Abstract
Rotator cuff repair is a common orthopaedic procedure. Despite advancements in the mechanics of rotator cuff repair, the re-tear rate post repair remains significant. This review assesses the available literature on usage of collagen bio-inductive scaffolds for rotator cuff repairs. Augmentation of biology is a key strategy to improving success of rotator cuff repair. Current evidence suggests that augmentation of rotator cuff repairs with a collagen bio-inductive scaffold improves the thickness of the rotator cuff. There is a favourable safety profile, and its usage may improve re-tear rates. However, there is currently no consensus on whether clinical outcomes are improved by the usage of collagen bio-inductive scaffolds. Further research is necessary to increase our understanding of the clinical effects of using collagen bio-inductive scaffolds and to determine which patient profiles will best benefit from its usage.
1. Introduction
Rotator cuff tears (RCTs) are a common orthopaedic condition with a reported prevalence of 20% to 30% in the general population [1,2,3,4]. The prevalence of rotator cuff tears increases with age [5]. There is considerable impact of RCTs on individuals and healthcare systems. Affected individuals experience a reduction in the quality of life due to pain and loss of function of the shoulder [6], with an economic cost from lost income due to limited working ability and missed workdays [7]. As such, it is important to minimise the impact of this disease with early and appropriate treatment initiation.
The rotator cuff tendon has a limited capacity for healing, undergoing a repair instead of a regenerative process. This complex process can be histologically divided into three overlapping stages—inflammation, repair and remodelling [8]. The first inflammatory phase occurs directly after injury, during which the various cytokines released attract inflammatory cells. These in turn release factors that induce the inflammatory cascade, including nuclear factor kappa B (NF-kB) and transforming growth factor-beta (TGF-β), which play a key role in promoting fibrogenesis over tenogenesis [9]. During the proliferative stage, tenocytes dominate and deposit a temporary matrix of type III collagen. Over the subsequent course of remodelling, type III collagen fibres are gradually converted to type I fibres, but there is an overall greater proportion of type III collagen fibres in scar tissue [10]. The healing process is also further compounded by several factors including diabetes, smoking status [11], poor healing at the tendon-bone interface [12] and increasing age [13]. This overall results in a structure that remains mechanically inferior.
Degenerative tears can be defined as full-thickness or partial-thickness tears [14]. Partial tears of the tendon tissue increase the strain on the remnant fibres [15], which is further compounded by the poor mechanical strength of scar tissue [16]. Subsequently, the natural progression of the disease often results in tear propagation, rotator cuff arthropathy and increasing disability. Whilst patients with rotator cuff tears can often be treated non-surgically with analgesia and physiotherapy, persistently symptomatic patients may require surgical intervention.
There has been a large focus on the biomechanical aspect of rotator cuff repair, with emphasis on restoration of native rotator cuff footprint, tension-free repairs and healing of the tendon–bone interface [13]. Despite advancements in rotator cuff repair techniques, post-operative re-tear remains one of the main complications and has been reported to affect 10% to 48% of patients [16,17,18]. Given that a significant portion of rotator cuff tears are degenerative in nature, recent interest in biological adjuncts has grown to improve the biology for healing.
Various biological adjuncts are currently available. These include platelet-rich plasma [19], bone marrow stimulation [20,21] and scaffold-based implants [13]. In particular, collagen-based bio-inductive implants synthesised from reconstituted type I bovine collagen have gained increasing attention over the past decade, with various studies highlighting its potential to augment conventional rotator cuff repair. This review aims to describe the current role of collagen-based implants in rotator cuff repair, with a focus on the histological and biomechanical properties, potential adverse effects, clinical outcomes and economic impact.
2. Methods
A literature search of the electronic databases PubMed, Embase and SCOPUS was performed with no data limit until June 2024. The search strategy used the following keywords “bioinductive patch AND rotator cuff”, “collagen patch AND rotator cuff”, “bovine patch AND rotator cuff”, “Regeneten AND rotator cuff”. In addition, a manual and a reference list search were performed.
2.1. Eligibility Criteria
The articles included in this systematic review had to meet the following inclusion criteria: (1) English language full-text articles; (2) level I to IV studies of patients undergoing surgical repair of rotator cuff tear with a collagen-based implant; (3) evaluated stiffness, re-tear rates and objective outcome measures including the American Shoulder and Elbow Surgeon score (ASES), Constant-Murley Score (CMS), Visual Analog Scale for pain (VAS), Single Assessment Numeric Evaluation (SANE), Veterans RAND 12-Item (VR-12) physical component and Western Ontario Rotator Cuff score (WORC), adverse effects or complications, and economic impact. Studies were excluded if they (1) involved non-collagen-based implants; (2) were technical notes or manufacturing articles.
2.2. Identification and Selection of Studies
A total of 272 articles were retrieved from the three databases. Duplicate articles were excluded before an initial screening of the abstract and title. Eligible articles were identified independently by two reviewers (WHDW, ZWNMT) by screening title and abstracts, following which the remaining articles were subject to full text screening to determine eligibility as per inclusion criteria.
The final number of articles included in the review was 41 (Figure 1). The shortlisted articles were separated based on study type and design (animal, human and others). The total number of animal studies was 2, the total number of human studies was 16 and the total number of other articles (economic evaluations, reviews, case reports etc.) was 23. The collagen-based implants included in this review are listed in Table 1.
