Optimization of the Factors Affecting Biogas Production Using the Taguchi Design of Experiment Method
Abstract
1. Introduction
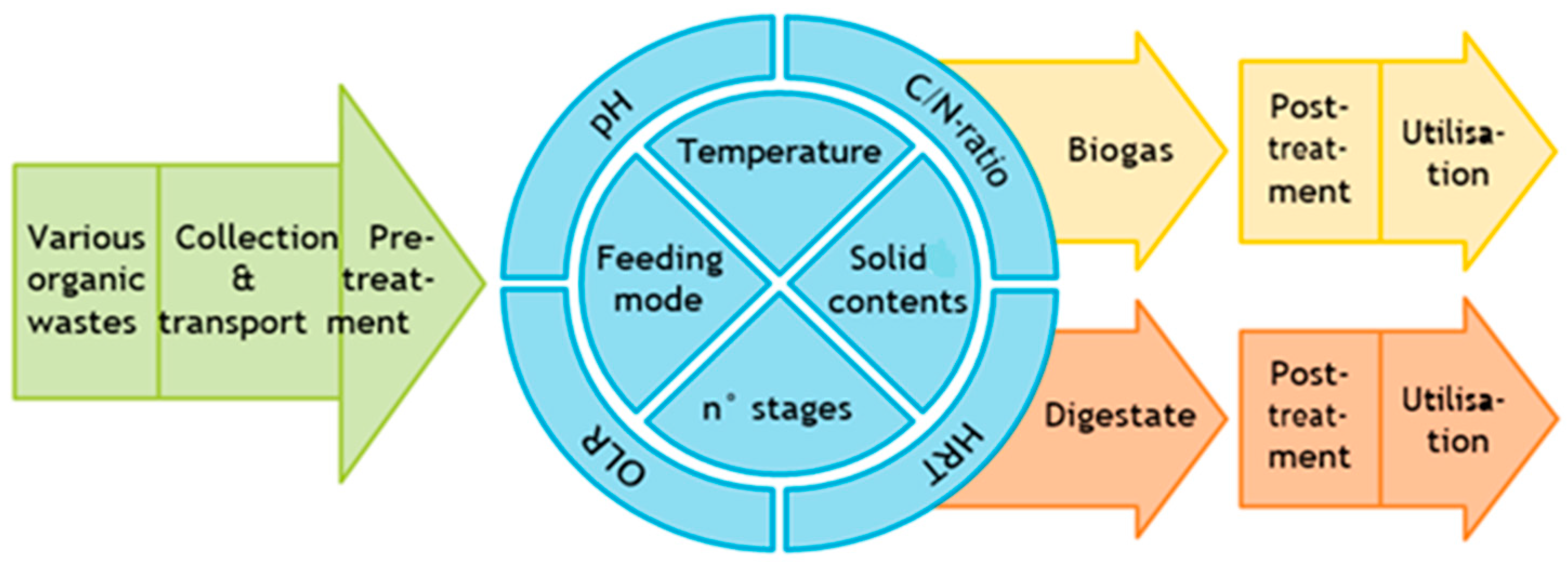
2. Factors Affecting Anaerobic Digestion
2.1. Temperature
2.2. Pre-Treatment
2.3. pH
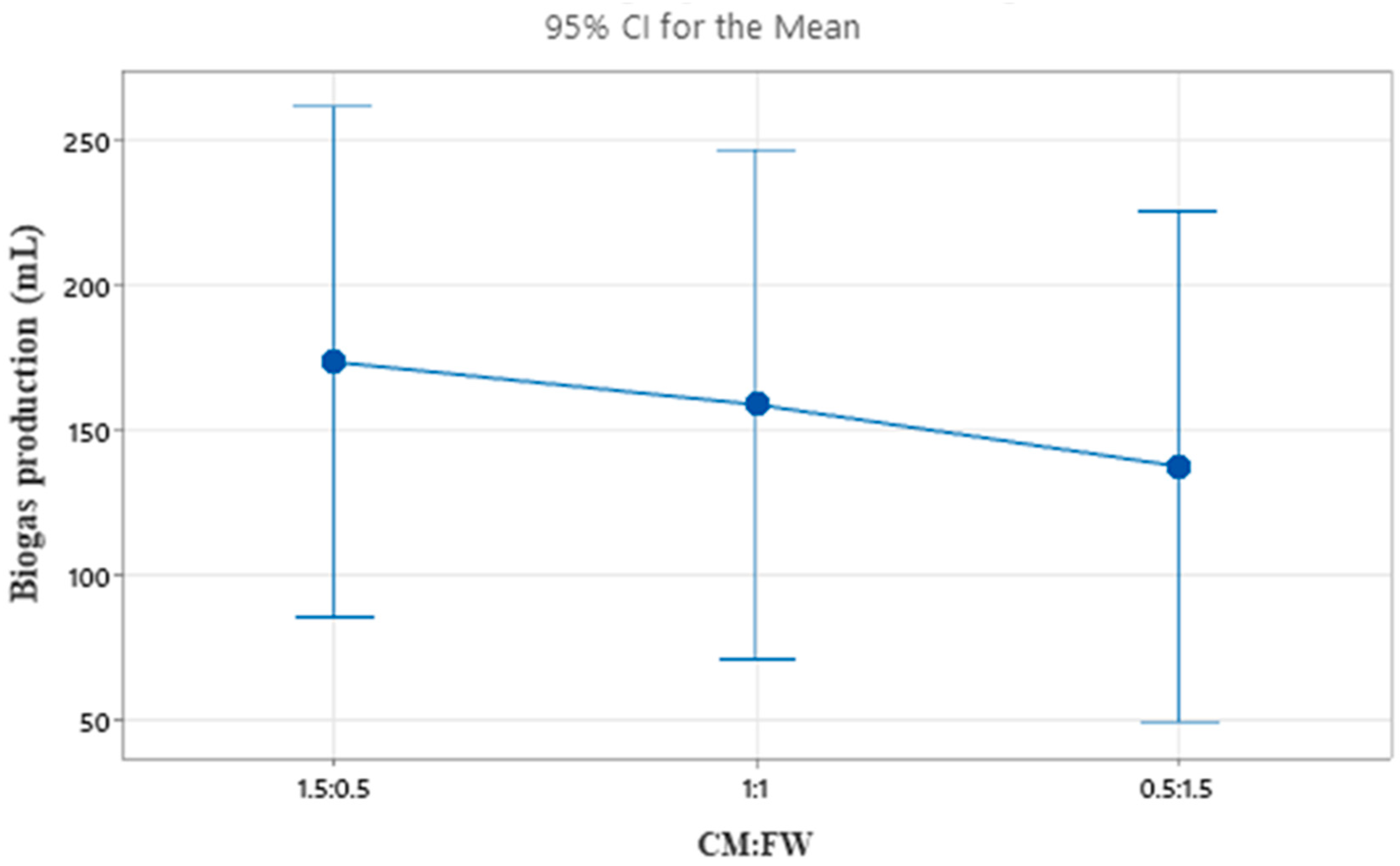
2.4. Mixing Ratio
3. Materials and Methods
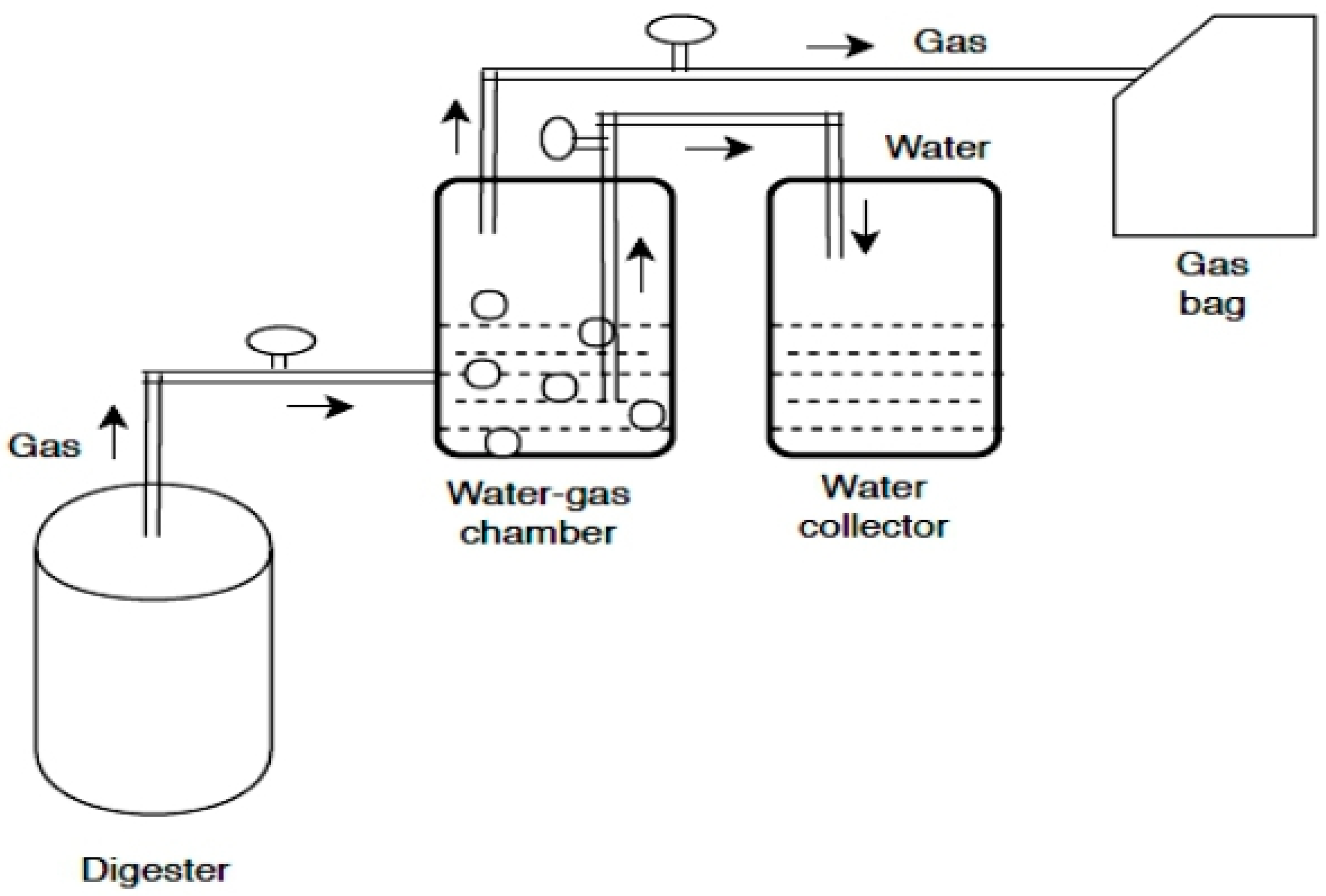
3.1. Experimental Set Up
3.2. Analysis of Methanogenic Performance
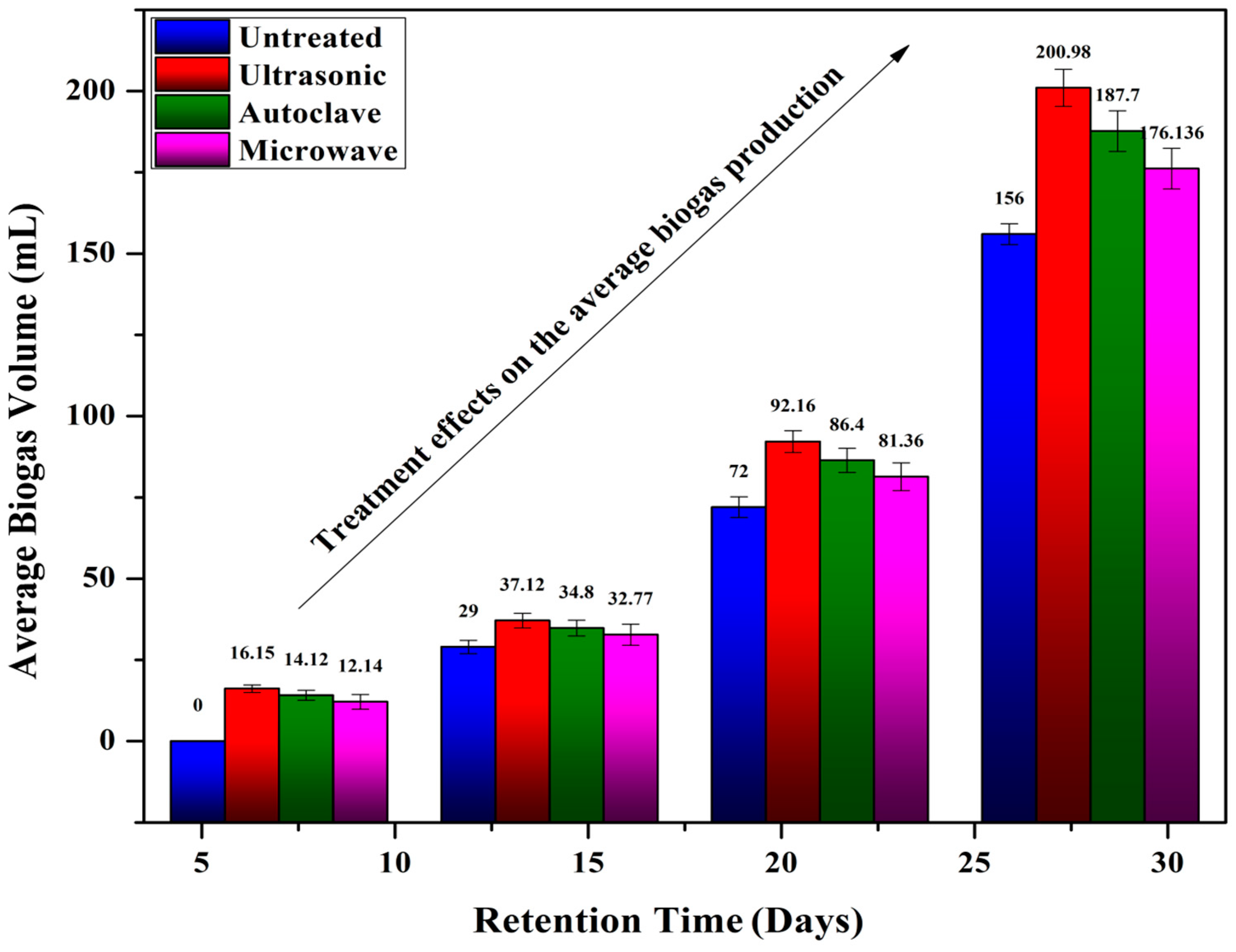
3.3. Pre-Treatment Methods Used
3.4. Taguchi Design of Experiments
- For the larger-the-better instant
3.5. Analysis of Variance (ANOVA)
4. Results and Discussions
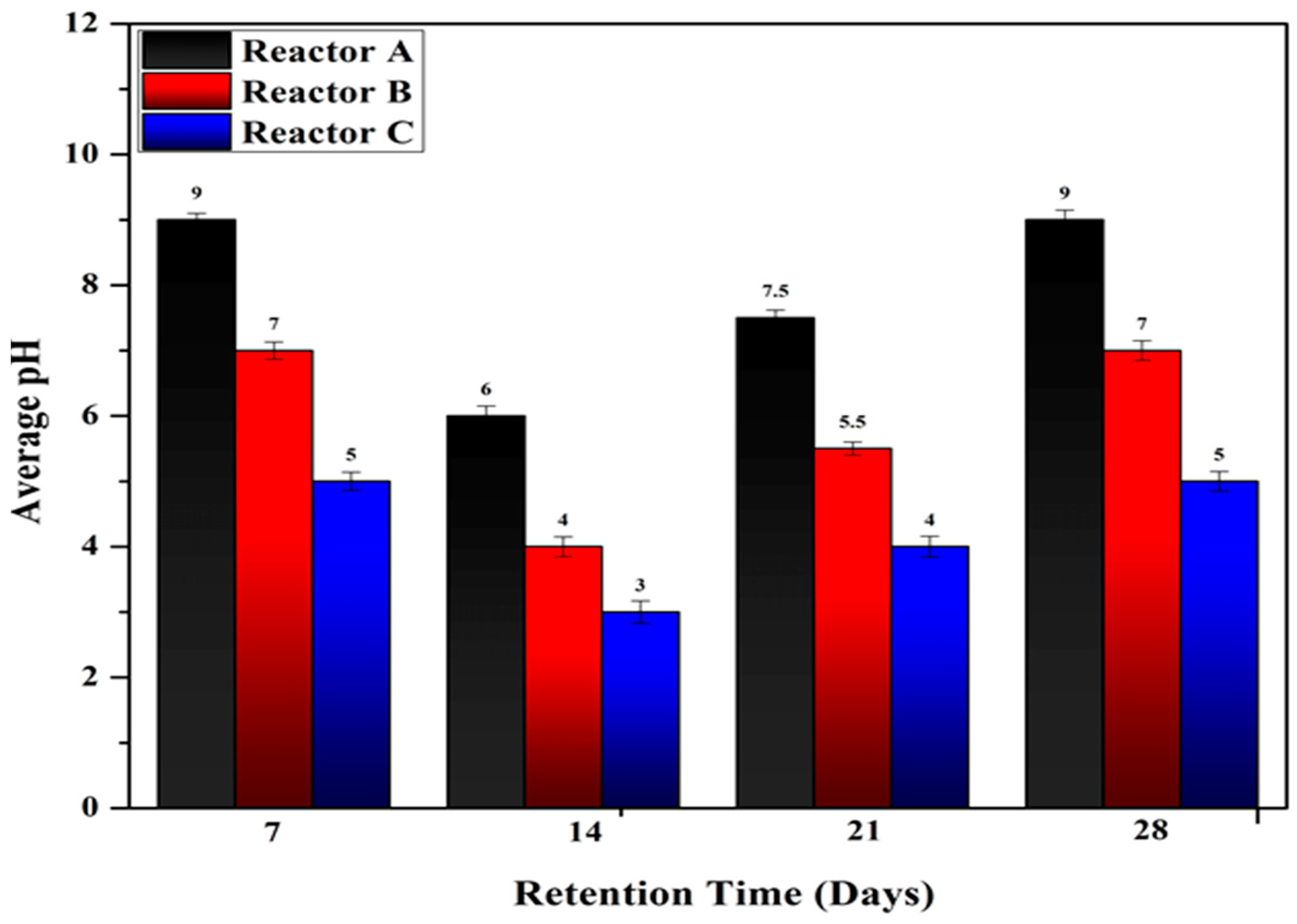
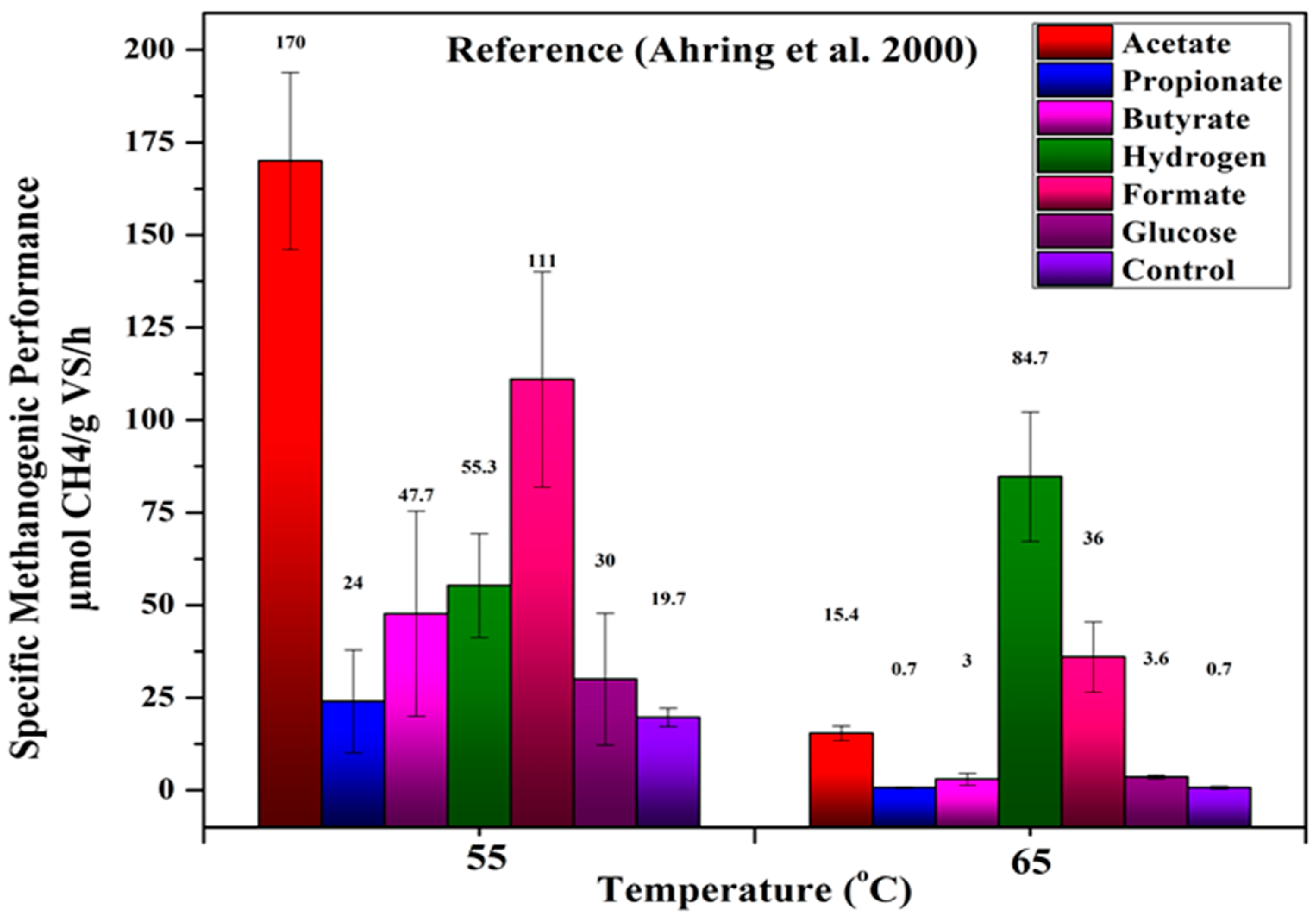
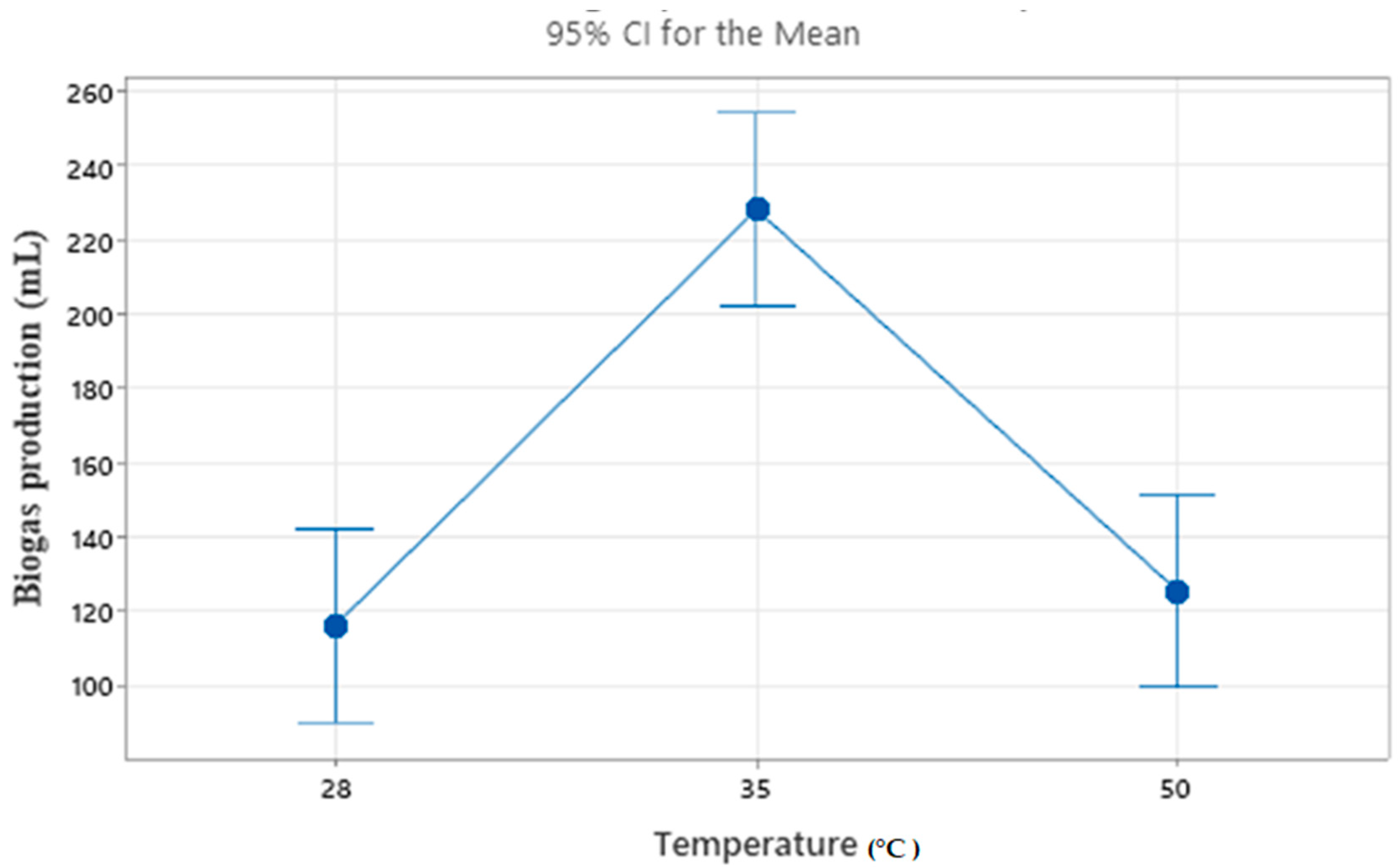
4.1. Effect of Temperature
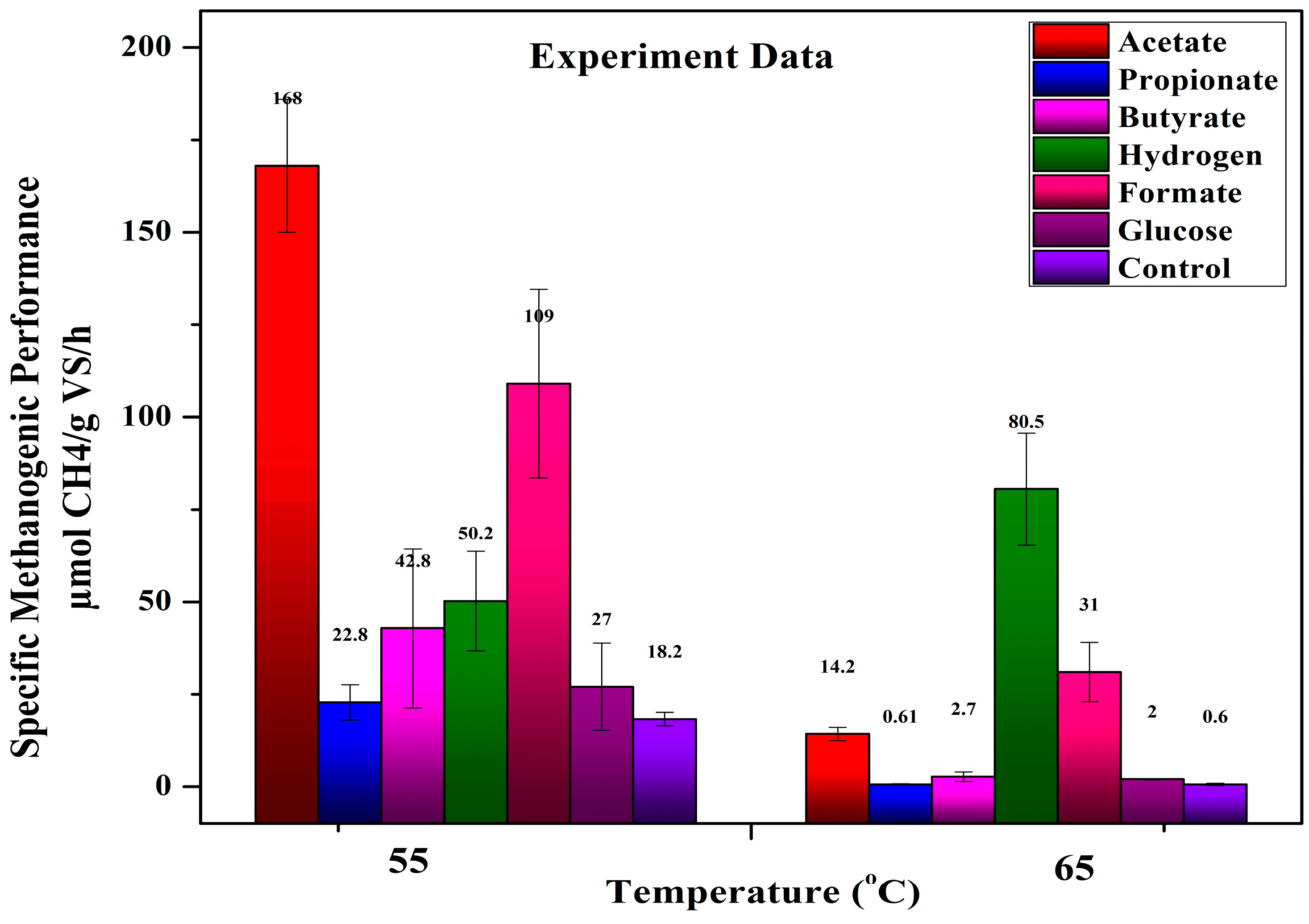
4.2. Effect of Microorganisms at Thermophilic Condition
4.3. Effect of Pre-Treatment on Biogas Production
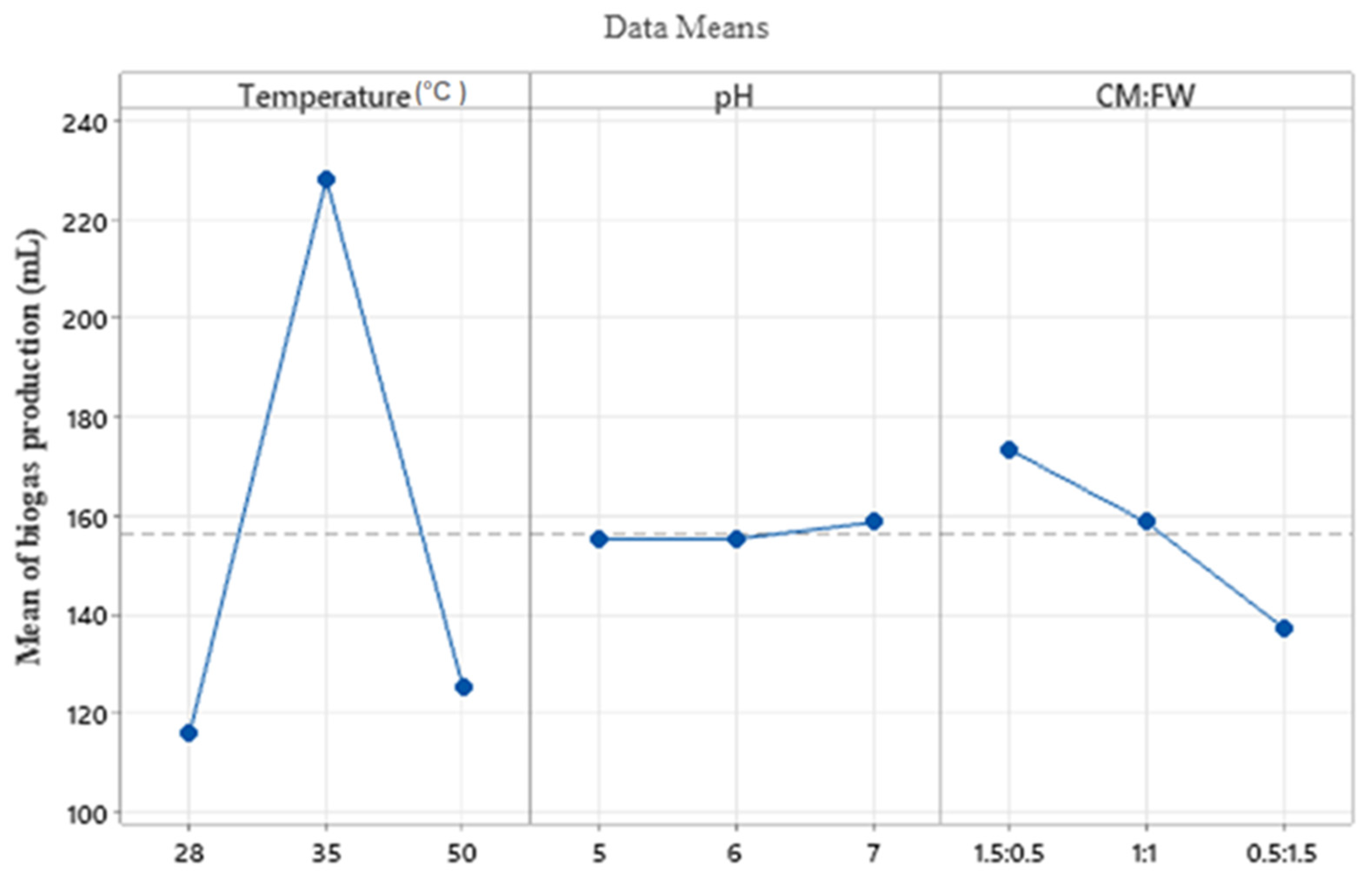
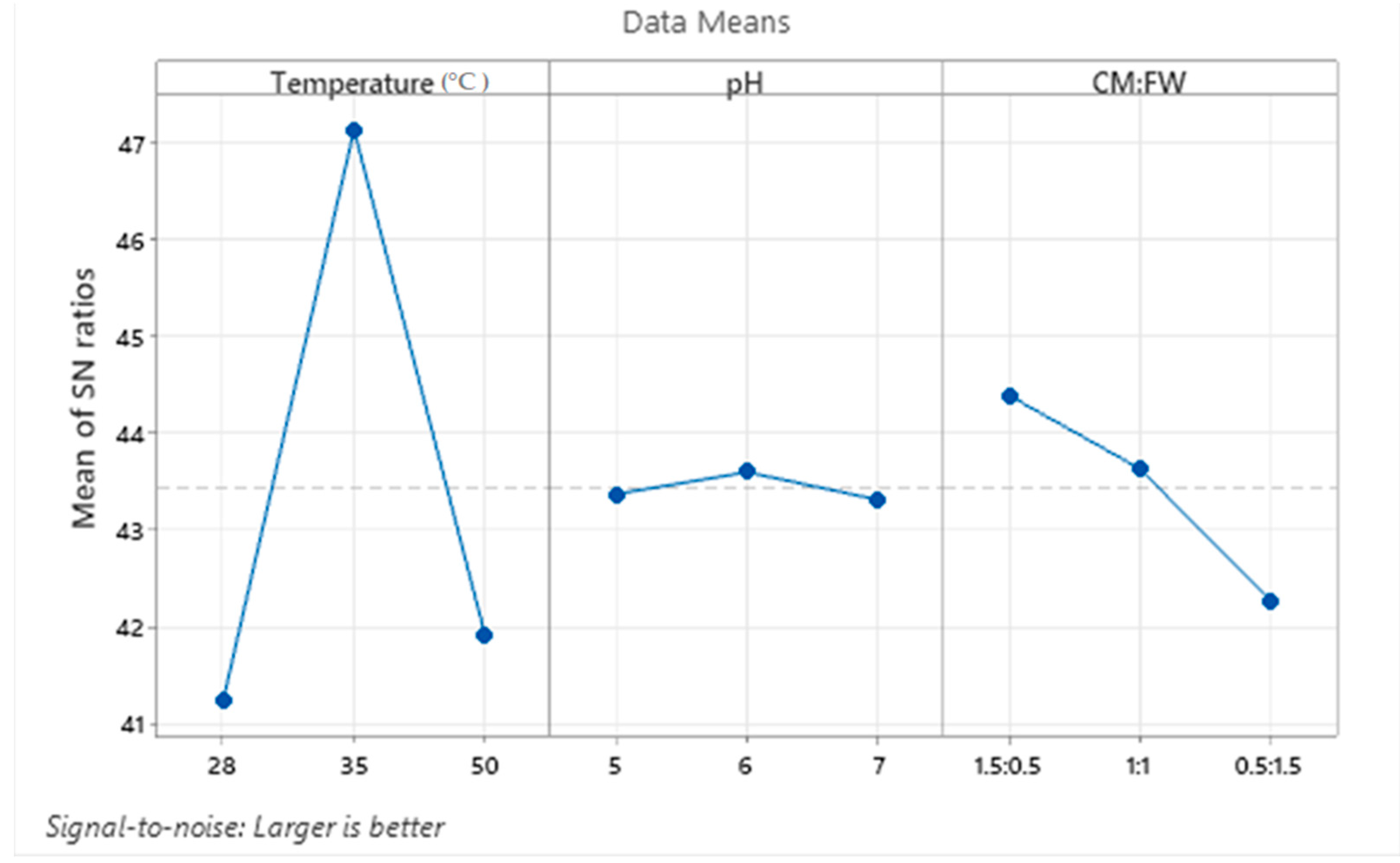
4.4. Optimization of the Parameters Using Taguchi Design of Experiments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdeshahian, P.; Lim, J.S.; Ho, W.S.; Hashim, H.; Lee, C.T. Potential of Biogas Production from Farm Animal Waste in Malaysia. Renew. Sustain. Energy Rev. 2016, 60, 714–723. [Google Scholar] [CrossRef]
