Differential Cytotoxicity of Flavored E-Liquids with and without Nicotine on Neonatal Human Melanocytes from Lightly and Darkly Pigmented Donors: A Preliminary Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. MTS Cytotoxicity Assay
2.4. Statistical Analysis
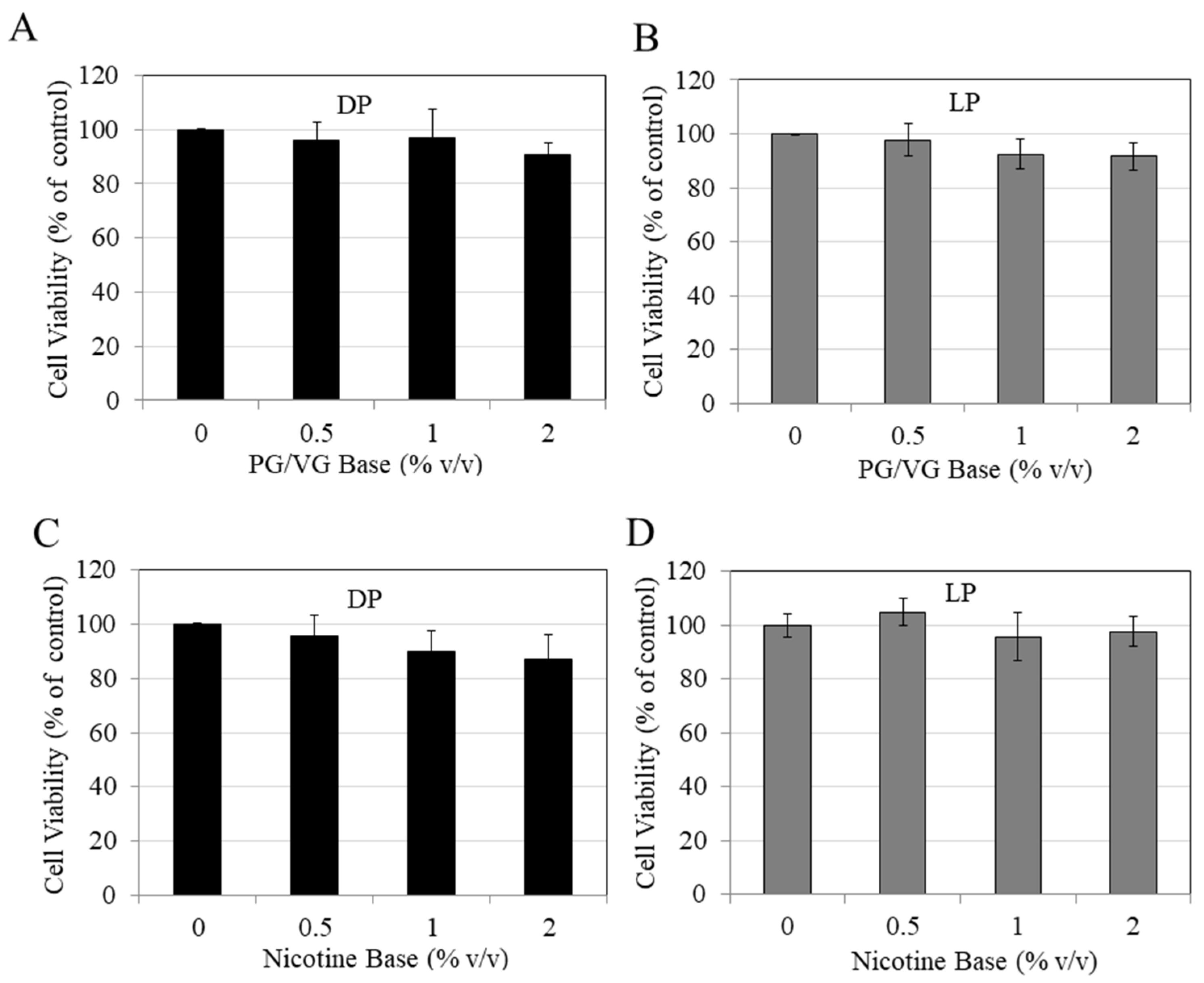
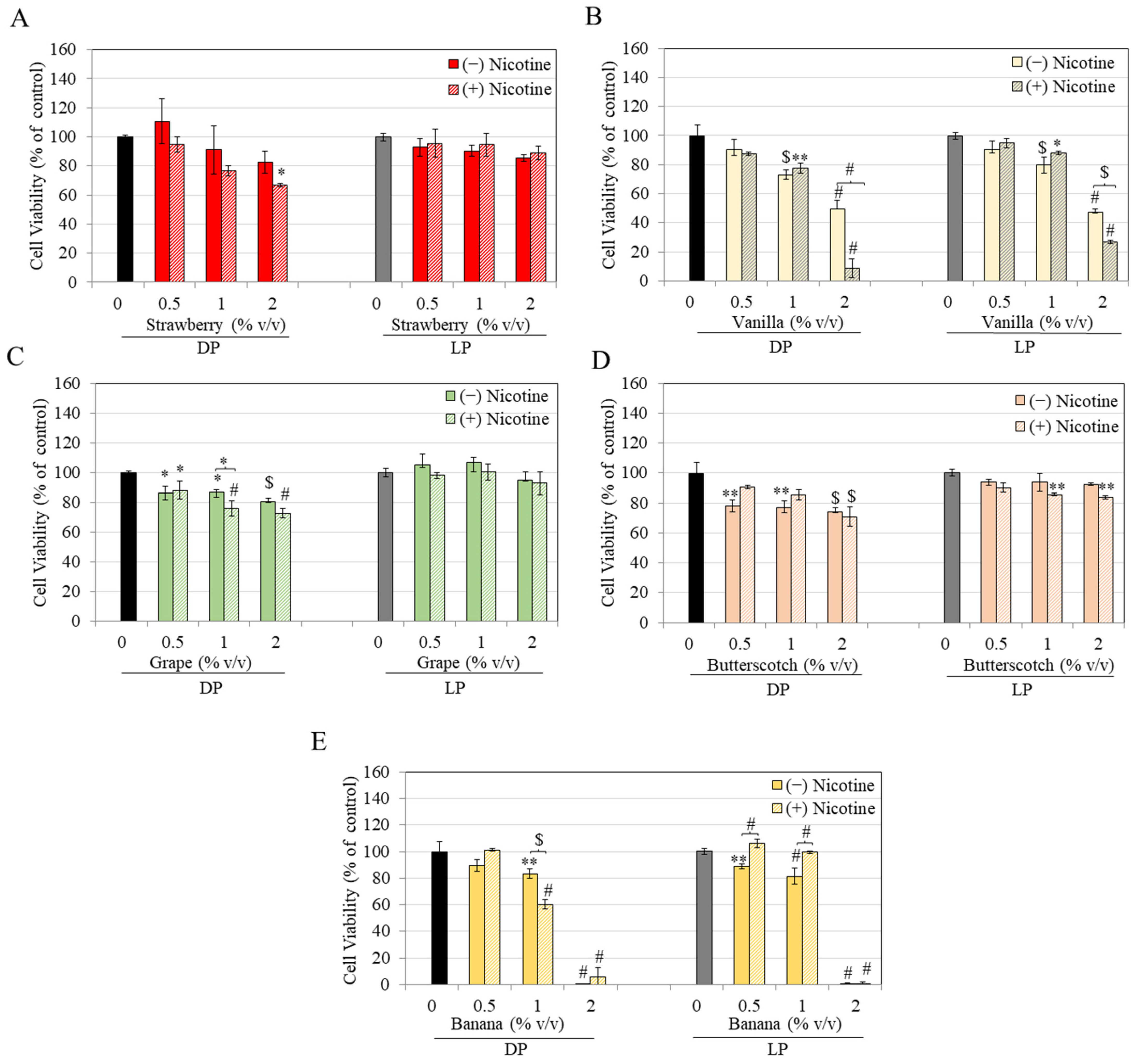
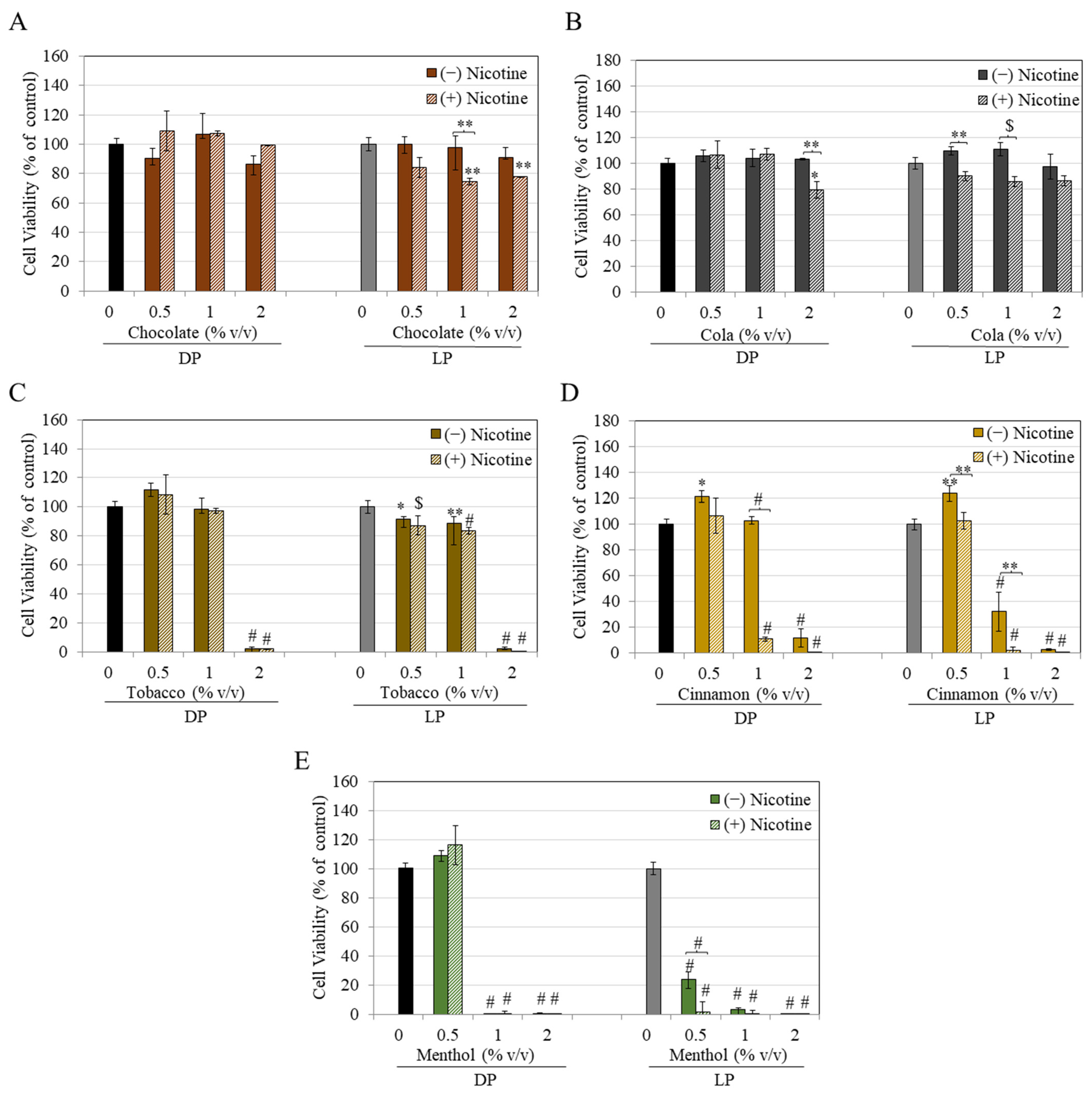
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rom, O.; Pecorelli, A.; Valacchi, G.; Reznick, A.Z. Are E-cigarettes a safe and good alternative to cigarette smoking? Ann. N. Y. Acad. Sci. 2015, 1340, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Breland, A.; Soule, E.; Lopez, A.; Ramôa, C.; El-Hellani, A.; Eissenberg, T. Electronic cigarettes: What are they and what do they do? Ann. N. Y. Acad. Sci. 2017, 1394, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Bertholon, J.; Becquemin, M.; Annesi-Maesano, I.; Dautzenberg, B. Electronic cigarettes: A short review. Respiration 2013, 86, 433–438. [Google Scholar] [CrossRef]
- Tierney, P.A.; Karpinski, C.D.; Brown, J.E.; Luo, W.; Pankow, J.F. Flavour chemicals in electronic cigarette fluids. Tob. Control 2016, 25, e10–e15. [Google Scholar] [CrossRef] [PubMed]
- Yoong, S.L.; Hall, A.; Leonard, A.; McCrabb, S.; Wiggers, J.; d’Espaignet, E.T.; Stockings, E.; Gouda, H.; Fayokun, R.; Commar, A. Prevalence of electronic nicotine delivery systems and electronic non-nicotine delivery systems in children and adolescents: A systematic review and meta-analysis. Lancet Public Health 2021, 6, e661–e673. [Google Scholar] [CrossRef]
