Two Coffee Diterpenes, Kahweol and Cafestol, Inhibit Extracellular Melanogenesis: An In Vitro Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
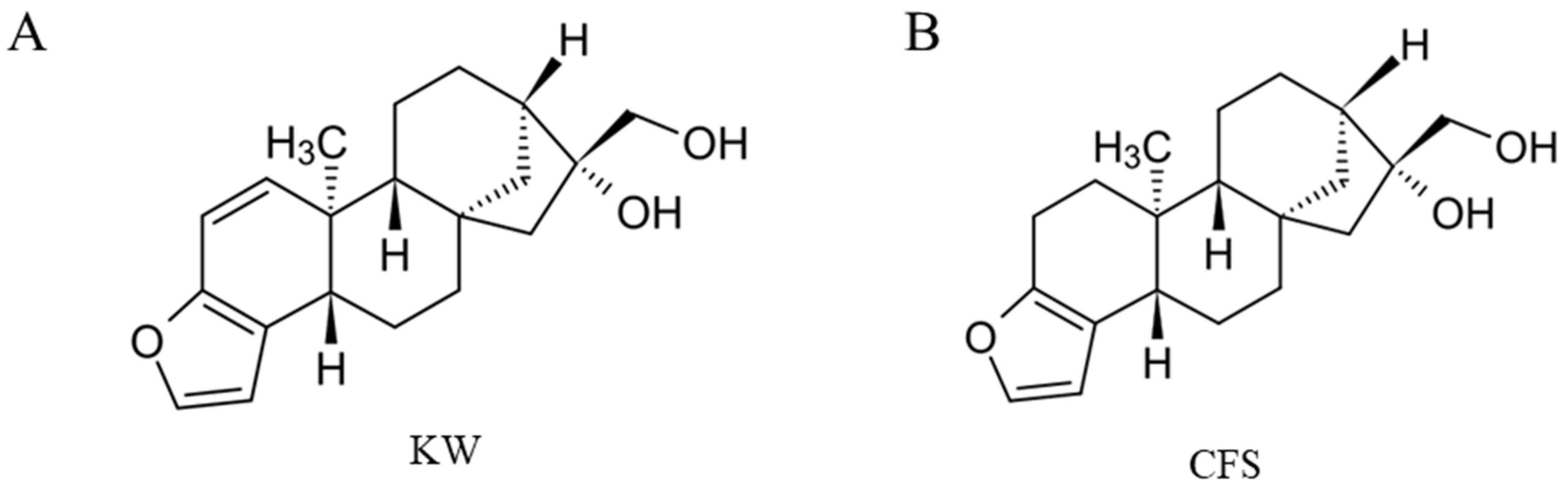
2.1. Materials
2.2. Cell Culture
2.3. MTS Cell Viability Assay
2.4. Determination of Intracellular and Extracellular Melanin
2.5. Intracellular Tyrosinase Activity
2.6. Quantitation of Melanocyte Dendricity Parameters
2.7. Cell-Free Tyrosinase Activity
2.8. Intracellular and Cell-Free α-Glucosidase Enzymatic Assay
2.9. ERK/AKT Phosphorylation ELISA Assay
2.10. Statistical Analysis
3. Results
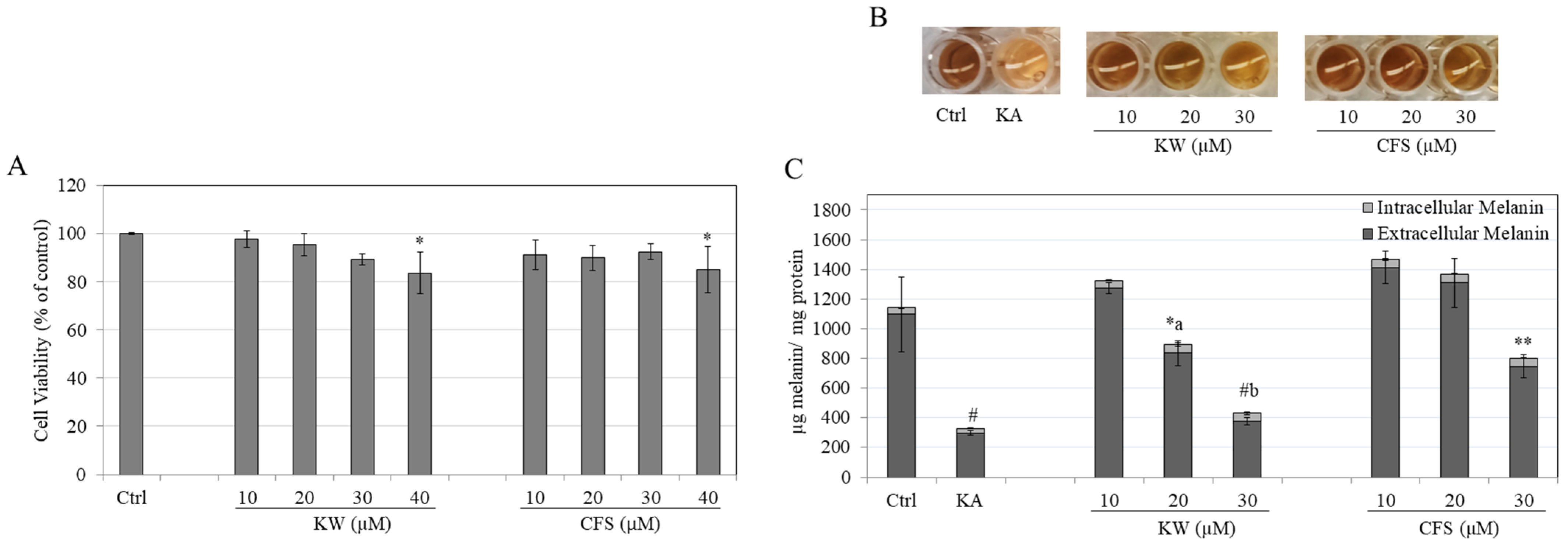
3.1. Effects of Compounds on B16F10 Cell Viability
3.2. Effects of Compounds on Melanin Content in B16F10 Cells
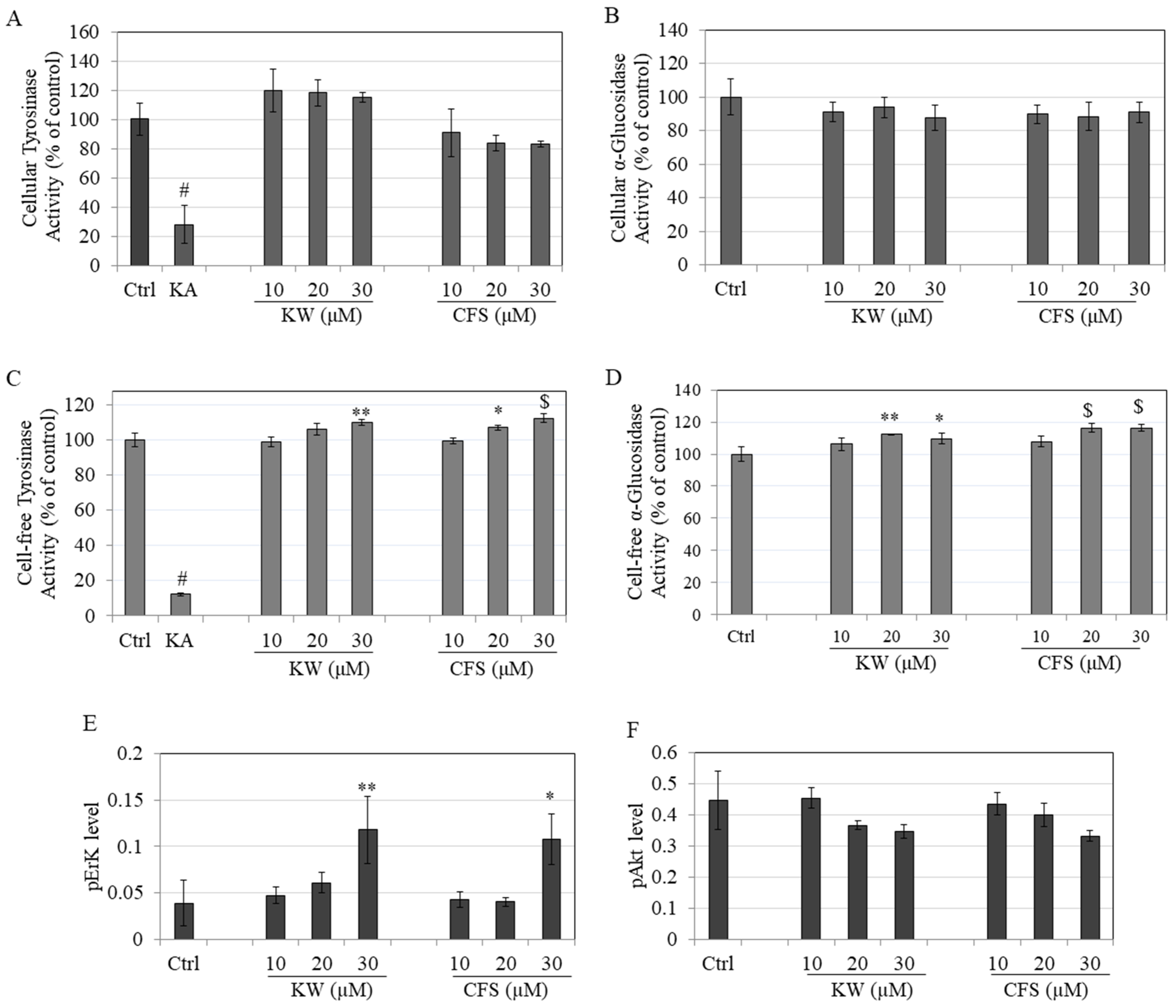
3.3. Effects on Tyrosinase and α-Glucosidase Enzymatic Activity
3.4. Effects on Phosphorylation of ERK/AKT
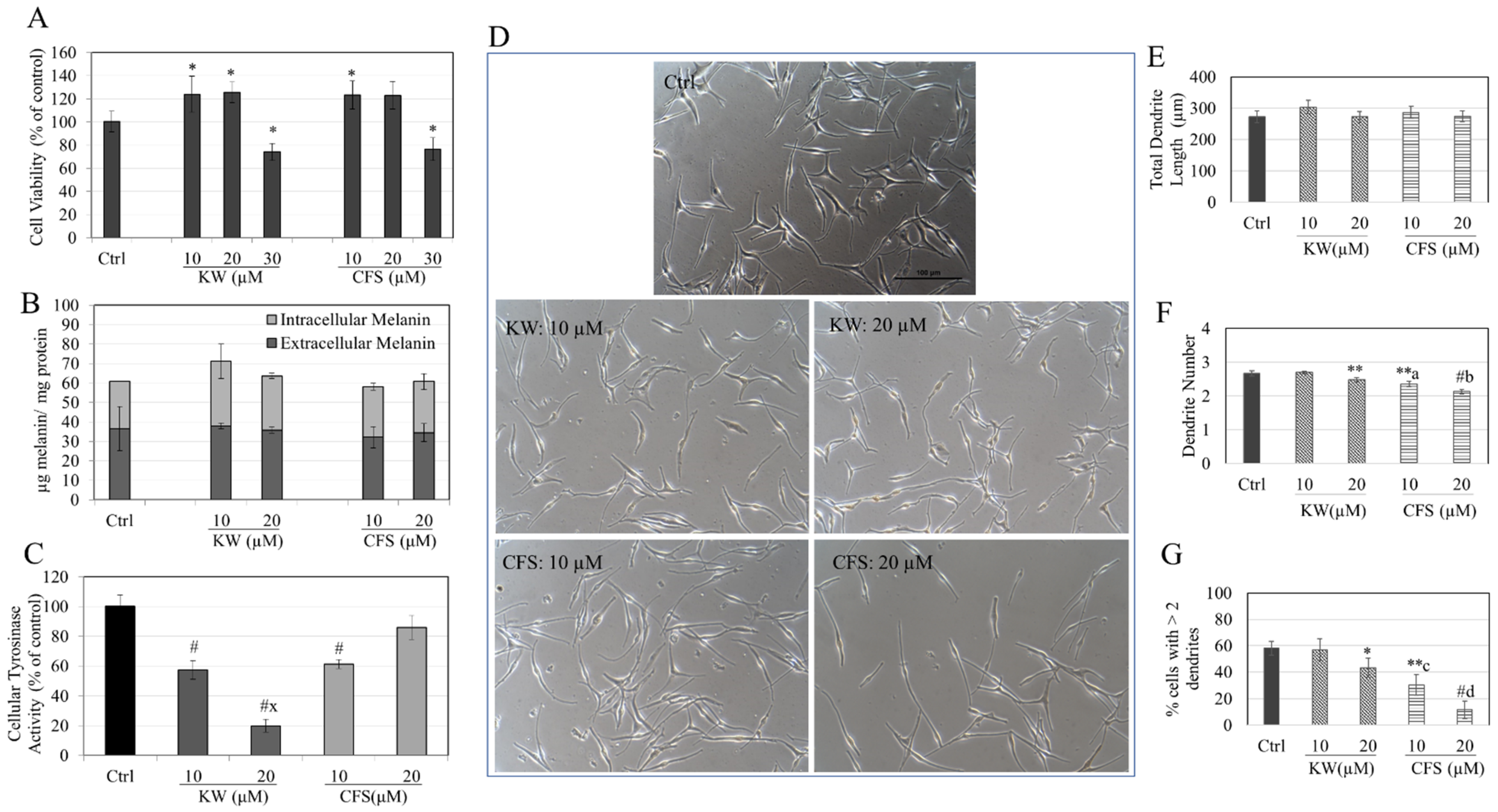
3.5. Effects of Compounds in HEMn-DP Cells
3.6. Effects of Compounds in HEMn-MP Cells
3.7. Effects of Compounds HaCaT Cell Viability
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoikidou, T.; Koidis, A. Coffee and tea bioactive compounds. In Functional Foods and Their Implications for Health Promotion; Elsevier: Amsterdam, The Netherlands, 2023; pp. 29–53. [Google Scholar]
- Moeenfard, M.; Silva, J.A.; Borges, N.; Santos, A.; Alves, A. Diterpenes in espresso coffee: Impact of preparation parameters. Eur. Food Res. Technol. 2015, 240, 763–773. [Google Scholar] [CrossRef]
- Lee, K.-A.; Chae, J.-I.; Shim, J.-H. Natural diterpenes from coffee, cafestol and kahweol induce apoptosis through regulation of specificity protein 1 expression in human malignant pleural mesothelioma. J. Biomed. Sci. 2012, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- De Roos, B.; Van Der Weg, G.; Urgert, R.; Van De Bovenkamp, P.; Charrier, A.; Katan, M.B. Levels of cafestol, kahweol, and related diterpenoids in wild species of the coffee plant Coffea. J. Agric. Food Chem. 1997, 45, 3065–3069. [Google Scholar] [CrossRef]
- Moeenfard, M.; Erny, G.L.; Alves, A. Variability of some diterpene esters in coffee beverages as influenced by brewing procedures. J. Food Sci. Technol. 2016, 53, 3916–3927. [Google Scholar] [CrossRef] [PubMed]
