Production of Anthocyanin-Rich Red Rose Petal Extract by Enzymatic Maceration
Abstract
1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Experimental
2.3. Analytical Methods
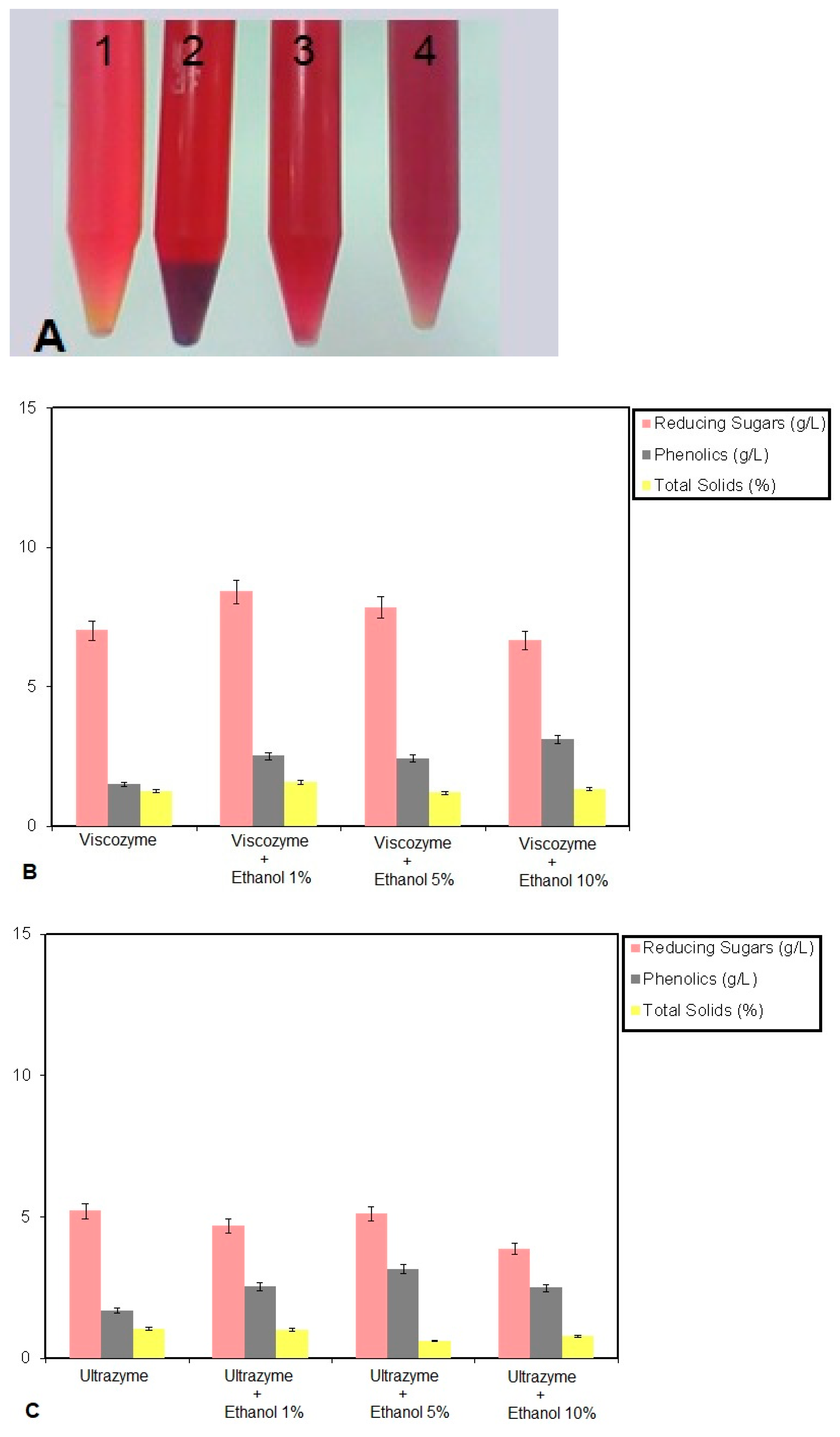
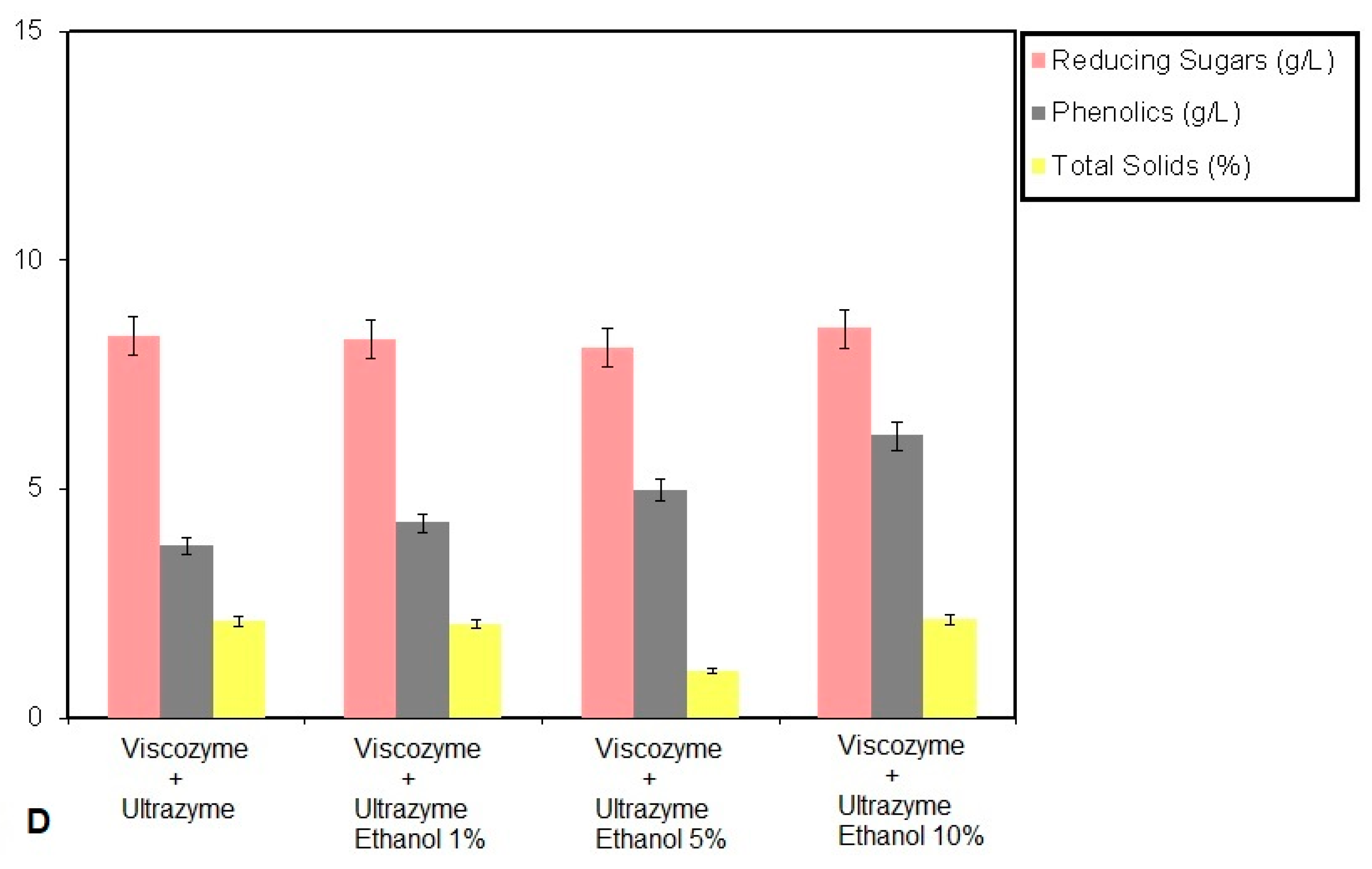
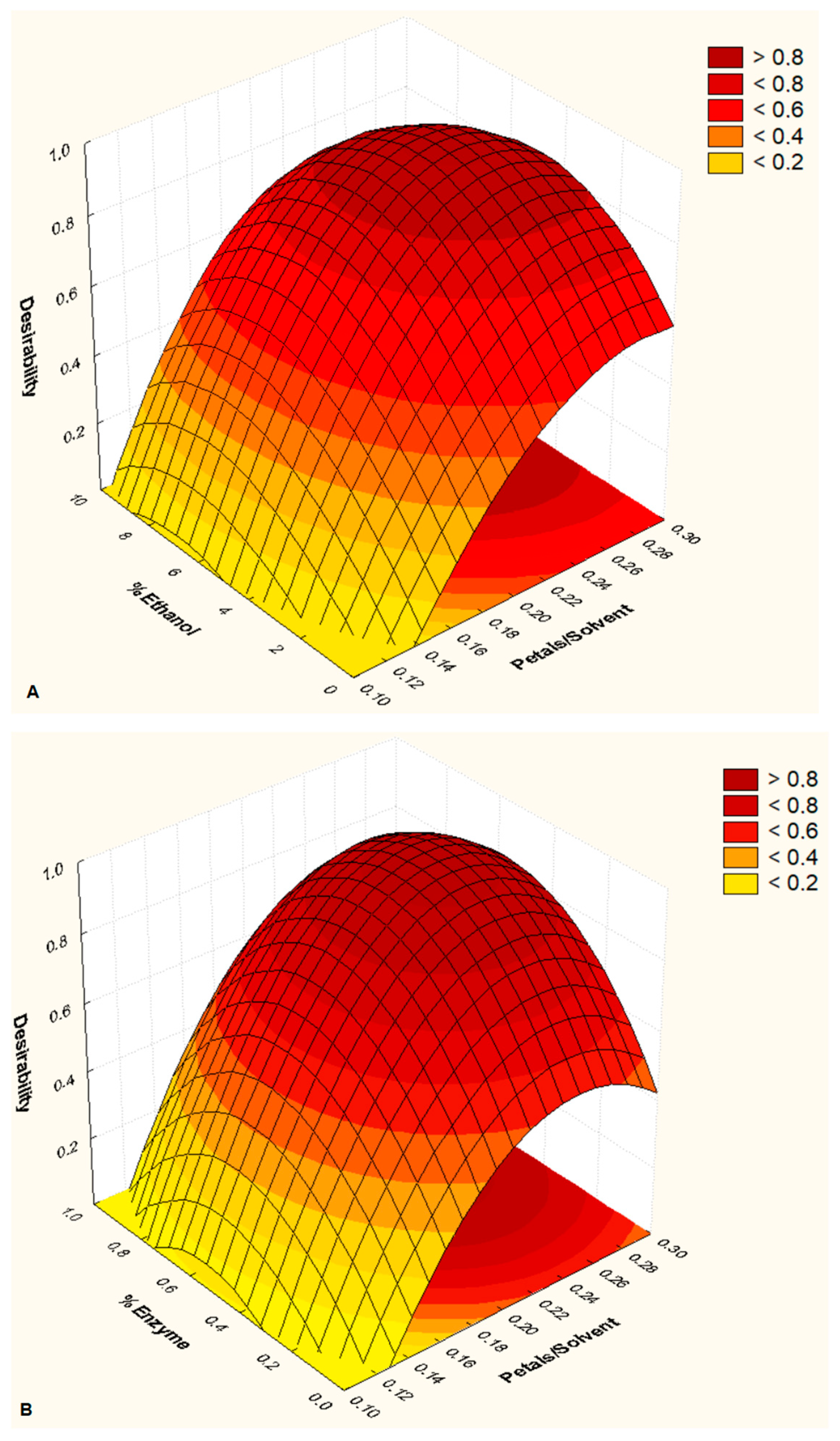
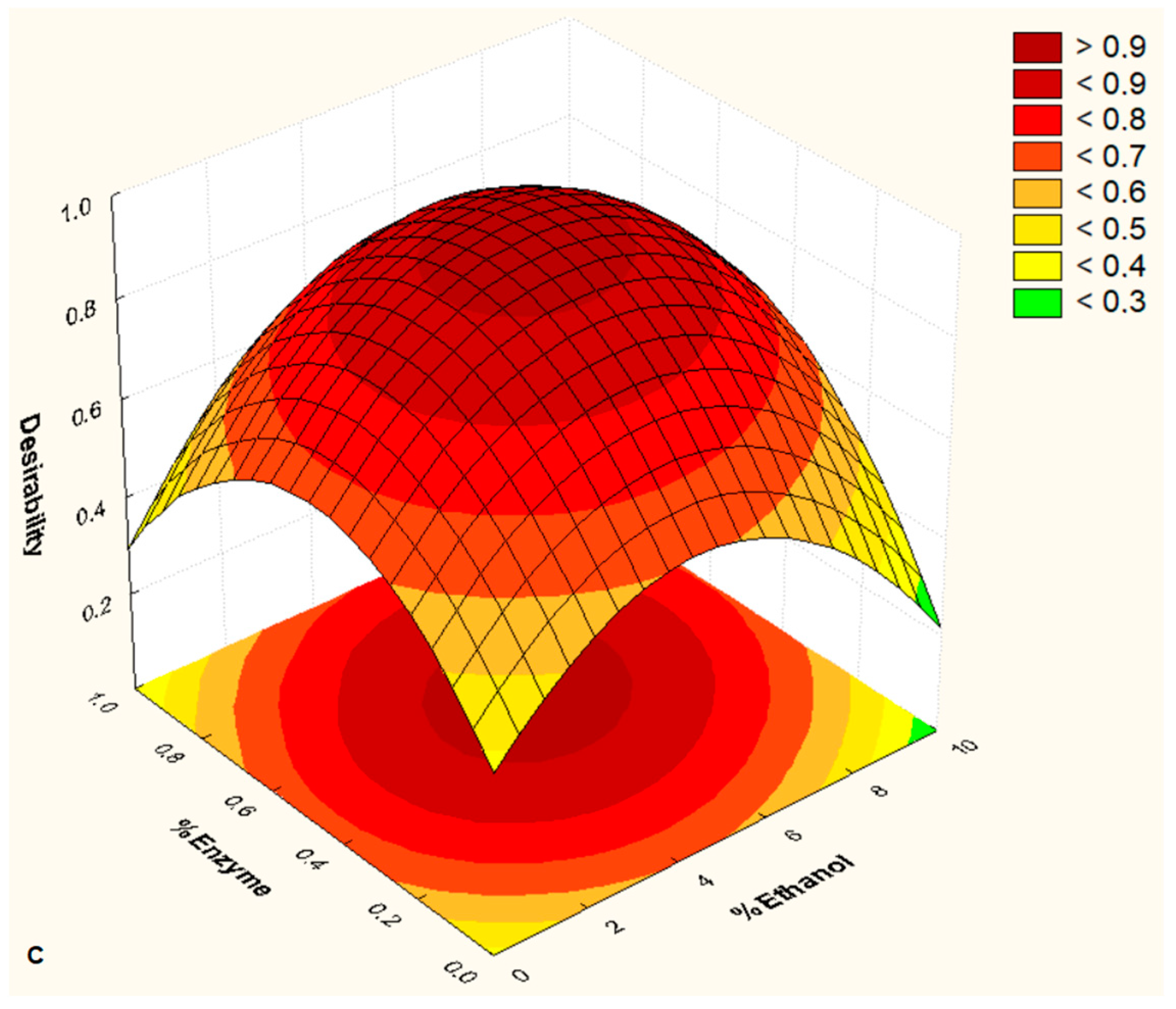
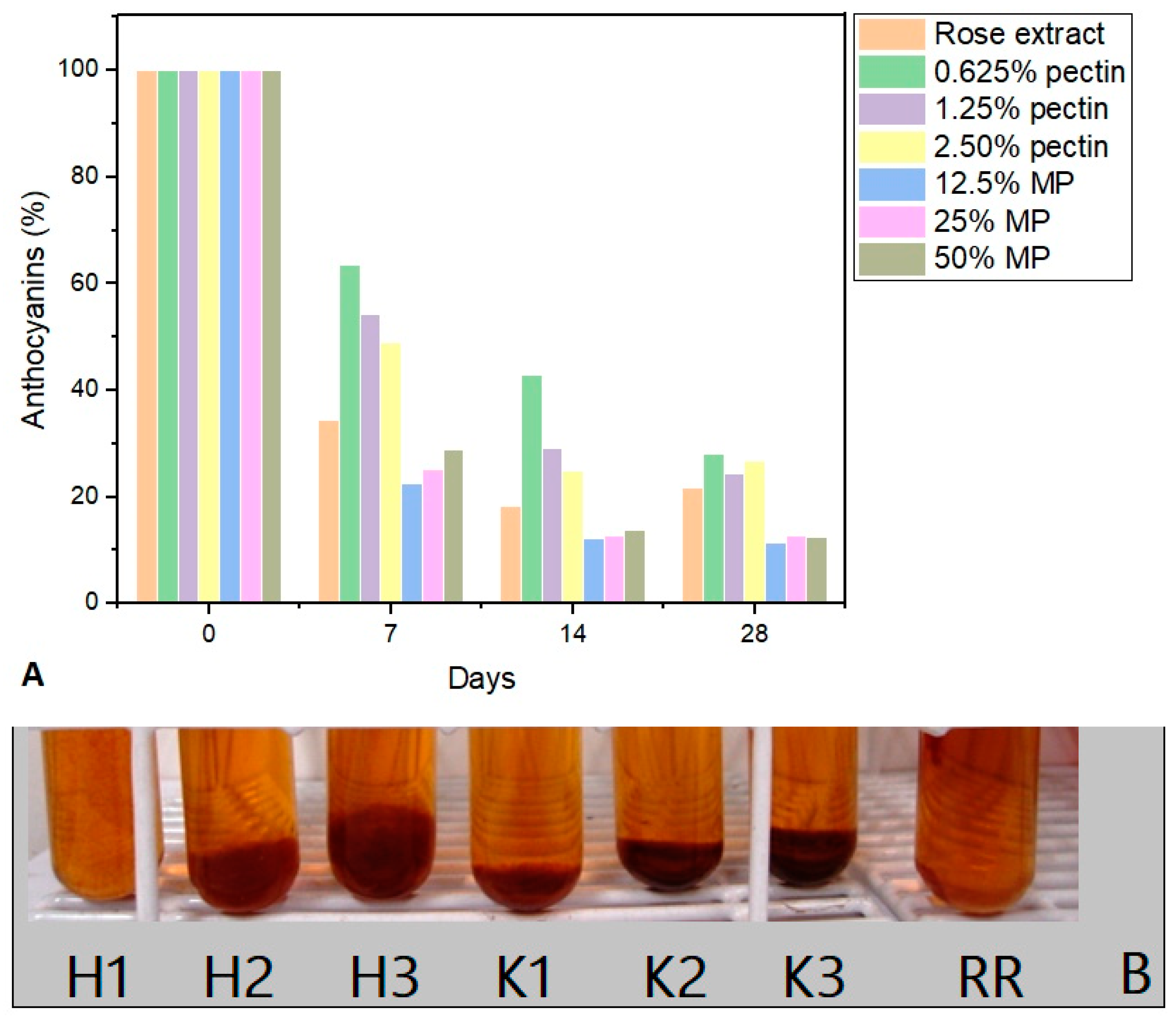
3. Results and Discussion
4. Limitations and Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erdogan Eliuz, E.A.; Yabalak, E. Chicken feather hydrochar incorporated with phenolic extract of Rosa damascena Mill. to enlarge the antibacterial performance against Acinobacter baumannii and Staphylococcus aureus. J. Environ. Chem. Eng. 2022, 10, 108289. [Google Scholar] [CrossRef]
- Nikolova, G.; Karamalakova, Y.; Kovacheva, N.; Stanev, S.; Zheleva, A.; Gadjeva, V. Protective effect of two essential oils isolated from Rosa damascena Mill. and Lavandula angustifolia Mill, and two classic antioxidants against L-dopa oxidative toxicity induced in healthy mice. Regul. Toxicol. Pharmacol. 2016, 81, 1–7. [Google Scholar] [CrossRef]
- Gupta, S.; Krishna Tewari, S.; Pathak, S. Chapter 16—Temple floral waste for various bio-products in India. In Recent Trends in Solid Waste Management; Ravindran, B., Gupta, S.K., Bhat, S.A., Chauhan, P.S., Tyagi, N., Eds.; Advances in Pollution Research; Elsevier: Amsterdam, The Netherlands, 2023; pp. 293–307. ISBN 978-0-443-15206-1. [Google Scholar]
- Falla, N.M.; Contu, S.; Demasi, S.; Caser, M.; Scariot, V. Environmental Impact of Edible Flower Production: A Case Study. Agronomy 2020, 10, 579. [Google Scholar] [CrossRef]
