Photodynamic Therapy for Thyroid Cancer
Abstract
:1. Introduction
2. Materials and Methods
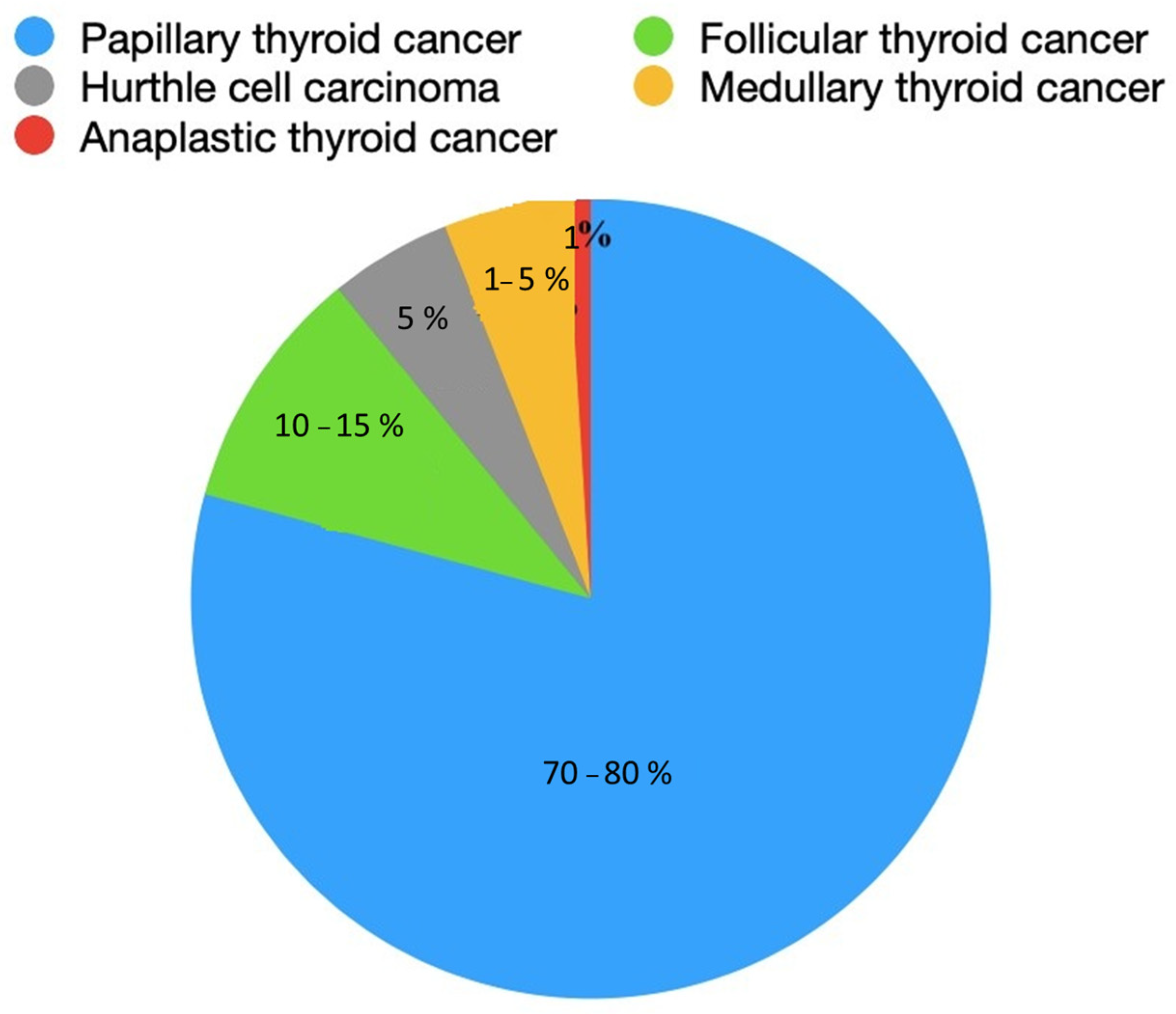
3. Genetic Background
4. Histopathological Picture
5. Diagnostics
6. Treatment
6.1. Photodynamic Therapy (PDT)
6.2. Translation
6.3. Mechanism of Photodynamic Therapy
6.4. Advantages
6.5. Defects
7. Application of Photodynamic Therapy in Thyroid Cancer
8. Future Directions
9. Summary
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-ALA | 5-aminolevulinic acid |
| AIF | apoptosis-inducing factor |
| AJCC | American Joint Committee on Cancer |
| ATA | American Thyroid Association |
| ATC | anaplastic thyroid cancer |
| BACC | fine-needle aspiration biopsy |
| DTC | differentiated thyroid cancer |
| EGFR | epidermal growth factor receptor |
| FTC | follicular thyroid cancer |
| H&E | hematoxylin and eosin |
| HCC | Hurthle cell carcinoma |
| HYP | hypericin |
| MAP, MAPK | mitogen-activated protein kinase |
| MEN | multiple endocrine neoplasia |
| MKI | multikinase inhibitors |
| MTC | medullary thyroid cancer |
| NIS | sodium–iodide symporter |
| PARP | poly(ADP-ribose) polymerase |
| PDT | photodynamic therapy |
| PI3K | 3-kinase phosphoinositide |
| PLP | porphyrin-HDL |
| PLP-PDT | PDT using PLP as PS |
| PS | photosensitizer |
| PTC | papillary thyroid cancer |
| PTEN | phosphatase and tensin homolog |
| RAI | radioactive iodine therapy |
| RFI | radiofrequency ablation |
| ROS | reactive oxygen species |
| SFE | sulforaphene |
| TNM | tumor, nodules, metastases |
| TSH | thyroid-stimulating hormone |
| USG | ultrasound examination |
References
- Lee, K.; Anastasopoulou, C.; Chandran, C.; Cassaro, S. Thyroid Cancer 2023 May 1. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Shonka, D.C., Jr.; Ho, A.; Chintakuntlawar, A.V.; Geiger, J.L.; Park, J.C.; Seetharamu, N.; Jasim, S.; Abdelhamid Ahmed, A.H.; Bible, K.C.; Brose, M.S.; et al. American Head and Neck Society Endocrine Surgery Section and International Thyroid Oncology Group consensus statement on mutational testing in thyroid cancer: Defining advanced thyroid cancer and its targeted treatment. Head. Neck. 2022, 44, 1277–1300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avram, A.M.; Zukotynski, K.; Nadel, H.R.; Giovanella, L. Management of Differentiated Thyroid Cancer: The Standard of Care. J. Nucl. Med. 2022, 63, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Lin, Y.; Liang, J. Radioactive Iodine-Refractory Differentiated Thyroid Cancer and Redifferentiation Therapy. Endocrinol. Metab. 2019, 34, 215–225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lechner, M.G.; Praw, S.S.; Angell, T.E. Treatment of Differentiated Thyroid Carcinomas. Surg. Pathol. Clin. 2019, 12, 931–942. [Google Scholar] [CrossRef] [PubMed]