- Urgert, R.; Katan, M. The cholesterol-raising factor from coffee beans. Annu. Rev. Nutr. 1997, 17, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Weusten-Van Der Wouw, M.; Katan, M.; Viani, R.; Huggett, A.; Liardon, R.; Lund-Larsen, P.; Thelle, D.; Ahola, I.; Aro, A. Identity of the cholesterol-raising factor from boiled coffee and its effects on liver function enzymes. J. Lipid Res. 1994, 35, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Urgert, R.; Van Der Weg, G.; Kosmeijer-Schuil, T.G.; Van De Bovenkamp, P.; Hovenier, R.; Katan, M.B. Levels of the cholesterol-elevating diterpenes cafestol and kahweol in various coffee brews. J. Agric. Food Chem. 1995, 43, 2167–2172. [Google Scholar] [CrossRef]
- Rustan, A.C.; Halvorsen, B.; Huggett, A.C.; Ranheim, T.; Drevon, C.A. Effect of coffee lipids (cafestol and kahweol) on regulation of cholesterol metabolism in HepG2 cells. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Pluchinotta, F.R.; Marventano, S.; Buscemi, S.; Li Volti, G.; Galvano, F.; Grosso, G. Coffee components and cardiovascular risk: Beneficial and detrimental effects. Int. J. Food Sci. Nutr. 2014, 65, 925–936. [Google Scholar] [CrossRef]
- De Roos, B.; Van Tol, A.; Urgert, R.; Scheek, L.; Van Gent, T.; Buytenhek, R.; Princen, H.; Katan, M. Consumption of french-press coffee raises cholesteryl ester transfer protein activity levels before LDL cholesterol in normolipidaemic subjects. J. Intern. Med. 2000, 248, 211–216. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.; Kim, H.S.; Kang, Y.J. Unraveling Connective Tissue Growth Factor as a Therapeutic Target and Assessing Kahweol as a Potential Drug Candidate in Triple-Negative Breast Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 16307. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, C.; Quesada, A.R.; Medina, M.A. Anti-angiogenic and anti-inflammatory properties of kahweol, a coffee diterpene. PLoS ONE 2011, 6, e23407. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, S.G.; Kang, Y.J.; Kwon, T.K.; Nam, J.-O. Kahweol inhibits adipogenesis of 3T3-L1 adipocytes through downregulation of PPARγ. Nat. Prod. Res. 2018, 32, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-Y.; Lee, S.-H.; Lee, J.-H.; Hwang, J.S.; Kim, M.K.; Jang, B.K. Kahweol activates the Nrf2/HO-1 pathway by decreasing Keap1 expression independently of p62 and autophagy pathways. PLoS ONE 2020, 15, e0240478. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Jeong, H.G. Protective effects of kahweol and cafestol against hydrogen peroxide-induced oxidative stress and DNA damage. Toxicol. Lett. 2007, 173, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Park, Y.C.; Kim, S.H.; Lee, J.; Cho, J.Y. Nuclear factor-κB/signal Transducers and activators of transcription-1-mediated inflammatory responses in Lipopolysaccharide-activated macrophages are a major inhibitory target of kahweol, a coffee diterpene. Biol. Pharm. Bull. 2010, 33, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hossain, M.A.; Kim, J.-H.; Cho, J.Y. Kahweol Exerts Skin Moisturizing Activities by Upregulating STAT1 Activity. Int. J. Mol. Sci. 2021, 22, 8864. [Google Scholar] [CrossRef] [PubMed]
