalcogenide Lett. 2010, 7, 485–489. [Google Scholar]
- Nasrolahi Shirazi, A.; Tiwari, R.K.; Oh, D.; Sullivan, B.; Kumar, A.; Beni, Y.A.; Parang, K. Cyclic peptide–selenium nanoparticles as drug transporters. Mol. Pharm. 2014, 11, 3631–3641. [Google Scholar] [CrossRef]
- Weekley, C.M.; Harris, H.H. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef]
- El-Bayoumy, K. The protective role of selenium on genetic damage and on cancer. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2001, 475, 123–139. [Google Scholar] [CrossRef]
- Combs Jr, G.F.; Gray, W.P. Chemopreventive agents: Selenium. Pharmacol. Ther. 1998, 79, 179–192. [Google Scholar] [CrossRef]
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium compounds, apoptosis and other types of cell death: An overview for cancer therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Cilloni, S.; Crespi, C.M. The Epidemiology of Selenium and Human Cancer. Adv. Cancer Res. 2017, 136, 1–48. [Google Scholar] [CrossRef]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Vinceti, M.; Filippini, T.; Wise, L.A.; Rothman, K.J. A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies. Environ. Res. 2021, 197, 111210. [Google Scholar] [CrossRef]
- Guan, B.; Yan, R.; Li, R.; Zhang, X. Selenium as a pleiotropic agent for medical discovery and drug delivery. Int J Nanomedicine 2018, 13, 7473–7490. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Combs, G.F., Jr. Selenium as an anticancer nutrient: Roles in cell proliferation and tumor cell invasion. J. Nutr. Biochem. 2008, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ganther, H.E.; Stewart, C.; Ip, C. Identification of molecular targets associated with selenium-induced growth inhibition in human breast cells using cDNA microarrays. Cancer Res. 2002, 62, 708–714. [Google Scholar] [PubMed]
- Lee, Y.K.; Park, S.Y.; Kim, Y.M.; Kim, D.C.; Lee, W.S.; Surh, Y.J.; Park, O.J. Suppression of mTOR via Akt-dependent and -independent mechanisms in selenium-treated colon cancer cells: Involvement of AMPKalpha1. Carcinogenesis 2010, 31, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Lancia, J.K.; Mercer, T.I.; Ip, C. Selenium compounds regulate p53 by common and distinctive mechanisms. Anticancer Res. 2004, 24, 1401–1408. [Google Scholar] [PubMed]
- Han, B.; Wei, W.; Hua, F.; Cao, T.; Dong, H.; Yang, T.; Yang, Y.; Pan, H.; Xu, C. Requirement for ERK activity in sodium selenite-induced apoptosis of acute promyelocytic leukemia-derived NB4 cells. J. Biochem. Mol. Biol. 2007, 40, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Wang, Z.; Ganther, H.; Lu, J. Distinct effects of methylseleninic acid versus selenite on apoptosis, cell cycle, and protein kinase pathways in DU145 human prostate cancer cells. Mol. Cancer Ther. 2002, 1, 1059–1066. [Google Scholar]
- Ren, Y.; Huang, F.; Liu, Y.; Yang, Y.; Jiang, Q.; Xu, C. Autophagy inhibition through PI3K/Akt increases apoptosis by sodium selenite in NB4 cells. BMB Rep. 2009, 42, 599–604. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Zeng, H.; Briske-Anderson, M.; Wu, M.; Moyer, M.P. Methylselenol, a selenium metabolite, plays common and different roles in cancerous colon HCT116 cell and noncancerous NCM460 colon cell proliferation. Nutr. Cancer 2012, 64, 128–135. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, M.; Botnen, J.H. Methylselenol, a selenium metabolite, induces cell cycle arrest in G1 phase and apoptosis via the extracellular-regulated kinase 1/2 pathway and other cancer signaling genes. J. Nutr. 2009, 139, 1613–1618. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishna, R.; Chen, Z.H.; Gundimeda, U. Selenocompounds induce a redox modulation of protein kinase C in the cell, compartmentally independent from cytosolic glutathione: Its role in inhibition of tumor promotion. Arch. Biochem. Biophys. 1997, 348, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.; Fleming, J.; El-Bayoumy, K.; Harrison, P.R. Enhanced sensitivity of human oral carcinomas to induction of apoptosis by selenium compounds: Involvement of mitogen-activated protein kinase and Fas pathways. Cancer Res. 2001, 61, 7479–7487. [Google Scholar] [PubMed]