- Mohammad, N.; Mohamad Ishak, W.W.; Mustapa, S.I.; Ayodele, B.V. Natural Gas as a Key Alternative Energy Source in Sustainable Renewable Energy Transition: A Mini Review. Front. Energy Res. 2021, 9, 625023. [Google Scholar] [CrossRef]
- Gürsan, C.; de Gooyert, V. The Systemic Impact of a Transition Fuel: Does Natural Gas Help or Hinder the Energy Transition? Renew. Sustain. Energy Rev. 2021, 138, 110552. [Google Scholar] [CrossRef]
- Kabeyi, M.J.; Olanrewaju, O.A. Sustainable Energy Transition for Renewable and Low Carbon Grid Electricity Generation and Supply. Front. Energy Res. 2022, 9, 743114. [Google Scholar] [CrossRef]
- Habib, S.S.; Torii, S. Biogas as Alternative to Liquefied Petroleum Gas in Mauritania: An Integrated Future Approach for Energy Sustainability and Socio-Economic Development. Clean Technol. 2024, 6, 453–470. [Google Scholar]
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for transformation of biogas to biomethane. J. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Xu, W.; Long, F.; Zhao, H.; Zhang, Y.; Liang, D.; Wang, L.; Lesnik, K.L.; Cao, H.; Zhang, Y.; Liu, H. Performance Prediction of Zvi-Based Anaerobic Digestion Reactor Using Machine Learning Algorithms. Waste Manag. 2021, 121, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sahota, S.; Shah, G.; Ghosh, P.; Kapoor, R.; Sengupta, S.; Singh, P.; Vijay, V.; Sahay, A.; Vijay, V.K.; Thakur, I.S. Review of Trends in Biogas Upgradation Technologies and Future Perspectives. Bioresour. Technol. Rep. 2018, 1, 79–88. [Google Scholar] [CrossRef]
