Production of Polyhydroxyalkanoates by Bacillus megaterium: Prospecting on Rice Hull and Residual Glycerol Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Inoculum
2.2. Culture Media
2.3. Microbial Production of Polyhydroxyalkanoates
2.4. Analytical Methods
2.4.1. Determination of Biomass
2.4.2. Quantification of Glucose, Total Sugars, and Glycerol
2.4.3. Extraction and Characterization of Polyhydroxyalkanoates
2.5. Data Analysis
3. Results
3.1. Bacterial Growth Rate
3.1.1. Glucose and Residual Glycerol as a Carbon Source
3.1.2. RHH as Alternative Carbon Source for the Polyhydroxyalkanoates Production
3.2. Extraction
3.3. Characterization of PHAs
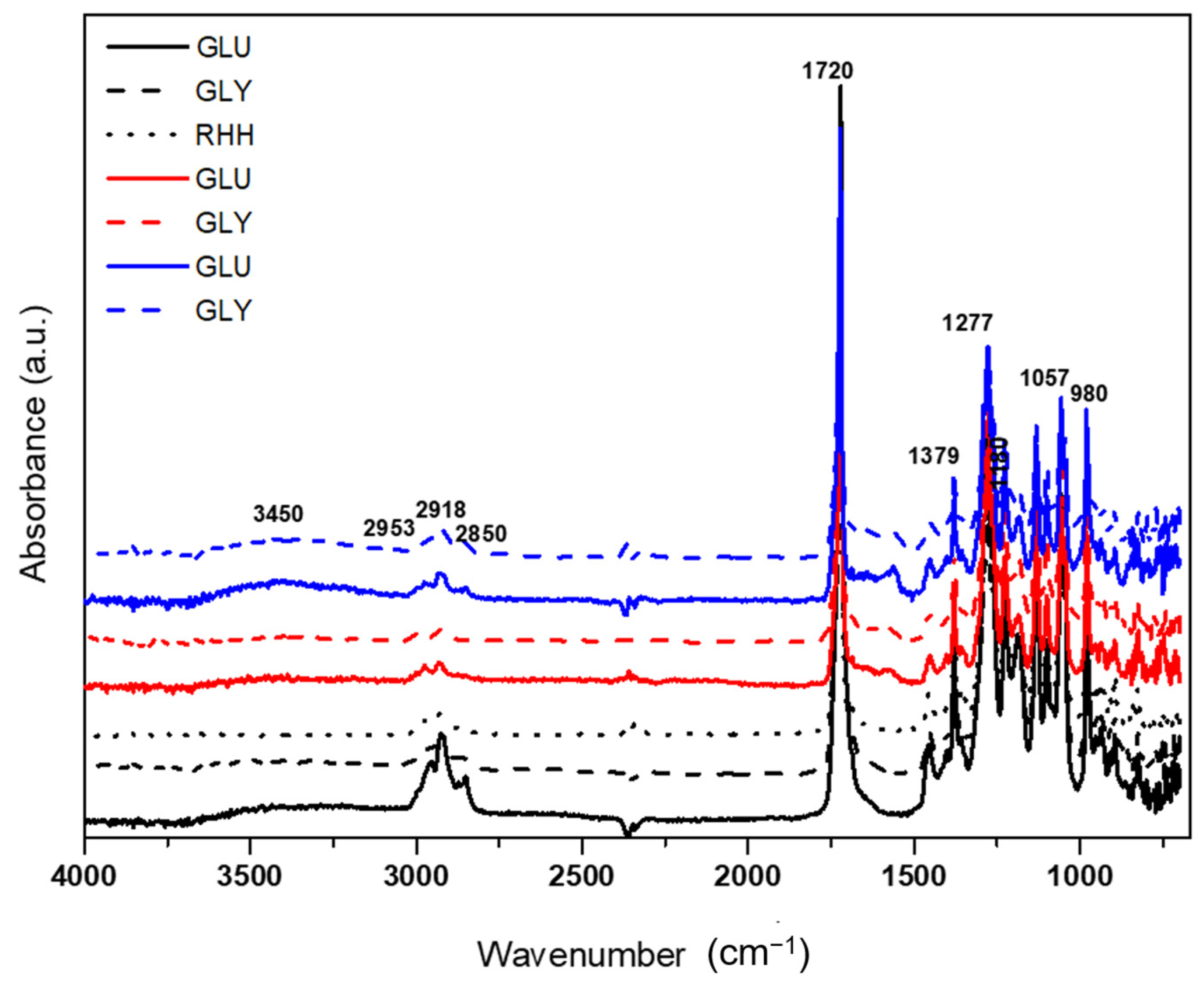
3.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
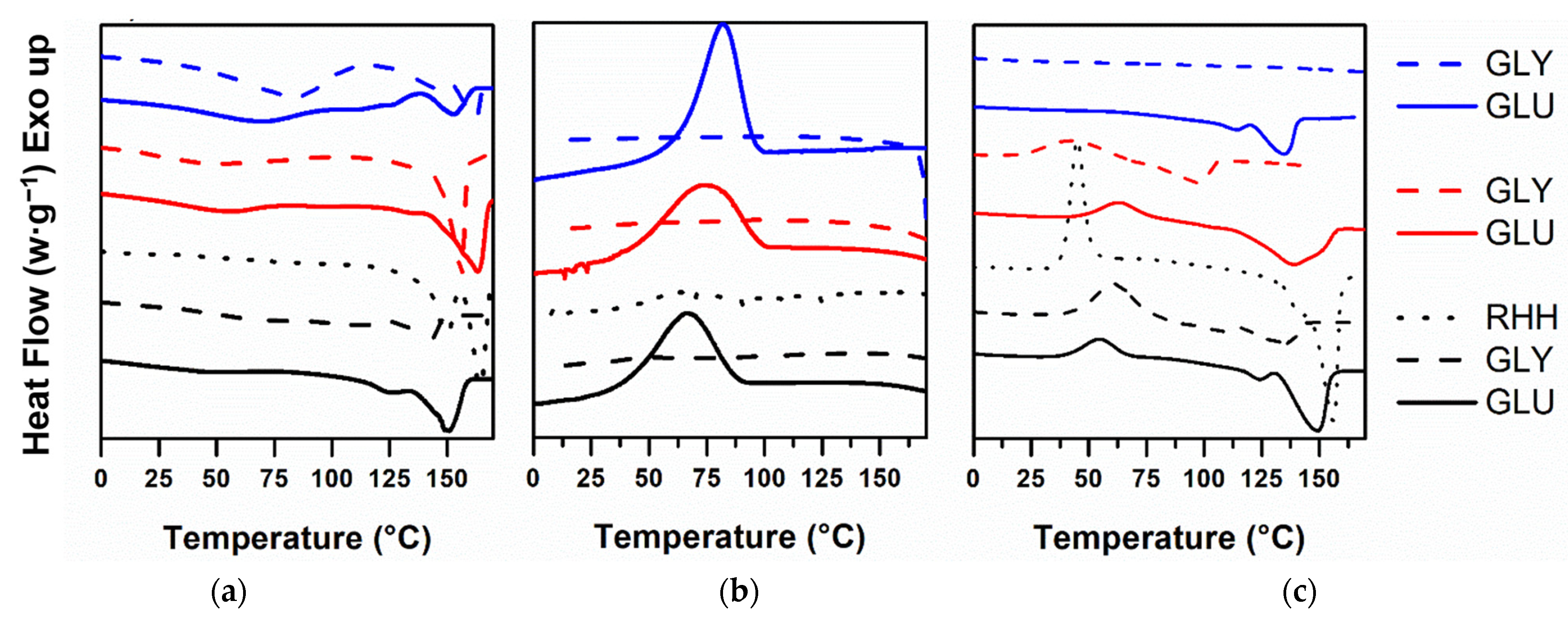
3.3.2. Differential Scanning Calorimetry (DSC)
4. Discussion
4.1. Bacterial Growth Rate
4.1.1. Glucose and Residual Glycerol as a Carbon Source
4.1.2. RHH as Alternative Carbon Source for the Polyhydroxyalkanoates Production
4.2. Extraction
4.3. Characterization of PHA
4.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