- Karpun, O. Conceptual model of floriculture supply chain management. Intellectualization Logist. Supply Chain. Manag. 2020, 1, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Utilization of Blackthorn Plums (Prunus spinosa) and Sweet Cherry (Prunus avium) Kernel Oil: Assessment of Chemical Composition, Antioxidant Activity, and Oxidative Stability. Biomass 2024, 4, 49–64. [Google Scholar] [CrossRef]
- Harborne, J. The anthocyanins of roses. Occurrence of peonin. Experientia 1961, 17, 72–73. [Google Scholar] [CrossRef]
- Trujillo-Reyes, Á.; Cubero-Cardoso, J.; Rodríguez-Gutiérrez, G.; García-Martín, J.F.; Rodríguez-Galán, M.; Borja, R.; Serrano, A.; Fermoso, F.G. Extraction of phenolic compounds and production of biomethane from strawberry and raspberry extrudates. Biochem. Eng. J. 2019, 147, 11–19. [Google Scholar] [CrossRef]
- Kendrick, A. 7-Coloring Aqueous Food Types. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2016; pp. 163–177. ISBN 978-0-08-100371-8. [Google Scholar]
- Perez, M.B.; Da Peña Hamparsomian, M.J.; Gonzalez, R.E.; Denoya, G.I.; Dominguez, D.L.E.; Barboza, K.; Iorizzo, M.; Simon, P.W.; Vaudagna, S.R.; Cavagnaro, P.F. Physicochemical properties, degradation kinetics, and antioxidant capacity of aqueous anthocyanin-based extracts from purple carrots compared to synthetic and natural food colorants. Food Chem. 2022, 387, 132893. [Google Scholar] [CrossRef]
- Cho, E.; Chung, E.Y.; Jang, H.-Y.; Hong, O.-Y.; Chae, H.S.; Jeong, Y.-J.; Kim, S.-Y.; Kim, B.-S.; Yoo, D.J.; Kim, J.-S.; et al. Anti-cancer Effect of Cyanidin-3-glucoside from Mulberry via Caspase-3 Cleavage and DNA Fragmentation in vitro and in vivo. Anticancer Agents Med. Chem. 2017, 17, 1519–1525. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Chai, Z.; Hutabarat, R.P.; Beta, T.; Feng, J.; Ma, K.; Li, D.; Huang, W. Hypoglycemic and hypolipidemic effects of blueberry anthocyanins by AMPK activation: In vitro and in vivo studies. Redox Biol. 2021, 46, 102100. [Google Scholar] [CrossRef]