- CShao, C.; Li, Z.; Zhang, C.; Zhang, W.; He, R.; Xu, J.; Cai, Y. Optical diagnostic imaging and therapy for thyroid cancer. Mater. Today Bio 2022, 17, 100441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agosto Salgado, S.; Kaye, E.R.; Sargi, Z.; Chung, C.H.; Papaleontiou, M. Management of Advanced Thyroid Cancer: Overview, Advances, and Opportunities. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389708. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Mondal, A.; Ahn, J.C. Deregulation of EGFR/PI3K and activation of PTEN by photodynamic therapy combined with carboplatin in human anaplastic thyroid cancer cells and xenograft tumors in nude mice. J. Photochem. Photobiol. B 2015, 148, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.W.; Seok, K.H.; Hwang, C.W.; Ahn, J.C.; Jin, J.O.; Kang, H.W. Hypericin-assisted photodynamic therapy against anaplastic thyroid cancer. Photodiagnosis Photodyn. Ther. 2018, 24, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Chung, P.S.; Moon, J.H.; Lee, S.H.; Ahn, J.C. Carboplatin synergistically triggers the efficacy of photodynamic therapy via caspase 3-, 8-, and 12-dependent pathways in human anaplastic thyroid cancer cells. Lasers Med. Sci. 2014, 29, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Filho, A.; Lortet-Tieulent, J.; Bray, F.; Cao, B.; Franceschi, S.; Vaccarella, S.; Dal Maso, L. Thyroid cancer incidence trends by histology in 25 countries: A population-based study. Lancet Diabetes Endocrinol. 2021, 9, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Genutis, L.K.; Tomsic, J.; Bundschuh, R.A.; Brock, P.L.; Williams, M.D.; Roychowdhury, S.; Reeser, J.W.; Frankel, W.L.; Alsomali, M.; Routbort, M.J.; et al. Microsatellite Instability Occurs in a Subset of Follicular Thyroid Cancers. Thyroid 2019, 29, 523–529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tiedje, V.; Fagin, J.A. Therapeutic breakthroughs for metastatic thyroid cancer. Nat. Rev. Endocrinol. 2020, 16, 77–78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muhanna, N.; Chan, H.H.L.; Townson, J.L.; Jin, C.S.; Ding, L.; Valic, M.S.; Douglas, C.M.; MacLaughlin, C.M.; Chen, J.; Zheng, G.; et al. Photodynamic therapy enables tumor-specific ablation in preclinical models of thyroid cancer. Endocr. Relat. Cancer 2020, 27, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, R.; Romei, C.; Ramone, T.; Prete, A.; Tacito, A.; Cappagli, V.; Bottici, V.; Viola, D.; Torregrossa, L.; Ugolini, C.; et al. Genetic Landscape of Somatic Mutations in a Large Cohort of Sporadic Medullary Thyroid Carcinomas Studied by Next-Generation Targeted Sequencing. iScience 2019, 20, 324–336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chatterjee, S.; Rhee, Y.; Chung, P.S.; Ge, R.F.; Ahn, J.C. Sulforaphene Enhances The Efficacy of Photodynamic Therapy In Anaplastic Thyroid Cancer Through Ras/RAF/MEK/ERK Pathway Suppression. J. Photochem. Photobiol. B 2018, 179, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Zafereo, M.E.; Dadu, R.; Ferrarotto, R.; Busaidy, N.L.; Lu, C.; Ahmed, S.; Gule-Monroe, M.K.; Williams, M.D.; Sturgis, E.M.; et al. Complete