4.3.2. Differential Scanning Calorimetry (DSC)
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maniglia, B.C.; Laroque, D.A.; de Andrade, L.M.; Carciofi, B.A.M.; Tenório, J.A.S.; de Andrade, C.J. Production of active cassava starch films; effect of adding a biosurfactant or synthetic surfactant. React. Funct. Polym. 2019, 144, 104368. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour Technol. 2021, 325, 124739. [Google Scholar] [CrossRef]
- Koller, M. Chemical and biochemical engineering approaches in manufacturing polyhydroxyalkanoate (PHA) biopolyesters of tailored structure with focus on the diversity of building blocks. Chem. Biochem. Eng. Q. 2018, 32, 413–438. [Google Scholar] [CrossRef]
- Dalton, B.; Bhagabati, P.; De Micco, J.; Padamati, R.B.; O’Connor, K. A Review on Biological Synthesis of the Biodegradable Polymers Polyhydroxyalkanoates and the Development of Multiple Applications. Catalysts 2022, 12, 319. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Gutierrez-Macias, P.; De Lourdes, M.; De Jesus, H.; Barragan-Huerta, B.E. The production of biomaterials from agro-industrial waste. Fresenius Environ. Bull. 2017, 26, 4128–4152. [Google Scholar]
- Dañez, J.C.A.; Requiso, P.J.; Alfafara, C.G.; Nayve, F.R.P.; Ventura, J.R.S. Optimization of fermentation factors for polyhydroxybutyrate (PHB) production using Bacillus megaterium PNCM 1890 in simulated glucose-xylose hydrolysates from agricultural residues. Philipp J. Sci. 2020, 149, 163–175. [Google Scholar] [CrossRef]
- USDA. United States Department of Agriculture. 2019. Available online: https://www.fas.usda.gov/commodities/rice (accessed on 19 August 2020).
- CONAB. Companhia Nacional de Abastecimento. Agricultural Perspectives, Brasília. 2019; p. 112. Available online: http://www.conab.gov.br (accessed on 19 August 2020).
- Foletto, E.L.; Hoffmann, R.; Hoffmann, R.S.; Portugal, U.L.; Jahn, S.L. Applicability of rice husk ash. Química Nova 2005, 28, 1055–1060. [Google Scholar] [CrossRef]
- ANP. Agência Nacional do Petróleo, Gás Natural e Biocombustíveis, Brazilian Statistical Yearbook of Petroleum, Natural Gas and Biofuels. 2019. Available online: http://www.anp.gov.br (accessed on 19 August 2020).
