Advance in Hydrothermal Bio-Oil Preparation from Lignocellulose: Effect of Raw Materials and Their Tissue Structures
Abstract
:1. Introduction
2. Structure of Lignocelluloses and Their Hydrothermal Liquefaction Mechanism
2.1. Structure of Plants
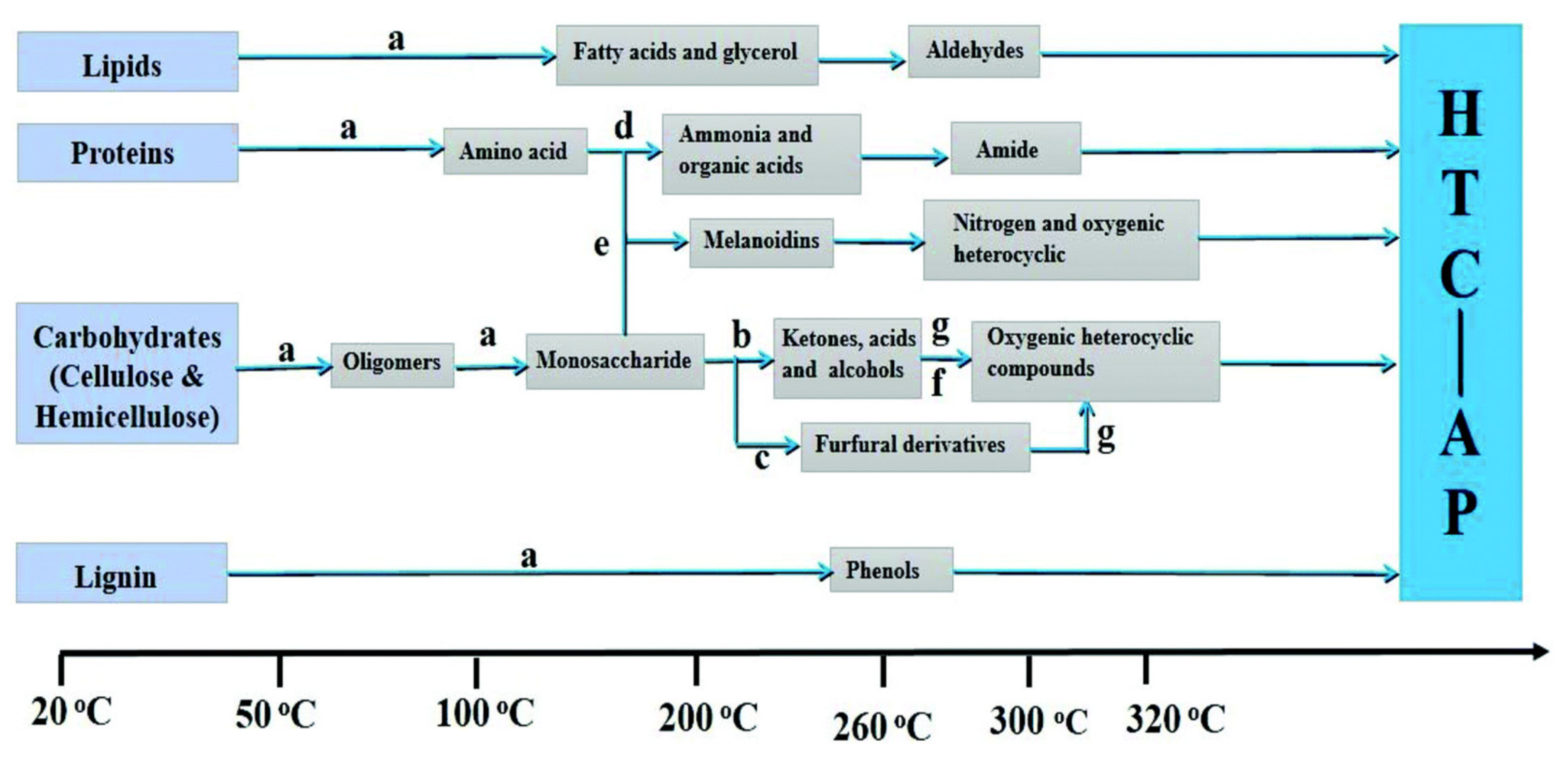
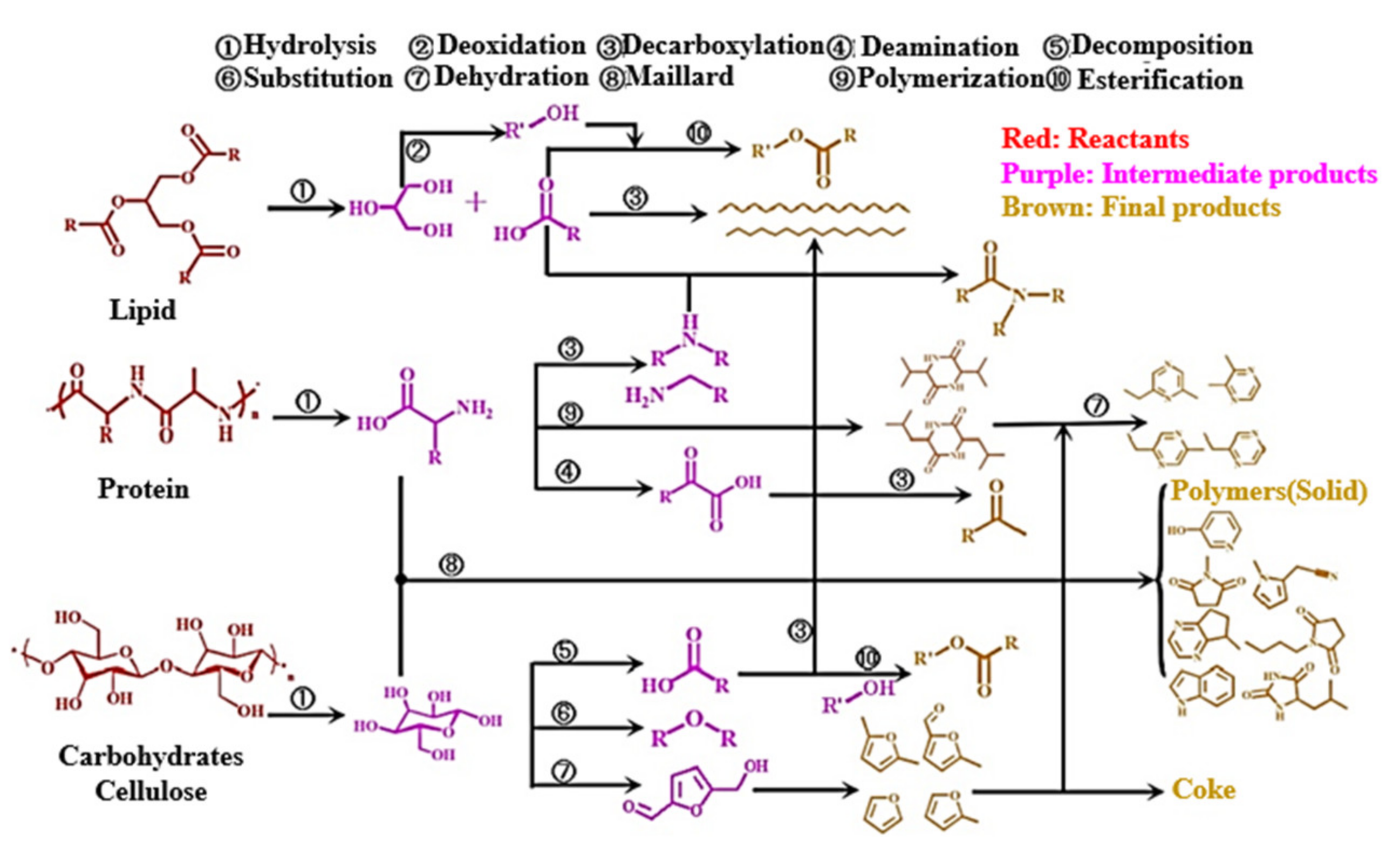
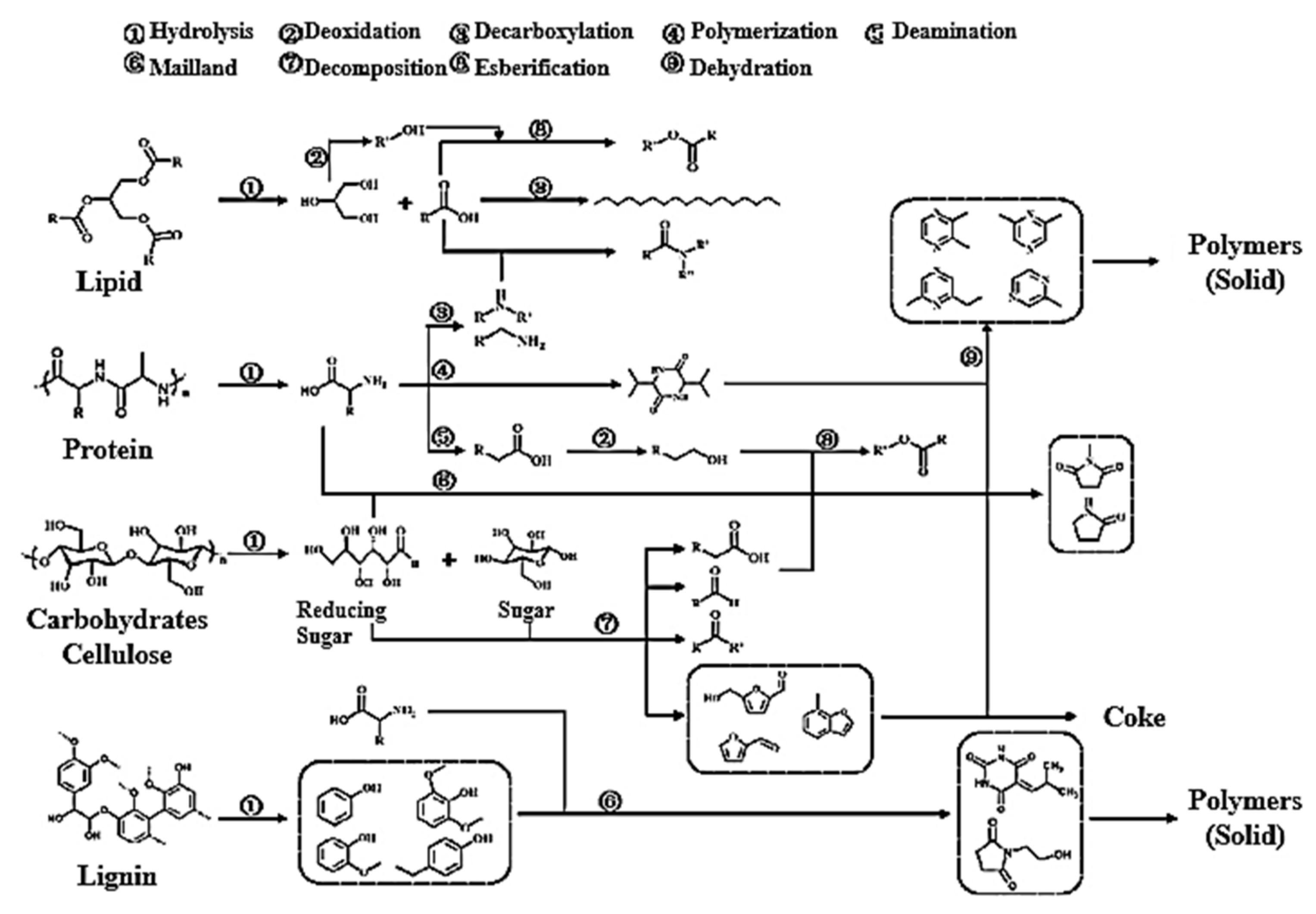
2.2. Hydrothermal Process and Product Distribution of Cellulose, Hemicellulose, and Lignin
3. Research Progress on the Effect of Lignocellulosic Tissue Structures on Their Hydrothermal Process
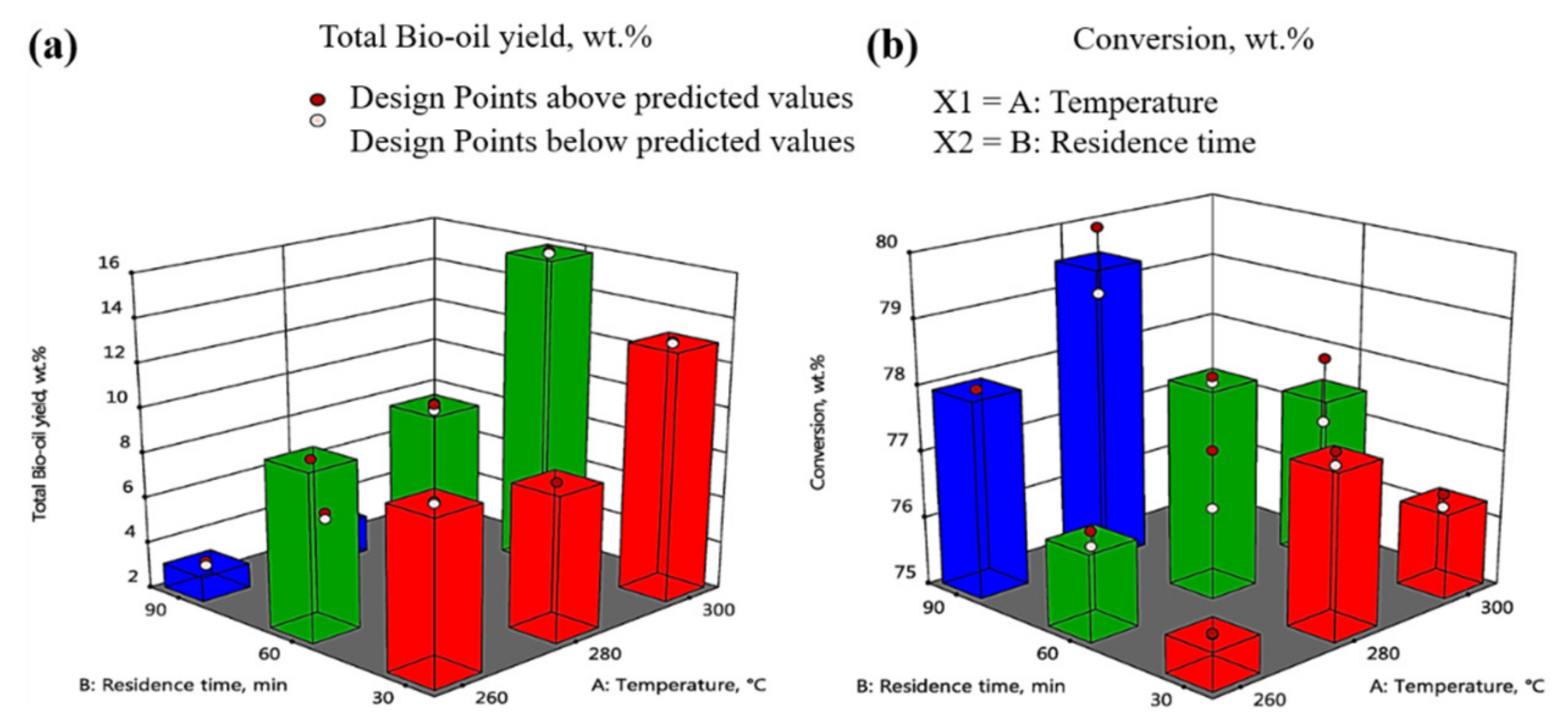
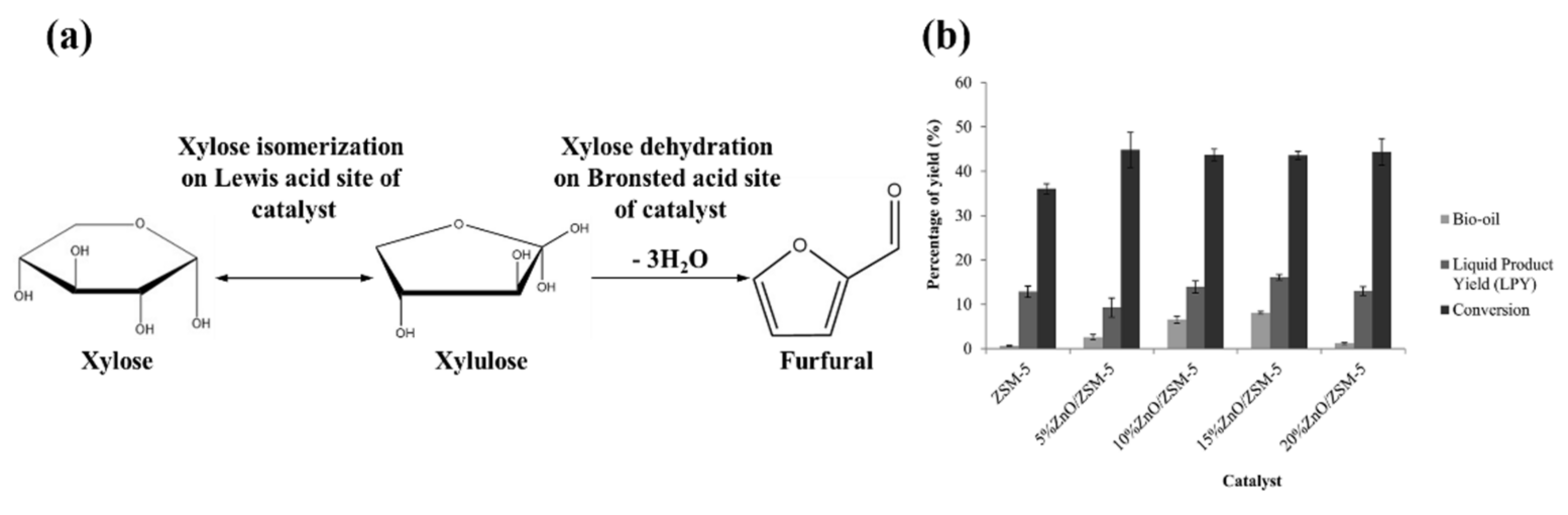
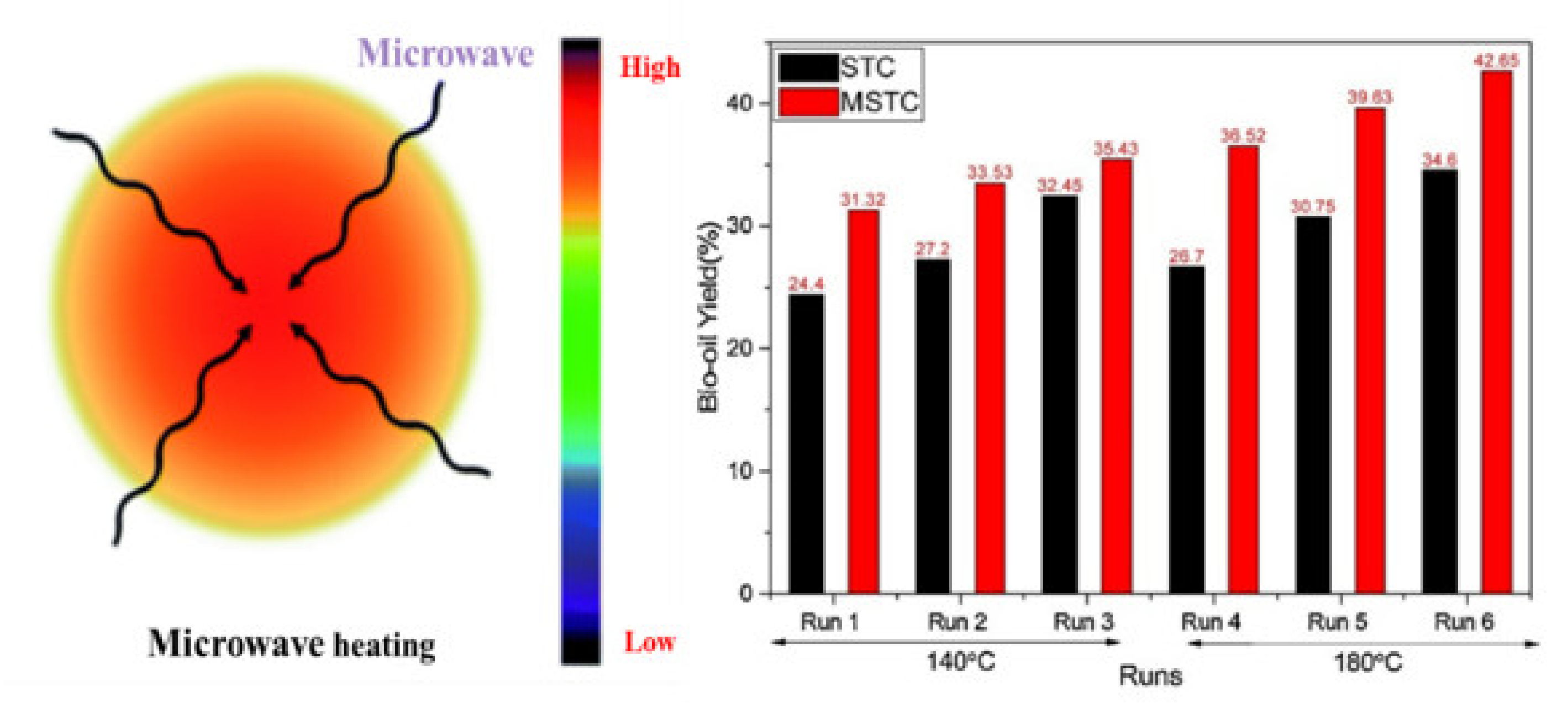
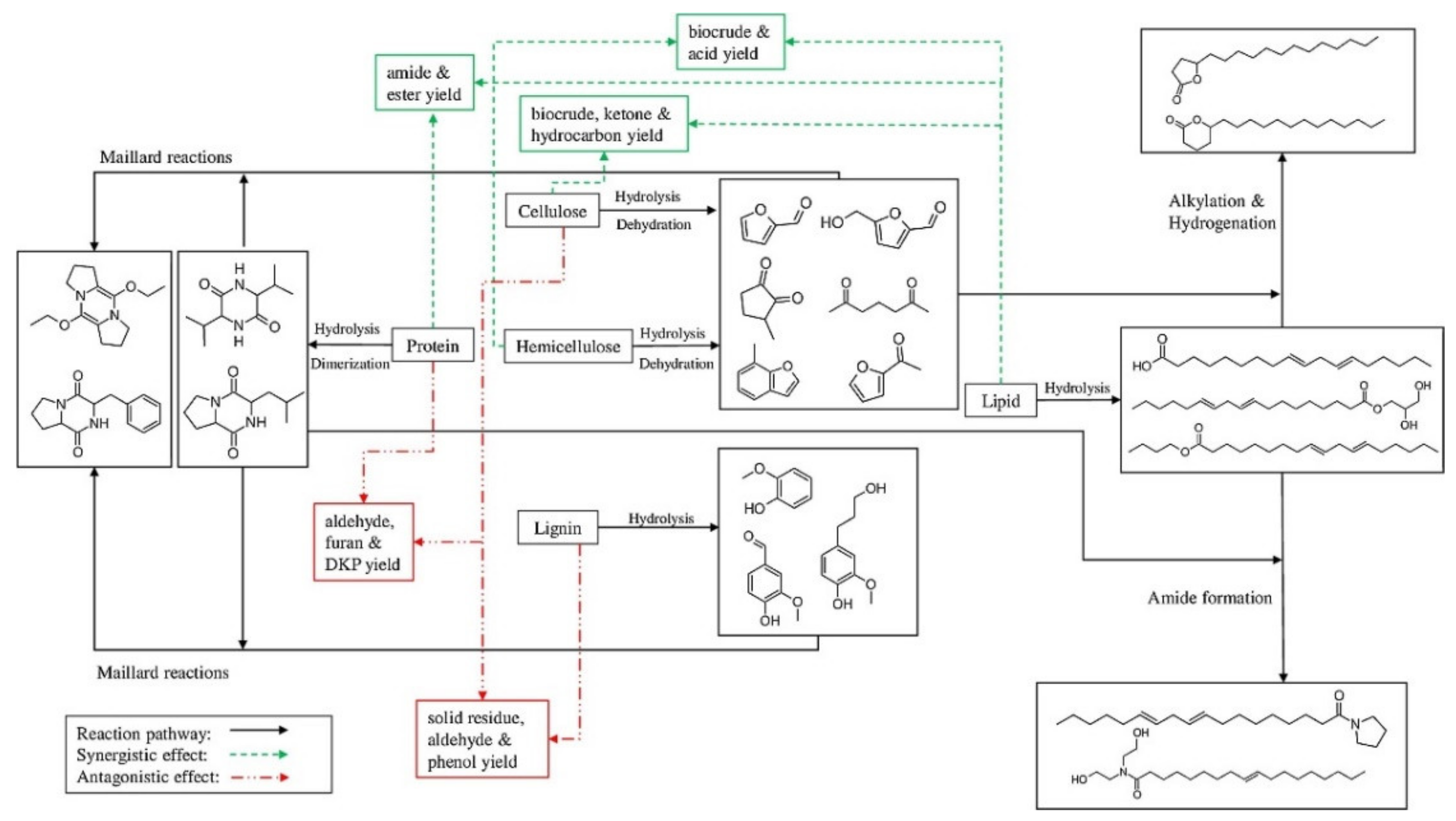
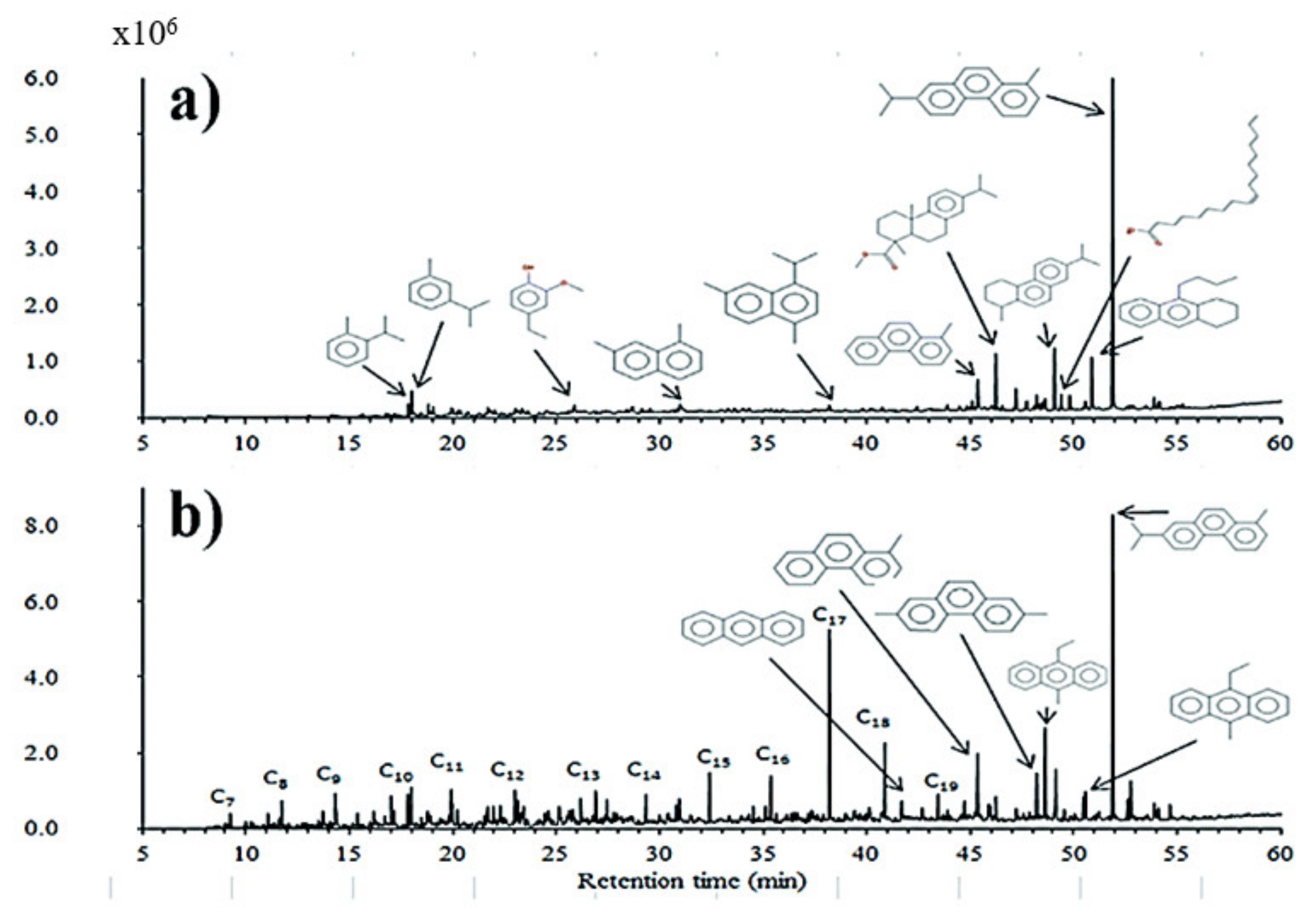
3.1. Interaction among Cellulose, Hemicellulose, and Lignin Components
59.8 × X2 X4 − 65.6 × X3 X4 − 25.46 × X3 X4 X5 − 18.93 × X1 X4 X6 − 38.63 × X1 X4 X7
4.349 × X3 X4 + 0.455 × X3 X5 − 2.957 × X1 X2 X5 − 3.396 × X2 X3 X5 − 1.838 × X1 X2 X6 −
0.339 × X2 X7 − 0.359 × X3 X7
3.2. Interaction between Cellulose, Hemicellulose, or Lignin Components and Real Lignocellulosic Raw Materials during the Thermal Process
3.3. Real Biomasses and Their Structure Effects on Their HT Bio-Oil Preparation
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cecilia, J.A.; García-Sancho, C.; Maireles-Torres, P.J.; Luque, R. Industrial Food Waste Valorization: A General Overview. In Biorefinery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 253–277. [Google Scholar] [CrossRef]
- Thombs, R.P. Has the relationship between non-fossil fuel energy sources and CO2 emissions changed over time? A cross-national study, 2000–2013. Clim. Change 2018, 148, 481–490. [Google Scholar] [CrossRef]
- Liu, D.; Vonwiller, M.; Li, J.; Liu, J.; Szidat, S.; Zhang, Y.; Tian, C.; Chen, Y.; Cheng, Z.; Zhong, G.J.A.; et al. Fossil and Non-fossil Fuel Sources of Organic and Elemental Carbonaceous Aerosol in Beijing, Shanghai, and Guangzhou: Seasonal Carbon Source Variation. Aerosol Air Qual. Res. 2020, 20, 2495–2506. [Google Scholar] [CrossRef]
- Zhou, M.; Hu, T. Analysis of carbon emission status under the carbon neutral target in China for Earth’s atmospheric balance. IOP Conf. Ser. Earth Environ. Sci. 2021, 804, 042082. [Google Scholar] [CrossRef]
- Xie, W.; Guo, W.; Shao, W.; Li, F.; Tang, Z. Environmental and Health Co-Benefits of Coal Regulation under the Carbon Neutral Target: A Case Study in Anhui Province, China. Sustainability 2021, 13, 6498. [Google Scholar] [CrossRef]