Surgical Resection Following Neoadjuvant Dabrafenib Plus Trametinib in BRAFV600E-Mutated Anaplastic Thyroid Carcinoma. Thyroid 2019, 29, 1036–1043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maniakas, A.; Zafereo, M.; Cabanillas, M.E. Anaplastic Thyroid Cancer: New Horizons and Challenges. Endocrinol. Metab. Clin. N. Am. 2022, 51, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Davis, A.; Verma, V. Thyroid Nodules: Advances in Evaluation and Management. Am. Fam. Physician 2020, 102, 298–304. [Google Scholar] [PubMed]
- LeClair, K.; Bell, K.J.L.; Furuya-Kanamori, L.; Doi, S.A.; Francis, D.O.; Davies, L. Evaluation of Gender Inequity in Thyroid Cancer Diagnosis: Differences by Sex in US Thyroid Cancer Incidence Compared With a Meta-analysis of Subclinical Thyroid Cancer Rates at Autopsy. JAMA Intern. Med. 2021, 181, 1351–1358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Getachew, G.; Korupalli, C.; Rasal, A.S.; Chang, J.Y. ROS generation/scavenging modulation of carbon dots as phototherapeutic candidates and peroxidase mimetics to integrate with polydopamine nanoparticles/GOx towards cooperative cancer therapy. Compos. Part B Eng. 2021, 226, 109364. [Google Scholar] [CrossRef]
- Getachew, G.; Wibrianto, A.; Rasal, A.S.; Dirersa, W.B.; Chang, J.Y. Metal halide perovskite nanocrystals for biomedical engineering: Recent advances, challenges, and future perspectives. Coord. Chem. Rev. 2023, 482, 215073. [Google Scholar] [CrossRef]
- Korupalli, C.; You, K.-L.; Getachew, G.; Rasal, A.S.; Dirersa, W.B.; Zakki Fahmi, M.; Chang, J.-Y. Engineering the Surface of Ti3C2 MXene Nanosheets for High Stability and Multimodal Anticancer Therapy. Pharmaceutics 2022, 14, 304. [Google Scholar] [CrossRef]
- Getachew, G.; Tien, Y.-C.; Kan, T.-C.; Batu Dirersa, W.; Wibrianto, A.; Orchirbat, S.; Chang, J.; Rasal, A.S.; Gurav, V.; Kizhepat, S.; et al. Defect-passivated metal halide perovskite quantum dots stabilized into biodegradable porous polydopamine nanoparticles for photothermal/chemodynamic/gas therapy of cancer. Chem. Eng. J. 2023, 467, 143560. [Google Scholar] [CrossRef]
- Getachew, G.; Hsiao, C.H.; Wibrianto, A.; Rasal, A.S.; Batu Dirersa, W.; Huang, C.C.; Vijayakameswara Rao, N.; Chen, J.H.; Chang, J.Y. High performance carbon dots based prodrug Platform: Image-Guided photodynamic and chemotherapy with On-Demand drug release upon laser irradiation. J. Colloid Interface Sci. 2023, 633, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Palot Manzil, F.F.; Kaur, H. Radioactive Iodine for Thyroid Malignancies. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Araque, K.A.; Gubbi, S.; Klubo-Gwiezdzinska, J. Updates on the Management of Thyroid Cancer. Horm. Metab. Res. 2020, 52, 562–577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alexander, L.F.; Patel, N.J.; Caserta, M.P.; Robbin, M.L. Thyroid Ultrasound: Diffuse and Nodular Disease. Radiol. Clin. N. Am. 2020, 58, 1041–1057. [Google Scholar] [CrossRef] [PubMed]