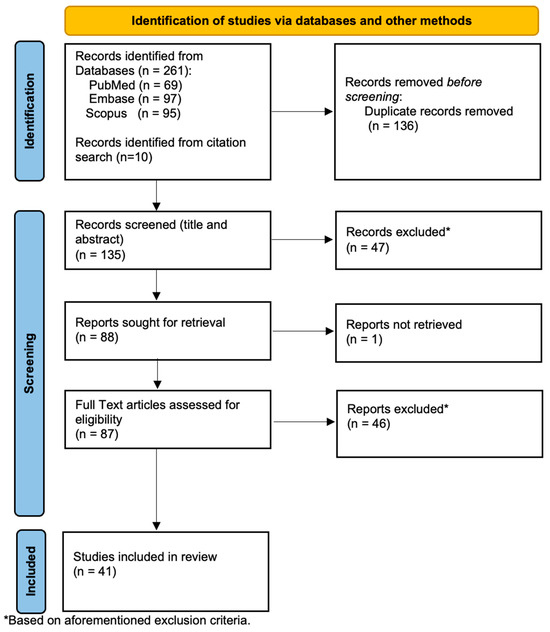
Figure 1.
Search flow diagram of the study selection process.

Table 1.
Types of commercial collagen-based implants.
3. Discussion
3.1. Properties
Collagen-based implants are designed to promote new host tissue regeneration at the site of the tear or repair. While the precise mechanism of tenogenesis remains unclear, various studies have shown the implant’s ability to induce mature tendon-like tissue. Kampen et al. conducted an animal study involving the augmentation of an infraspinatus tear repair in sheep [22]. The study was carried out using scaffolds produced with proprietary methods made from highly purified type I bovine tendons (Collagen Matrix, Inc., Oakland, NJ, USA). This study demonstrated the collagen implant’s ability to support formation and induce maturation of host tissue. Histologically, the new tissue composed of fibroblasts and regularly oriented collagen fibres that resembled regular tendon connective tissue. They also reported integration of the new tissue with the underlying tendon, increasing its overall thickness by 80% at 12 weeks. However, there were concerns that results from animal studies may not be replicable in human subjects, as unlike human tendons that tend to be poorly vascularized, animal tendons have a propensity for improved healing with neovascularization and fibrous tissue growth [23].
In recent years, a few studies have focused on biopsy specimens from human subjects. Arnoczky et al. assessed tendon biopsy samples from seven patients who underwent rotator cuff repair with bovine collagen implant (Rotation Medical Inc., Plymouth, MN, USA) augmentation [24]. At 5 and 8 weeks post-surgery, host fibroblasts were found to have invaded the interstices of the porous implant, with evidence of early collagen formation. By 3 months post-surgery, there was increased collagen formation, maturation and organisation over the implant. At 6 months post-surgery, the collagen implant had degraded and was replaced by full host tissue. A similar conclusion was also drawn by another study conducted by Camacho-Chacon et al. involving tendon biopsy samples from 29 patients who had undergone Regeneten (Smith & Nephew, Hertfordshire, UK) augmented cuff repairs [25], confirming the evolution of the healing process.
These histological results are supported by radiological evidence of tenogenesis and increase in tendon thickness. Both Camacho-Chacon et al.’s and Bokor et al.’s studies assessed tendon thickness with serial Magnetic Resonance Imaging (MRI) scans following arthroscopic repair and augmentation Regeneten [25,26]. They showed significant increase in new tissue induction over the repaired tendon that remained stable at 12 months. The new tissue signals were also found to be indistinguishable from the native tendon at 6 and 12 months, corresponding to the histological process of maturation and remodelling [26].
The increase in tendon thickness has been postulated to contribute to cuff healing by reducing local tendon strain [26,27] (Figure 2). After the initial injury, there is a significant increase in shear forces within the tendon, and this is thought to contribute to disease progression and impaired healing [28,29]. The collagen-based implant provides little to no structural support or tensile strength, but serves as a scaffold for fibroblast invasion and formation of collagen fibres, thus conferring the subsequent mechanical strength to the repair. A finite element model previously predicted that the induction of 2 mm of new host tissue on the PTRCT would significantly decrease the intratendinous strain of the bursal and articular tears by 47% and 40%, respectively [30]. In this manner, the implant can potentially represent a novel approach to improve healing and long-term durability of the repaired rotator cuff tendon.
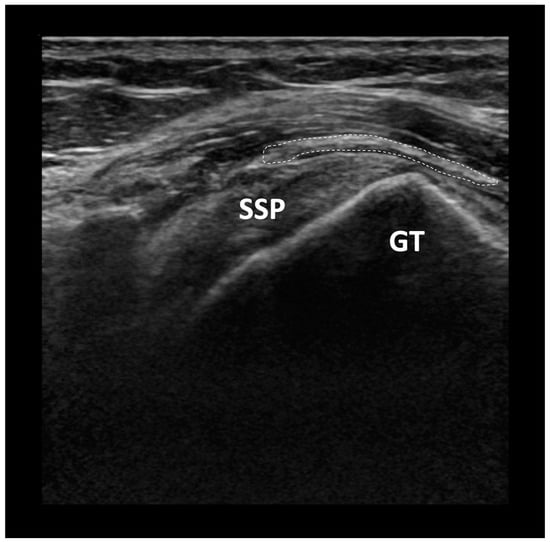
Figure 2.