- Birdsey, J. Tobacco Product Use among US Middle and High School Students—National Youth Tobacco Survey, 2023. MMWR. Morb. Mortal. Wkly. Rep. 2023, 72, 1173–1182. [Google Scholar] [CrossRef]
- Kong, G.; Morean, M.E.; Cavallo, D.A.; Camenga, D.R.; Krishnan-Sarin, S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob. Res. 2015, 17, 847–854. [Google Scholar] [CrossRef]
- Harrell, M.B.; Weaver, S.R.; Loukas, A.; Creamer, M.; Marti, C.; Jackson, C.D.; Heath, J.; Nayak, P.; Perry, C.; Pechacek, T. Flavored e-cigarette use: Characterizing youth, young adult, and adult users. Prev. Med. Rep. 2017, 5, 33–40. [Google Scholar] [CrossRef]
- Kechter, A.; Wong, M.; Mason, T.B.; Tackett, A.P.; Smith, C.E.; Leventhal, A.M.; Dunton, G.F.; Barrington-Trimis, J.L. E-cigarette weight and appetite control beliefs and e-cigarette initiation in young adults. Health Psychol. 2023, 42, 668. [Google Scholar] [CrossRef]
- Morean, M.E.; Bold, K.W.; Kong, G.; Camenga, D.R.; Simon, P.; Jackson, A.; Cavallo, D.A.; Krishnan-Sarin, S. High school students’ use of flavored e-cigarette e-liquids for appetite control and weight loss. Addict. Behav. 2020, 102, 106139. [Google Scholar] [CrossRef]
- Sapru, S.; Vardhan, M.; Li, Q.; Guo, Y.; Li, X.; Saxena, D. E-cigarettes use in the United States: Reasons for use, perceptions, and effects on health. BMC Public Health 2020, 20, 1518. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, M.; Petrescu, D.C.; Marteau, T.M. Impact of advertisements promoting candy-like flavoured e-cigarettes on appeal of tobacco smoking among children: An experimental study. Tob. Control 2016, 25, e107–e112. [Google Scholar] [CrossRef] [PubMed]
