The Production of High-Added-Value Bioproducts from Non-Conventional Biomasses: An Overview
Abstract
:1. Introduction
2. Bioproducts and Materials from Non-Conventional Sub-Products
2.1. Turmeric and Ginger Rhizome Biomass
2.2. Cereals, Nuts, and Seeds
2.3. Non-Conventional Fruit Biomass
2.4. Legumes
3. Collection and Application of the Bio-Compounds and Molecules from Biomass Agro-Foods
4. Biomass from Non-Conventional Agri-Foods and Sustainability: Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahari, W.A.W.; Waiho, K.; Fazhan, H.; Necibi, M.C.; Hafsa, J.; Ben Mrid, R.; Fal, S.; El Arroussi, H.; Peng, W.; Tabatabaei, M.; et al. Progress in valorisation of agriculture, aquaculture and shellfish biomass into biochemicals and biomaterials towards sustainable bioeconomy. Chemosphere 2021, 291, 133036. [Google Scholar] [CrossRef] [PubMed]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Saleem, M. Possibility of utilizing agriculture biomass as a renewable and sustainable future energy source. Heliyon 2022, 8, e08905. [Google Scholar] [CrossRef]
- Khandaker, M.M.; Abdullahi, U.A.; Abdulrahman, M.D.; Badaluddin, N.A.; Mohd, K.S. Bio-Ethanol Production from Fruit and Vegetable Waste by Using Saccharomyces cerevisiae; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Zou, X.; Li, S.; Wang, P.; Li, B.; Feng, Y.; Yang, S.-T. Sustainable production and biomedical application of polymalic acid from renewable biomass and food processing wastes. Crit. Rev. Biotechnol. 2020, 41, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Utilization of Food Waste Streams for the Production of Biopolymers|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S2405844020317345?token=413834F5E562D5196E33091A3AEFB52601CDE577ED3DCB45707A6A22396BA7AAEC96BAA8E21ADBACFECBB9ED46ED15FE&originRegion=us-east-1&originCreation=20230128121639 (accessed on 27 January 2023).
- Poomipuk, N.; Reungsang, A.; Plangklang, P. Poly-β-hydroxyalkanoates production from cassava starch hydrolysate by Cupriavidus sp. KKU38. Int. J. Biol. Macromol. 2014, 65, 51–64. [Google Scholar] [CrossRef]
- Havrysh, V.; Kalinichenko, A.; Mentel, G.; Mentel, U.; Vasbieva, D.G. Husk Energy Supply Systems for Sunflower Oil Mills. Energies 2020, 13, 361. [Google Scholar] [CrossRef]
- Kuntari, K.; Fajarwati, F. Utilization of bamboo leaves wastes for methylene blue dye absorption. In 2nd International Conference on Chemistry, Chemical Process and Engineering (IC3PE) AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2018; Volume 2026, p. 020062. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, J.; Zhang, J.; Brooks, M.S.-L. Potential for Value-Added Utilization of Bamboo Shoot Processing Waste—Recommendations for a Biorefinery Approach. Food Bioprocess Technol. 2018, 11, 901–912. [Google Scholar] [CrossRef]
- Nia, N.N.; Rahmani, M.; Kaykhaii, M.; Sasani, M. Evaluation of Eucalyptus leaves as an adsorbent for decolorization of Methyl Violet (2B) dye in contaminated waters: Thermodynamic and Kinetics model. Model. Earth Syst. Environ. 2017, 3, 825–829. [Google Scholar] [CrossRef]
- Nazar, M.; Xu, L.; Ullah, M.W.; Moradian, J.M.; Wang, Y.; Sethupathy, S.; Iqbal, B.; Nawaz, M.Z.; Zhu, D. Biological delignification of rice straw using laccase from Bacillus ligniniphilus L1 for bioethanol production: A clean approach for agro-biomass utilization. J. Clean. Prod. 2022, 360, 132171. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Órfão, J.J.D.M.; Pereira, M.F.R. Direct catalytic conversion of agro-forestry biomass wastes into ethylene glycol over CNT supported Ru and W catalysts. Ind. Crops Prod. 2021, 166, 113461. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Kolehmainen, A.; Liimatainen, H.; Niinimäki, J.; Hormi, O.E. Biocomposite cellulose-alginate films: Promising packaging materials. Food Chem. 2014, 151, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Doménech, P.; Duque, A.; Higueras, I.; Iglesias, R.; Manzanares, P. Biorefinery of the Olive Tree—Production of Sugars from Enzymatic Hydrolysis of Olive Stone Pretreated by Alkaline Extrusion. Energies 2020, 13, 4517. [Google Scholar] [CrossRef]