- Pace-Asciak, P.; Russell, J.O.; Tufano, R.P. The Treatment of Thyroid Cancer With Radiofrequency Ablation. Tech. Vasc. Interv. Radiol. 2022, 25, 100825. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Alzahrani, A.S. Risk Stratification in Differentiated Thyroid Cancer: From Detection to Final Follow-Up. J. Clin. Endocrinol. Metab. 2019, 104, 4087–4100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoon, B.H.; Lee, Y.; Oh, H.J.; Kim, S.H.; Lee, Y.K. Influence of Thyroid-stimulating Hormone Suppression Therapy on Bone Mineral Density in Patients with Differentiated Thyroid Cancer: A Meta-analysis. J. Bone Metab. 2019, 26, 51–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, M.; Kim, B.H. Current Guidelines for Management of Medullary Thyroid Carcinoma. Endocrinol. Metab. 2021, 36, 514–524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maharjan, P.S.; Bhattarai, H.K. Singlet Oxygen, Photodynamic Therapy, and Mechanisms of Cancer Cell Death. J. Oncol. 2022, 2022, 7211485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, C.N.; Hsu, R.; Chen, H.; Wong, T.W. Daylight Photodynamic Therapy: An Update. Molecules 2020, 25, 5195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, M.; Yang, T.; Mao, C. Enhancement of Photodynamic Cancer Therapy by Physical and Chemical Factors. Angew. Chem. Int. Ed. Engl. 2019, 58, 14066–14080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahn, J.C.; Biswas, R.; Chung, P.S. Combination with genistein enhances the efficacy of photodynamic therapy against human anaplastic thyroid cancer cells. Lasers Surg. Med. 2012, 44, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, X.; Lai, H.; Sun, Y.; Yan, X.; Ai, Q.; Lin, M.; Yang, S.; Yang, Y.; Chu, S.; et al. Novel antidepressant mechanism of hypericin: Role of connexin 43-based gap junctions. Biomed. Pharmacother. 2023, 167, 115545. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benov, L. Photodynamic therapy: Current status and future directions. Med. Princ. Pract. 2015, 24 (Suppl. 1), 14–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, F.; Wang, B.R.; Zhu, Z.F.; Wang, S.Q.; Chai, C.X.; Shang, D.; Li, M. Photodynamic therapy: A next alternative treatment strategy for hepatocellular carcinoma? World J. Gastrointest. Surg. 2021, 13, 1523–1535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kano, A.; Taniwaki, Y.; Nakamura, I.; Shimada, N.; Moriyama, K.; Maruyama, A. Tumor delivery of Photofrin® by PLL-g-PEG for photodynamic therapy. J. Control. Release 2013, 167, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.M.; Kim, Y.W.; Lee, J.M.; Namkoong, S.E.; Han, S.J.; Kim, J.K.; Lee, C.H.; Chun, H.J.; Jin, H.S.; Ahn, W.S. Photodynamic effects of Radachlorin on cervical cancer cells. Cancer Res. Treat. 