- Gorukanti, A.; Delucchi, K.; Ling, P.; Fisher-Travis, R.; Halpern-Felsher, B. Adolescents’ attitudes towards e-cigarette ingredients, safety, addictive properties, social norms, and regulation. Prev. Med. 2017, 94, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, B.K.; Rostron, B.L.; Johnson, S.E.; Portnoy, D.B.; Apelberg, B.J.; Kaufman, A.R.; Choiniere, C.J. Perceptions of the relative harm of cigarettes and e-cigarettes among US youth. Am. J. Prev. Med. 2014, 47, S53–S60. [Google Scholar] [CrossRef] [PubMed]
- Roditis, M.; Delucchi, K.; Cash, D.; Halpern-Felsher, B. Adolescents’ perceptions of health risks, social risks, and benefits differ across tobacco products. J. Adolesc. Health 2016, 58, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Amrock, S.M.; Lee, L.; Weitzman, M. Perceptions of e-cigarettes and noncigarette tobacco products among US youth. Pediatrics 2016, 138, e20154306. [Google Scholar] [CrossRef]
- Choi, K.; Forster, J.L. Beliefs and experimentation with electronic cigarettes: A prospective analysis among young adults. Am. J. Prev. Med. 2014, 46, 175–178. [Google Scholar] [CrossRef] [PubMed]
- FDA Finalizes Enforcement Policy on Unauthorized Flavored Cartridge-Based e-Cigarettes That Appeal to Children, Including Fruit and Mint. Available online: https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children (accessed on 5 December 2023).