- Al-Kenany, S.A.; Al-Shawi, N.N. Protective effect of cafestol against doxorubicin-induced cardiotoxicity in rats by activating the Nrf2 pathway. Front. Pharmacol. 2023, 14, 1206782. [Google Scholar] [CrossRef] [PubMed]
- Mellbye, F.B.; Jeppesen, P.B.; Shokouh, P.; Laustsen, C.; Hermansen, K.; Gregersen, S. Cafestol, a bioactive substance in coffee, has antidiabetic properties in KKAy mice. J. Nat. Prod. 2017, 80, 2353–2359. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, J.; Wang, Y.; Wang, X.; Ma, Q.; Li, Y.; Zhang, X.; Wang, S.; Zhang, Q.; Mi, F. Cafestol inhibits colon cancer cell proliferation and tumor growth in xenograft mice by activating LKB1/AMPK/ULK1-dependent autophagy. J. Nutr. Biochem. 2024, 129, 109623. [Google Scholar] [CrossRef]
- Pandya, A.G.; Guevara, I.L. Disorders of hyperpigmentation. Dermatol. Clin. 2000, 18, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase inhibitors from natural and synthetic sources as skin-lightening agents. Rev. Agric. Sci. 2019, 7, 41–58. [Google Scholar] [CrossRef]
- Oh, T.S.; Bae, E.J.; Ro, K.W.; Seo, S.H.; Son, S.W.; Kim, I.-H. Acral lentiginous melanoma developing during long-standing atypical melanosis: Usefulness of dermoscopy for detection of early acral melanoma. Ann. Dermatol. 2011, 23, 400. [Google Scholar] [CrossRef] [PubMed]
- Iriyama, S.; Ono, T.; Aoki, H.; Amano, S. Hyperpigmentation in human solar lentigo is promoted by heparanase-induced loss of heparan sulfate chains at the dermal–epidermal junction. J. Dermatol. Sci. 2011, 64, 223–228. [Google Scholar] [CrossRef]
- Ishack, S.; Lipner, S.R. Exogenous ochronosis associated with hydroquinone: A systematic review. Int. J. Dermatol. 2022, 61, 675–684. [Google Scholar] [CrossRef]
- Charoo, N.A. Hyperpigmentation: Looking beyond hydroquinone. J. Cosmet. Dermatol. 2022, 21, 4133–4145. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Jiang, L.; Geng, C.; Cao, J.; Zhong, L. Hydroquinone-induced genotoxicity and oxidative DNA damage in HepG2 cells. Chem.-Biol. Interact. 2008, 173, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kooyers, T.; Westerhof, W. Toxicology and health risks of hydroquinone in skin lightening formulations. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 777–780. [Google Scholar] [CrossRef]