2004, 36, 389–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva, Z.S., Jr.; Bussadori, S.K.; Fernandes, K.P.; Huang, Y.Y.; Hamblin, M.R. Animal models for photodynamic therapy (PDT). Biosci. Rep. 2015, 35, e00265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kochneva, E.V.; Filonenko, E.V.; Vakulovskaya, E.G.; Scherbakova, E.G.; Seliverstov, O.V.; Markichev, N.A.; Reshetnickov, A.V. Photosensitizer Radachlorin®: Skin cancer PDT phase II clinical trials. Photodiagnosis Photodyn. Ther. 2010, 7, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, M.M.; Islam, M.A.; Yamamoto, I.; Taniguchi, S.; Yonekura, S. 5-ALA Attenuates the Palmitic Acid-Induced ER Stress and Apoptosis in Bovine Mammary Epithelial Cells. Molecules 2021, 26, 1183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Watban, F.A.; Zhang, X.Y. Photodynamic therapy of human undifferentiated thyroid carcinoma-bearing nude mice using topical 5-aminolevulinic acid. Photomed. Laser Surg. 2005, 23, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H.; Kim, K.-W.; Chung, P.-S.; Lee, S.J. Photodynamic therapy for SNU-80 anaplastic thyroid cancer cells. Med. Lasers Eng. Basic Res. Clin. Appl. 2022, 11, 84–91. [Google Scholar] [CrossRef]
- Sangthong, S.; Weerapreeyakul, N. Simultaneous quantification of sulforaphene and sulforaphane by reverse phase HPLC and their content in Raphanus sativus L. var. caudatus Alef extracts. Food Chem. 2016, 201, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kuang, P.; Song, D.; Yuan, Q.; Lv, X.; Zhao, D.; Liang, H. Preparative separation and purification of sulforaphene from radish seeds by high-speed countercurrent chromatography. Food Chem. 2013, 136, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Tanley, S.W.; Diederichs, K.; Kroon-Batenburg, L.M.; Levy, C.; Schreurs, A.M.; Helliwell, J.R. Carboplatin binding to histidine. Acta Crystallogr. F Struct. Biol. Commun. 2014, 70 Pt 9, 1135–1142, Erratum in Acta Crystallogr. F Struct. Biol. Commun. 2016, 72 Pt 3, 251–252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxid. Med. Cell Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, G.; Wang, Q.; Liu, J.; Wu, M.; Ji, H.; Qin, Y.; Zhou, X.; Wu, L. Innovative strategies for enhanced tumor photodynamic therapy. J. Mater. Chem. B 2021, 9, 7347–7370. [Google Scholar] [CrossRef] [PubMed]
- Van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Z.; Zhang, L.; Zhang, Z.; Liu, Z. Advances in photosensitizer-related design for photodynamic therapy. Asian J. Pharm. Sci. 2021, 16, 668–686. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alvarez, N.; Sevilla, A. Current Advances in Photodynamic Therapy (PDT) and the Future Potential of PDT-Combinatorial Cancer Therapies. Int. J. Mol. Sci. 2024, 25, 1023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alsaab, H.O.; Alghamdi, M.S.; Alotaibi, A.S.; Alzhrani, R.; Alwuthaynani, F.; Althobaiti, Y.S.; Almalki, A.H.; Sau, S.; Iyer, A.K. Progress in Clinical Trials of Photodynamic Therapy for Solid Tumors and the Role of Nanomedicine. Cancers 2020, 12, 2793. [Google Scholar] [CrossRef] [PubMed]