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Albuquerque, P.B.S.; de Araújo, K.S.; de Silva, K.A.A.; da Houllou, L.M.; Locatelli, G.O.; Malafaia, C.B. Potential production of bioplastics polyhydroxyalkanoates using residual glycerol. J. Environ. Anal. Prog. 2018, 3, 055–060. [Google Scholar] [CrossRef]
- Faccin, D.J.; Martins, I.; Cardozo, N.S.; Rech, R.; Ayub, M.A.; Alves, T.L.; Gambetta, R.; Resende Secchi, A. Optimization of C:N ratio and minimal initial carbon source for poly(3-hydroxybutyrate) production by Bacillus megaterium. J. Chem. Technol. Biotechnol. 2009, 84, 1756–1761. [Google Scholar] [CrossRef]
- Aquino de Souza, E.; Rossi, D.M.; Záchia Ayub, M.A.Ô. Bioconversion of residual glycerol from biodiesel synthesis into 1,3-propanediol using immobilized cells of Klebsiella pneumoniae BLh-1. Renew. Energy 2014, 72, 253–257. [Google Scholar] [CrossRef]
- Hickert, L.R.; Da Cunha-Pereira, F.; De Souza-Cruz, P.B.; Rosa, C.A.; Ayub, M.A.Z. Ethanogenic fermentation of co-cultures of Candida shehatae HM 52.2 and Saccharomyces cerevisiae ICV D254 in synthetic medium and rice hull hydrolysate. Bioresour. Technol. 2013, 131, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schirmer-Michel, Â.C.; Flôres, S.H.; Hertz, P.F.; Matos, G.S.; Ayub, M.A.Z. Production of ethanol from soybean hull hydrolysate by osmotolerant Candida guilliermondii NRRL Y-2075. Bioresour. Technol. 2008, 99, 2898–2904. [Google Scholar] [CrossRef]
- Doran, P. Bioprocess Engineering Principles, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Cortivo, P.R.D.; Hickert, L.R.; Hector, R.; Ayub, M.A.Z. Fermentation of oat and soybean hull hydrolysates into ethanol and xylitol by recombinant industrial strains of Saccharomyces cerevisiae under diverse oxygen environments. Ind. Crops Prod. 2018, 113, 10–18. [Google Scholar] [CrossRef]
- Berger, E.; Ramsay, B.A.; Ramsay, J.A.; Chavarie, C.; Braunegg, G. PHB recovery by hypochlorite digestion of non-PHB biomass. Biotechnol. Tech. 1989, 3, 227–232. [Google Scholar] [CrossRef]
- Hahn, S.K.; Chang, Y.K.; Kim, B.S.; Chang, H.N. Optimization of microbial poly(3-hydroxybutyrate) recover using dispersions of sodium hypochlorite solution and chloroform. Biotechnol. Bioeng. 1994, 44, 256–261. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Lehninger, A.L.; Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 5th ed.; Worth Publishers: New York, NY, USA, 2000. [Google Scholar]
- Tanadchangsaeng, N.; Yu, J. Microbial synthesis of polyhydroxybutyrate from glycerol: Gluconeogenesis, molecular weight and material properties of biopolyester. Biotechnol. Bioeng. 2012, 109, 2808–2818. [Google Scholar] [CrossRef]
- Figueiredo, T.V.B.; Campos, M.I.; Sousa, L.S.; Da Silva, J.R.; Druzian, J.I. Produção e caracterização de polihidroxialcanoatos obtidos por fermentação da glicerina bruta residual do biodiesel. Química Nova 2014, 37, 1111–1117. [Google Scholar] [CrossRef]
- Gómez-Cardozo, J.R.; Velasco-Bucheli, R.; Marín-Pareja, N.; Ruíz-Villadiego, O.S.; Correa-Londoño, G.A.; Mora-Martínez, A.L. Fed-batch production and characterization of polyhydroxybutyrate by Bacillus megaterium lvn01 from residual glycerol. Dyna 2020, 87, 111–120. [Google Scholar] [CrossRef]