- Karanicola, P.; Patsalou, M.; Stergiou, P.-Y.; Kavallieratou, A.; Evripidou, N.; Christou, P.; Panagiotou, G.; Damianou, C.; Papamichael, E.M.; Koutinas, M. Ultrasound-assisted dilute acid hydrolysis for production of essential oils, pectin and bacterial cellulose via a citrus processing waste biorefinery. Bioresour. Technol. 2021, 342, 126010. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Wu, D.; Zhu, K.; Ye, X. Manosonication assisted extraction and characterization of pectin from different citrus peel wastes. Food Hydrocoll. 2021, 121, 106952. [Google Scholar] [CrossRef]
- Ntourtoglou, G.; Drosou, F.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Dourtoglou, V.G.; Elhakem, A.; Sami, R.; Ashour, A.A.; Shafie, A.; et al. Combination of Pulsed Electric Field and Ultrasound in the Extraction of Polyphenols and Volatile Compounds from Grape Stems. Appl. Sci. 2022, 12, 6219. [Google Scholar] [CrossRef]
- Fonseca, B.C.; Reginatto, V.; López-Linares, J.C.; Lucas, S.; García-Cubero, M.T.; Coca, M. Ideal conditions of microwave-assisted acid pretreatment of sugarcane straw allow fermentative butyric acid production without detoxification step. Bioresour. Technol. 2021, 329, 124929. [Google Scholar] [CrossRef]
- Singh, D.; Tembhare, M.; Machhirake, N.; Kumar, S. Biogas generation potential of discarded food waste residue from ultra-processing activities at food manufacturing and packaging industry. Energy 2023, 263, 126138. [Google Scholar] [CrossRef]
- Tosati, J.V.; Messias, V.C.; Carvalho, P.I.N.; Pollonio, M.A.R.; Meireles, M.A.A.; Monteiro, A.R. Antimicrobial Effect of Edible Coating Blend Based on Turmeric Starch Residue and Gelatin Applied onto Fresh Frankfurter Sausage. Food Bioprocess Technol. 2017, 10, 2165–2175. [Google Scholar] [CrossRef]
- Tosati, J.V.; de Oliveira, E.F.; Oliveira, J.V.; Nitin, N.; Monteiro, A.R. Light-activated antimicrobial activity of turmeric residue edible coatings against cross-contamination of Listeria innocua on sausages. Food Control 2018, 84, 177–185. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tapia-Blácido, D.R. Structural modification of fiber and starch in turmeric residue by chemical and mechanical treatment for production of biodegradable films. Int. J. Biol. Macromol. 2018, 126, 507–516. [Google Scholar] [CrossRef]
- Massimino, L.C.; Faria, H.A.; Yoshioka, S.A. Curcumin bioactive nanosizing: Increase of bioavailability. Ind. Crops Prod. 2017, 109, 493–497. [Google Scholar] [CrossRef]
- Dalsasso, R.R.; Valencia, G.A.; Monteiro, A.R. Impact of drying and extractions processes on the recovery of gingerols and shogaols, the main bioactive compounds of ginger. Food Res. Int. 2022, 154, 111043. [Google Scholar] [CrossRef] [PubMed]
- Liangshuo, L.I.; Lin, Q.; Xinyu, L.I.; Ming, D.; Xin, F. Preparation of biomass-based porous carbon derived from waste ginger slices and its electrochemical performance. Optoelectron. Adv. Mater. -Rapid Commu-Nications 2020, 14, 548–555. [Google Scholar]
- Gao, Y.; Ozel, M.Z.; Dugmore, T.; Sulaeman, A.; Matharu, A.S. A biorefinery strategy for spent industrial ginger waste. J. Hazard. Mater. 2020, 401, 123400. [Google Scholar] [CrossRef]
- Drzymała, K.; Mirończuk, A.M.; Pietrzak, W.; Dobrowolski, A. Rye and Oat Agricultural Wastes as Substrate Candidates for Biomass Production of the Non-Conventional Yeast Yarrowia lipolytica. Sustainability 2020, 12, 7704. [Google Scholar] [CrossRef]
- Zając, T.; Synowiec, A.; Oleksy, A.; Macuda, J.; Klimek-Kopyra, A.; Borowiec, F. Accumulation of biomass and bioenergy in culms of cereals as a factor of straw cutting height. Int. Agrophys. 2017, 31, 273–285. [Google Scholar] [CrossRef]