Ultrasound image of a collagen bio-inductive scaffold augmentation. SSP: Supraspinatus tendon, GT: Greater tuberosity of the humerus, Dashed line: outline of collagen bio-inductive scaffold.
3.2. Safety and Complications
The current literature on collagen-based implants has generally demonstrated a favourable safety profile. In previous histological [24,25] and radiological [31] evaluations, there was no evidence of inflammation or foreign body reaction. However, there have been a few reported cases of inflammation with use of various collagen-based implants [32,33], although the cause remains unclear. Root et al. reported a case of subacromial-subdeltoid bursitis 1 year following rotator cuff repair augmented with Regeneten implant [33]. The patient reported persistent pain and limited range of movement. A post-operative MRI showed significant swelling with debris in the subacromial bursa, and cystic-like structures eroding through the deltoid fascia into the inferior acromion. On arthroscopic examination, a large amount of rice bodies was found in the subacromial and bursal space. Rice bodies are small fibrinous nodules formed in response to synovial inflammation and have been reported to form in response to orthopaedic implants in the absence of underlying inflammatory joint conditions or infections [33,34]. The authors were unable to conclude the exact cause of the development of rice bodies and bursitis, but raised concern in view of the destruction of local tissue from the inflammation.
Barad et al. presented a parallel case of severe subacromial inflammation after the same procedure [32]. The patient had presented with acute massive swelling four months following the procedure, and rice bodies were also identified during the arthroscopic washout and debridement surgery. The swelling resolved four weeks after onset, and at three months the pain had nearly completely disappeared. The cause of inflammation was proposed to be secondary to a foreign body reaction to the medial staples, one of the three main components of the Regeneten implant (bovine collagen implant, medial and lateral staples) (Figure 3). The medial staples are made of polylactic acid and are dissolvable, and this process of degradation possibly caused a foreign body response that in turn evoked an inflammatory reaction [34]. However, Liu and Amin highlighted that it would have taken 12 months for resorption of these staples, which did not appear to coincide with the time frame of rice body formation at four months [35]. As such, there is still a lack of evidence to support direct causation. Overall, cases of inflammation reported in the present literature are few, and most of these studies have been single case reports or involving small sample sizes of fewer than 100 patients. Further research is required to ascertain the cause, true prevalence and predictive factors for occurrence and prevention.
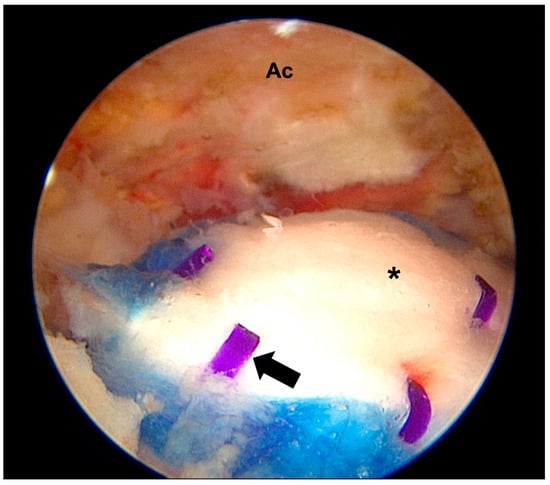
Figure 3.
Arthroscopic images of a collagen bio-inductive implant augmentation. Ac: Acromion, Black arrow: medial soft tissue anchors, *: collagen bio-inductive implant.
3.3. Stiffness
Stiffness of the shoulder joint is not uncommon in chronic cuff tears as well as post-rotator cuff repair. It often results from prolonged joint disuse and secondary muscle weakness. Post-operative rehabilitation programmes often balance the need for immobilisation to improve healing and the need for ranging of the shoulder to prevent post-operative stiffness. It is thus important to identify surgical factors that can minimise this peri-operative complication.
In their prospective multicentre study, Bushnell et al. reported that only 5 out of 241 patients who underwent either isolated bio-inductive repair or as a supplement to take-down and repair, required revision for shoulder stiffness or adhesive capsulitis [36]. Ting et al. performed a matched-cohort study on patients who underwent revision arthroscopic rotator cuff repair with and without Regeneten [37]. The primary outcome measures were that of patient-rated and surgeon-measured stiffness. The patients in the bio-inductive implant group reported less shoulder stiffness compared to the control group at 6 weeks and 3 months post-operatively. However, in terms of objective range of movement, the control group had greater passive external rotation at 6 weeks and forward flexion at 6 weeks and 3 months, with no other significant differences between groups at all other time points up to 6 months. In another matched cohort comparison of 64 patients with high-grade partial-thickness supraspinatus tears, Yeazell et al. also reported stiffness in 8 out of 32 patients in the bioinductive collagen patch group [38], which they defined as “loss of motion in ≥2 planes with cutoff values of forward elevation to 120°, external rotation to 30°, or internal rotation to the buttock”. This contrasted with 1 out of 32 patients in the control group. Six patients in the patch group underwent reoperations compared with no patients in the control group, of which all six were performed to address stiffness. They suggested that the aetiology of stiffness was likely multifactorial, including immunogenic response to the patch or foreign body, as well as a slower rehabilitation protocol that led to prolonged immobilisation after the repair.