- Savvides, P.; Nagaiah, G.; Lavertu, P.; Fu, P.; Wright, J.J.; Chapman, R.; Wasman, J.; Dowlati, A.; Remick, S.C. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid 2013, 23, 600–604. [Google Scholar] [CrossRef]
- Elisei, R.; Schlumberger, M.J.; Müller, S.P.; Schöffski, P.; Brose, M.S.; Shah, M.H.; Licitra, L.; Jarzab, B.; Medvedev, V.; Kreissl, M.C.; et al. Cabozantinib in Progressive Medullary Thyroid Cancer. J. Clin. Oncol. 2013, 31, 3639–3646. [Google Scholar] [CrossRef]
- Baldini, E.; D’Armiento, M.; Ulisse, S. A New Aurora in Anaplastic Thyroid Cancer Therapy. Int. J. Endocrinol. 2014, 2014, 11. [Google Scholar] [CrossRef]
- Bible, K.C.; Suman, V.J.; Molina, J.R.; Smallridge, R.C.; Maples, W.J.; Menefee, M.E.; Rubin, J.; Karlin, N.; Sideras, K.; Morris, J.C.; et al. A multicenter phase 2 trial of pazopanib in metastatic and progressive medullary thyroid carcinoma: MC057H. J. Clin. Endocrinol. Metab. 2014, 99, 1687–1693. [Google Scholar] [CrossRef]
- Sosa, J.A.; Elisei, R.; Jarzab, B.; Balkissoon, J.; Lu, S.P.; Bal, C. Randomized safety and efficacy study of fosbretabulin with paclitaxel/carboplatin against anaplastic thyroid carcinoma. Thyroid 2014, 24, 232–240. [Google Scholar] [CrossRef]
- Locati, L.D.; Licitra, L.; Agate, L.; Ou, S.I.; Boucher, A.; Jarzab, B.; Qin, S.; Kane, M.A.; Wirth, L.J.; Chen, C.; et al. Treatment of advanced thyroid cancer with axitinib: Phase 2 study with pharmacokinetic/pharmacodynamic and quality-of-life assessments. Cancer 2014, 120, 2694–2703. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, J.; Olczyk, T.; Jarzab, B. Cabozantinib for the treatment of progressive metastatic medullary thyroid cancer. Expert Rev. Clin. Pharmacol. 2016, 9, 69–79. [Google Scholar] [CrossRef]
- Ryder, M.; Gild, M.; Hohl, T.M.; Pamer, E.; Knauf, J.; Ghossein, R.; Joyce, J.A.; Fagin, J.A. Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid Cancer progression. PLoS ONE 2013, 8, e54302. [Google Scholar] [CrossRef] [PubMed]

| Features Indicating a Malignant Process in an Ultrasound Examination of the Thyroid Gland |
|---|
| Hypoechogenicity |
| Irregular margins |
| Shape taller than wide |
| Central vascularization |
| Extension of the lesion beyond the thyroid gland |
| Solid structure |
| Type of Treatment | Type of Cancer |
|---|---|
| Active supervision | DTC |
| Lobectomy | DTC |
| Thyroidectomy with/without nodulectomy | DTC, MTC, ATC |
| Chemotherapy | DTC, MTC, ATC |
| Radiotherapy | DTC, MTC, ATC |
| Radioiodine ablation | DTC |
| Thyroid hormone suppression therapy | DTC |
| Radiofrequency ablation (RFA) | PTC, small recurrence, symptomatic in non-resectable cancers, MTC |
| Advantages of PDT | Disadvantages of PDT |
|---|---|
| Low invasiveness | It causes pain |
| Low costs compared to other oncological treatment methods | Dependence on the accuracy of pathological tissue irradiation |
| Possibility of outpatient use | High costs of preparation, transportation, and maintenance of equipment needed for PDT |
