Assessing Lymph Node Involvement in Muscle-Invasive Bladder Cancer: Proposal of a Predictive Model Using Clinical Variables
Abstract
:1. Introduction
2. Materials and Methods
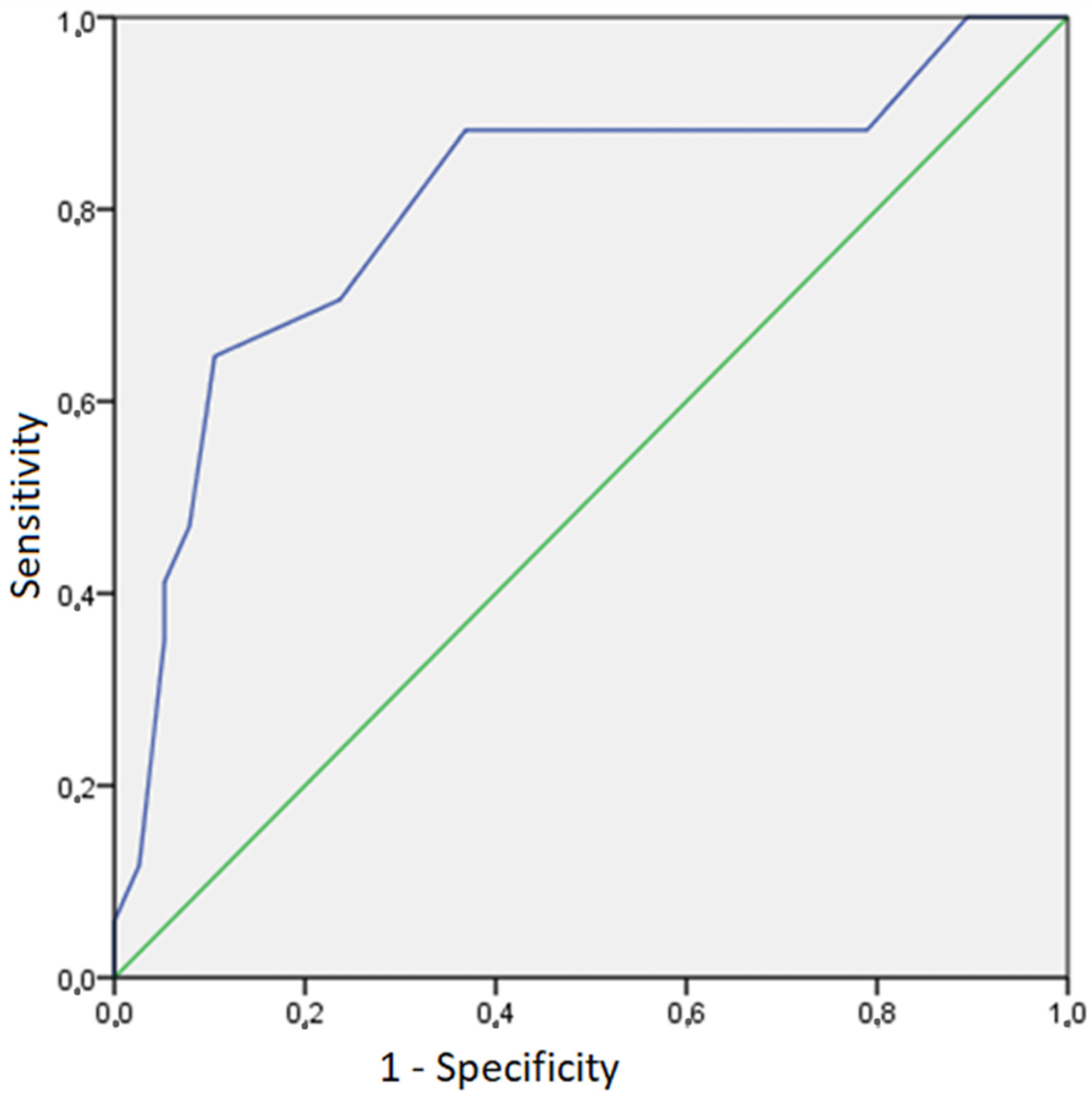
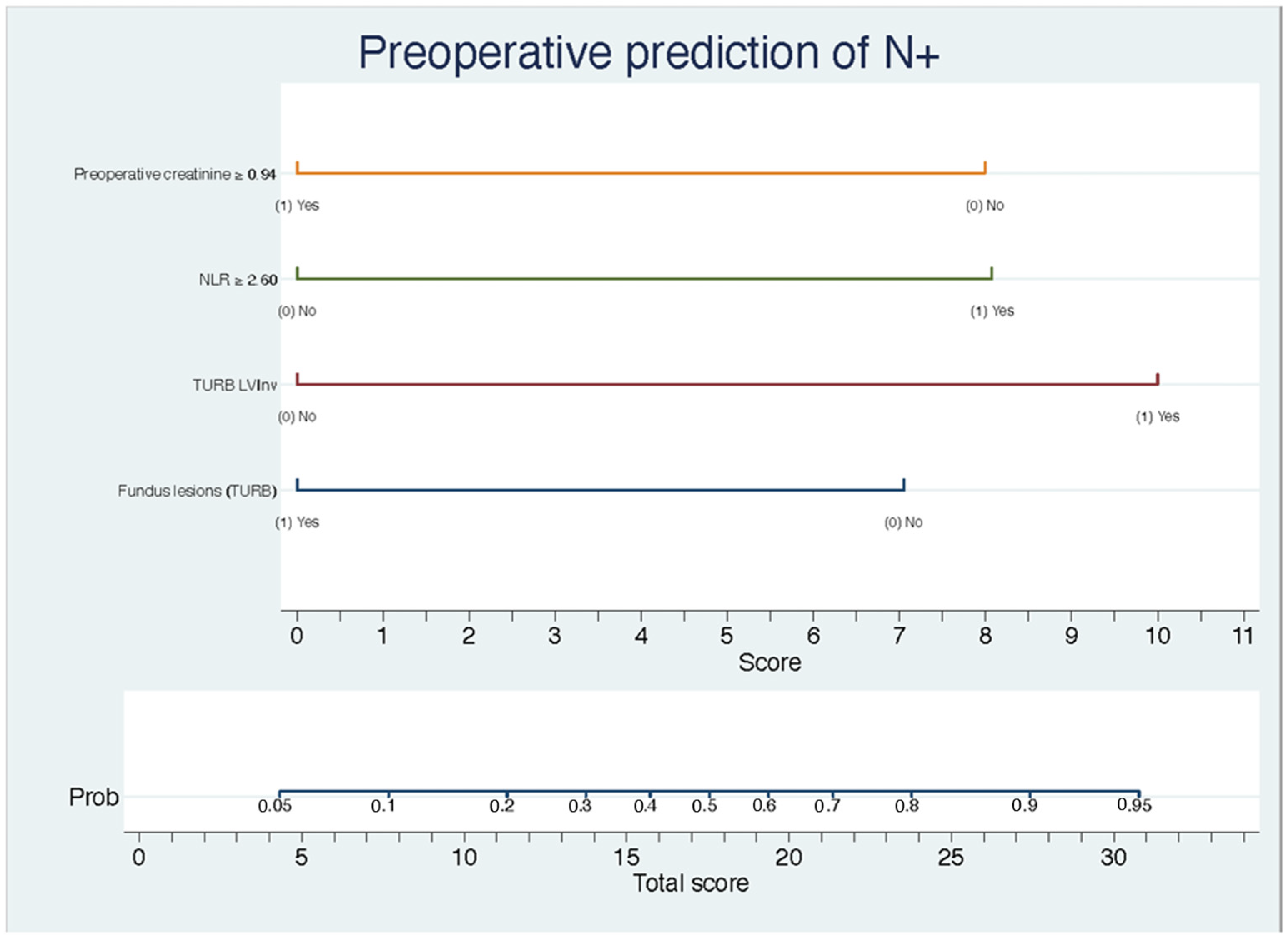
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.D.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. Lymphovascular invasion have a similar prognostic value as lymph node involvement in patients undergoing radical cystectomy with urothelial carcinoma. Sci. Rep. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Yafi, F.A.; Aprikian, A.G.; Chin, J.L.; Fradet, Y.; Izawa, J.; Estey, E.; Fairey, A.; Rendon, R.; Cagiannos, I.; Lacombe, L.; et al. Impact of concomitant carcinoma in situ on upstaging and outcome following radical cystectomy for bladder cancer. World J. Urol. 2014, 32, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, B.; Zha, Z.; Qu, W.; Zhao, H.; Yuan, J. Clinicopathological factors in bladder cancer for cancer-specific survival outcomes following radical cystectomy: A systematic review and meta-analysis. BMC Cancer 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cha, E.K.; Sfakianos, J.P.; Sukhu, R.; Yee, A.M.; Sjoberg, D.D.; Bochner, B.H. Poor prognosis of bladder cancer patients with occult lymph node metastases treated with neoadjuvant chemotherapy. BJU Int. 2018, 122, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Green, D.A.; Rink, M.; Hansen, J.; Cha, E.K.; Robinson, B.; Tian, Z.; Chun, F.K.; Tagawa, S.; Karakiewicz, P.I.; Fisch, M.; et al. Accurate preoperative prediction of non-organ-confined bladder urothelial carcinoma at cystectomy. BJU Int. 2012, 111, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Seiler, R.; Lam, L.L.; Erho, N.; Takhar, M.; Mitra, A.P.; Buerki, C.; Davicioni, E.; Skinner, E.C.; Daneshmand, S.; Black, P.C. Prediction of Lymph Node Metastasis in Patients with Bladder Cancer Using Whole Transcriptome Gene Expression Signatures. J. Urol. 2016, 196, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, M.; Cathomas, R.; Compérat, E.; Cowan, N.C.; Gakis, G.; Thalmann, G.N. EAU Guidelines on: Muscle-Invasive and Metastatic Bladder Cancer; European Association of Urology: Arnhem, The Netherlands, 2019. [Google Scholar]
- Chang, S.S.; Bochner, B.H.; Chou, R.; Dreicer, R.; Kamat, A.M.; Lerner, S.P.; Holzbeierlein, J.M. Tratamiento del Cáncer de Vejiga Músculo Invasivo y No Metastásico: Guía de AUA/ASCO/ASTRO/SUO; Spanish Version; American Urological Association Education and Research: Linthicum, MD, USA, 2019. [Google Scholar]
- Madersbacher, S.; Hochreiter, W.; Burkhard, F.; Thalmann, G.N.; Danuser, H.; Markwalder, R.; Studer, U.E. Radical cystectomy for bladder cancer today—A homogeneous series without neoadjuvant therapy. J. Clin. Oncol. 2003, 21, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Karakiewicz, P.I.; Shariat, S.F.; Palapattu, G.S.; Perrotte, P.; Lotan, Y.; Rogers, C.G.; Amiel, G.E.; Vazina, A.; Gupta, A.; Bastian, P.J.; et al. Precystectomy Nomogram for Prediction of Advanced Bladder Cancer Stage. Eur. Urol. 2006, 50, 1254–1262. [Google Scholar] [CrossRef]
- Vartolomei, M.D.; Porav-Hodade, D.; Ferro, M.; Mathieu, R.; Abufaraj, M.; Foerster, B.; Kimura, S.; Shariat, S.F. Prognostic Role of Pretreatment Neutrophil-To-Lymphocyte Ratio (NLR) in Patients With Non-Muscle-Invasive Bladder Cancer (NMIBC): A Systematic Review and Meta-Analysis. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Takano, M.; Miyamoto, M.; Yoshikawa, T.; Kato, K.; Sakamoto, T.; Takasaki, K.; Matsuura, H.; Soyama, H.; Hirata, J.; et al. Pretreatment Neutrophil-to-Lymphocyte Ratio Was a Predictor of Lymph Node Metastasis in Endometrial Cancer Patients. Oncology 2019, 96, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Kluth, L.A.; Black, P.C.; Bochner, B.H.; Catto, J.; Lerner, S.P.; Stenzl, A.; Sylvester, R.; Vickers, A.J.; Xylinas, E.; Shariat, S.F. Prognostic and Prediction Tools in Bladder Cancer: A Comprehensive Review of the Literature. Eur. Urol. 2015, 68, 238–253. [Google Scholar] [CrossRef] [PubMed]
| Variables | N° (%) Patients | |
|---|---|---|
| Age | Mean (Median) | 65 (66) |
| Range | 33–83 | |
| Gender | Male | 58 (93.50) |
| Female | 4 (6.50) | |
| Presurgical creatinine | Mean (Median) | 1.04 (0.94) |
| Range | 0.46–2.31 | |
| TURB T stage | Tx | 1 (1.60) |
| Tis | 0 (0) | |
| Ta | 0 (0) | |
| T1 | 5 (8.20) | |
| T2 | 55 (90.20) | |
| CIS presence after TURB | 17 (27.40) | |
| LVInv presence after TURB | 8 (12.90) | |
| cT stage | Tx | 5 (8.50) |
| T0-T1-T2 | 27 (45.70) | |
| T3 | 20 (33.90) | |
| T4 | 7 (11.90) | |
| cN stage | N0 | 46 (79.30) |
| N1 | 6 (10.30) | |
| N2 | 3 (5.20) | |
| N3 | 3 (5.20) | |
| Presurgical NLR | Mean (Median) | 2.94 (2.25) |
| Range | 0.59–13.50 | |
| Neoadjuvant Chemotherapy | 5 (8.10) | |
| Lymph nodes obtained | Mean (Median) | 19.84 (19) |
| pT stage | Tis | 8 (12.90) |
| T0 | 5 (8.10) | |
| T1 | 8 (12.90) | |
| T2 | 7 (11.30) | |
| T3 | 21 (33.90) | |
| T4 | 13 (21) | |
| pN stage | N0 | 44 (71) |
| N1 | 6 (9.70) | |
| N2 | 12 (19.40) | |
| N3 | 0 (0) | |
| pT3 ≥ stage | ||||
| No | Yes | Total | ||
| cT3 ≥ stage | No | 18 | 14 | 32 |
| Yes | 8 | 20 | 28 | |
| Total | 26 | 34 | 60 | |
| pN+ | ||||
| No | Yes | Total | ||