- Zampaligré, N.; Yoda, G.; Delma, J.; Sanfo, A.; Balehegn, M.; Rios, E.; Dubeux, J.C.; Boote, K.; Adesogan, A.T. Fodder biomass, nutritive value, and grain yield of dual-purpose improved cereal crops in Burkina Faso. Agron. J. 2021, 114, 115–125. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Bergamo, T.F.; Kikas, T. Potential of cereal-based agricultural residues available for bioenergy production. Data Brief 2019, 23, 103829. [Google Scholar] [CrossRef]
- Hassan, G.; Shabbir, M.A.; Ahmad, F.; Pasha, I.; Aslam, N.; Ahmad, T.; Rehman, A.; Manzoor, M.F.; Inam-Ur-Raheem, M.; Aadil, R.M. Cereal processing waste, an environmental impact and value addition perspectives: A comprehensive treatise. Food Chem. 2021, 363, 130352. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Poojary, M.M.; Barba, F.J.; Lorenzo, J.M.; Mañes, J.; Moltó, J.C. Tiger nut and its by-products valorization: From extraction of oil and valuable compounds to development of new healthy products. Innov. Food Sci. Emerg. Technol. 2018, 45, 306–312. [Google Scholar] [CrossRef]
- Verdú, S.; Barat, J.M.; Alava, C.; Grau, R. Effect of tiger-nut (Cyperus esculentus) milk co-product on the surface and diffusional properties of a wheat-based matrix. Food Chem. 2017, 224, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.M.; Lima, J.R.; Wurlitzer, N.; Rodrigues, T.C. Non-dairy cashew nut milk as a matrix to deliver probiotic bacteria. Food Sci. Technol. 2020, 40, 604–607. [Google Scholar] [CrossRef]
- Mgaya, J.; Shombe, G.B.; Masikane, S.C.; Mlowe, S.; Mubofu, E.B.; Revaprasadu, N. Cashew nut shell: A potential bio-resource for the production of bio-sourced chemicals, materials and fuels. Green Chem. 2019, 21, 1186–1201. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Chemistry and Specialty Industrial Applications of Lignocellulosic Biomass. Waste Biomass Valoriz. 2020, 12, 2145–2169. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Falcao, N.; Damaceno, J.B.D.; Guerrini, I.A. Biochar Yield from Shell of Brazil Nut Fruit and Its Effects on Soil Acidity and Phosphorus Availability in Central Amazonian Yellow Oxisol. J. Agric. Sci. 2020, 12, 222. [Google Scholar] [CrossRef]
- Gomes, S.; Finotelli, P.V.; Sardela, V.F.; Pereira, H.M.; Santelli, R.E.; Freire, A.S.; Torres, A.G. Microencapsulated Brazil nut (Bertholletia excelsa) cake extract powder as an added-value functional food ingredient. LWT 2019, 116, 108495. [Google Scholar] [CrossRef]
- Leandro, R.I.M.; Abreu, J.J.D.C.; Martins, C.D.S.; Santos, I.S.; Bianchi, M.L.; Nobre, J.R.C. Elementary, Chemical and Energy Characteristics of Brazil Nuts Waste (Bertholletia excelsa) in the State of Pará. Floresta Ambient. 2019, 26, e20180436. [Google Scholar] [CrossRef]
- Sartori, A.G.D.O.; Regitano-D’arce, M.A.B.; Skibsted, L.H. Brazil nuts: Nutritional benefits from a unique combination of antioxidants. J. Food Bioact. 2020, 9, 36–39. [Google Scholar] [CrossRef]
- Pena, D.W.P.; Tonoli, G.H.D.; Protásio, T.D.P.; de Souza, T.M.; Ferreira, G.C.; Vale, I.D.; Ferreira, I.M.; Bufalino, L. Exfoliating Agents for Skincare Soaps Obtained from the Crabwood Waste Bagasse, a Natural Abrasive from Amazonia. Waste Biomass Valoriz. 2021, 12, 4441–4461. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Xing, C.; El Ouahabi, H.; Kamińska, A.; Sreńscek-Nazzal, J. Activated carbons from the Amazonian biomass andiroba shells applied as a CO2 adsorbent and a cheap semiconductor material. J. CO2 Util. 2022, 62, 102071. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Cruz, O.F.; Sreńscek-Nazzal, J.; Gómez, I.C.; Azar, F.-Z.; Mafull, C.A.R.; Hotza, D.; Rambo, C.R. Conversion of fruit waste-derived biomass to highly microporous activated carbon for enhanced CO2 capture. Waste Manag. 2021, 136, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Becker, F.S.; Damiani, C.; De Melo, A.A.M.; Borges, P.R.S.; Boas, E.V.D.B.V. Incorporation of Buriti Endocarp Flour in Gluten-free Whole Cookies as Potential Source of Dietary Fiber. Plant Foods Hum. Nutr. 2014, 69, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Adriana, I.T.D.O.; Jhonatha, B.C.; Talal, S.M.; Guilherme, N.L.D.N.; Juliana, F.M.D.S.; Raphael, S.P.; Paula, B.D.M.; De Oliveira, A.I.T.; Cabral, J.B.; Mahmoud, T.S.; et al. In vitro antimicrobial activity and fatty acid composition through gas chromatography-mass spectrometry (GC-MS) of ethanol extracts of Mauritia flexuosa (Buriti) fruits. J. Med. Plants Res. 2017, 11, 635–641. [Google Scholar] [CrossRef]