While the evidence appears to suggest increased stiffness rates with the use of the bioinductive patch, a commentary by Bushnell et al. [39] raised several pertinent points on the Yeazell et al. [38] study. Bushnell pointed out several discrepancies in the reported demographics, including important risk factors for stiffness post-repair such as diabetes and smoking. If diabetes and smoking were not matched, this could potentially affect the interpretation of the results.
Another concern highlighted was the heterogeneity of surgical treatment between and within the groups in the study. The patients had either undergone debridement or repair of the supraspinatus tendon, as well as concomitant procedures such as subacromial debridement, distal clavicle excision and biceps tenotomy or tenodesis. The authors had justified the inclusion of this heterogenous population based on prior studies that showed comparable clinical results in debridement versus repair techniques [40,41]; however, the interplay between combination procedures is not well evaluated. Overall, these factors are important limitations of the study by Yeazell et al. [38] Future studies are needed to confirm the relationship between bio-inductive scaffolds and post-operative stiffness.
3.4. Re-Tear Rates
Rotator cuff re-tear is one of the main post-operative complications of conventional arthroscopic repairs and have been reported to range from 5% to 40% [16,42,43]. Prior studies on patch augmentation with synthetic and biological scaffolds have shown lower re-tear rates [44], despite no improvement in time zero mechanical stability. Given that collagen patches have the additional advantage of biological induction, there is great interest in whether collagen patch augmentation techniques can also reduce re-tear rates.
Iban et al. compared re-tear rates with MRI evaluation between bio-inductive patch augmented repairs (Regeneten) with transosseous equivalent (TOE) repairs and TOE repairs alone. The patch group was reported to have an 8.3% re-tear rate compared to 25.8% in the TOE only group at 12 months post-operatively. The structural continuity of the repaired tendon according to Sugaya was also better in the patch group. A 2023 study by De Barros et al. also compared standard arthroscopic repair with knotless transosseous equivalent technique against the same repair technique augmented with patch. He found 0 re-tears in the augmented repair group compared to 4 re-tears in the standard repair group at 12 months [45].
There are a few studies that drew a contrasting conclusion on the re-tear rates. A comparative study by Burdick in 2023 showed a 13% re-tear rate in in bovine collagen patch augmented repairs versus no re-tears in a conventional repair group within 12 months [46]. On the other hand, Ting et al. reported no significant difference in repair integrity at 6 months and at final follow-up between the bio-inductive implant group and control group [37].
It is important to note that these studies had a relatively short follow-up period not exceeding 12 months, while it has been shown that re-tears can primarily occur between 6 and 26 weeks after repair [47]. Whilst the present literature does seem to suggest that rotator cuff repairs augmented with bio-inductive collagen scaffolds have decreased re-tear rates, further level I evidence is required to make a conclusive verdict.
3.5. Clinical Outcomes
Clinical outcomes after bio-inductive collagen patch augmentation for rotator cuff repair has yielded encouraging results. One of the largest multicentre studies involved a cohort of 173 patients (83 partial-thickness tears, 90 full-thickness tears) and reported patient outcomes up to 1 year post-surgery [48]. For both partial and full-thickness RCTs, they reported statistically significant improvements to VAS, SANE, VR-12 physical component, WORC, and ASES scores at 12 months, with majority of patients meeting or exceeding the minimal clinically important difference (MCID) of VAS pain and ASES scores. The authors also reported shorter average time in sling, time to return to driving and sports of their cohort compared to that found in the literature for conventional surgical method, although this observation was not further analysed for significance or cohort matched.
Schlegel et al. performed a prospective study on 33 patients with intermediate-grade or high-grade partial-thickness tears. He reported significant improvement in CMS and ASES scores after 12 months [27]. With favourable results in smaller tears, Bushnell et al. evaluated the same technique of collagen-based augmentation in a prospective study on more difficult-to-heal medium and large full-thickness RCTs [49]. They reported the achievement of MCID for both ASES and CMS in more than 90% of patients at 2 years. These preliminary evaluations demonstrate the potential of augmented arthroscopic repair in varying degrees of RCTs and may be the solution to achieving good outcomes even in challenging cases. However, a major limitation of these papers is the lack of a control group to offer comparison to conventional repair techniques without augmentation.
Zhang et al. reported clinical outcomes in a consecutive series of 24 patients undergoing arthroscopic repair of retracted large and massive rotator cuff tears and compared them against a control group matched by tear size who underwent similar repair but without patch augmentation [50]. Despite achievement of MCID, substantial clinical benefit (SCB) and patient-acceptable symptomatic state (PASS) for ASES and VASp, the improvement in patient-reported outcomes for both groups was comparable. Similarly, Iban et al. also reported no difference in pain, CMS, ASES or EQ-5D-5L scores between both groups in their cohort of patients with full-thickness cuff tear within 12 months of follow up [12]. Warren et al. performed a systematic review of randomized controlled trials comparing clinical outcomes of rotator cuff repair with and without patch augmentation. They reported that patient-reported outcome measure improvements were similar to historical improvements in standard rotator cuff repair, despite a lower overall re-tear rate [51]. This discrepancy could be because re-tears can be less symptomatic than the original injury, hence this difference in re-tear rates do not correlate directly with superior outcomes.