- Cardozoa, J.R.; Buchelib, R.V.; del Cerro Sánchezc, C.; de la Mata, I.; Riescod, A.L. Engineering of Bacillus megaterium for improving PHA production from glycerol. Asia-Pac. J. Mol. Biol. Biotechnol. 2019, 27, 64–72. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Lütke-Eversloh, T. Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem. Eng. J. 2003, 16, 81–96. [Google Scholar] [CrossRef]
- Andrade CJ de Andrade LM de Bution, M.L.; Heidi Dolder, M.A.; Cavalcante Barros, F.F.; Pastore, G.M. Optimizing alternative substrate for simultaneous production of surfactin and 2,3-butanediol by Bacillus subtilis LB5a. Biocatal. Agric. Biotechnol. 2016, 6, 209–218. [Google Scholar] [CrossRef]
- Mothes, G.; Schnorpfeil, C.; Ackermann, J.U. Production of PHB from crude glycerol. Eng. Life Sci. 2007, 7, 475–479. [Google Scholar] [CrossRef]
- Sweet, G.; Gandor, C.; Voegele, R.; Wittekindt, N.; Beuerle, J.; Truniger, V.; Lin, E.C.; Boos, W. Glycerol facilitator of Escherichia coli: Cloning of glpF and identification of the glpF product. J. Bacteriol. 1990, 172, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Pappalardo, F.; Fragalà, M.; Mineo, P.G.; Damigella, A.; Catara, A.F.; Palmeri, R.; Rescifina, A. Production of filmable medium-chain-length polyhydroxyalkanoates produced from glycerol by Pseudomonas mediterranea. Int. J. Biol. Macromol. 2014, 65, 89–96. [Google Scholar] [CrossRef]
- Taidi, B.; Mansfield, D.A.; Anderson, A.J. Turnover of poly(3-hydroxybutyrate) (PHB) and its influence on the molecular mass of the polymer accumulated by Alcaligenes eutrophus during batch culture. FEMS Microbiol. Lett. 1995, 129, 201–205. [Google Scholar] [CrossRef]
- Braunegg, G.; Genser, K.; Bona, R.; Haage, G.; Schellauf, F.; Winkler, E. Production of PHAs from agricultural waste material. Macromol. Symp. 1999, 144, 375–383. [Google Scholar] [CrossRef]
- de Oliveira Schmidt, V.K.; de Souza Carvalho, J.; de Oliveira, D.; de Andrade, C.J. Biosurfactant inducers for enhanced production of surfactin and rhamnolipids: An overview. World J. Microbiol. Biotechnol. 2021, 37, 1–5. [Google Scholar] [CrossRef]
- Sabapathy, P.C.; Devaraj, S.; Parthiban, A.; Pugazhendhi, A.; Kathirvel, P. Aegle marmelos: A novel low cost substrate for the synthesis of polyhydroxyalkanoate by Bacillus aerophilus RSL-7. Biocatal. Agric. Biotechnol. 2019, 18, 101021. [Google Scholar] [CrossRef]
- Gourlate, P.G. Comparação da Produção de Poli-Hidroxibutirato por Cupriavidus necator Parental e Recombinante a Partir de Glicerol e Glicose Combinados. Universidade Federal de Santa Catarina. 2018. Available online: https://repositorio.ufsc.br/bitstream/handle/123456789/198865/PEAL0336-T.pdf?sequence=-1&isAllowed=y (accessed on 28 August 2020).
- Cavalheiro, J.M.B.T.; de Almeida, M.C.M.D.; Grandfils, C.; da Fonseca, M.M.R. Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochem. 2009, 44, 509–515. [Google Scholar] [CrossRef]
- Naranjo, J.M.; Posada, J.A.; Higuita, J.C.; Cardona, C.A. Valorization of glycerol through the production of biopolymers: The PHB case using Bacillus megaterium. Bioresour. Technol. 2013, 133, 38–44. [Google Scholar] [CrossRef]