- Koop, B.L.; Knapp, M.A.; Di Luccio, M.; Pinto, V.Z.; Tormen, L.; Valencia, G.A.; Monteiro, A.R. Bioactive Compounds from Jambolan (Syzygium cumini (L.)) Extract Concentrated by Ultra- and Nan-ofiltration: A Potential Natural Antioxidant for Food. Plant Foods Hum. Nutr. 2021, 76, 90–97. [Google Scholar] [CrossRef]
- Nascimento-Silva, N.R.R.D.; Bastos, R.P.; da Silva, F.A. Jambolan (Syzygium cumini (L.) Skeels): A review on its nutrients, bioactive compounds and health benefits. J. Food Compos. Anal. 2022, 109, 104491. [Google Scholar] [CrossRef]
- Freita, B.F.D.; Magalhães, G.L.; Júnior, M.S.S.; Caliari, M. Produção de corante natural extraído de jambolão (Syzygium cumini). Res. Soc. Dev. 2021, 10, e27410212600. [Google Scholar] [CrossRef]
- Soares, J.; Júnior, M.S.S.; Ferreira, K.C.; Caliari, M. Physicochemical characteristics and sensory acceptance of jambolan nectars (Syzygium cumini). Food Sci. Technol. 2019, 39, 8–14. [Google Scholar] [CrossRef]
- Silva, C.A.D.A.; Fonseca, G.G. Brazilian savannah fruits: Characteristics, properties, and potential applications. Food Sci. Biotechnol. 2016, 25, 1225–1232. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mariutti, L.R.B.; Mercadante, A.Z. Two-step cleanup procedure for the identification of carotenoid esters by liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J. Chromatogr. A 2016, 1457, 116–124. [Google Scholar] [CrossRef]
- Araújo, A.C.M.A.; Menezes, E.G.T.; Terra, A.W.C.; Dias, B.O.; de Oliveira, É.R.; Queiroz, F. Bioactive compounds and chemical composition of Brazilian Cerrado fruits’ wastes: Pequi almonds, murici, and sweet passionfruit seeds. Food Sci. Technol. 2018, 38, 203–214. [Google Scholar] [CrossRef]
- Nyirenda, J.; Zombe, K.; Kalaba, G.; Siabbamba, C.; Mukela, I. Exhaustive valorization of cashew nut shell waste as a potential bioresource material. Sci. Rep. 2021, 11, 11986. [Google Scholar] [CrossRef] [PubMed]
- Mubofu, E.B.; Mgaya, J.E. Chemical Valorization of Cashew Nut Shell Waste. Top. Curr. Chem. 2018, 376, 8. [Google Scholar] [CrossRef] [PubMed]
- Mubofu, E.B. From cashew nut shell wastes to high value chemicals. Pure Appl. Chem. 2015, 88, 17–27. [Google Scholar] [CrossRef]
- da Silva, J.; de Brito, E.S.; Ferreira, S.R.S. Biorefinery of Cashew By-Products: Recovery of Value-Added Compounds. Food Bioprocess Technol. 2022, 16, 944–960. [Google Scholar] [CrossRef]
- Zafeer, M.K.; Bhat, K.S. Valorisation of agro-waste cashew nut husk (Testa) for different value-added products. Sustain. Chem. Clim. Action 2023, 2, 100014. [Google Scholar] [CrossRef]
- Runjala, S.; Kella, L. Cashew apple (Anacardium occidentale L.) therapeutic benefits, processing and product development: An over view. Pharma Innov. 2017, 6, 260–264. [Google Scholar]
- Kamani, M.H.; Martin, A.; Meera, M.S. Valorization of By-products Derived from Milled Moth Bean: Evaluation of Chemical Composition, Nutritional Profile and Functional Characteristics. Waste Biomass Valoriz. 2019, 11, 4895–4906. [Google Scholar] [CrossRef]
- Gençdağ, E.; Görgüç, A.; Yılmaz, F.M. Recent Advances in the Recovery Techniques of Plant-Based Proteins from Agro-Industrial By-Products. Food Rev. Int. 2020, 37, 447–468. [Google Scholar] [CrossRef]