- Gaiha, S.M.; Lempert, L.K.; McKelvey, K.; Halpern-Felsher, B. E-Cigarette devices, brands, and flavors attract youth: Informing FDA’s policies and priorities to close critical gaps. Addict. Behav. 2022, 126, 107179. [Google Scholar] [CrossRef] [PubMed]
- Williams, R. The rise of disposable JUUL-type e-cigarette devices. Tob. Control 2020, 29, e134–e135. [Google Scholar] [CrossRef]
- Amalia, B.; Fu, M.; Tigova, O.; Ballbè, M.; Paniello-Castillo, B.; Castellano, Y.; Vyzikidou, V.K.; O’donnell, R.; Dobson, R.; Lugo, A. Exposure to secondhand aerosol from electronic cigarettes at homes: A real-life study in four European countries. Sci. Total Environ. 2023, 854, 158668. [Google Scholar] [CrossRef]
- Ballbè, M.; Fu, M.; Masana, G.; Pérez-Ortuño, R.; Gual, A.; Gil, F.; Olmedo, P.; García-Algar, Ó.; Pascual, J.A.; Fernández, E. Passive exposure to electronic cigarette aerosol in pregnancy: A case study of a family. Environ. Res. 2023, 216, 114490. [Google Scholar] [CrossRef] [PubMed]
- Quintana, P.J.; Lopez-Galvez, N.; Dodder, N.G.; Hoh, E.; Matt, G.E.; Zakarian, J.M.; Vyas, M.; Chu, L.; Akins, B.; Padilla, S. Nicotine, cotinine, and tobacco-specific nitrosamines measured in children’s silicone wristbands in relation to secondhand smoke and E-cigarette vapor exposure. Nicotine Tob. Res. 2021, 23, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Lee, L. Electronic cigarettes are a source of thirdhand exposure to nicotine. Nicotine Tob. Res. 2015, 17, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, P.; Spiller, H.A.; Casavant, M.J.; Chounthirath, T.; Smith, G.A. E-cigarette and liquid nicotine exposures among young children. Pediatrics 2018, 141, e20173361. [Google Scholar] [CrossRef] [PubMed]
- Jackler, R.K.; Ramamurthi, D. Nicotine arms race: JUUL and the high-nicotine product market. Tob. Control 2019, 28, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Maina, G.; Castagnoli, C.; Ghione, G.; Passini, V.; Adami, G.; Filon, F.L.; Crosera, M. Skin contamination as pathway for nicotine intoxication in vapers. Toxicol. Vitr. 2017, 41, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Plonka, P.M.; Passeron, T.; Brenner, M.; Tobin, D.J.; Shibahara, S.; Thomas, A.; Slominski, A.; Kadekaro, A.L.; Hershkovitz, D.; Peters, E. What are melanocytes really doing all day long…? Exp. Dermatol. 2009, 18, 799–819. [Google Scholar] [CrossRef] [PubMed]
- ElObeid, A.S.; Kamal-Eldin, A.; Abdelhalim, M.A.K.; Haseeb, A.M. Pharmacological properties of melanin and its function in health. Basic Clin. Pharmacol. Toxicol. 2017, 120, 515–522. [Google Scholar] [CrossRef]
- Tolleson, W.H. Human melanocyte biology, toxicology, and pathology. J. Environ. Sci. Health Part C 2005, 23, 105–161. [Google Scholar] [CrossRef]
- Jin, W.; Stehbens, S.J.; Barnard, R.T.; Blaskovich, M.A.; Ziora, Z.M. Dysregulation of tyrosinase activity: A potential link between skin disorders and neurodegeneration. J. Pharm. Pharmacol. 2023, 76, 13–22. [Google Scholar] [CrossRef]
- Whittington, J.R.; Simmons, P.M.; Phillips, A.M.; Gammill, S.K.; Cen, R.; Magann, E.F.; Cardenas, V.M. The use of electronic cigarettes in pregnancy: A review of the literature. Obstet. Gynecol. Surv. 2018, 73, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Li, G.E.; Chen, H.; Cranfield, C.G.; McGrath, K.C.; Gorrie, C.A. Maternal e-cigarette exposure results in cognitive and epigenetic alterations in offspring in a mouse model. Chem. Res. Toxicol. 2018, 31, 601–611. [Google Scholar] [CrossRef] [PubMed]
- England, L.J.; Bunnell, R.E.; Pechacek, T.F.; Tong, V.T.; McAfee, T.A. Nicotine and the developing human: A neglected element in the electronic cigarette debate. Am. J. Prev. Med. 2015, 49, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, P.T.; Holloway, A.C.; Vijayan, M.M. Vape flavourants dull sensory perception and cause hyperactivity in developing zebrafish embryos. Biol. Lett. 2020, 16, 20200361. [Google Scholar] [CrossRef] [PubMed]