- Numata, T.; Tobita, R.; Tsuboi, R.; Okubo, Y. Contact dermatitis caused by arbutin contained in skin-whitening cosmetics. Contact Dermat. 2016, 75, 187–188. [Google Scholar] [CrossRef]
- Nakagawa, M.; Kawai, K.; Kawai, K. Contact allergy to kojic acid in skin care products. Contact Dermat. 1995, 32, 9–13. [Google Scholar] [CrossRef]
- Lim, J.T.E. Treatment of melasma using kojic acid in a gel containing hydroquinone and glycolic acid. Dermatol. Surg. 1999, 25, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Scott, G. Rac and rho: The story behind melanocyte dendrite formation. Pigment. Cell Res. 2002, 15, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Investig. Dermatol. 2012, 132, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bossche, K.; Naeyaert, J.M.; Lambert, J. The quest for the mechanism of melanin transfer. Traffic 2006, 7, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Lee, J.H. Antibacterial and whitening activities of Coffea arabica ethanol extract. Korean Chem. Eng. Res. 2018, 56, 245–251. [Google Scholar]
- Sung, H.M.; Jung, H.J.; Sin, J.S.; Kim, K.M.; Wee, J.-H. Skin whitening activity of supercritical fluid extract from spent coffee in B16F10 melanoma cell. Food Sci. Biotechnol. 2015, 24, 1087–1096. [Google Scholar] [CrossRef]
- Elfiah, U.; Perdanakusuma, D.S.; Saputro, I.D. Robusta BP-42 coffee bean extract is a new anti-tyrosinase candidate to reduce melanogenesis activity. Bali Med. J. 2022, 11, 2022–2026. [Google Scholar]
- Fukushima, Y.; Takahashi, Y.; Hori, Y.; Kishimoto, Y.; Shiga, K.; Tanaka, Y.; Masunaga, E.; Tani, M.; Yokoyama, M.; Kondo, K. Skin photoprotection and consumption of coffee and polyphenols in healthy middle-aged Japanese females. Int. J. Dermatol. 2015, 54, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Kawakami, F.; Lwin, T.-T.; Imai, M.; Shamsa, F. Biochemical characterization of ferulic acid and caffeic acid which effectively inhibit melanin synthesis via different mechanisms in B16 melanoma cells. Biol. Pharm. Bull. 2018, 41, 806–810. [Google Scholar] [CrossRef]
- Yamate, Y.; Hiramoto, K.; Sato, E.F. The preventive effect of coffee compounds on dermatitis and epidermal pigmentation after ultraviolet irradiation in mice. Ski. Pharmacol. Physiol. 2017, 30, 24–35. [Google Scholar] [CrossRef]
- De Mello, V.; De Mesquita Júnior, G.A.; Alvim, J.G.E.; Costa, J.D.C.D.; Vilela, F.M.P. Recent patent applications for coffee and coffee by-products as active ingredients in cosmetics. Int. J. Cosmet. Sci. 2023, 45, 267–287. [Google Scholar] [CrossRef]