- Andrade, T.A.; Hamerski, F.; López Fetzer, D.E.; Roda-Serrat, M.C.; Corazza, M.L.; Norddahl, B.; Errico, M. Ultrasound-assisted pressurized liquid extraction of anthocyanins from Aronia melanocarpa pomace. Sep. Purif. Technol. 2021, 276, 119290. [Google Scholar] [CrossRef]
- Sidor, A.; Drożdżyńska, A.; Gramza-Michałowska, A. Black chokeberry (Aronia melanocarpa) and its products as potential health-promoting factors—An overview. Trends Food Sci. Technol. 2019, 89, 45–60. [Google Scholar] [CrossRef]
- Song, J.; Yu, Y.; Chen, M.; Ren, Z.; Chen, L.; Fu, C.; Li, Z. Advancement of protein-and polysaccharide-based biopolymers for anthocyanin encapsulation. Front. Nutr. 2022, 9, 938829. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; He, Y.; Fan, K. Recent advances in stability improvement of anthocyanins by efficient methods and its application in food intelligent packaging: A review. Food Biosci. 2023, 56, 103164. [Google Scholar] [CrossRef]
- Li, J.; Guo, X.; Wang, R.; Geng, Z.; Jia, J.; Pang, S.; Du, Y.; Jia, S.; Cui, J. Ultrasonic assisted extraction of anthocyanins from rose flower petal in DES system and enzymatic acylation. LWT 2023, 180, 114693. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Wondra, A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Didion, Y.P.; Tjalsma, T.G.; Su, Z.; Malankowska, M.; Pinelo, M. What is next? the greener future of solid liquid extraction of biobased compounds: Novel techniques and solvents overpower traditional ones. Sep. Purif. Technol. 2023, 320, 124147. [Google Scholar] [CrossRef]