- Massarsky, A.; Abdel, A.; Glazer, L.; Levin, E.D.; Di Giulio, R.T. Neurobehavioral effects of 1, 2-propanediol in zebrafish (Danio rerio). Neurotoxicology 2018, 65, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Massarsky, A.; Abdel, A.; Glazer, L.; Levin, E.D.; Di Giulio, R.T. Exposure to 1, 2-propanediol impacts early development of zebrafish (Danio rerio) and induces hyperactivity. Zebrafish 2017, 14, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Delijewski, M.; Wrześniok, D.; Beberok, A.; Rok, J.; Otręba, M.; Buszman, E. The effect of simultaneous exposure of HEMn-DP and HEMn-LP melanocytes to nicotine and UV-radiation on the cell viability and melanogenesis. Environ. Res. 2016, 151, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Delijewski, M.; Wrześniok, D.; Otręba, M.; Beberok, A.; Buszman, E. Nicotine impact on melanogenesis and antioxidant defense system in HEMn-DP melanocytes. Mol. Cell. Biochem. 2014, 395, 109–116. [Google Scholar] [CrossRef]
- Delijewski, M.; Beberok, A.; Otręba, M.; Wrześniok, D.; Rok, J.; Buszman, E. Effect of nicotine on melanogenesis and antioxidant status in HEMn-LP melanocytes. Environ. Res. 2014, 134, 309–314. [Google Scholar] [CrossRef]
- Goenka, S. Biological impact of the ratio of e-cigarette liquid base constituents, propylene glycol and vegetable glycerin, on primary human melanocytes. Oral 2023, 3, 40–56. [Google Scholar] [CrossRef]
- Cambron, C. Racial/Ethnic Differences in Vaping Product Use among Youth: A State-Level Analysis. Int. J. Environ. Res. Public Health 2023, 20, 5729. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Ramos, A.K.; Faseru, B.; Hill, J.L.; Sussman, S.Y. Racial disparities of e-cigarette use among US youths: 2014–2019. Am. J. Public Health 2021, 111, 2050–2058. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Bluestein, M.; Chen, B.; Perry, C.L.; Harrell, M.B. Prospectively estimating the age of initiation of e-cigarettes among US youth: Findings from the population assessment of tobacco and health (PATH) study, 2013–2017. J. Biom. Biostat. 2020, 11. [Google Scholar]
- Harlow, A.F.; Stokes, A.; Brooks, D.R. Socioeconomic and racial/ethnic differences in e-cigarette uptake among cigarette smokers: Longitudinal analysis of the population assessment of tobacco and health (PATH) study. Nicotine Tob. Res. 2019, 21, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Lippert, A.M. Do adolescent smokers use e-cigarettes to help them quit? The sociodemographic correlates and cessation motivations of US adolescent e-cigarette use. Am. J. Health Promot. 2015, 29, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Dutra, L.M.; Glantz, S.A. Electronic cigarettes and conventional cigarette use among US adolescents: A cross-sectional study. JAMA Pediatr. 2014, 168, 610–617. [Google Scholar] [CrossRef]
- Baumann, A.W.; Kohler, C.; Kim, Y.-i.; Cheong, J.; Hendricks, P.; Bailey, W.C.; Harrington, K.F. Differences in electronic cigarette awareness, use history, and advertisement exposure between black and white hospitalized cigarette smokers. J. Cancer Educ. 2015, 30, 648–654. [Google Scholar] [CrossRef]
- Muscat, J.; Richie, J.; Stellman, S. Mentholated cigarettes and smoking habits in whites and blacks. Tob. Control 2002, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Marumoto, S.; Takahashi, T.; Nakahashi, H.; Haigou, R.; Nakanishi, K. Metabolism of (+)-and (−)-menthols by CYP2A6 in human liver microsomes. J. Oleo Sci. 2011, 60, 127–132. [Google Scholar] [CrossRef]
- Winters, B.R.; Kochar, T.K.; Clapp, P.W.; Jaspers, I.; Madden, M.C. Impact of e-cigarette liquid flavoring agents on activity of microsomal recombinant CYP2A6, the primary nicotine-metabolizing enzyme. Chem. Res. Toxicol. 2020, 33, 1689–1697. [Google Scholar] [CrossRef]
- King, G.; Yerger, V.B.; Whembolua, G.-L.; Bendel, R.B.; Kittles, R.; Moolchan, E.T. Link between facultative melanin and tobacco use among African Americans. Pharmacol. Biochem. Behav. 2009, 92, 589–596. [Google Scholar] [CrossRef]
- Yerger, V.B.; Malone, R.E. Melanin and nicotine: A review of the literature. Nicotine Tob. Res. 2006, 8, 487–498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larsson, B.S. Interaction between chemicals and melanin. Pigment Cell Res. 1993, 6, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Effects of e-cigarette refill liquid flavorings with and without nicotine on human retinal pigment epithelial cells: A preliminary study. Int. J. Environ. Res. Public Health 2021, 18, 11655. [Google Scholar] [CrossRef]
- Human Epidermal Melanocytes, Neonatal, Lightly Pigmented Donor, (HEMn-LP). Available online: https://www.thermofisher.com/order/catalog/product/C0025C?SID=srch-srp-C0025C (accessed on 23 October 2023).
- Human Epidermal Melanocytes, Neonatal, Darkly Pigmented Donor, (HEMn-DP). Available online: https://www.thermofisher.com/order/catalog/product/C2025C (accessed on 19 December 2023).