- Pedrosa, M.M.; Varela, A.; Domínguez-Timón, F.; Tovar, C.A.; Moreno, H.M.; Borderías, A.J.; Díaz, M.T. Comparison of Bioactive Compounds Content and Techno-Functional Properties of Pea and Bean Flours and their Protein Isolates. Plant Foods Hum. Nutr. 2020, 75, 642–650. [Google Scholar] [CrossRef]
- Kasar, C.; Thanushree, M.P.; Gupta, S.; Inamdar, A.A. Milled fractions of common buckwheat (Fagopyrum esculentum) from the Himalayan regions: Grain characteristics, functional properties and nutrient composition. J. Food Sci. Technol. 2020, 58, 3871–3881. [Google Scholar] [CrossRef] [PubMed]
- Maniglia, B.; Domingos, J.; de Paula, R.; Tapia-Blácido, D. Development of bioactive edible film from turmeric dye solvent extraction residue. LWT 2014, 56, 269–277. [Google Scholar] [CrossRef]
- Malacrida, C.R.; Ferreira, S.; Zuanon, L.A.C.; Telis, V.R.N. Freeze-Drying for Microencapsulation of Turmeric Oleoresin Using Modified Starch and Gelatin. J. Food Process. Preserv. 2014, 39, 1710–1719. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F.; Carvalho, P.I.; Rostagno, M.A.; Petenate, A.J.; Meireles, M.A.A. Extraction of curcuminoids from deflavored turmeric (Curcuma longa L.) using pressurized liquids: Process integration and economic evaluation. J. Supercrit. Fluids 2014, 95, 167–174. [Google Scholar] [CrossRef]
- Suleiman, M.S.; Olajide, J.E.; Omale, J.A.; Abbah, O.C.; Ejembi, D.O. Proximate composition, mineral and some vitamin contents of tigernut (Cyperus esculentus). Clin. Investig. 2018, 8, 161–165. [Google Scholar] [CrossRef]
- de Brito, R.C.M.; Junior, J.B.P.; Dantas, K.d.G.F. Quantification of inorganic constituents in Brazil nuts and their products by inductively coupled plasma optical emission spectrometry. LWT 2019, 116, 108383. [Google Scholar] [CrossRef]
- Pagno, V.; Módenes, A.N.; Dragunski, D.C.; Fiorentin-Ferrari, L.D.; Caetano, J.; Guellis, C.; Gonçalves, B.C.; dos Anjos, E.V.; Pagno, F.; Martinelli, V. Heat treatment of polymeric PBAT/PCL membranes containing activated carbon from Brazil nutshell biomass obtained by electrospinning and applied in drug removal. J. Environ. Chem. Eng. 2020, 8, 104159. [Google Scholar] [CrossRef]
- de Oliveira, J.M.; de Alencar, E.R.; Blum, L.E.B.; Ferreira, W.F.D.S.; Botelho, S.D.C.C.; Racanicci, A.M.C.; Leandro, E.D.S.; Mendonça, M.A.; Moscon, E.S.; Bizerra, L.V.A.D.S.; et al. Ozonation of Brazil nuts: Decomposition kinetics, control of Aspergillus flavus and the effect on color and on raw oil quality. LWT 2020, 123, 109106. [Google Scholar] [CrossRef]
- Rojas-Bringas, P.M.; De-La-Torre, G.E.; Torres, F.G. Influence of the source of starch and plasticizers on the environmental burden of starch-Brazil nut fiber biocomposite production: A life cycle assessment approach. Sci. Total. Environ. 2020, 769, 144869. [Google Scholar] [CrossRef]
- Darnet, S.H.; Silva, L.H.M.; Rodrigues, A.M.C.; Lins, R.T. Nutritional composition, fatty acid and tocopherol contents of buriti (Mauritia flexuosa) and patawa (Oenocarpus bataua) fruit pulp from the Amazon region. Ciência e Tecnoogia de. Alimentos. 2011, 31, 488–491. [Google Scholar] [CrossRef]
- de Oliveira, A.I.T.; Mahmoud, T.S.; Nascimento, G.N.L.D.; da Silva, J.F.M.; Pimenta, R.S.; de Morais, P.B. Chemical Composition and Antimicrobial Potential of Palm Leaf Extracts from Babaçu (Attalea speciosa), Buriti (Mauritia flexuosa), and Macaúba (Acrocomia aculeata). Sci. World J. 2016, 2016, 9734181. [Google Scholar] [CrossRef]
- Guimarães, M.G.; Evaristo, R.B.W.; Brasil, A.C.D.M.; Ghesti, G.F. Green energy technology from buriti (Mauritia flexuosa L. f.) for Brazilian agro-extractive communities. SN Appl. Sci. 2021, 3, 283. [Google Scholar] [CrossRef]
- Brandão, T.S.O.; Pinho, L.S.; Silva-Hughes, A.F.; Souza, J.L.; Rosa, C.A.; Teshima, E.; Brandão, H.N.; David, J.M. Characterization of the jambolan (Syzygium cumini L.) fruit wine processing. BioResources 2017, 12, 7069–7083. [Google Scholar] [CrossRef]