- Das, S.; Nadar, S.S.; Rathod, V.K. Integrated strategies for enzyme assisted extraction of bioactive molecules: A review. Int. J. Biol. Macromol. 2021, 191, 899–917. [Google Scholar] [CrossRef]
- Wijesinghe, W.; Jeon, Y.-J. Enzyme-assistant extraction (EAE) of bioactive components: A useful approach for recovery of industrially important metabolites from seaweeds: A review. Fitoterapia 2012, 83, 6–12. [Google Scholar] [CrossRef]
- Barzana, E.; Rubio, D.; Santamaria, R.; Garcia-Correa, O.; Garcia, F.; Ridaura Sanz, V.; López-Munguía, A. Enzyme-mediated solvent extraction of carotenoids from marigold flower (Tagetes erecta). J. Agric. Food Chem. 2002, 50, 4491–4496. [Google Scholar] [CrossRef]
- Passos, C.P.; Yilmaz, S.; Silva, C.M.; Coimbra, M.A. Enhancement of grape seed oil extraction using a cell wall degrading enzyme cocktail. Food Chem. 2009, 115, 48–53. [Google Scholar] [CrossRef]
- Ruiz-Terán, F.; Perez-Amador, I.; López-Munguia, A. Enzymatic extraction and transformation of glucovanillin to vanillin from vanilla green pods. J. Agric. Food Chem. 2001, 49, 5207–5209. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.; Li, J.; Zu, Y.-G.; Fu, Y.-J.; Luo, M.; Wu, N.; Liu, X.-L. Optimisation of microwave-assisted enzymatic extraction of corilagin and geraniin from Geranium sibiricum Linne and evaluation of antioxidant activity. Food Chem. 2010, 122, 373–380. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, E.; Golkar, P.; Vahabi, M.R.; Taghizadeh, M. Elicitation Effects on Some Secondary Metabolites and Antioxidant Activity in Callus Cultures of Allium jesdianum Boiss. & Buhse.: Methyl Jasmonate and Putrescine. Appl. Biochem. Biotechnol. 2022, 194, 601–619. [Google Scholar] [CrossRef] [PubMed]