| Protection of surrounding tissues | Lack of specific standards and quality control |
| No long-term side effects | No use in case of disseminated cancers |
| Leaves minor scars or no scars at all. | Not applicable in the case of deeper, less oxygenated tumors |
| High effectiveness | |
| Also affecting the vascularization of the tumor—long-term effects | |
| Short therapy time | |
| Can be used multiple times and combined with other methods |
| Name | Formula | Ref. | |
|---|---|---|---|
| Hypericin | C30H16O8 | [38] | 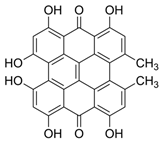 |
| Porphyrin | C44H34N8 | [39,40,41] |  |
| Photophrin | C68H74N8O11 | [42,43] |  |
| Radachlorin | C38H41N5O9 | [44,45] |  |
| 5-Aminolevulinic acid (ALA) | C5H9NO3 | [43,46] | 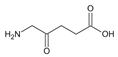 |
| Name | Formula | Name | Structure | Ref. |
|---|---|---|---|---|
| Sulforaphane | C6H9NOS2 | 4-methylsulfinyl-3-butenyl isothiocyanate | 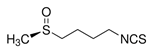 | [16,49,50] |
| Carboplatin | C6H12N2O2Pt | (SP-4-2)-diamino[cyclobutane-1,1-dicarboxylate(2-)-O,O′]-platinum | 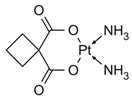 | [51] |
| Genistein | C15H10O5 | 5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one | 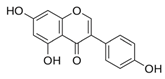 | [52] |
| No. | Clinical Trial | Status | Sponsor | |
|---|---|---|---|---|
| 1 | NCT00587314 | Barrett’s esophagus; early esophageal adenocarcinoma | Biopsy | Mayo Clinic |
| 2 | Phase I (NCT01366833) | Malignant dysphagia; esophageal cancer | Radiation: Brachytherapy; Procedure: Stent insertion | McGill University Health Center |
| 3 | Phase II (NCT00217087) | Early-stage esophageal adenocarcinoma. Barrett esophagus | Porfimer sodium 2 mg/kg | Mayo Clinic |
| 4 | Phase III (NCT02628665) | Stage I, II, and III of esophageal adenocarcinoma and esophageal squamous cell carcinoma | Photosensitizer(photophrin) device: 630 nm laser irradiation (DIOMED) | The First Affiliated Hospital of Henan University of Science and Technology |
| 5 | Phase I (NCT01043016) | Skin cancer and esophageal cancer | Photocyanine injection | Fujian Longhua Pharmaceutical Co. Ltd. |
| 6 | Phase II (NCT01086488) | Nasopharyngeal carcinoma | FOSCAN | Ministry of Health, Malaysia |
| 7 | Phase I, II (NCT00060268) | Esophageal cancer | HPPH | Roswell Park Cancer Institute |
| 8 | Phase II (NCT00002935) | Esophageal cancer | Porfimer sodium | Roswell Park Cancer Institute |
| 9 | Phase I, II (NCT02070432) | Head and neck cancer | LUZ11 | Luzitin SA |
| 10 | Phase I (NCT00978081) | Head and neck cancer Precancerous condition | Aminolevulinic acid hydrochloride | Abramson Cancer Center of the University of Pennsylvania |
| 11 | Phase II (NCT00003856) | Head and neck cancer | Temoporfin | Quintiles, Inc. |
| 12 | Phase I (NCT01019954) | Head and neck tumors | Levulan | Abramson Cancer Center of the University of Pennsylvania |