- Sharma, P.K.; Munir, R.I.; Kievit T de Levin, D.B. Synthesis of Polyhydroxyalkanoates (PHAs) from vegetable oils and free fatty acids by wild-type and mutant strains of Pseudomonas chlororaphis. Can. J. Microbiol. 2017, 63, 1009–1024. [Google Scholar] [CrossRef] [Green Version]
- Tiedje, J.M. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. Environ. Anaerobes. Microorg. 1988, 717, 179–244. [Google Scholar]
- Rodríguez-Contreras, A.; Koller, M.; Miranda-de Sousa Dias, M.; Calafell-Monfort, M.; Braunegg, G.; Marqués-Calvo, M.S. High production of poly(3-hydroxybutyrate) from a wild Bacillus megaterium Bolivian strain. J. Appl. Microbiol. 2013, 114, 1378–1387. [Google Scholar] [CrossRef]
- Yu, J.; Stahl, H. Microbial utilization and biopolyester synthesis of bagasse hydrolysates. Bioresour. Technol. 2008, 99, 8042–8048. [Google Scholar] [CrossRef]
- Nielsen, C.; Rahman, A.; Rehman, A.U.; Walsh, M.K.; Miller, C.D. Food waste conversion to microbial polyhydroxyalkanoates. Microb. Biotechnol. 2017, 10, 1338–1352. [Google Scholar] [CrossRef]
- Kucera, D.; Benesova, P.; Ladicky, P.; Pekar, M.; Sedlacek, P.; Obruca, S. Production of polyhydroxyalkanoates using hydrolyzates of spruce sawdust: Comparison of hydrolyzates detoxification by application of overliming, active carbon, and lignite. Bioengineering 2017, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Ramsay, J.A.; Ramsay, B.A. Acetone extraction of mcl-PHA from Pseudomonas putida KT2440. J. Microbiol. Methods 2006, 67, 212–219. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. Polyhydroxyalkanoates—Linking properties, applications, and end-of-life options. Chem. Biochem. Eng. Q. 2020, 34, 115–129. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.Y. Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl. Microbiol. Biotechnol. 1999, 51, 13–21. [Google Scholar] [CrossRef]
- Jacquel, N.; Lo, C.W.; Wei, Y.H.; Wu, H.S.; Wang, S.S. Isolation and purification of bacterial poly(3-hydroxyalkanoates). Biochem. Eng. J. 2008, 39, 15–27. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Zhou, Q.; Chen, J.; Gu, G. Cleaner recovery of poly(3-hydroxybutyric acid) synthesized in Alcaligenes eutrophus. Process Biochem. 2001, 36, 501–506. [Google Scholar] [CrossRef]
- López-Abelairas, M.; García-Torreiro, M.; Lú-Chau, T.; Lema, J.M.; Steinbüchel, A. Comparison of several methods for the separation of poly(3-hydroxybutyrate) from Cupriavidus necator H16 cultures. Biochem. Eng. J. 2015, 93, 250–259. [Google Scholar] [CrossRef]
- Barud, H.S.; Souza, J.L.; Santos, D.B.; Crespi, M.S.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J. Bacterial cellulose/poly(3-hydroxybutyrate) composite membranes. Carbohydr. Polym. 2011, 83, 1279–1284. [Google Scholar] [CrossRef]
- Pereira, S.M.F.; Rodriguez, R.S.; Gomes, J.G.C. Biosynthesis and characterization of biodegradable Poly(3-hydroxybutyrate) from renewable sources. Matéria 2008, 13, 1–11. [Google Scholar] [CrossRef]
- Tripathi, A.D.; Raj Joshi, T.; Kumar Srivastava, S.; Darani, K.K.; Khade, S.; Srivastava, J. Effect of nutritional supplements on bio-plastics (PHB) production utilizing sugar refinery waste with potential application in food packaging. Prep. Biochem. Biotechnol. 2019, 49, 567–577. [Google Scholar] [CrossRef]