- Ma, C.; Liu, J.; Ye, M.; Zou, L.; Qian, G.; Li, Y.-Y. Towards Utmost Bioenergy Conversion Efficiency of Food Waste: Pretreatment, Co-Digestion, and Reactor Type. Renew. Sustain. Energy Rev. 2018, 90, 700–709. [Google Scholar] [CrossRef]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Semi-Continuous Anaerobic Co-Digestion of Sugar Beet Byproduct and Pig Manure: Effect of the Organic Loading Rate (OLR) on Process Performance. Bioresour. Technol. 2015, 194, 283–290. [Google Scholar] [CrossRef]
- Dhar, H.; Kumar, P.; Kumar, S.; Mukherjee, S.; Vaidya, A.N. Effect of Organic Loading Rate during Anaerobic Digestion of Municipal Solid Waste. Bioresour. Technol. 2016, 217, 56–61. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Effect of Substrate Pretreatment on Biogas Production through Anaerobic Digestion of Food Waste. Int. J. Hydrogen Energy 2017, 42, 26522–26528. [Google Scholar] [CrossRef]
- Ajiboye, O.K.; Ochiegbu, C.V.; Ofosu, E.A.; Gyamfi, S. A Review of Hybrid Renewable Energies Optimisation: Design, Methodologies, and Criteria. Int. J. Sustain. Energy 2023, 42, 648–684. [Google Scholar] [CrossRef]
- Yuan, Y.; Bian, A.; Zhang, L.; Chen, T.; Pan, M.; He, L.; Wang, A.; Ding, C. A combined process for efficient biomethane production from corn straw and cattle manure: Optimizing C/N ratio of mixed hydrolysates. BioResources 2019, 14, 1347–1363. [Google Scholar] [CrossRef]