| 13 | Phase I (NCT00670397) | Head and neck cancer Precancerous/nonmalignant condition | Porfimer sodium + PDT | Roswell Park Cancer Institute |
| 14 | Phase I (NCT00028405) | Liver metastasis Pelvic cancer Head and neck Breast, colorectal, rectal, and mouth cancer, sarcoma | Drug: LS 11 (Taporfin Sodium) Device: Lumaflex Light Delivery Catheter | Light Sciences LLC |
| S. No | Drug | Phase | Clinical Trial No. |
|---|---|---|---|
| 1 | MLN0128 | II | NCT02244463 Dana-Farber Cancer Institute, USA |
| 2 | Lenvatinib | II | NCT02726503 Translational Research Informatics Center, Kobe, Hyogo, Japan |
| 3 | Trametinib in combination with Paclitaxel | I | NCT03085056 Memorial Sloan Kettering Cancer Center, USA |
| 4 | Pembrolizumab | II | NCT02688608 University of Texas Southwestern Medical Center, USA |
| 5 | Inolitazone dihydrochloride (Efutazone) and Paclitaxel | II | NCT02152137 Alliance for Clinical Trials in Oncology, USA |
| 6 | Combination of Durvalumab (MEDI4736) or Tremelimumab with stereotactic body radiotherapy (SBRT) | I | NCT03122496 Memorial Sloan Kettering Cancer Center, USA |
| 7 | Intensity-modulated radiation therapy and Paclitaxel with or without Pazopanib hydrochloride | II | NCT01236547 National Cancer Institute (NCI), USA |
| No. | Drug | Phase | Thyroid Cancer Type | Reference |
|---|---|---|---|---|
| 1 | Sorafenib (Bay43–9006, Nexavar) | II | Advanced thyroid cancer | [58] |
| 2 | Carbozantinib | III | Metastatic thyroid cancer | [59] |
| 3 | Efatutazone+ Paclitaxel | I | Advanced thyroid cancer | [60] |
| 4 | Pazopanib | II | Advanced and progressive medullary | [61] |
| 5 | Fosbretabulin + Paclitaxel/Carboplatin | II | Advanced thyroid cancer | [62] |
| 6 | Vemurafenib | BRAFV600E positive, metastatic, radioiodine refractory PTC | [62] | |
| 7 | Axitinib | II | Advanced thyroid cancer | [62] |
| 8 | Levatinib | III | Iodine refractory thyroid cancer | [63] |
| 9 | Sunitinib (second line of therapy) | II | Progressive, radioiodine refractory thyroid cancer | [64] |
| 10 | Cabozantinib (XL-184) | III | Advanced metastatic thyroid cancer | [65] |
| 11 | Dabrafenib plus trametinib | II | BRAF V600E-mutated anaplastic thyroid cancer | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inglot, J.; Strzelczyk, J.K.; Bartusik-Aebisher, D.; Aebisher, D. Photodynamic Therapy for Thyroid Cancer. BioMed 2025, 5, 8. https://doi.org/10.3390/biomed5010008
Inglot J, Strzelczyk JK, Bartusik-Aebisher D, Aebisher D. Photodynamic Therapy for Thyroid Cancer. BioMed. 2025; 5(1):8. https://doi.org/10.3390/biomed5010008
Chicago/Turabian StyleInglot, Julia, Joanna Katarzyna Strzelczyk, Dorota Bartusik-Aebisher, and David Aebisher. 2025. "Photodynamic Therapy for Thyroid Cancer" BioMed 5, no. 1: 8. https://doi.org/10.3390/biomed5010008
APA StyleInglot, J., Strzelczyk, J. K., Bartusik-Aebisher, D., & Aebisher, D. (2025). Photodynamic Therapy for Thyroid Cancer. BioMed, 5(1), 8. https://doi.org/10.3390/biomed5010008