The current evidence suggests that further work is required to identify indications for augmentation that can maximise its potential in cuff healing. Jackson et al. utilised the Rotator Cuff Healing Index (RoHI) score to determine the need for augmentation of rotator cuff repairs [52]. The Rotator Cuff Healing Index (RoHI) was developed by Kwon et al. and is a numerical scoring system that integrates various independent prognostic factors to predict healing after rotator cuff repair [53]. The results show a drop in healing rates from 66% at 6 points to 38% at 7 points. As such, they propose the use of patch augmentation when the patient’s RoHI score was 7 or greater. However, RoHI was mainly based on radiological observation of cuff healing and did not demonstrate direct correlation to patient outcomes. In addition, the patch augmentation suggested by the authors [43] were not specific to collagen-based scaffolds. There are to date no studies that have investigated the appropriate usage of collagen-based implants in rotator cuff repair as determined by the RoHI score. Future studies should focus on comparing clinical outcomes after collagen patch augmentation for rotator cuff repair versus isolated rotator cuff repair. Patient and injury characteristics should be studied to determine if there is a subgroup of patients that would best benefit from collagen patch augmentation.
3.6. Economic Impact
The direct and indirect costs of rotator cuff tear treatment are important considerations as they impact individual financial burden and societal resource allocation. The direct costs are associated with the treatment, while indirect costs reflect the estimated losses due to factors associated with productivity loss. There are currently only two studies that compare the cost-effectiveness of Regeneten to the existing standard of care [54,55]. Both studies utilised a decision analytic model comparing the expecting incremental cost and clinical consequences for a cohort of patients with full-thickness RCTs, considering resources including various specialist visits, imaging studies, surgery, implant costs and physiotherapy sessions. From the patient’s perspective, the use of the collagen-based implant could improve healing rates but inevitably result in an incremental initial cost. Following the inclusion of indirect costs, however, the authors predicted that there would be overall lower treatment costs, particularly when used in patients with larger tears and at higher risk of poor healing or re-tears. These were based on the assumptions of improved healing rates enabling faster return to work as well as decreased risk of tear recurrence, which would save on the substantial cost of revision surgeries or shoulder replacements.
There are several limitations of these previous studies. Firstly, the identification and measurement of healthcare resource utilisation were based on the clinical experiences of the clinicians selected in the study. However, clinical pathways can be complex with multiple factors, and the model may not capture the true representation of the cost profile. Secondly, the existing studies were restricted to 12 months post-surgery, but complications such as tear recurrence may occur after this period and potentially alter overall costs of either arm. Thirdly, the assumptions including failure rates (recurrence of tear and failure of healing) and time to return to work have been based on non-comparative single-arm studies. As discussed in the previous sections, the potential impact of the collagen-based implant remains contentious, and the values utilised in these economic analyses may over- or underestimate the true cost value. Hence, future economic studies should focus on longitudinal data and involve data collection directly from patients, which may provide a more comprehensive picture of the cost-effectiveness of this novel treatment method.
4. Conclusions
Collagen patch augmentation of rotator cuff repair appears to be safe and effective. Collagen patch augmentation of rotator cuff repair appears to reduce re-tear rates but its effect on improving clinical outcomes is uncertain. Future studies are needed to determine if there is a particular group of patients that would best benefit from collagen patch augmentation.
Author Contributions
Conceptualization, J.H.Y. and S.W.L.H.; methodology, W.H.D.W. and Z.W.N.M.T.; formal analysis, C.X.Q.; investigation, C.X.Q.; data curation W.H.D.W. and Z.W.N.M.T.; writing—original draft preparation, W.H.D.W. and Z.W.N.M.T.; writing—review and editing, C.X.Q. and S.W.L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data was created.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hinsley, H.; Ganderton, C.; Arden, N.K.; Carr, A.J. Prevalence of rotator cuff tendon tears and symptoms in a Chingford general population cohort, and the resultant impact on UK health services: A cross-sectional observational study. BMJ Open 2022, 12, e059175. [Google Scholar] [CrossRef] [PubMed]