- Sankaralingam, B.; Balan, L.; Chandrasekaran, S.; Muthu Selvam, A. Anthocyanin: A Natural Dye Extracted from Hibiscus Sabdariffa (L.) for Textile and Dye Industries. Appl. Biochem. Biotechnol. 2023, 195, 2648–2663. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, L.; Kalbasi-Ashtari, A.; Hamedi, M.; Ghorbani, F. Effects of enzymatic extraction on anthocyanins yield of saffron tepals (Crocos sativus) along with its color properties and structural stability. J. Food Drug Anal. 2015, 23, 210–218. [Google Scholar] [CrossRef]
- Serea, D.; Râpeanu, G.; Constantin, O.E.; Bahrim, G.E.; Stănciuc, N.; Croitoru, C. Ultrasound and enzymatic assisted extractions of bioactive compounds found in red grape skins băbească neagră (vitis vinifera) variety. Ann. Univ. Dunarea Jos Galati Fascicle VI-Food Technol. 2021, 45, 9–25. [Google Scholar] [CrossRef]
- Li, X.; Zhu, F.; Zeng, Z. Effects of different extraction methods on antioxidant properties of blueberry anthocyanins. Open Chem. 2021, 19, 138–148. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Silva, T.O.M.; Barros, R.G.C.; de Oliveira, C.S.; Cunha, G.C.; Gualberto, N.C.; Rajan, M.; Narain, N. Enzymatic and ultrasonic-assisted pretreatment in the extraction of bioactive compounds from Monguba (Pachira aquatic Aubl) leaf, bark and seed. Food Res. Int. 2021, 140, 109869. [Google Scholar] [CrossRef]
- Oancea, S.; Perju, M. Influence of enzymatic and ultrasonic extraction on phenolics content and antioxidant activity of Hibiscus Sabdariffa, L. flowers. Bulg. Chem. Commun. 2020, 52, 25–29. [Google Scholar]
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Ultrasound-assisted enzymatic extraction of anthocyanins from raspberry wine residues: Process optimization, isolation, purification, and bioactivity determination. Food Anal. Methods 2021, 14, 1369–1386. [Google Scholar] [CrossRef]