- Grande, L.; Pedroarena, I.; Korili, S.A.; Gil, A. Hydrothermal Liquefaction of Biomass as One of the Most Promising Alternatives for the Synthesis of Advanced Liquid Biofuels: A Review. Materials 2021, 14, 5286. [Google Scholar] [CrossRef]
- Biller, P.; Roth, A. Hydrothermal Liquefaction: A Promising Pathway Towards Renewable Jet Fuel. In Biokerosene: Status and Prospects; Kaltschmitt, M., Neuling, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 607–635. [Google Scholar]
- Sharma, R.; Jasrotia, K.; Singh, N.; Ghosh, P.; Srivastava, S.; Sharma, N.R.; Singh, J.; Kanwar, R.; Kumar, A. A Comprehensive Review on Hydrothermal Carbonization of Biomass and its Applications. Chem. Afr. 2020, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Ponnusamy, V.K.; Nagappan, S.; Bhosale, R.R.; Lay, C.-H.; Nguyen, D.D.; Pugazhendhi, A.; Chang, S.W.; Kumar, G. Review on sustainable production of biochar through hydrothermal liquefaction: Physico-chemical properties and applications. Bioresour. Technol. 2020, 310, 123414. [Google Scholar] [CrossRef]
- Beims, R.F.; Hu, Y.; Shui, H.; Xu, C. Hydrothermal liquefaction of biomass to fuels and value-added chemicals: Products applications and challenges to develop large-scale operations. Biomass Bioenergy 2020, 135, 105510. [Google Scholar] [CrossRef]
- Leng, L.; Zhang, W.; Peng, H.; Li, H.; Jiang, S.; Huang, H. Nitrogen in bio-oil produced from hydrothermal liquefaction of biomass: A review. Chem. Eng. J. 2020, 401, 126030. [Google Scholar] [CrossRef]
- Hao, B.; Xu, D.; Jiang, G.; Sabri, T.A.; Jing, Z.; Guo, Y. Chemical reactions in the hydrothermal liquefaction of biomass and in the catalytic hydrogenation upgrading of biocrude. Green Chem. 2021, 23, 1562–1583. [Google Scholar] [CrossRef]
- De Blasio, C. Notions of Biomass Gasification. In Fundamentals of Biofuels Engineering and Technology; De Blasio, C., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 307–334. [Google Scholar]
- Özdenkçi, K.; Prestipino, M.; Björklund-Sänkiaho, M.; Galvagno, A.; De Blasio, C. Alternative energy valorization routes of black liquor by stepwise supercritical water gasification: Effect of process parameters on hydrogen yield and energy efficiency. Renew. Sustain. Energy Rev. 2020, 134, 110146. [Google Scholar] [CrossRef]
- Hossain, M.M.; Scott, I.M.; Berruti, F.; Briens, C. Application of Novel Pyrolysis Reactor Technology to Concentrate Bio-oil Components with Antioxidant Activity from Tobacco, Tomato and Coffee Ground Biomass. Waste Biomass Valorization 2018, 9, 1607–1617. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Z.; Chen, X.; Hasan, M.R.M. Preparation process of bio-oil and bio-asphalt, their performance, and the application of bio-asphalt: A comprehensive review. J. Traffic Transp. Eng. 2020, 7, 137–151. [Google Scholar] [CrossRef]