- Stroot, P.G.; McMohan, K.D.; Mackie, R.I.; Raskin, L. Anaerobic co-digestion of solid wastes and biosolids under various mixing conditions. Water Res. 2001, 35, 1804–1816. [Google Scholar] [CrossRef]
- Mol, S.K.; Sadiq, A. Material Parametric Optimisation of Wing Leading Edge Profile against Soft Body Impact. Int. J. Crashworthiness 2020, 27, 677–687. [Google Scholar] [CrossRef]
- Kavitha Mol, S.; Salem, S.C.; Sadiq, A. Crashworthiness enhancement of aluminum alloy used for leading edges of wing and empennage structures. J. Aerosp. Eng. 2022, 35. [Google Scholar] [CrossRef]
- Demollari, E. Temperature and Stirring Effect of Biogas Production from Two Different Systems. Am. J. Energy Eng. 2017, 5, 6. [Google Scholar] [CrossRef]
- El-Mashad, H.M.; van Loon, W.K.P.; Zeeman, G. A Model of Solar Energy Utilisation in the Anaerobic Digestion of Cattle Manure. Biosyst. Eng. 2003, 84, 231–238. [Google Scholar] [CrossRef]
- Trzcinski, A.P.; Stuckey, D.C. Treatment of Municipal Solid Waste Leachate Using a Submerged Anaerobic Membrane Bioreactor at Mesophilic and Psychrophilic Temperatures: Analysis of Recalcitrants in the Permeate Using GC-MS. Water Res. 2010, 44, 671–680. [Google Scholar] [CrossRef]
- Kim, J.K.; Oh, B.R.; Chun, Y.N.; Kim, S.W. Effects of Temperature and Hydraulic Retention Time on Anaerobic Digestion of Food Waste. J. Biosci. Bioeng. 2006, 102, 328–332. [Google Scholar] [CrossRef]