- Miranda, M.R.D.S.; Veras, C.A.G.; Ghesti, G.F. Charcoal production from waste pequi seeds for heat and power generation. Waste Manag. 2019, 103, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.H.; Yusup, S.; Kadir, W.N.A.; Wong, C.Y.; Rosli, S.S.; Ruslan, M.S.H.; Chin, B.L.F.; Yiin, C.L. Valorization of Tropical Biomass Waste by Supercritical Fluid Extraction Technology. Sustainability 2020, 13, 233. [Google Scholar] [CrossRef]
- Tzima, S.; Georgiopoulou, I.; Louli, V.; Magoulas, K. Recent Advances in Supercritical CO2 Extraction of Pigments, Lipids and Bioactive Compounds from Microalgae. Molecules 2023, 28, 1410. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, Z. A review of current progress of supercritical fluid technologies for e-waste treatment. J. Clean. Prod. 2019, 227, 794–809. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Ultra-sonication assisted cross-linking of cellulose polymers. Ultrason. Sonochem. 2018, 42, 567–576. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.; Mohee, R. Ultrasound-assisted biological conversion of biomass and waste materials to biofuels: A review. Ultrason. Sonochem. 2018, 40, 298–313. [Google Scholar] [CrossRef]
- Flores, E.M.; Cravotto, G.; Bizzi, C.A.; Santos, D.; Iop, G.D. Ultrasound-assisted biomass valorization to industrial interesting products: State-of-the-art, perspectives and challenges. Ultrason. Sonochem. 2021, 72, 105455. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing Biostimulants from Agro-Food and Industrial By-Products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef] [PubMed]
- Koop, B.L.; Zenin, E.; Cesca, K.; Valencia, G.A.; Monteiro, A.R. Intelligent labels manufactured by thermo-compression using starch and natural biohybrid based. Int. J. Biol. Macromol. 2022, 220, 964–972. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.N.; Fonseca, J.D.M.; Feldhaus, H.K.; Soares, L.S.; Valencia, G.A.; de Campos, C.E.M.; Di Luccio, M.; Monteiro, A.R. Physical and morphological properties of hydroxypropyl methylcellulose films with curcumin polymorphs. Food Hydrocoll. 2019, 97, 105217. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.; Shirzadifar, A. A review on cellulose nanocrystals as promising biocompounds for the synthesis of nanocomposite hydrogels. Carbohydr. Polym. 2019, 216, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2011, 132, 799–807. [Google Scholar] [CrossRef]
- Abbas, S.; Chang, D.; Riaz, N.; Maan, A.A.; Khan, M.K.I.; Ahmad, I.; Alsagaby, S.A.; El-Ghorab, A.; Ali, M.; Imran, M.; et al. In-vitro stress stability, digestibility and bioaccessibility of curcumin-loaded polymeric nanocapsules. J. Exp. Nanosci. 2021, 16, 229–245. [Google Scholar] [CrossRef]
- Lee, N.Y.; Kim, Y.; Kim, Y.S.; Shin, J.-H.; Rubin, L.P.; Kim, Y. β-Carotene exerts anti-colon cancer effects by regulating M2 macrophages and activated fibroblasts. J. Nutr. Biochem. 2020, 82, 108402. [Google Scholar] [CrossRef]
- Huang, W.; Feng, Z.; Aila, R.; Hou, Y.; Carne, A.; Bekhit, A.E.-D.A. Effect of pulsed electric fields (PEF) on physico-chemical properties, β-carotene and antioxidant activity of air-dried apricots. Food Chem. 2019, 291, 253–262. [Google Scholar] [CrossRef]
- Moser, P.; Ferreira, S.; Nicoletti, V.R. Buriti oil microencapsulation in chickpea protein-pectin matrix as affected by spray drying parameters. Food Bioprod. Process. 2019, 117, 183–193. [Google Scholar] [CrossRef]
- Li, W.; Qiu, Z.; Ma, Y.; Zhang, B.; Li, L.; Li, Q.; He, Q.; Zheng, Z. Preparation and Characterization of Ginger Peel Polysaccharide–Zn (II) Complexes and Evaluation of Anti-Inflammatory Activity. Antioxidants 2022, 11, 2331. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Wang, F.; Xu, J.; Tang, X.; Li, N. Structural characterization and antioxidant activity of polysaccharide from ginger. Int. J. Biol. Macromol. 2018, 111, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Nagavekar, N.; Singhal, R.S. Supercritical fluid extraction of Curcuma longa and Curcuma amada oleoresin: Optimization of extraction conditions, extract profiling, and comparison of bioactivities. Ind. Crops Prod. 2019, 134, 134–145. [Google Scholar] [CrossRef]