- Zhang, Z.; Macquarrie, D.J.; De Bruyn, M.; Budarin, V.L.; Hunt, A.J.; Gronnow, M.J.; Fan, J.; Shuttleworth, P.S.; Clark, J.H.; Matharu, A.S. Low-temperature microwave-assisted pyrolysis of waste office paper and the application of bio-oil as an Al adhesive. Green Chem. 2015, 17, 260–270. [Google Scholar] [CrossRef] [Green Version]
- De Blasio, C. Integrated Biorefinery Concepts. In Fundamentals of Biofuels Engineering and Technology; Springer International Publishing: Cham, Switzerland, 2019; pp. 155–171. [Google Scholar]
- Ozdenkci, K.; De Blasio, C.; Muddassar, H.R.; Melin, K.; Oinas, P.; Koskinen, J.; Sarwar, G.; Järvinen, M. A novel biorefinery integration concept for lignocellulosic biomass. Energy Convers. Manag. 2017, 149, 974–987. [Google Scholar] [CrossRef] [Green Version]
- Ahamed, T.S.; Anto, S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Upgrading of bio-oil from thermochemical conversion of various biomass–Mechanism, challenges and opportunities. Fuel 2021, 287, 119329. [Google Scholar] [CrossRef]
- Sahoo, A.; Saini, K.; Jindal, M.; Bhaskar, T.; Pant, K.K. Co-Hydrothermal Liquefaction of algal and lignocellulosic biomass: Status and perspectives. Bioresour. Technol. 2021, 342, 125948. [Google Scholar] [CrossRef]
- Cheng, F.; Cui, Z.; Chen, L.; Jarvis, J.; Paz, N.; Schaub, T.; Nirmalakhandan, N.; Brewer, C.E. Hydrothermal liquefaction of high- and low-lipid algae: Bio-crude oil chemistry. Appl. Energy 2017, 206, 278–292. [Google Scholar] [CrossRef]
- Chen, H.; Luo, G.; Zhang, S. Hydrothermal Liquefaction of Lignocellulosic Biomass for Bioenergy Production. In Sustainable Resource Management: Technologies for Recovery and Reuse of Energy and Waste Materials; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Optimization of process parameters for hydrothermal conversion of castor residue. Sci. Total Environ. 2019, 686, 641–647. [Google Scholar] [CrossRef]
- Çolak, U.; Durak, H.; Genel, S. Hydrothermal liquefaction of Syrian mesquite (Prosopis farcta): Effects of operating parameters on product yields and characterization by different analysis methods. J. Supercrit. Fluids 2018, 140, 53–61. [Google Scholar] [CrossRef]