- Talbi, E.-G. Metaheuristics: From Design to Implementation; John Wiley & Sons: New York, NY, USA, 2009; ISBN 0-470-49690-8. [Google Scholar]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef]
- Folin, O.; Denis, W. On phosphotungstic-phosphomolybdic compounds as color reagents. J. Biol. Chem. 1912, 12, 239–243. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the AOAC International; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- da Silva, C.; Herdeiro, R.; Mathias, C.; Panek, A.; Silveira, C.; Rodrigues, V.; Rennó, M.; Falcão, D.; Cerqueira, D.; Minto, A. Evaluation of antioxidant activity of Brazilian plants. Pharmacol. Res. 2005, 52, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Özgür, M.Ü.; Çimen, E. Ultrasound-Assisted Extraction of Anthocyanins from Red Rose Petals and New Spectrophotometric Methods for the Determination of Total Monomeric Anthocyanins. J. AOAC Int. 2018, 101, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.; Xavier, M.; Quadri, M.G.; Quadri, M. Antocianinas: Uma breve revisão das características estruturais e da estabilidade. Curr. Agric. Sci. Technol. 2007, 13, 112861433. [Google Scholar]
- García-Aparicio, M.P.; Ballesteros, M.; Manzanares, P.; Ballesteros, I.; González, A.; Negro, M.J. Xylanase Contribution to the Efficiency of Cellulose Enzymatic Hydrolysis of Barley Straw; Springer: Berlin/Heidelberg, Germany, 2007; pp. 353–365. [Google Scholar]
- Huang, H.-L.; Tsai, I.-L.; Lin, C.; Hang, Y.-H.; Ho, Y.-C.; Tsai, M.-L.; Mi, F.-L. Intelligent films of marine polysaccharides and purple cauliflower extract for food packaging and spoilage monitoring. Carbohydr. Polym. 2023, 299, 120133. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.; Lee, J.; Sop Nam, H.; Gyu Lee, H. Stabilization of black soybean anthocyanin by chitosan nanoencapsulation and copigmentation. J. Food Biochem. 2017, 41, e12316. [Google Scholar] [CrossRef]