- Kashyap, D.R.; Dadhich, K.S.; Sharma, S.K. Biomethanation under Psychrophilic Conditions: A Review. Bioresour. Technol. 2003, 87, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Fezzani, B.; Ben Cheikh, R. Two-Phase Anaerobic Co-Digestion of Olive Mill Wastes in Semi-Continuous Digesters at Mesophilic Temperature. Bioresour. Technol. 2010, 101, 1628–1634. [Google Scholar] [CrossRef]
- Briški, F.; Vuković, M.; Papa, K.; Gomzi, Z.; Domanovac, T. Modelling of Composting of Food Waste in a Column Reactor. Chem. Pap. 2007, 61, 24–29. [Google Scholar] [CrossRef]
- Zhu, B.; Gikas, P.; Zhang, R.; Lord, J.; Jenkins, B.; Li, X. Characteristics and Biogas Production Potential of Municipal Solid Wastes Pretreated with a Rotary Drum Reactor. Bioresour. Technol. 2009, 100, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Molnar, L.; Bartha, I. High Solids Anaerobic Fermentation for Biogas and Compost Production. Biomass 1988, 16, 173–182. [Google Scholar] [CrossRef]
- Anand, R.C.; Singh, R. A Simple Technique, Charcoal Coating around the Digester, Improves Biogas Production in Winter. Bioresour. Technol. 1993, 45, 151–152. [Google Scholar] [CrossRef]
- Desai, M.; Madamwar, D. Anaerobic Digestion of a Mixture of Cheese Whey, Poultry Waste and Cattle Dung: A Study of the Use of Adsorbents to Improve Digester Performance. Environ. Pollut. 1994, 86, 337–340. [Google Scholar] [CrossRef]