- Wang, X.; Xie, X.-A.; Sun, J.; Liao, W. Effects of liquefaction parameters of cellulose in supercritical solvents of methanol, ethanol and acetone on products yield and compositions. Bioresour. Technol. 2019, 275, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Yücedağ, E.; Durak, H. Bio-oil and bio-char from lactuca scariola: Significance of catalyst and temperature for assessing yield and quality of pyrolysis. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 1–14. [Google Scholar] [CrossRef]
- Rachel-Tang, D.Y.; Islam, A.; Taufiq-Yap, Y.H. Bio-oil production via catalytic solvolysis of biomass. RSC Adv. 2017, 7, 7820–7830. [Google Scholar] [CrossRef] [Green Version]
- Remón, J.; Randall, J.; Budarin, V.L.; Clark, J.H. Production of bio-fuels and chemicals by microwave-assisted, catalytic, hydrothermal liquefaction (MAC-HTL) of a mixture of pine and spruce biomass. Green Chem. 2019, 21, 284–299. [Google Scholar] [CrossRef]
- Yang, J.; Niu, H.; Corscadden, K.; He, Q.; Zhou, N. MW-assisted hydrothermal liquefaction of spent coffee grounds. Can. J. Chem. Eng. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Gao, Y.; Remón, J.; Matharu, A.S. Microwave-assisted hydrothermal treatments for biomass valorisation: A critical review. Green Chem. 2021, 23, 3502–3525. [Google Scholar] [CrossRef]
- Siddiqui, M.T.H.; Chan, F.L.; Nizamuddin, S.; Baloch, H.A.; Kundu, S.; Czajka, M.; Griffin, G.; Tanksale, A.; Shah, K.; Srinivasan, M. Comparative study of microwave and conventional solvothermal synthesis for magnetic carbon nanocomposites and bio-oil from rice husk. J. Environ. Chem. Eng. 2019, 7, 103266. [Google Scholar] [CrossRef]
- Yang, C.; Wang, S.; Ren, M.; Li, Y.; Song, W. Hydrothermal Liquefaction of an Animal Carcass for Biocrude Oil. Energy Fuels 2019, 33, 11302–11309. [Google Scholar] [CrossRef]
- Montesantos, N.; Maschietti, M. Supercritical Carbon Dioxide Extraction of Lignocellulosic Bio-Oils: The Potential of Fuel Upgrading and Chemical Recovery. Energies 2020, 13, 1600. [Google Scholar] [CrossRef] [Green Version]
- Pang, S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Anastasakis, K.; Biller, P.; Madsen, R.B.; Glasius, M.; Johannsen, I. Continuous Hydrothermal Liquefaction of Biomass in a Novel Pilot Plant with Heat Recovery and Hydraulic Oscillation. Energies 2018, 11, 2695. [Google Scholar] [CrossRef] [Green Version]
- Lane, J. The Silver in Silva: The Story of Steeper Energy and SGF’s $59M Advanced Biofuels Project in Norway. Available online: http://www.biofuelsdigest.com/bdigest/2018/01/16/the-silver-in-silva-thestory-of-steeper-energys-59m-advanced-biofuels-project-in-norway (accessed on 17 April 2018).