- Grigolon, G.; Nowak, K.; Poigny, S.; Hubert, J.; Kotland, A.; Waldschütz, L.; Wandrey, F. From Coffee Waste to Active Ingredient for Cosmetic Applications. Int. J. Mol. Sci. 2023, 24, 8516. [Google Scholar] [CrossRef]
- Human Epidermal Melanocytes, Neonatal, Darkly Pigmented Donor, (Hemn-DP). Available online: https://www.thermofisher.com/order/catalog/product/C2025C?SID=srch-srp-C2025C (accessed on 3 April 2024).
- Human Epidermal Melanocytes, Neonatal, Moderately Pigmented Donor, (Hemn-MP). Available online: https://www.thermofisher.com/order/catalog/product/C2025C (accessed on 3 April 2024).
- Goenka, S.; Simon, S.R. Cmt-308, a nonantimicrobial chemically-modified tetracycline, exhibits anti-melanogenic activity by suppression of melanosome export. Biomedicines 2020, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Inhibitory effects of the bioactive thermorubin isolated from the fungus thermoactinomyces antibioticus on melanogenesis. Cosmetics 2020, 7, 61. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Comparative study of curcumin and its hydrogenated metabolites, tetrahydrocurcumin, hexahydrocurcumin, and octahydrocurcumin, on melanogenesis in B16F10 and MNT-1 cells. Cosmetics 2021, 8, 4. [Google Scholar] [CrossRef]
- Goenka, S. Comparative study of Δ9-tetrahydrocannabinol and cannabidiol on melanogenesis in human epidermal melanocytes from different pigmentation phototypes: A pilot study. J. Xenobiot. 2022, 12, 131–144. [Google Scholar] [CrossRef]
- Goenka, S. Exploring the effect of butyric acid, a metabolite from periodontopathic bacteria, on primary human melanocytes: An in vitro study. J. Oral Biosci. 2024, 66, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Golub, L.M. In Vitro Study of the Effects of Five Chemically Modified Tetracycline (CMT) Analogs on Human Epidermal Melanogenesis: Potential as Novel Anti-Melanogenic Agents. Drugs Drug Candidates 2023, 2, 810–826. [Google Scholar] [CrossRef]
- Mikami, M.; Sonoki, T.; Ito, M.; Funasaka, Y.; Suzuki, T.; Katagata, Y. Glycosylation of tyrosinase is a determinant of melanin production in cultured melanoma cells. Mol. Med. Rep. 2013, 8, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Hebert, D.N. Tyrosinase maturation through the mammalian secretory pathway: Bringing color to life. Pigment Cell Res. 2006, 19, 3–18. [Google Scholar] [CrossRef]
- Goenka, S.; Nagabhushanam, K.; Majeed, M.; Simon, S.R. Calebin-A, a Curcuminoid analog inhibits α-MSH-induced melanogenesis in B16F10 mouse melanoma cells. Cosmetics 2019, 6, 51. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B.; Breathnach, A. The epidermal melanin unit system. Dermatol. Wochenschr. 1963, 147, 481–489. [Google Scholar] [PubMed]