- Hren, R.; Petrovič, A.; Čuček, L.; Simonič, M. Determination of Various Parameters during Thermal and Biological Pretreatment of Waste Materials. Energies 2020, 13, 2262. [Google Scholar] [CrossRef]
- Hamdi, M. Effects of Agitation and Pretreatment on the Batch Anaerobic Digestion of Olive Mil. Bioresour. Technol. 1991, 36, 173–178. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, D.; Wu, S.; Wang, C. Alkali Pretreatment Enhances Biogas Production in the Anaerobic Digestion of Pulp and Paper Sludge. J. Hazard. Mater. 2009, 170, 366–373. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Macé, S.; Llabrés, P. Anaerobic Digestion of Organic Solid Wastes. an Overview of Research Achievements and Perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The Anaerobic Digestion of Solid Organic Waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the Anaerobic Digestion of Agricultural Resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Behera, S.K.; Kim, J.W.; Park, H.-S. Methane Production Potential of Leachate Generated from Korean Food Waste Recycling Facilities: A Lab-Scale Study. Waste Manag. 2009, 29, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Bai, X.; Zhou, X.; Cheng, S.; Gao, R.; Sun, J. Study on Improving Anaerobic Co-Digestion of Cow Manure and Corn Straw by Fruit and Vegetable Waste: Methane Production and Microbial Community in CSTR Process. Bioresour. Technol. 2018, 249, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Hills, D.J. Effects of Carbon: Nitrogen Ratio on Anaerobic Digestion of Dairy Manure. Agric. Wastes 1979, 1, 267–278. [Google Scholar] [CrossRef]