- Muradel Pty Ltd. Corporate Website. Available online: http://www.muradel.com.au (accessed on 26 April 2018).
- Chinnasamy, S.; Bhaskar, S.; Nallasivam, J.; Kumar Ratha, S.; Lewis, D.M.; Meenakshisundaram, A.; Lavanya, M.; Selvavathi, C. Method for Processing Algae, Carbonaceous Feedstocks, and Their Mixtures to Biocrude and Its Conversion into Biofuel Products. U.S. Patent 2017/0198223 A1, 13 July 2017. [Google Scholar]
- Xu, Y.-H.; Li, M.-F. Hydrothermal liquefaction of lignocellulose for value-added products: Mechanism, parameter and production application. Bioresour. Technol. 2021, 342, 126035. [Google Scholar] [CrossRef]
- De Caprariis, B.; Bavasso, I.; Bracciale, M.P.; Damizia, M.; De Filippis, P.; Scarsella, M. Enhanced bio-crude yield and quality by reductive hydrothermal liquefaction of oak wood biomass: Effect of iron addition. J. Anal. Appl. Pyrolysis 2019, 139, 123–130. [Google Scholar] [CrossRef]
- Ding, Y.-J.; Zhao, C.-X.; Liu, Z.-C. Catalytic hydrothermal liquefaction of rice straw for production of monomers phenol over metal supported mesoporous catalyst. Bioresour. Technol. 2019, 294, 122097. [Google Scholar] [CrossRef]
- Wu, X.-F.; Zhou, Q.; Li, M.-F.; Li, S.-X.; Bian, J.; Peng, F. Conversion of poplar into bio-oil via subcritical hydrothermal liquefaction: Structure and antioxidant capacity. Bioresour. Technol. 2018, 270, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Mohanty, K. Co-HTL of domestic sewage sludge and wastewater treatment derived microalgal biomass—An integrated biorefinery approach for sustainable biocrude production. Energy Convers. Manag. 2020, 204, 112312. [Google Scholar] [CrossRef]
- Lozano, E.M.; Petersen, S.B.; Paulsen, M.M.; Rosendahl, L.A.; Pedersen, T.H. Techno-economic evaluation of carbon capture via physical absorption from HTL gas phase derived from woody biomass and sewage sludge. Energy Convers. Manag. X 2021, 11, 100089. [Google Scholar] [CrossRef]
- Cuello, C.; Marchand, P.; Laurans, F.; Grand-Perret, C.; Lainé-Prade, V.; Pilate, G.; Déjardin, A. ATR-FTIR Microspectroscopy Brings a Novel Insight into the Study of Cell Wall Chemistry at the Cellular Level. Front. Plant Sci. 2020, 11, 105. [Google Scholar] [CrossRef] [Green Version]
- Marmont, L.S.; Bernhardt, T.G. A conserved subcomplex within the bacterial cytokinetic ring activates cell wall synthesis by the FtsW-FtsI synthase. Proc. Natl. Acad. Sci. USA 2020, 117, 23879–23885. [Google Scholar] [CrossRef] [PubMed]
- Maaß, M.-C.; Saleh, S.; Militz, H.; Volkert, C.A. The Structural Origins of Wood Cell Wall Toughness. Adv. Mater. 2020, 32, 1907693. [Google Scholar] [CrossRef] [Green Version]
- Mika, L.T.; Cséfalvay, E.; Nemeth, A. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef] [PubMed]
- Gírio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Lukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef]
- Kobayashi, H.; Fukuoka, A. Development of Solid Catalyst–Solid Substrate Reactions for Efficient Utilization of Biomass. Bull. Chem. Soc. Jpn. 2018, 91, 29–43. [Google Scholar] [CrossRef]
- Usman, M.; Chen, H.; Chen, K.; Ren, S.; Clark, J.H.; Fan, J.; Luo, G.; Zhang, S. Characterization and utilization of aqueous products from hydrothermal conversion of biomass for bio-oil and hydro-char production: A review. Green Chem. 2019, 21, 1553–1572. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, J.; Xing, G.; Dou, X.; Guo, X. Cotton stalk-derived hydrothermal carbon for methylene blue dye removal: Investigation of the raw material plant tissues. Bioresour. Bioprocess. 2021, 8, 10. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.S.; Niu, H.; Astatkie, T.; Corscadden, K.; Shi, R. Statistical Clarification of the Hydrothermal Co-Liquefaction Effect and Investigation on the Influence of Process Variables on the Co-Liquefaction Effect. Ind. Eng. Chem. Res. 2020, 59, 2839–2848. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Özdenkci, K.; Muddassar, H.R.; Melin, K.; Golam, S.; Oinas, P. Hydrothermal Liquefaction of Softwood: Selective Chemical Production Under Oxidative Conditions. ACS Sustain. Chem. Eng. 2016, 4, 3978–3984. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Nazari, L.; Yuan, Z.; Corscadden, K.; Xu, C.C. Hydrothermal liquefaction of spent coffee grounds in water medium for bio-oil production. Biomass Bioenergy 2016, 86, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Balagurumurthy, B.; Prakash, A.; Bhaskar, T. Catalytic hydrothermal liquefaction of water hyacinth. Bioresour. Technol. 2015, 178, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Luo, G.; Zhang, S.; Chen, J. Bio-oil production from eight selected green landscaping wastes through hydrothermal liquefaction. RSC Adv. 2016, 6, 15260–15270. [Google Scholar] [CrossRef]