- Jayathilake, A.L.; Jayasinghe, M.A.; Walpita, J. Development of ginger, turmeric oleoresins and pomegranate peel extracts incorporated pasteurized milk with pharmacologically important active compounds. Appl. Food Res. 2022, 2, 100063. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Wang, Z.; Kan, J. A comparison of a polysaccharide extracted from ginger (Zingiber officinale) stems and leaves using different methods: Preparation, structure characteristics, and biological activities. Int. J. Biol. Macromol. 2020, 151, 635–649. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Jia, H.-J.; Li, X.-D.; Liu, Y.-L.; Wei, A.-C.; Zhu, W.-X. Effect of drying methods on the quality of tiger nuts (Cyperus esculents L.) and its oil. LWT 2022, 167, 113827. [Google Scholar] [CrossRef]
- Ferreira, L.M.d.M.C.; Pereira, R.R.; de Carvalho, F.B.; Santos, A.S.; Ribeiro-Costa, R.M.; Júnior, J.O.C.S. Green Extraction by Ultrasound, Microencapsulation by Spray Drying and Antioxidant Activity of the Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds. Biomolecules 2021, 11, 545. [Google Scholar] [CrossRef]
- AlNimer, M. Ultraviolet light assisted extraction of flavonoids and allantoin from aqueous and alcoholic extracts of Symphytum officinale. J. Intercult. Ethnopharmacol. 2017, 6, 280–283. [Google Scholar] [CrossRef]
- Mohammadi, A.S.; Alemtabriz, A.; Pishvaee, M.S.; Zandieh, M. A multi-stage stochastic programming model for sustainable closed-loop supply chain network design with financial decisions: A case study of plastic production and recycling supply chain. Sci. Iran. 2019, 27, 377–395. [Google Scholar] [CrossRef]
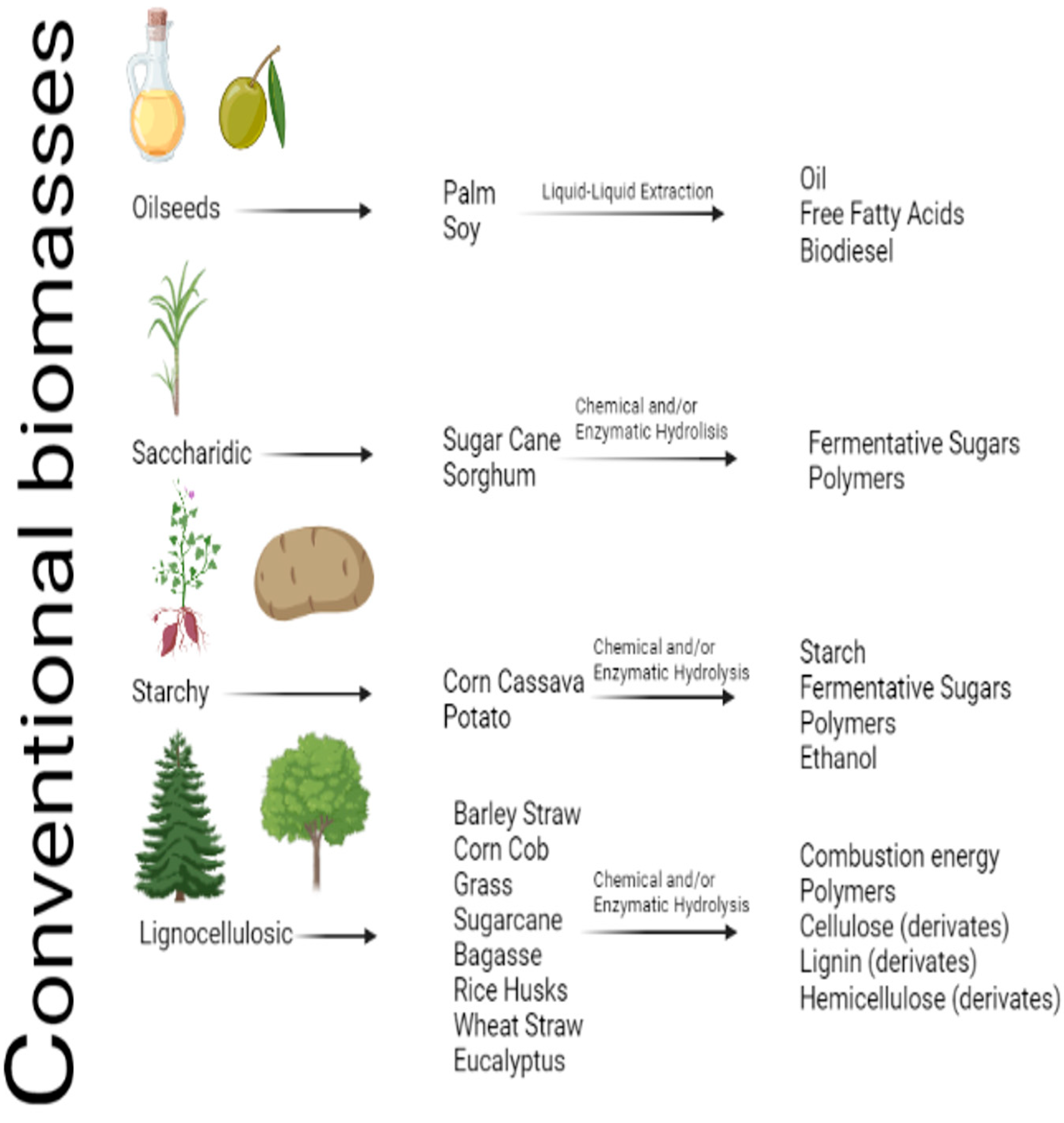
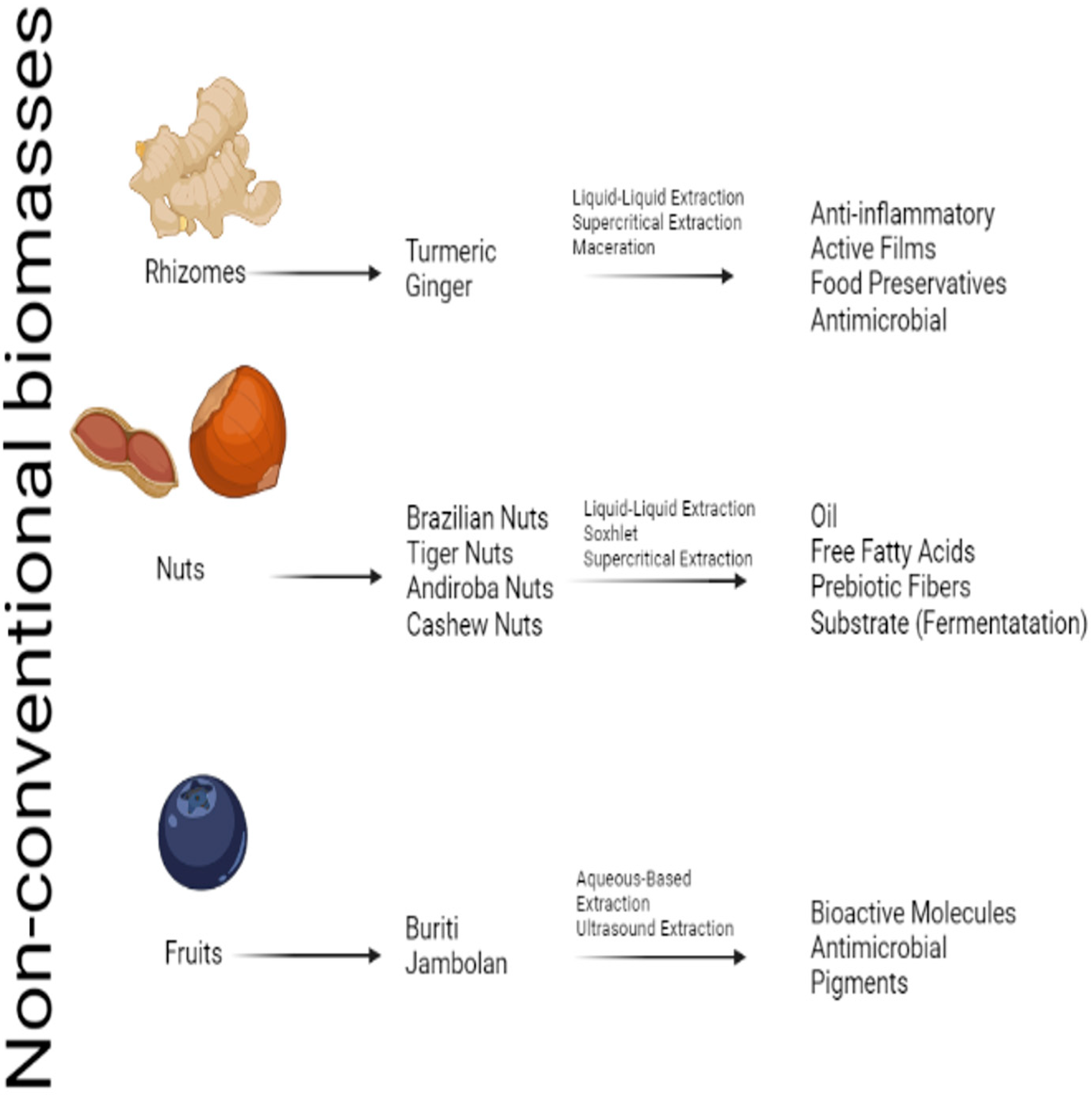
| Biomass | Conversion | References |
|---|---|---|
| Sunflower seed residue oil extraction | Fuel to produce energy | [8] |
| Bamboo leaf waste | Dye adsorption (wastewater), hardwood floors, furniture | [9,10] |
| Eucalyptus leaves | Dye adsorption from contaminated waters | [11] |
| Rice straw | Delignification for bioethanol production | [12] |
| Agro-forestry biomass waste | Ethylene glycol production (direct catalytic conversion) | [13] |
| Sugarcane bagasse waste | Nanocellulose extraction for food-packaging products (biofilms) | [14] |
| Olive stones | Extrusion pretreatment for sugar production in food sector | [15] |
| Orange peel waste | Extraction of essential oils, pectin, and bacterial cellulose for the food industry | [16,17] |
| Grape stems | Extraction of polyphenol and volatile compounds | [18] |
| Sugarcane straw | Production of bio-butyric acid | [19] |
| Discarded food-waste residue | Biogas generation | [20] |
| Biomass | Main Composition | Trends for Applications in the Food Sector | References |
|---|---|---|---|
| (Rhizome) turmeric residue: from volatile and oleoresin extractions | Saturated fat, calcium, phosphorus, sodium potassium, thiamin, iron, riboflavin, dietary fibers, sugars and proteins, curcumin, starch | Macro- and nano-molecules in food: anti-inflammatory, antimicrobial food packaging (active biopolymer): active films and coatings | [64,65,66] |
| (Rhizome) ginger from volatile and oleoresin extractions | Total carbohydrates, dietary fibers, sugars, proteins, and starch Vitamins: B (thiamine) and E Essential oils: monoterpenes and sesquiterpénes; oleoresin: shogaols, gingerols | Macro- and nano-molecules in the food sector: anti-inflammatory and antimicrobial Packaging (biopolymer): active films and coatings | [25,27] |
| Tiger nuts | Minerals (protective nutrients), fibers, fat, proteins (higher than cassava), vitamins | Food formulation; green energy | [59,67] |