| cN+ | No | 33 | 14 | 47 |
| Yes | 9 | 4 | 13 | |
| Total | 42 | 18 | 60 | |
| Variables | OR | I.C. 95% | p-Value |
|---|---|---|---|
| Preoperative creatinine | 0.17 | 0.03–0.80 | 0.02 |
| NLR | 6.03 | 1.29–28.30 | 0.02 |
| LVInv (TURB) | 9.26 | 1.11–77.30 | 0.04 |
| Fundus lesion (RTU) | 0.21 | 0.05–0.93 | 0.04 |
| Model Calibration | X2 = 16.84 | p = 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barragán Flores, W.A.; Carrillo George, C.; Sandoval, J.M.; Cívico Sánchez, C.; Flores, C.; Muñoz, V.; Fernández Aparicio, T. Assessing Lymph Node Involvement in Muscle-Invasive Bladder Cancer: Proposal of a Predictive Model Using Clinical Variables. BioMed 2024, 4, 213-219. https://doi.org/10.3390/biomed4030017
Barragán Flores WA, Carrillo George C, Sandoval JM, Cívico Sánchez C, Flores C, Muñoz V, Fernández Aparicio T. Assessing Lymph Node Involvement in Muscle-Invasive Bladder Cancer: Proposal of a Predictive Model Using Clinical Variables. BioMed. 2024; 4(3):213-219. https://doi.org/10.3390/biomed4030017
Chicago/Turabian StyleBarragán Flores, William A., Carlos Carrillo George, José María Sandoval, Claudia Cívico Sánchez, Cristina Flores, Victoria Muñoz, and Tomás Fernández Aparicio. 2024. "Assessing Lymph Node Involvement in Muscle-Invasive Bladder Cancer: Proposal of a Predictive Model Using Clinical Variables" BioMed 4, no. 3: 213-219. https://doi.org/10.3390/biomed4030017
APA StyleBarragán Flores, W. A., Carrillo George, C., Sandoval, J. M., Cívico Sánchez, C., Flores, C., Muñoz, V., & Fernández Aparicio, T. (2024). Assessing Lymph Node Involvement in Muscle-Invasive Bladder Cancer: Proposal of a Predictive Model Using Clinical Variables. BioMed, 4(3), 213-219. https://doi.org/10.3390/biomed4030017