- De Luca, M. Keratinocyte-melanocyte interactions in in vitro reconstituted normal human epidermis. Chron. Dermatol. 1994, 4, 935–940. [Google Scholar]
- Yamauchi, K.; Mitsunaga, T.; Itakura, Y.; Batubara, I. Extracellular melanogenesis inhibitory activity and the structure-activity relationships of ugonins from Helminthostachys zeylanica roots. Fitoterapia 2015, 104, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Natsume, M.; Yamaguchi, K.; Batubara, I.; Mitsunaga, T. Structure-activity relationship for vanilloid compounds from extract of Zingiber officinale var rubrum rhizomes: Effect on extracellular melanogenesis inhibitory activity. Med. Chem. Res. 2019, 28, 1402–1412. [Google Scholar] [CrossRef]
- Yamauchi, K.; Mitsunaga, T.; Muddathir, A.M. Screening for melanogenesis-controlled agents using Sudanese medicinal plants and identification of active compounds in the methanol extract of Terminalia brownii bark. J. Wood Sci. 2016, 62, 285–293. [Google Scholar] [CrossRef]
- Chang, H.; Choi, H.; Joo, K.M.; Kim, D.; Lee, T.R. Manassantin B inhibits melanosome transport in melanocytes by disrupting the melanophilin–myosin Va interaction. Pigment. Cell Melanoma Res. 2012, 25, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Comparative study of doxycycline, sancycline, and 4-dedimethylamino sancycline (CMT-3) on epidermal melanogenesis. Arch. Dermatol. Res. 2021, 315, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.K.; Subedi, L.; Jeong, M.; Park, Y.U.; Kim, C.Y.; Kim, H.; Kim, S.Y. Gomisin N Inhibits Melanogenesis through Regulating the PI3K/Akt and MAPK/ERK Signaling Pathways in Melanocytes. Int. J. Mol. Sci. 2017, 18, 471. [Google Scholar] [CrossRef]
- D’mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, J.H.; Kang, B.W.; Chung, K.T.; Choi, Y.H.; Choi, B.T. Dichloromethane fraction of Cimicifuga heracleifolia decreases the level of melanin synthesis by activating the ERK or AKT signaling pathway in B16F10 cells. Exp. Dermatol. 2009, 18, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, L.; Sun, Y.; Zhou, J.; Gu, Y.; Li, Y. Baicalein inhibits melanogenesis through activation of the ERK signaling pathway. Int. J. Mol. Med. 2010, 25, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Balcos, M.C.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Kim, M.K.; Kim, D.S. ERK Activation by Fucoidan Leads to Inhibition of Melanogenesis in Mel-Ab Cells. Korean J. Physiol. Pharmacol. 