- Muthumalage, T.; Prinz, M.; Ansah, K.O.; Gerloff, J.; Sundar, I.K.; Rahman, I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front. Physiol. 2017, 8, 1130. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, D.; Minor, M.; Chu, H.W. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS ONE 2014, 9, e108342. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S. Effects of serotype and species dependency of bacterial lipopolysaccharides in human melanocytes from lightly and darkly-pigmented skin. BBA Adv. 2022, 2, 100042. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, K.D.; Lu, W.; Ferdowsi, P.V.; Myers, S.; Markos, J.; Larby, J.; Chia, C.; Weber, H.C.; Haug, G.; Eapen, M.S. Electronic cigarette aerosol is cytotoxic and increases ACE2 expression on human airway epithelial cells: Implications for SARS-CoV-2 (COVID-19). J. Clin. Med. 2021, 10, 1028. [Google Scholar] [CrossRef]
- Golli, N.E.; Dallagi, Y.; Rahali, D.; Rejeb, I.; Fazaa, S.E. Neurobehavioral assessment following e-cigarette refill liquid exposure in adult rats. Toxicol. Mech. Methods 2016, 26, 425–432. [Google Scholar] [CrossRef]
- Thomas, A.J.; Erickson, C.A. The making of a melanocyte: The specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008, 21, 598–610. [Google Scholar] [CrossRef]
- Sommer, L. Generation of melanocytes from neural crest cells. Pigment Cell Melanoma Res. 2011, 24, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.E.; Kandalam, S.; Olivares-Navarrete, R.; Dickinson, A.J. E-cigarette aerosol exposure can cause craniofacial defects in Xenopus laevis embryos and mammalian neural crest cells. PLoS ONE 2017, 12, e0185729. [Google Scholar] [CrossRef] [PubMed]
- Ween, M.P.; Moshensky, A.; Thredgold, L.; Bastian, N.A.; Hamon, R.; Badiei, A.; Nguyen, P.T.; Herewane, K.; Jersmann, H.; Bojanowski, C.M. E-cigarettes and health risks: More to the flavor than just the name. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 320, L600–L614. [Google Scholar] [CrossRef]
- Sherwood, C.L.; Boitano, S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir. Res. 2016, 17, 57. [Google Scholar] [CrossRef]
- Kechter, A.; Ceasar, R.C.; Simpson, K.A.; Schiff, S.J.; Dunton, G.F.; Bluthenthal, R.N.; Barrington-Trimis, J.L. A chocolate cake or a chocolate vape? Young adults describe their relationship with food and weight in the context of nicotine vaping. Appetite 2022, 175, 106075. [Google Scholar] [CrossRef] [PubMed]
- Litt, M.D.; Duffy, V.; Oncken, C. Cigarette smoking and electronic cigarette vaping patterns as a function of e-cigarette flavourings. Tob. Control 2016, 25, ii67–ii72. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Narain, V.; Bondesson, M. E-cigarette vaping liquids and the flavoring chemical cinnamaldehyde perturb bone, cartilage and vascular development in zebrafish embryos. Aquat. Toxicol. 2021, 240, 105995. [Google Scholar] [CrossRef]
- Behar, R.; Davis, B.; Wang, Y.; Bahl, V.; Lin, S.; Talbot, P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol. Vitr. 2014, 28, 198–208. [Google Scholar] [CrossRef]
- Clapp, P.W.; Pawlak, E.A.; Lackey, J.T.; Keating, J.E.; Reeber, S.L.; Glish, G.L.; Jaspers, I. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L278–L292. [Google Scholar] [CrossRef]
- Cabello, C.M.; Bair III, W.B.; Lamore, S.D.; Ley, S.; Bause, A.S.; Azimian, S.; Wondrak, G.T. The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic. Biol. Med. 2009, 46, 220–231. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, J.; Cheng, C.; Zhang, M.; Liu, W.; Ma, X.; Lei, W.; Hao, E.; Hou, X.; Hou, Y. Cinnamaldehyde enhances antimelanoma activity through covalently binding ENO1 and exhibits a promoting effect with dacarbazine. Cancers 2020, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Behar, R.Z.; Luo, W.; Lin, S.C.; Wang, Y.; Valle, J.; Pankow, J.F.; Talbot, P. Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob. Control 2016, 25, ii94–ii102. [Google Scholar] [CrossRef] [PubMed]
- Behar, R.Z.; Wang, Y.; Talbot, P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob. Control 2018, 27, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Omaiye, E.E.; McWhirter, K.J.; Luo, W.; Tierney, P.A.; Pankow, J.F.; Talbot, P. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci. Rep. 2019, 9, 2468. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lim, K.-M. Melanocytotoxic chemicals and their toxic mechanisms. Toxicol. Res. 2022, 38, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, B.; Wilkinson, D. Cinnamic aldehyde in toothpaste. 1. Clinical aspects and patch tests. Contact Dermat. 1975, 1, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Isaac-Renton, M.; Li, M.K.; Parsons, L.M. Cinnamon spice and everything not nice: Many features of intraoral allergy to cinnamic aldehyde. Dermatitis 2015, 26, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Mathias, C.T.; Maibach, H.I.; Conant, M.A. Perioral leukoderma simulating vitiligo from use of a toothpaste containing cinnamic aldehyde. Arch. Dermatol. 1980, 116, 1172–1173. [Google Scholar] [CrossRef]