- De Caprariis, B.; De Filippis, P.; Petrullo, A.; Scarsella, M. Hydrothermal liquefaction of biomass: Influence of temperature and biomass composition on the bio-oil production. Fuel 2017, 208, 618–625. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, F.; Djandja, J.O.; Zhang, S.-L.; Xu, Y.-P.; Duan, P.-G. Hydrothermal liquefaction of crop straws: Effect of feedstock composition. Fuel 2020, 265, 116946. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Rosendahl, L.A. Production of fuel range oxygenates by supercritical hydrothermal liquefaction of lignocellulosic model systems. Biomass Bioenergy 2015, 83, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Liu, Z.; Zhang, Y.; Savage, P.E. Synergistic and Antagonistic Interactions during Hydrothermal Liquefaction of Soybean Oil, Soy Protein, Cellulose, Xylose, and Lignin. ACS Sustain. Chem. Eng. 2018, 6, 14501–14509. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Z.; Song, S.; Chen, H.; Wang, X.; Cheng, J.; Sun, R.; Han, J. Understanding catalytic mechanisms of HZSM-5 in hydrothermal liquefaction of algae through model components: Glucose and glutamic acid. Biomass Bioenergy 2019, 130, 105356. [Google Scholar] [CrossRef]
- Yang, J.; Niu, H.; Corscadden, K.; Astatkie, T. Hydrothermal liquefaction of biomass model components for product yield prediction and reaction pathways exploration. Appl. Energy 2018, 228, 1618–1628. [Google Scholar] [CrossRef]
- Yang, J.; Corscadden, K.; Niu, H.; Lin, J.; Astatkie, T. Advanced models for the prediction of product yield in hydrothermal liquefaction via a mixture design of biomass model components coupled with process variables. Appl. Energy 2019, 233, 906–915. [Google Scholar] [CrossRef]
- Chen, H.; He, Z.; Zhang, B.; Feng, H.; Kandasamy, S.; Wang, B. Effects of the aqueous phase recycling on bio-oil yield in hydrothermal liquefaction of Spirulina Platensis, α-cellulose, and lignin. Energy 2019, 179, 1103–1113. [Google Scholar] [CrossRef]
- Déniel, M.; Haarlemmer, G.; Roubaud, A.; Weiss-Hortala, E.; Fages, J. Hydrothermal liquefaction of blackcurrant pomace and model molecules: Understanding of reaction mechanisms. Sustain. Energy Fuels 2017, 1, 555–582. [Google Scholar] [CrossRef] [Green Version]
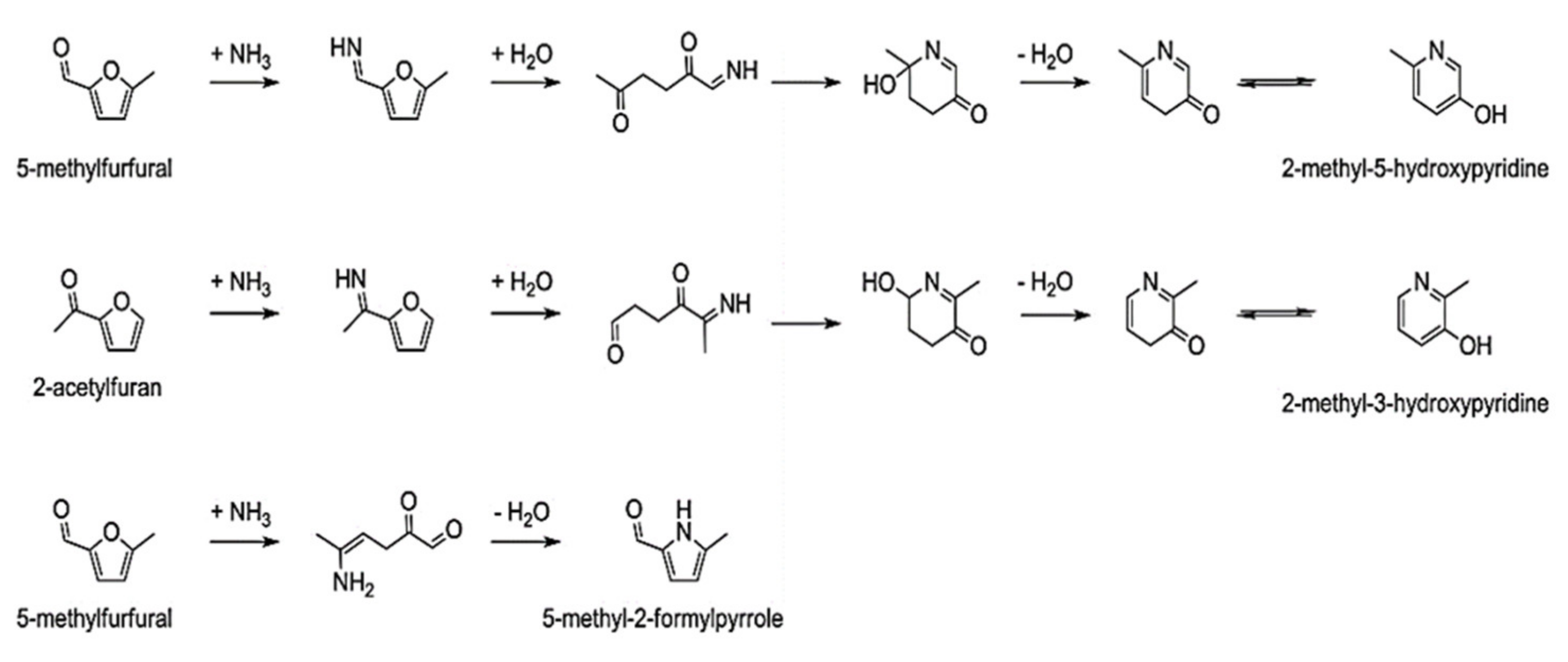
- Fan, Y.; Hornung, U.; Dahmen, N.; Kruse, A. Hydrothermal liquefaction of protein-containing biomass: Study of model compounds for Maillard reactions. Biomass Convers. Biorefinery 2018, 8, 909–923. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.; Niu, H.; Dalai, A.; Corscadden, K.; Zhou, N. Microwave-assisted hydrothermal liquefaction of biomass model components and comparison with conventional heating. Fuel 2020, 277, 118202. [Google Scholar] [CrossRef]
- Qiu, Y.; Aierzhati, A.; Cheng, J.; Guo, H.; Yang, W.; Zhang, Y. Biocrude Oil Production through the Maillard Reaction between Leucine and Glucose during Hydrothermal Liquefaction. Energy Fuels 2019, 33, 8758–8765. [Google Scholar] [CrossRef]
- Fan, Y.; Hornung, U.; Raffelt, K.; Dahmen, N. The influence of lipids on the fate of nitrogen during hydrothermal liquefaction of protein-containing biomass. J. Anal. Appl. Pyrolysis 2020, 147, 104798. [Google Scholar] [CrossRef]
- Croce, A.; Battistel, E.; Chiaberge, S.; Spera, S.; De Angelis, F.; Reale, S. A Model Study to Unravel the Complexity of Bio-Oil from Organic Wastes. ChemSusChem 2017, 10, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; He, Z.; Zhang, B.; Chen, H.; Wang, Q.; Kandasamy, S. Synergistic bio-oil production from hydrothermal co-liquefaction of Spirulina platensis and α-Cellulose. Energy 2019, 174, 1283–1291. [Google Scholar] [CrossRef]
- Jadhav, A.; Ahmed, I.; Baloch, A.G.; Jadhav, H.; Nizamuddin, S.; Siddiqui, M.T.H.; Baloch, H.A.; Qureshi, S.S.; Mubarak, N.M. Utilization of oil palm fronds for bio-oil and bio-char production using hydrothermal liquefaction technology. Biomass Convers. Biorefinery 2019, 11, 1465–1473. [Google Scholar] [CrossRef]