2015, 19, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Su, T.R.; Lin, J.J.; Tsai, C.C.; Huang, T.K.; Yang, Z.Y.; Wu, M.O.; Zheng, Y.Q.; Su, C.C.; Wu, Y.J. Inhibition of melanogenesis by gallic acid: Possible involvement of the PI3K/Akt, MEK/ERK and Wnt/beta-catenin signaling pathways in B16F10 cells. Int. J. Mol. Sci. 2013, 14, 20443–20458. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Brenner, M.; Hearing, V.J. The regulation of skin pigmentation. J. Biol. Chem. 2007, 282, 27557–27561. [Google Scholar] [CrossRef]
- Widlund, H.R.; Fisher, D.E. Microphthalamia-associated transcription factor: A critical regulator of pigment cell development and survival. Oncogene 2003, 22, 3035–3041. [Google Scholar] [CrossRef] [PubMed]
- Chiaverini, C.; Beuret, L.; Flori, E.; Abbe, P.; Bille, K.; Bahadoran, P.; Ortonne, J.-P.; Bertolotto, C.; Ballotti, R. Microphthalmia-associated transcription factor regulates RAB27A gene expression and controls melanosome transport. J. Biol. Chem. 2008, 283, 12635–12642. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.L.; Evans, R.D.; Sivarasa, K.; Ramalho, J.S.; Briggs, D.A.; Hume, A.N. The adaptor protein melanophilin regulates dynamic myosin-Va: Cargo interaction and dendrite development in melanocytes. Mol. Biol. Cell 2019, 30, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Strom, M.; Hume, A.N.; Tarafder, A.K.; Barkagianni, E.; Seabra, M.C. A family of Rab27-binding proteins: Melanophilin links Rab27a and myosin Va function in melanosome transport. J. Biol. Chem. 2002, 277, 25423–25430. [Google Scholar] [CrossRef]
- Bicho, N.C.; Leitao, A.E.; Ramalho, J.C.; Lidon, F.C. Identification of chemical clusters discriminators of the roast degree in Arabica and Robusta coffee beans. Eur. Food Res. Technol. 2011, 233, 303–311. [Google Scholar] [CrossRef]
- Kitzberger, C.S.G.; Dos Santos Scholz, M.B.; Pereira, L.F.P.; Vieira, L.G.E.; Sera, T.; Silva, J.B.G.D.; De Toledo Benassi, M. Diterpenes in green and roasted coffee of Coffea arabica cultivars growing in the same edapho-climatic conditions. J. Food Compos. Anal. 2013, 30, 52–57. [Google Scholar] [CrossRef]
- Dias, R.C.E.; De Faria-Machado, A.F.; Mercadante, A.Z.; Bragagnolo, N.; Benassi, M.D.T. Roasting process affects the profile of diterpenes in coffee. Eur. Food Res. Technol. 2014, 239, 961–970. [Google Scholar] [CrossRef]