| Brazil nuts: shell and seed residues from oil extraction, milk, husks | Carbohydrates: starch, sugars; dietary fiber; fat: saturated, monounsaturated, polyunsaturated; protein: glutamic acid, arginine, leucine; vitamins: B, C, and E; minerals: calcium, iron, magnesium, manganese, phosphorus, potassium, sodium, zinc | Active food packaging; biomaterials for food applications; biopolymers; effluents treatment; biodiesel; and green energy | [40,41,68,69,70] |
| Cashew shell (nuts) and pulp residue from juice extraction | Protein, ash, sodium (Na), lipids, phenolic compounds, lignin, fibers, starch | Food formulation, active food package (biopolymer), synthesis material production, biodiesel | [54,56,57,71] |
| Buriti: skin, pulp waste from oil extraction | Lipids, proteins, ashes, dietary fibers, carbohydrates | Food industry: bioactive molecules, antimicrobial pigments | [72,73,74] |
| Jambolan: pulp | Monomeric anthocyanins: phenolics and tannins | Food colorant, meat control, food packaging | [47,48,49,75] |
| Pequi: skin and seeds | Furanic compounds, organic acids, and derivatives, such as levulinic acid | Biochar, food pigments and ingredients | [53,76] |
| Bean and pea flours | Protein and lipids | Emulsion stability and foaming | [62] |
| Buckwheat grain flour milled fractions | Protein, carbohydrates, and minerals | Suplementary food: gluten-free food products | [63] |
| Biomass | Technology | Biomolecules or Coproducts Obtained | Main Results | References |
|---|---|---|---|---|
| Curcuma longa (CL) and Curcuma (CA) amada | Supercritical fluid | Oleoresins, curcuminoids, and total volatiles | CL have better bioactivity than CA because of the high concentration of curcuminoids. In vivo anti-inflammatory activity os CA is greater than CL | [95] |
| Turmeric, ginger residue, and pomegranate peel | Soxhlet extraction with absolute ethanol | Essential oil and oleoresin | Milk incorporated with ginger; turmeric and pomegranate peel extracts with high biological properties | [96] |
| Ginger leaves and stems | Extraction methods: hot water (HWE), ultrasound-assisted extraction (UAE), alkaline solution extraction (ASE), and enzyme-assisted extraction (EAE) | Active polysaccharides | ASE produces the highest yield and lower hypoglycemic and antioxidant activities | [97] |
| Tiger nuts | Soxhlet extraction with petroleum ether | Oil: oleic acid, palmitic acid, linoleic acid, stearic acid, tocopherols, tocotrienols, phytosterol Residues: fibers and polyphenols | Short extraction time and high yield with residues in oil | [98] |
| Tucuman (coproduct of tucuma kernels) | Ultrasonic-assisted extraction and spray dryer | Oily extract and microparticles | Total carotenoids contained in oily extract was higher than microparticles | [99] |
| Symphytum officinale | Ultraviolet-light-assisted extraction | Flavonoids and allantoin | UV radiation enhances the yields of active ingredients | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, A.R.; Battisti, A.P.; Valencia, G.A.; de Andrade, C.J. The Production of High-Added-Value Bioproducts from Non-Conventional Biomasses: An Overview. Biomass 2023, 3, 123-137. https://doi.org/10.3390/biomass3020009
Monteiro AR, Battisti AP, Valencia GA, de Andrade CJ. The Production of High-Added-Value Bioproducts from Non-Conventional Biomasses: An Overview. Biomass. 2023; 3(2):123-137. https://doi.org/10.3390/biomass3020009
Chicago/Turabian StyleMonteiro, Alcilene Rodrigues, Andrei Pavei Battisti, Germán Ayala Valencia, and Cristiano José de Andrade. 2023. "The Production of High-Added-Value Bioproducts from Non-Conventional Biomasses: An Overview" Biomass 3, no. 2: 123-137. https://doi.org/10.3390/biomass3020009
APA StyleMonteiro, A. R., Battisti, A. P., Valencia, G. A., & de Andrade, C. J. (2023). The Production of High-Added-Value Bioproducts from Non-Conventional Biomasses: An Overview. Biomass, 3(2), 123-137. https://doi.org/10.3390/biomass3020009