- Reilly, P.; Macleod, I.; Macfarlane, R.; Windley, J.; Emery, R. Dead men and radiologists don’t lie: A review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann. R. Coll. Surg. Engl. 2006, 88, 116–121. [Google Scholar] [CrossRef]
- Minagawa, H.; Yamamoto, N.; Abe, H.; Fukuda, M.; Seki, N.; Kikuchi, K.; Kijima, H.; Itoi, E. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: From mass-screening in one village. J. Orthop. 2013, 10, 8–12. [Google Scholar] [CrossRef]
- Khoschnau, S.; Milosavjevic, J.; Sahlstedt, B.; Rylance, R.; Rahme, H.; Kadum, B. High prevalence of rotator cuff tears in a population who never sought for shoulder problems: A clinical, ultrasonographic and radiographic screening study. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Teunis, T.; Lubbers, B.; Reilly, B.T.; Ring, D. A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. J. Shoulder Elb. Surg. 2014, 23, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- MacDermid, J.C.; Ramos, J.; Drosdowech, D.; Faber, K.; Patterson, S. The impact of rotator cuff pathology on isometric and isokinetic strength, function, and quality of life. J. Shoulder Elb. Surg. 2004, 13, 593–598. [Google Scholar] [CrossRef]
- Parikh, N.; Martinez, D.J.; Winer, I.; Costa, L.; Dua, D.; Trueman, P. Direct and indirect economic burden associated with rotator cuff tears and repairs in the US. Curr. Med. Res. Opin. 2021, 37, 1199–1211. [Google Scholar] [CrossRef]
- Rajalekshmi, R.; Agrawal, D.K. Understanding Fibrous Tissue in the Effective Healing of Rotator Cuff Injury. J. Surg. Res. 2024, 7, 215–228. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Z.; Song, W.; Cai, Z.; Ding, Z.; Chen, D.; Xia, F.; He, Y. Inhibition of IKKβ/NF-κB signaling facilitates tendinopathy healing by rejuvenating inflamm-aging induced tendon-derived stem/progenitor cell senescence. Mol. Ther. Nucleic Acids 2022, 27, 562–576. [Google Scholar] [CrossRef]
- Chaudhury, S.; Holland, C.; Thompson, M.S.; Vollrath, F.; Carr, A.J. Tensile and shear mechanical properties of rotator cuff repair patches. J. Shoulder Elb. Surg. 2012, 21, 1168–1176. [Google Scholar] [CrossRef]
- Zumstein, M.-A.; Lädermann, A.; Raniga, S.; Schär, M.-O. The biology of rotator cuff healing. Orthop. Traumatol. Surg. Res. 2017, 103 (Suppl. S1), S1–S10. [Google Scholar] [CrossRef]
- Ruiz Ibán, M.Á.; García, N.M.; Moros, M.S.; Diaz, H.J.; Hernando, S.A.; Ruiz, D.R.; Vaquero, C.C.; Rosas, O.M.L.; Del Monte, B.G.; Ávila, L.J.L. Augmentation of a Transosseous-Equivalent Repair in Posterosuperior Nonacute Rotator Cuff Tears with a Bioinductive Collagen Implant Decreases the Retear Rate at One Year: A Randomized Controlled Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2023, 40, 1760–1773. [Google Scholar] [CrossRef] [PubMed]
- Rohman, L.; Snow, M. Use of biologics in rotator cuff disorders: Current concept review. J. Clin. Orthop. Trauma 2021, 19, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.; Davies, M. Rotator Cuff Disease: Treatment Options and Considerations. Sports Med. Arthrosc. Rev. 2018, 26, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Reilly, P.; Amis, A.A.; Wallace, A.L.; Emery, R.J. Supraspinatus tears: Propagation and strain alteration. J. Shoulder Elb. Surg. 2003, 12, 134–138. [Google Scholar] [CrossRef]
- Longo, U.G.; Carnevale, A.; Piergentili, I.; Berton, A.; Candela, V.; Schena, E.; Denaro, V. Retear rates after rotator cuff surgery: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2021, 22, 749. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, M.; Pan, J.; Liang, G.; Feng, W.; Zeng, L.; Yang, W.; Liu, J. Risk factors affecting rotator cuff retear after arthroscopic repair: A meta-analysis and systematic review. J. Shoulder Elb. Surg. 2021, 30, 2660–2670. [Google Scholar] [CrossRef]
- Routledge, J.C.; Saber, A.Y.; Pennington, N.; Gupta, N. Re-Tear Rates Following Rotator Cuff Repair Surgery. Cureus 2023, 15, e34426. [Google Scholar] [CrossRef]
- Barber, F.A. PRP as an Adjunct to Rotator Cuff Tendon Repair. Sports Med. Arthrosc. Rev. 2018, 26, 42–47. [Google Scholar] [CrossRef]