- Ahring, B.K.; Ibrahim, A.A.; Mladenovska, Z. Effect of temperature increase from 55 to 65 °C on performance and microbial population dynamics of an anaerobic reactor treating cattle manure. Water Res. 2001, 35, 2446–2452. [Google Scholar] [CrossRef]
- Stams, A.J. Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie Leeuwenhoek 1994, 66, 271–294. [Google Scholar] [CrossRef]
- Ahring, B.K. Status on science and application of thermophilic anaerobic digestion. Water Sci. Technol. 1994, 30, 241–249. [Google Scholar] [CrossRef]
- Sorensen, A.; Sørensen, A.H. Measurements of the specific methanogenic activity of anaerobic digestor biomass. Appl. Microbiol. Biotechnol. 1993, 40, 427–431. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Experimental and kinetic study on anaerobic digestion of food waste: The effect of total solids and ph. J. Renew. Sustain. Energy 2015, 7, 063104. [Google Scholar] [CrossRef]



| Control Parameters | Level 1 | Level 2 | Level 3 |
|---|---|---|---|
| Temperature (°C) | 28 | 35 | 50 |
| pH | 5 | 6 | 7 |
| Mixing ratio (CM:FW) | 1.5:0.5 | 1:1 | 0.5:1.5 |
| Factors Affecting Anaerobic Digestion | Results | ||||
|---|---|---|---|---|---|
| Exp. No. | Temperature (°C) | pH | Mixing Ratio | Biogas Production (mL) | S/N Ratio |