- Last, K.; Maharaj, L.; Perry, J.; Strauss, S.; Fitzgibbon, J.; Lister, T.A.; Joel, S. The activity of methylated and non-methylated selenium species in lymphoma cell lines and primary tumours. Ann. Oncol. 2006, 17, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jiang, C. Antiangiogenic activity of selenium in cancer chemoprevention: Metabolite-specific effects. Nutr. Cancer 2001, 40, 64–73. [Google Scholar] [CrossRef]
- Juliger, S.; Goenaga-Infante, H.; Lister, T.A.; Fitzgibbon, J.; Joel, S.P. Chemosensitization of B-cell lymphomas by methylseleninic acid involves nuclear factor-kappaB inhibition and the rapid generation of other selenium species. Cancer Res. 2007, 67, 10984–10992. [Google Scholar] [CrossRef] [Green Version]
- Gopee, N.V.; Johnson, V.J.; Sharma, R.P. Sodium selenite-induced apoptosis in murine B-lymphoma cells is associated with inhibition of protein kinase C-delta, nuclear factor kappaB, and inhibitor of apoptosis protein. Toxicol. Sci. 2004, 78, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Tomar, M.S.; Acharya, A. Carboxylic group-induced synthesis and characterization of selenium nanoparticles and its anti-tumor potential on Dalton’s lymphoma cells. Colloids Surf. B Biointerfaces 2015, 126, 546–552. [Google Scholar] [CrossRef]
- Gautam, P.K.; Kumar, S.; Tomar, M.S.; Singh, R.K.; Acharya, A.; Kumar, S.; Ram, B. Selenium nanoparticles induce suppressed function of tumor associated macrophages and inhibit Dalton’s lymphoma proliferation. Biochem. Biophys. Rep. 2017, 12, 172–184. [Google Scholar] [CrossRef]
- Liao, G.; Tang, J.; Wang, D.; Zuo, H.; Zhang, Q.; Liu, Y.; Xiong, H. Selenium nanoparticles (SeNPs) have potent antitumor activity against prostate cancer cells through the upregulation of miR-16. World J. Surg. Oncol. 2020, 18, 81. [Google Scholar] [CrossRef]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Golla, U.; Sesham, K.; Dallavalasa, S.; Manda, N.K.; Unnam, S.; Sanapala, A.K.; Nalla, S.; Kondam, S.; Kumar, R. ABHD11-AS1: An Emerging Long Non-Coding RNA (lncRNA) with Clinical Significance in Human Malignancies. Noncoding RNA 2022, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. 2021, 6, 425. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moreno, O.; Segura, V.; Serrano, D.; Nguewa, P.; de las Rivas, J.; Calvo, A. Methylseleninic acid enhances the effect of etoposide to inhibit prostate cancer growth in vivo. Int. J. Cancer 2007, 121, 1197–1204. [Google Scholar] [CrossRef]
- Baud, V.; Karin, M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, A.S., Jr. The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karin, M. How NF-kappaB is activated: The role of the IkappaB kinase (IKK) complex. Oncogene 1999, 18, 6867–6874. [Google Scholar] [CrossRef] [Green Version]
- Bentires-Alj, M.; Barbu, V.; Fillet, M.; Chariot, A.; Relic, B.; Jacobs, N.; Gielen, J.; Merville, M.P.; Bours, V. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene 2003, 22, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, C.; Toi, M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat. Rev. Cancer 2005, 5, 297–309. [Google Scholar] [CrossRef]