- Ajrawat, P.; Dwyer, T.; Almasri, M.; Veillette, C.; Romeo, A.; Leroux, T.; Theodoropoulos, J.; Nauth, A.; Henry, P.; Chahal, J. Bone marrow stimulation decreases retear rates after primary arthroscopic rotator cuff repair: A systematic review and meta-analysis. J. Shoulder Elb. Surg. 2019, 28, 782–791. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y. Efficacy of bone marrow stimulation in arthroscopic repair of full thickness rotator cuff tears: A meta-analysis. J. Orthop. Surg. Res. 2019, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, C.; Arnoczky, S.; Parks, P.; Hackett, E.; Ruehlman, D.; Turner, A.; Schlegel, T. Tissue-engineered augmentation of a rotator cuff tendon using a reconstituted collagen scaffold: A histological evaluation in sheep. Muscle Ligaments Tendons J. 2019, 3, 229–235. [Google Scholar] [CrossRef]
- Turner, A.S. Experiences with sheep as an animal model for shoulder surgery: Strengths and shortcomings. J. Shoulder Elb. Surg. 2007, 16, S158–S163. [Google Scholar] [CrossRef] [PubMed]
- Arnoczky, S.P.; Bishai, S.K.; Schofield, B.; Sigman, S.; Bushnell, B.D.; Hommen, J.P.; Van Kampen, C. Histologic Evaluation of Biopsy Specimens Obtained After Rotator Cuff Repair Augmented with a Highly Porous Collagen Implant. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Chacon, J.A.; Cuenca-Espierrez, J.; Roda-Rojo, V.; Martin-Martinez, A.; Calderon-Meza, J.M.; Alvarez-Alegret, R.; Martin-Hernandez, C. Bioinductive collagen implants facilitate tendon regeneration in rotator cuff tears. J. Exp. Orthop. 2022, 9, 53. [Google Scholar] [CrossRef]
- Bokor, D.J.; Sonnabend, D.; Deady, L.; Cass, B.; Young, A.; Van Kampen, C.; Arnoczky, S. Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: A 2-year MRI follow-up. Muscles Ligaments Tendons J. 2016, 6, 16–25. [Google Scholar] [CrossRef]
- Schlegel, T.F.; Abrams, J.S.; Bushnell, B.D.; Brock, J.L.; Ho, C.P. Radiologic and clinical evaluation of a bioabsorbable collagen implant to treat partial-thickness tears: A prospective multicenter study. J. Shoulder Elb. Surg. 2018, 27, 242–251. [Google Scholar] [CrossRef]
- Sano, H.; Wakabayashi, I.; Itoi, E. Stress distribution in the supraspinatus tendon with partial-thickness tears: An analysis using two-dimensional finite element model. J. Shoulder Elb. Surg. 2006, 15, 100–105. [Google Scholar] [CrossRef]
- Bey, M.J.; Ramsey, M.L.; Soslowsky, L.J. Intratendinous strain fields of the supraspinatus tendon: Effect of a surgically created articular-surface rotator cuff tear. J. Shoulder Elb. Surg. 2002, 11, 562–569. [Google Scholar] [CrossRef]
- Chen, Q. Two-dimensional finite element proof-of-concept modeling on rotator cuff tear scaffold efficacy. In Technical Report from the Material and Structural Testing Core; Mayo Clinic: Rochester, MN, USA, 2011. [Google Scholar]
- Thon, S.G.; O’malley, L.; O’brien, M.J.; Savoie, F.H. Evaluation of Healing Rates and Safety with a Bioinductive Collagen Patch for Large and Massive Rotator Cuff Tears: 2-Year Safety and Clinical Outcomes. Am. J. Sports Med. 2019, 47, 1901–1908. [Google Scholar] [CrossRef]
- Barad, S.J. Severe subacromial-subdeltoid inflammation with rice bodies associated with implantation of a bio-inductive collagen scaffold after rotator cuff repair. J. Shoulder Elb. Surg. 2019, 28, e190–e192. [Google Scholar] [CrossRef] [PubMed]
- Root, K.T.; Wright, J.O.; Mandato, N.; Stewart, B.D.; Moser, M.W. Subacromial-Subdeltoid Bursitis with Rice Bodies After Rotator Cuff Repair with a Collagen Scaffold Implant: A Case Report. JBJS Case Connect. 2023, 13, e22.00565. [Google Scholar] [CrossRef]
- Ciccone, W.J.; Motz, C.; Bentley, C.; Tasto, J.P. Bioabsorbable implants in orthopaedics: New developments and clinical applications. J. Am. Acad. Orthop. Surg. 2001, 9, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.N.; Amin, N.H. Letter to the Editor regarding Barad: “Severe subacromial-subdeltoid inflammation with rice bodies associated with implantation of a bio-inductive collagen scaffold after rotator cuff repair”. J. Shoulder Elb. Surg. 2020, 29, e93–e94. [Google Scholar] [CrossRef]
- Bushnell, B.D.; Bishai, S.K.; Krupp, R.J.; McMillan, S.; Schofield, B.A.; Trenhaile, S.W.; McIntyre, L.F. Treatment of Partial-Thickness Rotator Cuff Tears with a Resorbable Bioinductive Bovine Collagen Implant: 1-Year Results from a Prospective Multicenter Registry. Orthop. J. Sports Med. 2021, 9, 23259671211027850. [Google Scholar] [CrossRef] [PubMed]
- Ting, R.S.; Loh, Y.C.; Rosenthal, R.; Zhong, K.; Al-Housni, H.S.; Shenouda, M.; Hackett, L.; Lam, P.H.; Murrell, G.A. Revision Rotator Cuff Repair with versus without an Arthroscopically Inserted Onlay Bioinductive Implant in Workers’ Compensation Patients. Orthop. J. Sports Med. 2023, 11, 23259671231175883. [Google Scholar] [CrossRef] [PubMed]
- Yeazell, S.; Lutz, A.; Bohon, H.; Shanley, E.; Thigpen, C.A.; Kissenberth, M.J.; Pill, S.G. Increased stiffness and reoperation rate in partial rotator cuff repairs treated with a bovine patch: A propensity-matched trial. J. Shoulder Elb. Surg. 2022, 31, S131–S135. [Google Scholar] [CrossRef]