- Gross, G.; Jaccaud, E.; Huggett, A. Analysis of the content of the diterpenes cafestol and kahweol in coffee brews. Food Chem. Toxicol. 1997, 35, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Moeenfard, M.; Alves, A. New trends in coffee diterpenes research from technological to health aspects. Food Res. Int. 2020, 134, 109207. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and kahweol: A review on their bioactivities and pharmacological properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Izumi, K.; Hiratsuka, K.; Kano, H.; Shimada, T.; Nakano, T.; Kadomoto, S.; Naito, R.; Iwamoto, H.; Yaegashi, H. Anti-proliferative and anti-migratory properties of coffee diterpenes kahweol acetate and cafestol in human renal cancer cells. Sci. Rep. 2021, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-H.; Seo, Y.-K.; Yoon, H.-H.; Song, K.-Y.; Park, J.-K. Effect of keratinocytes on regulation of melanogenesis in culture of melanocytes. Biotechnol. Bioprocess Eng. 2012, 17, 203–210. [Google Scholar] [CrossRef]
- Chen, N.; Hu, Y.; Li, W.H.; Eisinger, M.; Seiberg, M.; Lin, C.B. The The role of keratinocyte growth factor in melanogenesis: A possible mechanism for the initiation of solar lentigines. Exp. Dermatol. 2010, 19, 865–872. [Google Scholar] [CrossRef]
- Prospéri, M.-T.; Giordano, C.; Gomez-Duro, M.; Hurbain, I.; Macé, A.-S.; Raposo, G.; D’angelo, G. Extracellular vesicles released by keratinocytes regulate melanosome maturation, melanocyte dendricity, and pigment transfer. Proc. Natl. Acad. Sci. USA 2024, 121, e2321323121. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Izumi, K.; Natsagdorj, A.; Naito, R.; Makino, T.; Kadomoto, S.; Hiratsuka, K.; Shigehara, K.; Kadono, Y.; Narimoto, K. Coffee diterpenes kahweol acetate and cafestol synergistically inhibit the proliferation and migration of prostate cancer cells. Prostate 2019, 79, 468–479. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenka, S. Two Coffee Diterpenes, Kahweol and Cafestol, Inhibit Extracellular Melanogenesis: An In Vitro Pilot Study. Biologics 2024, 4, 202-217. https://doi.org/10.3390/biologics4020014
Goenka S. Two Coffee Diterpenes, Kahweol and Cafestol, Inhibit Extracellular Melanogenesis: An In Vitro Pilot Study. Biologics. 2024; 4(2):202-217. https://doi.org/10.3390/biologics4020014
Chicago/Turabian StyleGoenka, Shilpi. 2024. "Two Coffee Diterpenes, Kahweol and Cafestol, Inhibit Extracellular Melanogenesis: An In Vitro Pilot Study" Biologics 4, no. 2: 202-217. https://doi.org/10.3390/biologics4020014
APA StyleGoenka, S. (2024). Two Coffee Diterpenes, Kahweol and Cafestol, Inhibit Extracellular Melanogenesis: An In Vitro Pilot Study. Biologics, 4(2), 202-217. https://doi.org/10.3390/biologics4020014