| S. No. | SeP | Localization | Tissue Distribution | Classification |
|---|---|---|---|---|
| 1 | GPx1 | Cytosol | Ubiquitous | Antioxidant enzymes |
| 2 | GPx2 | Cytosol | Epithelium of the gastrointestinal | |
| 3 | GPx3 | Plasma | Plasma | |
| 4 | GPx4 | Cytosol; mitochondria; nucleus | Ubiquitous | |
| 5 | GPx6 | Cytosol | Olfactory epithelium and embryonic tissues | |
| 6 | SelK | ER | Heart, skeletal muscle, and endoplasmic reticulum | |
| 7 | SelR | Cytosol | Cytosol, nucleus | |
| 8 | SelW | Cytosol | Ubiquitous | |
| 9 | TrxR1 | Cytosol | Cytosol, nucleus | Redox signaling |
| 10 | TrxR2 | Cytosol | Mitochondria | |
| 11 | TrxR3 | Mitochondria | Testis | |
| 12 | DIO1 | Plasma Membrane | Liver, kidney, thyroid, and pituitary gland | Thyroid hormone metabolism |
| 13 | DIO2 | ER | Thyroid, the central nervous system, the pituitary gland, and skeletal muscle | |
| 14 | DIO3 | Plasma membrane | Pregnant uterus, placenta, embryonic liver, embryonic and neonatal brain, and neonatal skin | |
| 15 | SPS2 | Cytosol | Liver, kidney, and testis | Sec synthesis |
| 16 | SelP | Plasma | Plasma | Transport and storage of Se |
| 17 | Sep15 | ER | Brain, lung, testis, liver, thyroid, and kidney | Protein folding (potential) |
| 18 | SelN | ER membrane | Tissues | |
| 19 | SelM | ER | Endoplasmic reticulum | |
| 20 | SelS | ER | Endoplasmic reticulum, plasma membrane | |
| 21 | SelH | Nucleus | — | |
| 22 | SelI | Membrane | ||
| 23 | SelO | Mitochondria | ||
| 24 | SelT | ER and golgi | ||
| 25 | SelV | Cytosol |
| S. No. | Se/SeCs | Antitumor Mechanism | Outcome of Effect |
|---|---|---|---|
| 1 | Selenate | Up regulation of p-53 Down regulation of Bcl2 Suppression of mTOR | Activation of Apoptotic pathway [89] |
| 2 | Se-met | Up regulation of p-53 dependent and non-dependent proteins Up regulation of PARP Cleavage | Activation of Apoptotic pathway [90] |
| 3 | Selenite | Up regulation of Bax Down regulation of Bcl2 G2/M Phase arrest slight effects on p38 | Activation of Apoptotic pathway and Cell death [91] |
| 4 | Methylseleninic acid (MSA) | Activation of caspase activity, cytotoxicity, Dephosphorylation of Akt and involvement of PI3k, ERK1/2, and p38 | DNA damage [92] |
| 5 | Sodium selenite | Induction of autophagy | Cell death [93] |
| 6 | Ebselen | Activation of caspase activity | Activation of Apoptotic pathway [94] |
| 7 | Ethaselen | Activation of caspase activity | Activation of Apoptotic pathway [94] |
| 8 | Methylselenol | Inhibition of the ERK1/2 pathway activation and c-myc expression | Cell death [95,96] |
| 9 | Selenocystine | Catalyze the oxidation of active site Cys thiols in protein kinase C (PKC) | Cell death [97] |
| 10 | selenodiglutathione (SDG) | JNK and p38 kinases; activate ERKs and Akt | Activation of Apoptotic pathway [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golla, U.; Dallavalasa, S. Chemopreventive Effects of Selenium and Selenocompounds in the Treatment of Lymphoma. BioMed 2022, 2, 310-327. https://doi.org/10.3390/biomed2030025
Golla U, Dallavalasa S. Chemopreventive Effects of Selenium and Selenocompounds in the Treatment of Lymphoma. BioMed. 2022; 2(3):310-327. https://doi.org/10.3390/biomed2030025
Chicago/Turabian StyleGolla, Upendarrao, and Siva Dallavalasa. 2022. "Chemopreventive Effects of Selenium and Selenocompounds in the Treatment of Lymphoma" BioMed 2, no. 3: 310-327. https://doi.org/10.3390/biomed2030025
APA StyleGolla, U., & Dallavalasa, S. (2022). Chemopreventive Effects of Selenium and Selenocompounds in the Treatment of Lymphoma. BioMed, 2(3), 310-327. https://doi.org/10.3390/biomed2030025