- Yu, J.; Biller, P.; Mamahkel, A.; Klemmer, M.; Becker, J.; Glasius, M.; Iversen, B.B. Catalytic hydrotreatment of bio-crude produced from the hydrothermal liquefaction of aspen wood: A catalyst screening and parameter optimization study. Sustain. Energy Fuels 2017, 1, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Ellersdorfer, M. Hydrothermal co-liquefaction of chlorella vulgaris with food processing residues, green waste and sewage sludge. Biomass Bioenergy 2020, 142, 105796. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, R.; Yan, C.; Han, L.; Lei, H.; Ruan, R.; Zhang, X. Fast hydrothermal co-liquefaction of corn stover and cow manure for biocrude and hydrochar production. Bioresour. Technol. 2021, 340, 125630. [Google Scholar] [CrossRef]



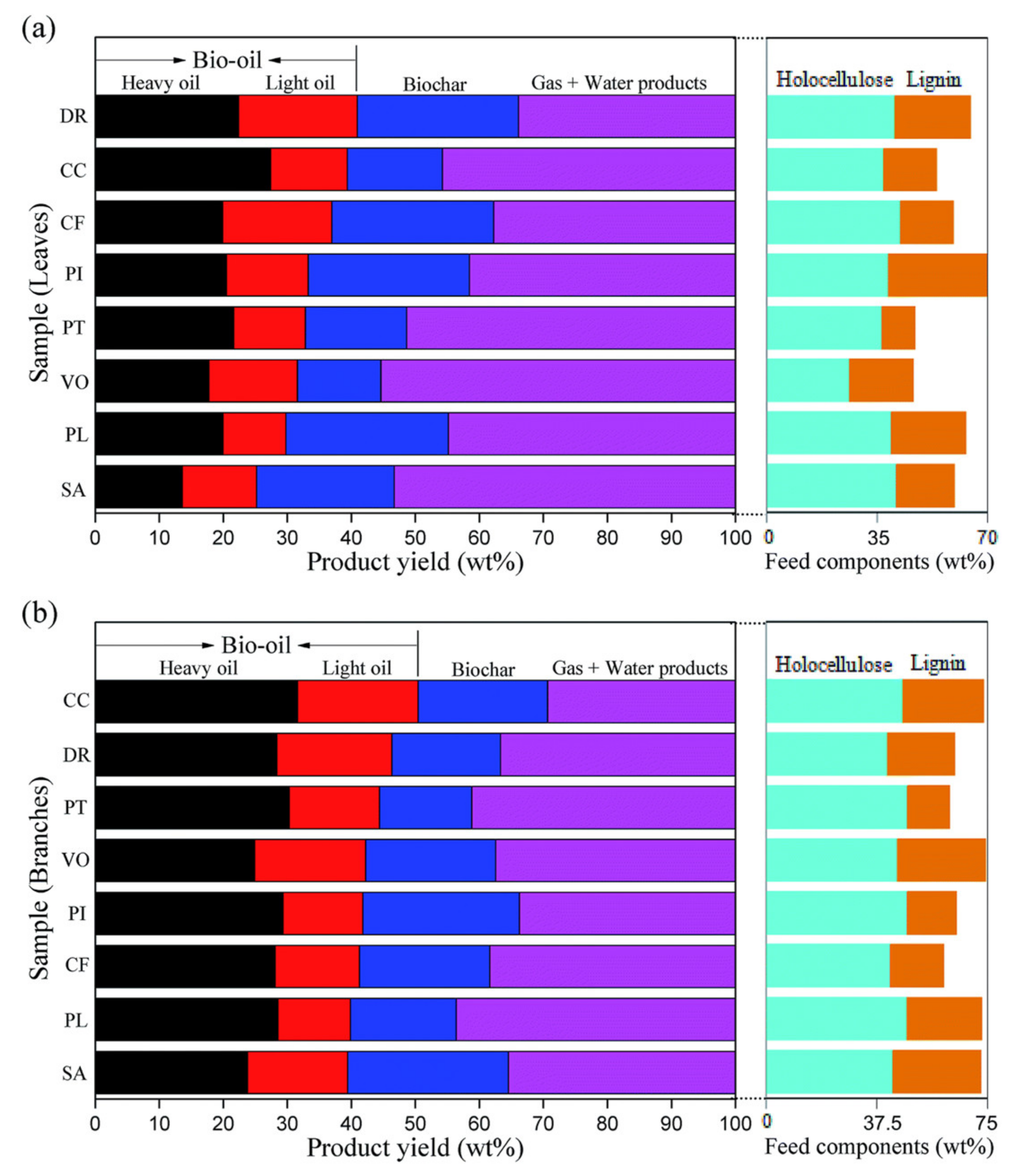

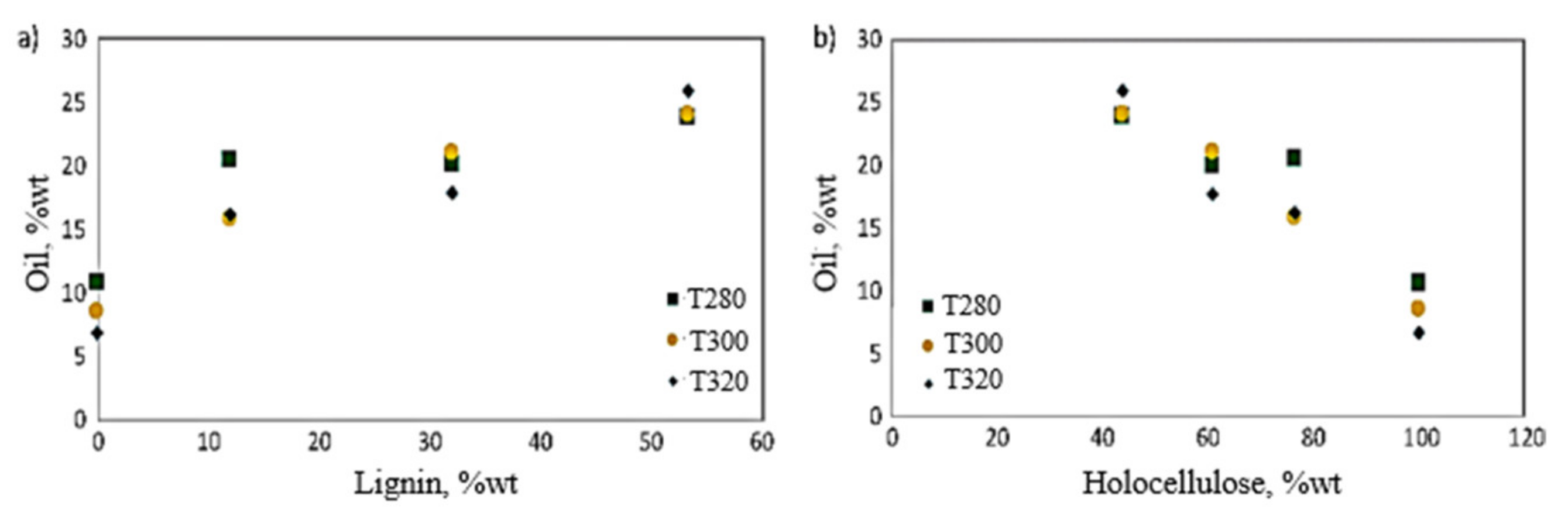
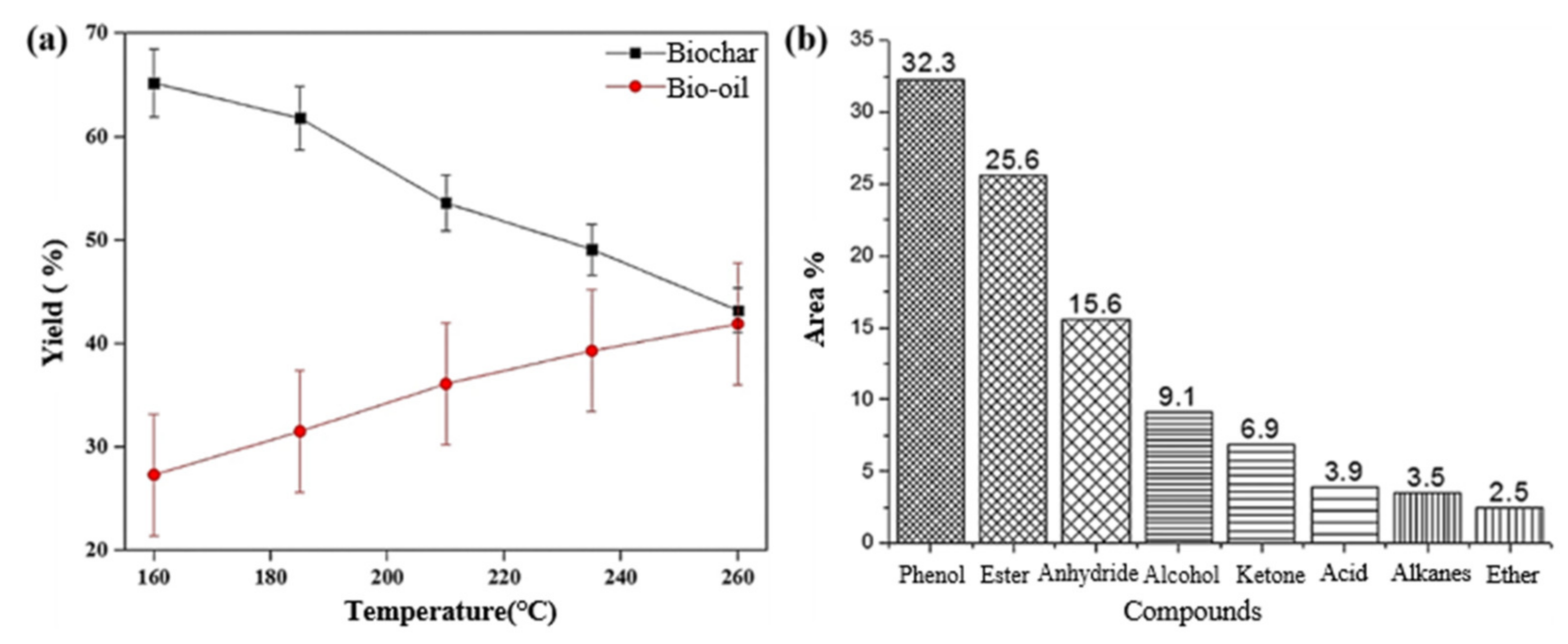
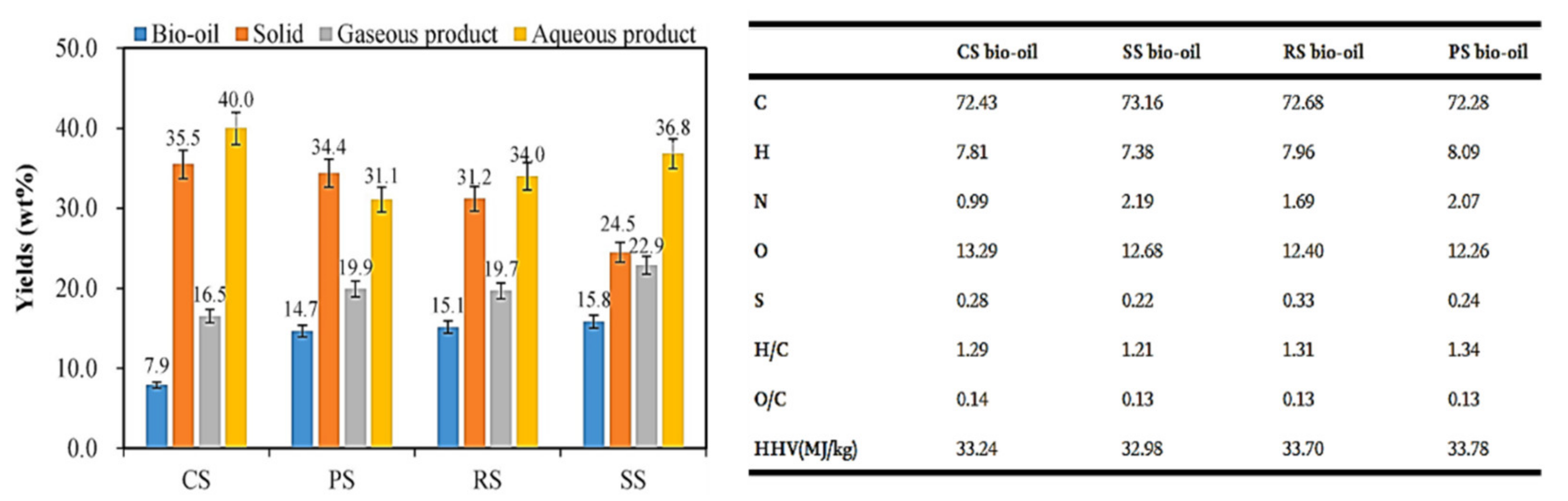
| Feedstock | Feedstock Composition or Element Composition (wt.%) | Operating Conditions | Solid–Liquid Mass Ratio | Catalyst | Bio-Oil Yield (wt.%) | Main Components of Bio-Oil | Ref. |
|---|---|---|---|---|---|---|---|
| Softwood | Lignin 28.2%, polysaccharide 70.6% ± (including 64.8% glucose, 24.5% mannose and arabinose, 6.6% xylose, and 4.1% galactose) | 270 °C, 30 min | 14:62.5 | Na2CO3 | 7.4% | Sugars, furans, organic acids, and phenolic | [55] |
| Coffee grounds | Volatile matter 82.3%, ash 1.4%, moisture 4.0%, C 50.4%, H 7.2%, N 2.1%, O 40.3% | 275 °C, 10 min | 1:20 | / | 43.7% | Long-chain aliphatic acids and esters | [56] |
| Water hyacinth | Moisture 9.54%, ash 21.80, volatile 77.85%, fixed carbon 0.35% | 280 °C, 15 min | 1:6 | KOH | 23% | Aliphatic and aromatic | [57] |
| Pinus | Branches: cellulose 26.9%, hemicellulose 20.8%, lignin 16.8%, ash 2.8%, ethanol extract 7.0%, others 25.7%; Leaves: cellulose 22.9%, hemicellulose 15.0%, lignin 31.0%, ash 1.3%, ethanol extract 8.5%, others 21.4% | 300 °C, 30 min | 1:10 | / | 29% (Branches), 20% (Leaves) | Branches: phenols, aliphatics, esters, acids, ketones, alcohols, and furans; Leaves: phenols, cyclones, ketones, and alcohols | [58] |
| Cupressus funebris | Branches: cellulose 23.0%, hemicellulose 19.0%, lignin 18.3%, ash 2.9%, ethanol extract 5.9%, others 31.0%; Leaves: cellulose 24.8%, hemicellulose 16.9%, lignin 16.8%, ash 7.4%, ethanol extract 8.5%, others 25.6% | 300 °C, 30 min | 1:10 | / | 28% (Branches), 19% (Leaves) | Branches: phenols, aliphatics, esters, acids, ketones, alcohols, and furans; Leaves: phenols, cyclones, ketones, and alcohols | [58] |
| Platanus | Branches: cellulose 27.7%, hemicellulose 19.9%, lignin 18.3%, ash 2.1%, ethanol extract 2.6%, others 22.1%; Leaves: cellulose 22.7%, hemicellulose 16.1%, lignin 23.6%, ash 6.0%, ethanol extract 5.4%, others 26.3% | 300 °C, 30 min | 1:10 | / | 29% (Branches), 19% (Leaves) | Branches: phenols, aliphatics, esters, acids, ketones, alcohols, and furans; Leaves: phenols, cyclones, ketones, and alcohols | [58] |
| Cinnamomum camphora | Branches: cellulose 27.8%, hemicellulose 18.4%, lignin 27.7%, ash 2.0%, ethanol extract 4.9%, others 19.2%; Leaves: cellulose 23.5%, hemicellulose 12.9%, lignin 16.8%, ash 8.0%, ethanol extract 5.4%, others 33.5% | 300 °C, 30 min | 1:10 | / | 32% (Branches), 26% (Leaves) | Branches: phenols, aliphatics, esters, acids, ketones, alcohols, and furans; Leaves: phenols, cyclones, ketones, and alcohols | [58] |
| Pittosporum tobira | Branches: cellulose 26.7%, hemicellulose 21.2%, lignin 14.5%, ash 5.4%, ethanol extract 5.6%, others 26.6%; Leaves: cellulose 18.2%, hemicellulose 17.6%, lignin 10.6%, ash 10.7%, ethanol extract 9.1%, others 33.8% | 300 °C, 30 min | 1:10 | / | 30% (Branches), 21% (Leaves) | Branches: phenols, aliphatics, esters, acids, ketones, alcohols, and furans; Leaves: phenols, cyclones, ketones, and alcohols | [58] |
| Distylium racemosum | Branches: cellulose 24.5%, hemicellulose 16.4%, lignin 23.1%, ash 4.6%, ethanol extract 4.5%, others 26.9%; Leaves: cellulose 23.7%, hemicellulose 16.3%, lignin 23.8%, ash 10.0%, ethanol extract 6.1%, others 20.1% | 300 °C, 30 min | 1:10 | / | 28% (Branches), 22% (Leaves) | Branches: phenols, aliphatics, esters, acids, ketones, alcohols, and furans; Leaves: phenols, cyclones, ketones, and alcohols | [58] |