- Muneer, F.; Rasul, I.; Azeem, F.; Siddique, M.H.; Zubair, M.; Nadeem, H. Microbial Polyhydroxyalkanoates (PHAs): Efficient Replacement of Synthetic Polymers. J. Polym. Environ. 2020, 28, 2301–2323. [Google Scholar] [CrossRef]

| Substrate | YX/S (g·g−1) | YP/S (g·g−1) | Px (g·L−1h−1) | PHA (g·L−1) |
|---|---|---|---|---|
| Glucose | 0.52 ± * 0.18 | 0.18 ± 0.04 | 0.06 ± 0.00 | 1.19 ± 0.40 |
| Glycerol | 0.05 ± 0.07 | 0.02 ± 0.01 | 0.06 ± 0.00 | 0.66 ± 0.38 |
| Solvent | Substrate | %PHA |
|---|---|---|
| Chloroform | Glucose | 19.6 ± * 1.56 |
| Glycerol | 22.3 ± 1.78 | |
| RHH | 11.0 ± 2.51 | |
| SH ** | Glucose | 52.5 ± 3.45 |
| Glycerol | 16.0 ± 2.34 | |
| Chloroform:SH | Glucose | 27.8 ± 5.75 |
| Glycerol | 33.3 ± 2.24 |
| Carbon Source | Solvent | Tg * | Tc * | ΔHc | Tcc * | ΔHcc | Tm * | ΔHm | Xc (%) |
|---|---|---|---|---|---|---|---|---|---|
| Glucose | Chloroform | −19 | 66 | 29.4 | 55 | 16.9 | 124–150 | 49.1 | 32 |
| SH *** | −10 | 73 | 37.8 | 64 | 15.8 | 139 | 56.3 | 33 | |
| Chloroform:SH | −2 | 82 | 39.0 | nd ** | nd | 126–147 | 49.8 | 34 | |
| Glycerol | Chloroform | nd | nd | 29.4 | 55 | 16.9 | 107–138 | 18.5 | 13 |
| SH | −13 | nd | nd | 61 | 44.4 | 129 | 27.6 | 19 | |
| Chloroform:SH | −14 | nd | nd | nd | nd | nd | nd | 0 | |
| RHH | Chloroform | −1 | nd | nd | 54 | 53 | 163 | 95 | 65 |
| Assignment of Bands | PHB * | PHA ** |
|---|---|---|
| Axial deformation of the connection C=O (cm−1) | 1735–1721 | 1721 |
| Axial deformation of the connection C–C (cm−1) | 978 | 978 |
| Binding stretch C–H (cm−1) | 2972–2850 | 2925 |
| Asymmetrical and symmetrical stretching of the group C–O–C (cm−1) | 1272 and 1058 | 1262 and 1055 |
| Symmetrical angular deformation in the plane of the groups CH3 (cm−1) | 1380 | 1379 |
| Physical Properties | ||
| Melting temperature (°C) | 175 | 163 |
| Glass transition temperature (°C) | 5 | −1 |
| Degree of crystallinity (%) | 55–80 | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Schmidt, V.K.; Santos, E.F.d.; de Oliveira, D.; Ayub, M.A.Z.; Cesca, K.; Cortivo, P.R.D.; de Andrade, C.J.; Hickert, L.R. Production of Polyhydroxyalkanoates by Bacillus megaterium: Prospecting on Rice Hull and Residual Glycerol Potential. Biomass 2022, 2, 412-425. https://doi.org/10.3390/biomass2040026
de Oliveira Schmidt VK, Santos EFd, de Oliveira D, Ayub MAZ, Cesca K, Cortivo PRD, de Andrade CJ, Hickert LR. Production of Polyhydroxyalkanoates by Bacillus megaterium: Prospecting on Rice Hull and Residual Glycerol Potential. Biomass. 2022; 2(4):412-425. https://doi.org/10.3390/biomass2040026
Chicago/Turabian Stylede Oliveira Schmidt, Vanessa Kristine, Evelise Fonseca dos Santos, Débora de Oliveira, Marco Antônio Záchia Ayub, Karina Cesca, Paulo Roberto Dall Cortivo, Cristiano José de Andrade, and Lilian Raquel Hickert. 2022. "Production of Polyhydroxyalkanoates by Bacillus megaterium: Prospecting on Rice Hull and Residual Glycerol Potential" Biomass 2, no. 4: 412-425. https://doi.org/10.3390/biomass2040026
APA Stylede Oliveira Schmidt, V. K., Santos, E. F. d., de Oliveira, D., Ayub, M. A. Z., Cesca, K., Cortivo, P. R. D., de Andrade, C. J., & Hickert, L. R. (2022). Production of Polyhydroxyalkanoates by Bacillus megaterium: Prospecting on Rice Hull and Residual Glycerol Potential. Biomass, 2(4), 412-425. https://doi.org/10.3390/biomass2040026