- Vahav, I.; Thon, M.; van den Broek, L.J.; Spiekstra, S.W.; Atac, B.; Lindner, G.; Schimek, K.; Marx, U.; Gibbs, S. Proof-of-concept organ-on-chip study: Topical cinnamaldehyde exposure of reconstructed human skin with integrated neopapillae cultured under dynamic flow. Pharmaceutics 2022, 14, 1529. [Google Scholar] [CrossRef]
- Chang, K.; Zeng, N.; Ding, Y.; Zhao, X.; Gao, C.; Li, Y.; Wang, H.; Liu, X.; Niu, Y.; Sun, Y. Cinnamaldehyde causes developmental neurotoxicity in zebrafish via the oxidative stress pathway that is rescued by astaxanthin. Food Funct. 2022, 13, 13028–13039. [Google Scholar] [CrossRef]
- Holden, L.L.; Truong, L.; Simonich, M.T.; Tanguay, R.L. Assessing the hazard of E-Cigarette flavor mixtures using zebrafish. Food Chem. Toxicol. 2020, 136, 110945. [Google Scholar] [CrossRef] [PubMed]
- Berkelhamer, S.K.; Helman, J.M.; Gugino, S.F.; Leigh, N.J.; Lakshminrusimha, S.; Goniewicz, M.L. In vitro consequences of electronic-cigarette flavoring exposure on the immature lung. Int. J. Environ. Res. Public Health 2019, 16, 3635. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.; Kelty, J.S.; Laskin, J.D.; Laskin, D.L.; Gow, A.J. Menthol flavoring in e-cigarette condensate causes pulmonary dysfunction and cytotoxicity in precision cut lung slices. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2023, 324, L345–L357. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.C.; Donovan, E.M.; Schillo, B.A.; Vallone, D. Menthol e-cigarette sales rise following 2020 FDA guidance. Tob. Control 2021, 30, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Diomede, L. The soothing effect of menthol, eucalyptol and high-intensity cooling agents. Int. J. Nutraceuticals Funct. Foods Nov. Foods 2017, 16, 153–157. [Google Scholar]
- Davis, D.R.; Morean, M.E.; Bold, K.W.; Camenga, D.; Kong, G.; Jackson, A.; Simon, P.; Krishnan-Sarin, S. Cooling e-cigarette flavors and the association with e-cigarette use among a sample of high school students. PLoS ONE 2021, 16, e0256844. [Google Scholar] [CrossRef] [PubMed]
- Paschke, M.; Tkachenko, A.; Ackermann, K.; Hutzler, C.; Henkler, F.; Luch, A. Activation of the cold-receptor TRPM8 by low levels of menthol in tobacco products. Toxicol. Lett. 2017, 271, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Chen, H.; Zhang, X.; Liu, T.; Fu, Y.n. Levels of selected groups of compounds in refill solutions for electronic cigarettes. Nicotine Tob. Res. 2016, 18, 708–714. [Google Scholar] [CrossRef]
- Ramamurthi, D.; Chau, C.; Berke, H.Y.; Tolba, A.M.; Yuan, L.; Kanchan, V.; Santos, G.; Jackler, R.K. Flavour spectrum of the Puff family of disposable e-cigarettes. Tob. Control 2022, 32, e71–e77. [Google Scholar] [CrossRef] [PubMed]
- Churchill, V.; Fairman, R.T.; Brown, D.; Massey, Z.B.; Ashley, D.L.; Popova, L. “I get the flavors and it makes me love vaping more:” How and why youth users modify electronic nicotine delivery systems. Nicotine Tob. Res. 2023, 25, 1791–1797. [Google Scholar] [CrossRef]
- Bengalli, R.; Ferri, E.; Labra, M.; Mantecca, P. Lung toxicity of condensed aerosol from E-CIG liquids: Influence of the flavor and the in vitro model used. Int. J. Environ. Res. Public Health 2017, 14, 1254. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthi, D.; Chau, C.; Jackler, R.K. JUUL and other stealth vaporisers: Hiding the habit from parents and teachers. Tob. Control 2019, 28, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Kerber, P.J.; Duell, A.K.; Powers, M.; Strongin, R.M.; Peyton, D.H. Effects of common e-liquid flavorants and added nicotine on toxicant formation during vaping analyzed by 1H NMR spectroscopy. Chem. Res. Toxicol. 2022, 35, 1267–1276. [Google Scholar] [CrossRef]
- Barltrop, J.A.; Owen, T.C.; Cory, A.H.; Cory, J.G. 5-(3-carboxymethoxyphenyl)-2-(4, 5-dimethylthiazolyl)-3-(4-sulfophenyl) tetrazolium, inner salt (MTS) and related analogs of 3-(4, 5-dimethylthiazolyl)-2, 5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators. Bioorg. Med. Chem. Lett. 1991, 1, 611–614. [Google Scholar]
- Malich, G.; Markovic, B.; Winder, C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef]
- Beard, J.M.; Collom, C.; Liu, J.Y.; Obiako, P.; Strongin, R.M.; Zavala, J.; Sayes, C.M. In vitro toxicity and chemical analysis of e-cigarette aerosol produced amid dry hitting. Toxicology 2024, 506, 153865. [Google Scholar] [CrossRef] [PubMed]
- Baldovinos, Y.; Archer, A.; Salamanca, J.; Strongin, R.M.; Sayes, C.M. Chemical interactions and cytotoxicity of terpene and diluent vaping ingredients. Chem. Res. Toxicol. 2022, 36, 589–597. [Google Scholar] [CrossRef]
- Rickard, B.P.; Ho, H.; Tiley, J.B.; Jaspers, I.; Brouwer, K.L. E-cigarette flavoring chemicals induce cytotoxicity in HepG2 cells. ACS Omega 2021, 6, 6708–6713. [Google Scholar] [CrossRef]
- Chan, F.K.-M.; Moriwaki, K.; De Rosa, M.J. Detection of necrosis by release of lactate dehydrogenase activity. In Immune Homeostasis: Methods and Protocols; Springer: New York, NY, USA, 2013; pp. 65–70. [Google Scholar]