- Bushnell, B.D.; Angelo, R.L.; Bishai, S.K.; Bravman, J.T.; Connor, P.M.; Getelman, M.H.; Harris, H.W.; McIntyre, L.F.; McMillan, S.; Trenhaile, S.W. Letter to the Editor regarding Yeazell et al: "Increased stiffness and reoperation rate in partial rotator cuff repairs treated with a bovine patch: A propensity-matched trial". J. Shoulder Elb. Surg. 2022, 31, E569–E571. [Google Scholar] [CrossRef]
- Castagna, A.; Borroni, M.; Garofalo, R.; Rose, G.D.; Cesari, E.; Padua, R.; Conti, M.; Gumina, S. Deep partial rotator cuff tear: Transtend on repair or tear completion and repair? A randomized clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 460–463. [Google Scholar] [CrossRef]
- Franceschi, F.; Papalia, R.; Del Buono, A.; Vasta, S.; Costa, V.; Maffulli, N.; Denaro, V. Articular-sided rotator cuff tears: Which is the best repair? A three-year prospective randomised controlled trial. Int. Orthop. 2013, 37, 1487–1493. [Google Scholar] [CrossRef]
- Jeong, H.J.; Nam, K.P.; Yeo, J.H.; Rhee, S.-M.; Oh, J.H. Retear After Arthroscopic Rotator Cuff Repair Results in Functional Outcome Deterioration Over Time. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 2399–2412. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, H.; Maeda, K.; Matsuki, K.; Moriishi, J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J. Bone Jt. Surg. 2007, 89, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.W.; Kim, J.Y.; Lee, H.W.; Yoon, J.H.; Noh, K.-C. Clinical and Anatomical Outcomes of Arthroscopic Repair of Large Rotator Cuff Tears with Allograft Patch Augmentation: A Prospective, Single-Blinded, Randomized Controlled Trial with a Long-term Follow-up. Clin. Orthop. Surg. 2022, 14, 263–271. [Google Scholar] [CrossRef]
- De Barros, A.A.F. Bioinductive Scafold Augmentation in Complete and Massive Rotator Cuff Tears—Prospective Randomized Trial. J. Shoulder Elb. Surg. 2023, 32, e251. [Google Scholar] [CrossRef]
- Burdick, G.; Fathima, B.; Gaudiani, M.; Wolternik, T.; Haan, J.; Kasto, J.; Kolowich, P.; Muh, S.; Castle, J. Poster 174: Arthroscopic rotator cuff repair with bioinductive patch achieves equivalent patient- reported outcomes at 1 year. Orthop. J. Sports Med. 2023, 11 (7_Suppl. S3), 2325967123S00160. [Google Scholar] [CrossRef]
- Iannotti, J.P.; Deutsch, A.; Green, A.; Rudicel, S.; Christensen, J.; Marraffino, S.; Rodeo, S. Time to failure after rotator cuff repair: A prospective imaging study. J. Bone Jt. Surg. 2013, 95, 965–971. [Google Scholar] [CrossRef]
- McIntyre, L.F.; Bishai, S.K.; Brown, P.B.; Bushnell, B.D.; Trenhaile, S.W. Patient-Reported Outcomes After Use of a Bioabsorbable Collagen Implant to Treat Partial and Full-Thickness Rotator Cuff Tears. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 2262–2271. [Google Scholar] [CrossRef]
- Bushnell, B.D.; Connor, P.M.; Harris, H.W.; Ho, C.P.; Trenhaile, S.W.; Abrams, J.S. Two-year outcomes with a bioinductive collagen implant used in augmentation of arthroscopic repair of full-thickness rotator cuff tears: Final results of a prospective multicenter study. J. Shoulder Elb. Surg. 2022, 31, 2532–2541. [Google Scholar] [CrossRef]
- Zhang, T.; Ajayi, A.; Hajjar, M.; Fleckenstein, C.M.; Nolan, J.; Hasan, S.S. Arthroscopic Repair of Retracted Large and Massive Rotator Cuff Tears with and without Augmentation with a Bio-Inductive Collagen Implant Reveals Substantial and Comparable Clinical Improvement. Arthroscopy 2023, 40, 1434–1442. [Google Scholar] [CrossRef]
- Warren, J.R.; Domingo-Johnson, E.; Sorensen, A.; Cheng, A.-L.; Latz, K.; Cil, A. Bioinductive Patch as an Augmentation for Rotator Cuff Repair, a Systematic Review and Meta-Analysis. J. Shoulder Elb. Surg. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Jackson, G.R.; Bedi, A.; Denard, P.J. Graft Augmentation of Repairable Rotator Cuff Tears: An Algorithmic Approach Based on Healing Rates. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, S.H.; Lee, Y.H.; Kim, T.I.; Oh, J.H. The Rotator Cuff Healing Index: A New Scoring System to Predict Rotator Cuff Healing After Surgical Repair. Am. J. Sports Med. 2019, 47, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, C.; Nherera, L.M.; Garofalo, R.; Guerra, E.; Longo, U.G.; Taverna, E.; Tarricone, R. Economic Evaluation of a Bioinductive Implant for the Repair of Rotator Cuff Tears Compared with Standard Surgery in Italy. Adv. Ther. 2023, 40, 5271–5284. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, L.F.; Nherera, L.M.; Schlegel, T.F. Resorbable Bioinductive Collagen Implant Is Cost Effective in the Treatment of Rotator Cuff Tears. Arthrosc. Sports Med. Rehabil. 2023, 5, e367–e374. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).