- Hubbermann, E.M.; Heins, A.; Stöckmann, H.; Schwarz, K. Influence of acids, salt, sugars and hydrocolloids on the colour stability of anthocyanin rich black currant and elderberry concentrates. Eur. Food Res. Technol. 2006, 223, 83–90. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Sachdev, A.; Kaur, C.; Varghese, E.; Saha, S.; Sairam, K.V.S.S. Evaluation of enzyme and microwave-assisted conditions on extraction of anthocyanins and total phenolics from black soybean (Glycine max L.) seed coat. Int. J. Biol. Macromol. 2019, 135, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.M.D.C.; Chaud, M.V.; Balcão, V.M. Chapter 19—Microencapsulation of Natural Anti-Oxidant Pigments. In Microencapsulation and Microspheres for Food Applications; Sagis, L.M.C., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 369–389. ISBN 978-0-12-800350-3. [Google Scholar]
- Ryu, D.; Koh, E. Application of response surface methodology to acidified water extraction of black soybeans for improving anthocyanin content, total phenols content and antioxidant activity. Food Chem. 2018, 261, 260–266. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, P.; Zhu, Y.; Lou, Q.; He, S. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato (Ipomoea batatas (L.) Lam.). Sci. Rep. 2018, 8, 5018. [Google Scholar] [CrossRef]


| Factors | −1.68 | −1 | 0 | +1 | +1.68 |
|---|---|---|---|---|---|
| Rose petals/solvent | 0.10 | 0.14 | 0.2 | 0.26 | 0.30 |
| %Ethanol (v/v) | 0 | 2 | 5 | 8 | 10 |
| %Enzyme | 0 | 0.2 | 0.5 | 0.8 | 1.0 |
| Assays | Petals/Liq. | % Ethanol | %Enzyme | Sugars (g/L) | Phenols (g/L) | Total Solids (%) |
|---|---|---|---|---|---|---|