| Viburnum odoratissinum | Branches: cellulose 25.4%, hemicellulose 18.9%, lignin 30.1%, ash 2.2%, ethanol extract 3.4%, others 19.9%; Leaves: cellulose 8.3%, hemicellulose 17.5%, lignin 20.1%, ash 9.8%, ethanol extract 8.1%, others 36.2% | 300 °C, 30 min | 1:10 | / | 25% (Branches), 16% (Leaves) | Branches: phenols, aliphatics, esters, acids, ketones, alcohols, and furans; Leaves: phenols, cyclones, ketones, and alcohols | [58] |
| Salix babylonica | Branches: cellulose 25.9%, hemicellulose 17.0%, lignin 30.1%, ash 1.4%, ethanol extract 3.8%, others 21.9%; Leaves: cellulose 23.2%, hemicellulose 17.3%, lignin 18.3%, ash 8.8%, ethanol extract 4.9%, others 27.6% | 300 °C, 30 min | 1:10 | / | 24% (Branches), 14% (Leaves) | Branches: phenols, aliphatics, esters, acids, ketones, alcohols, and furans; Leaves: phenols, cyclones, ketones, and alcohols | [58] |
| Poplar | N 0.91%, C 47.04%, H 5.60 %, O 43.20% | 260 °C, 30 min | 1:10 | / | 13% | Alcohols, ethers, lipids, alkanes, aldehydes, and phenols | [43] |
| Oil palm empty fruit bunch | Moisture 8.99%, dry matter 91.01%, ash 2.42%, hemicellulose 41.32%, cellulose 39.07%, lignin 34.82%, C 45.44%, H 6.22%, N 1.58%, S 0.36%, O 46.40% | 180 °C, 90 min | 1:10 | Zn/ZSM-5 | 43.6% | Furfural, ketones, aldehydes, phenol, benzoic acid, and other aromatic hydrocarbons | [28] |
| Walnut shell | Moisture 5–12%, ash 1.5%, cellulose 23.3%, hemicellulose 20.4%, lignin 53.5%, extract 2.8%, C 45.6%, H 4.3%, O 50.1% | 320 °C, 30 min | 1:5 | / | 25% | Phenols, alcohols, ketones, aldehydes, and phenol | [59] |
| Corn straw | Ash 7.00%, volatile 68.50%, fixed carbon 24.50%, cellulose 30.81%, hemicellulose 25.52%, lignin 16.76% | 320 °C, 60 min | 3:8 | / | 7.9% | Phenols, ketones, aldehydes, carboxylic acids, nitrogen-containing heterocyclic compounds, ethanol, and aromatic compounds | [60] |
| Soybean straw | Ash 4.43%, volatile 73.10%, fixed carbon 22.47%, cellulose 42.39%, hemicellulose 22.05%, lignin 18.93% | 320 °C, 60 min | 3:8 | / | 15.8% | Phenols, ketones, aldehydes, carboxylic acids, nitrogen-containing heterocyclic compounds, and olefins | [60] |
| Peanut straw | Ash 13.05%, volatile 65.50%, fixed carbon 21.45%, cellulose 36.56%, hemicellulose 20.27%, lignin 18.36% | 320 °C, 60 min | 3:8 | / | 14.6% | Phenols, ketones, aldehydes, carboxylic acids, nitrogen-containing heterocyclic compounds, ethanol, alkanes, and olefins | [60] |
| Rice straw | Ash 15.10%, volatile 64.00%, fixed carbon 20.91%, cellulose 46.33%, hemicellulose 31.09%, lignin 10.17% | 320 °C, 60 min | 3:8 | / | 15.1% | Phenols, ketones, aldehydes, carboxylic acids, nitrogen-containing heterocyclic compounds, ethanol, alkanes, and olefins | [60] |
| Oak wood | Moisture 7–15%, ash 2.0%, cellulose 38.1%, hemicellulose 23.0%, lignin 32.0%, extract 6.9%, C 50.2%, H 7.0%, O 42.8% | 320 °C, 15 min | 1:5 | Fe | 38% | Phenols, ketones, and aliphatic | [41] |
| Castor plant | Moisture 11.14%, ash 5.40%, volatile 74.30%, fixed carbon 9.16%, extract 16.40%. cellulose 38.42%, hemicellulose 22.40%, lignin 20.20% | 300 °C, 60 min | 1:6 | / | 15.9% | / | [24] |
| Rice straw | Moisture 11.8%, volatile matter 79.7%, fixed carbon 6.3%, ash 10.5%, C 37.8%, H 5.4%, O 55.8%, N 0.7%, S0.3% | 280 °C, 15 min | 1:6 | Ni-Al/SBA-15 | 56.6% | Aliphatic, phenolic, and phenol | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Dou, X.; Yang, Z.; Yang, X.; Guo, X. Advance in Hydrothermal Bio-Oil Preparation from Lignocellulose: Effect of Raw Materials and Their Tissue Structures. Biomass 2021, 1, 74-93. https://doi.org/10.3390/biomass1020006
Zhang L, Dou X, Yang Z, Yang X, Guo X. Advance in Hydrothermal Bio-Oil Preparation from Lignocellulose: Effect of Raw Materials and Their Tissue Structures. Biomass. 2021; 1(2):74-93. https://doi.org/10.3390/biomass1020006
Chicago/Turabian StyleZhang, Libo, Xintong Dou, Zhilin Yang, Xiao Yang, and Xuqiang Guo. 2021. "Advance in Hydrothermal Bio-Oil Preparation from Lignocellulose: Effect of Raw Materials and Their Tissue Structures" Biomass 1, no. 2: 74-93. https://doi.org/10.3390/biomass1020006
APA StyleZhang, L., Dou, X., Yang, Z., Yang, X., & Guo, X. (2021). Advance in Hydrothermal Bio-Oil Preparation from Lignocellulose: Effect of Raw Materials and Their Tissue Structures. Biomass, 1(2), 74-93. https://doi.org/10.3390/biomass1020006