- Puig-Herreros, C.; Sanz, J.L.; García-Bernal, D.; Rodríguez-Lozano, F.J.; Murcia, L.; Forner, L.; Ghilotti, J.; Oñate-Sánchez, R.E.; López-García, S. Comparative Cytotoxicity of Menthol and Eucalyptol: An In Vitro Study on Human Gingival Fibroblasts. Pharmaceutics 2024, 16, 521. [Google Scholar] [CrossRef]
- Alshareef, M.; Alrafiah, A.; Abed, S.; Basingab, F.; Alrofaidi, A. Effect of e-cigarette flavoring agents on the neural retina of chick embryo: Histological and gene expression study. Folia Histochem. Cytobiol. 2021, 59, 245–258. [Google Scholar] [CrossRef]
- Abouassali, O.; Chang, M.; Chidipi, B.; Martinez, J.L.; Reiser, M.; Kanithi, M.; Soni, R.; McDonald, T.V.; Herweg, B.; Saiz, J. In vitro and in vivo cardiac toxicity of flavored electronic nicotine delivery systems. Am. J. Physiol.-Heart Circ. Physiol. 2021, 320, H133–H143. [Google Scholar] [CrossRef] [PubMed]
- Girvalaki, C.; Tzatzarakis, M.; Kyriakos, C.N.; Vardavas, A.I.; Stivaktakis, P.D.; Kavvalakis, M.; Tsatsakis, A.; Vardavas, C. Composition and chemical health hazards of the most common electronic cigarette liquids in nine European countries. Inhal. Toxicol. 2018, 30, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Krüsemann, E.J.; Pennings, J.L.; Cremers, J.W.; Bakker, F.; Boesveldt, S.; Talhout, R. GC–MS analysis of e-cigarette refill solutions: A comparison of flavoring composition between flavor categories. J. Pharm. Biomed. Anal. 2020, 188, 113364. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Go, Y.Y.; Mun, J.Y.; Lee, S.; Im, G.J.; Kim, Y.Y.; Lee, J.H.; Chang, J. Effect of electronic cigarettes on human middle ear. Int. J. Pediatr. Otorhinolaryngol. 2018, 109, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Dunn, K.; Turfus, S. A review of nicotine-containing electronic cigarettes—Trends in use, effects, contents, labelling accuracy and detection methods. Drug Test. Anal. 2021, 13, 242–260. [Google Scholar] [CrossRef] [PubMed]
- Gschwend, G.; Jenkins, C.; Jones, A.; Kelso, C.; Morgan, J. A wide range of flavoring–carrier fluid adducts form in e-cigarette liquids. Chem. Res. Toxicol. 2023, 36, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Suarez, I.; Marescotti, D.; Martin, F.; Scotti, E.; Guedj, E.; Acali, S.; Dulize, R.; Baumer, K.; Peric, D.; Frentzel, S. In vitro systems toxicology assessment of nonflavored e-cigarette liquids in primary lung epithelial cells. Appl. Vitr. Toxicol. 2017, 3, 41–55. [Google Scholar] [CrossRef]
- De Martin, S.; Gabbia, D.; Bogialli, S.; Biasioli, F.; Boschetti, A.; Gstir, R.; Rainer, D.; Cappellin, L. Refill liquids for electronic cigarettes display peculiar toxicity on human endothelial cells. Toxicol. Rep. 2021, 8, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Rowell, T.R.; Reeber, S.L.; Lee, S.L.; Harris, R.A.; Nethery, R.C.; Herring, A.H.; Glish, G.L.; Tarran, R. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L52–L66. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Boykan, R.; Messina, C.R.; Eliscu, A.; Tolentino, J. High exposure to nicotine among adolescents who use Juul and other vape pod systems (‘pods’). Tob. Control 2019, 28, 676–677. [Google Scholar] [CrossRef]
- Talih, S.; Salman, R.; El-Hage, R.; Karam, E.; Karaoghlanian, N.; El-Hellani, A.; Saliba, N.; Shihadeh, A. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob. Control 2019, 28, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Mr. Fog E-Liquids. Available online: https://www.mrfog.com/e-liquid/?f=nav&site=com (accessed on 12 December 2023).
- Marland Vape Store: Mr. Fog Switch 5500 E-Liquids (20 mg/mL). Available online: https://marlandvape.com/?product_cat=&s=Fog&post_type=product (accessed on 12 December 2023).

| Cells | Nicotine | Tobacco | Cinnamon | Menthol | Vanilla | Banana | Others |
|---|---|---|---|---|---|---|---|
| DP | (−) | 1.48 ± 0.28 | 1.77 ± 0.14 | 0.89 ± 0.03 | 1.97 ± 0.22 | 1.17 ± 0.02 | |
| (+) | 1.57 ± 0.24 | 0.86 ± 0.11 a | 0.87 ± 0.03 | 1.26 ± 0.03 b,d | 1.09 ± 0.09 | ND | |
| LP | (−) | 1.27 ± 0.03 | 0.98 ± 0.02 p | 0.34 ± 0.04 q | 1.94 ± 0.08 | 1.18 ± 0.03 | |
| (+) | 1.19 ± 0.02 | 0.82 ± 0.10 | 0.04 ± 0.06 x,c | 1.58 ± 0.04 y | 1.38 ± 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenka, S. Differential Cytotoxicity of Flavored E-Liquids with and without Nicotine on Neonatal Human Melanocytes from Lightly and Darkly Pigmented Donors: A Preliminary Report. BioMed 2024, 4, 237-255. https://doi.org/10.3390/biomed4030019
Goenka S. Differential Cytotoxicity of Flavored E-Liquids with and without Nicotine on Neonatal Human Melanocytes from Lightly and Darkly Pigmented Donors: A Preliminary Report. BioMed. 2024; 4(3):237-255. https://doi.org/10.3390/biomed4030019
Chicago/Turabian StyleGoenka, Shilpi. 2024. "Differential Cytotoxicity of Flavored E-Liquids with and without Nicotine on Neonatal Human Melanocytes from Lightly and Darkly Pigmented Donors: A Preliminary Report" BioMed 4, no. 3: 237-255. https://doi.org/10.3390/biomed4030019
APA StyleGoenka, S. (2024). Differential Cytotoxicity of Flavored E-Liquids with and without Nicotine on Neonatal Human Melanocytes from Lightly and Darkly Pigmented Donors: A Preliminary Report. BioMed, 4(3), 237-255. https://doi.org/10.3390/biomed4030019