| 1 | 0.14 | 2.0 | 0.20 | 6.03 | 5.33 | 1.08 |
| 2 | 0.14 | 2.0 | 0.80 | 6.13 | 4.36 | 1.25 |
| 3 | 0.14 | 8.0 | 0.20 | 6.77 | 6.50 | 1.78 |
| 4 | 0.14 | 8.0 | 0.80 | 7.40 | 6.69 | 1.44 |
| 5 | 0.26 | 2.0 | 0.20 | 9.07 | 8.76 | 2.43 |
| 6 | 0.26 | 2.0 | 0.80 | 9.20 | 7.26 | 2.37 |
| 7 | 0.26 | 8.0 | 0.20 | 8.76 | 8.82 | 2.27 |
| 8 | 0.26 | 8.0 | 0.80 | 8.50 | 9.99 | 2.71 |
| 9 | 0.10 | 5.0 | 0.50 | 5.92 | 6.07 | 1.19 |
| 10 | 0.30 | 5.0 | 0.50 | 9.06 | 6.44 | 2.56 |
| 11 | 0.20 | 0.0 | 0.50 | 8.39 | 6.95 | 1.80 |
| 12 | 0.20 | 10.0 | 0.50 | 8.29 | 5.80 | 2.06 |
| 13 | 0.20 | 5.0 | 0.00 | 6.79 | 7.90 | 1.51 |
| 14 | 0.20 | 5.0 | 1.00 | 8.87 | 7.66 | 1.57 |
| 15 | 0.20 | 5.0 | 0.50 | 9.16 | 8.13 | 2.68 |
| 16 | 0.20 | 5.0 | 0.50 | 9.07 | 8.14 | 2.65 |
| 17 | 0.20 | 5.0 | 0.50 | 9.10 | 8.11 | 2.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, B.D.; Ferreira, R.d.M.; Coelho, L.A.B.; Barreto, D.W. Production of Anthocyanin-Rich Red Rose Petal Extract by Enzymatic Maceration. Biomass 2024, 4, 429-441. https://doi.org/10.3390/biomass4020021
Ribeiro BD, Ferreira RdM, Coelho LAB, Barreto DW. Production of Anthocyanin-Rich Red Rose Petal Extract by Enzymatic Maceration. Biomass. 2024; 4(2):429-441. https://doi.org/10.3390/biomass4020021
Chicago/Turabian StyleRibeiro, Bernardo Dias, Rachel de Moraes Ferreira, Liliana Areia Bastos Coelho, and Daniel Weingart Barreto. 2024. "Production of Anthocyanin-Rich Red Rose Petal Extract by Enzymatic Maceration" Biomass 4, no. 2: 429-441. https://doi.org/10.3390/biomass4020021
APA StyleRibeiro, B. D., Ferreira, R. d. M., Coelho, L. A. B., & Barreto, D. W. (2024). Production of Anthocyanin-Rich Red Rose Petal Extract by Enzymatic Maceration. Biomass, 4(2), 429-441. https://doi.org/10.3390/biomass4020021