| 1 | 28 | 5 | 1.5:0.5 | 130 | 42.2789 |
| 2 | 28 | 6 | 1:1 | 120 | 41.5836 |
| 3 | 28 | 7 | 05:1:5 | 98 | 39.8245 |
| 4 | 35 | 5 | 1:1 | 228 | 47.1587 |
| 5 | 35 | 6 | 0.5:1.5 | 206 | 46.2773 |
| 6 | 35 | 7 | 1.5:0.5 | 250 | 47.9588 |
| 7 | 50 | 5 | 0.5:1.5 | 108 | 40.6685 |
| 8 | 50 | 6 | 1.5:0.5 | 140 | 42.9226 |
| 9 | 50 | 7 | 1:1 | 128 | 42.1442 |
| Level | Temperature (°C) | pH | Mixing Ratio (CM:FW) |
|---|---|---|---|
| 1 | 116.0 | 155.3 | 173.3 |
| 2 | 228.0 | 155.3 | 158.7 |
| 3 | 125.3 | 158.7 | 137.3 |
| Delta | 112.0 | 3.3 | 36.0 |
| Rank | 1 | 3 | 2 |
| Level | Temperature (°C) | pH | Mixing Ratio (CM:FW) |
|---|---|---|---|
| 1 | 41.23 | 43.37 | 44.39 |
| 2 | 47.13 | 43.59 | 43.63 |
| 3 | 41.91 | 43.31 | 42.26 |
| Delta | 5.90 | 0.29 | 2.13 |
| Rank | 1 | 3 | 2 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value | R2 |
|---|---|---|---|---|---|---|
| Temperature | 2 | 23,171.6 | 11,585.8 | 34.3 | 0.001 | 91.96 |
| pH | 2 | 22.2 | 11.1 | 0 | 0.997 | 0.09 |
| CM:FW | 2 | 1966.2 | 983.1 | 0.25 | 0.784 | 7.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidi Habib, S.; Torii, S.; S., K.M.; Charivuparampil Achuthan Nair, A. Optimization of the Factors Affecting Biogas Production Using the Taguchi Design of Experiment Method. Biomass 2024, 4, 687-703. https://doi.org/10.3390/biomass4030038
Sidi Habib S, Torii S, S. KM, Charivuparampil Achuthan Nair A. Optimization of the Factors Affecting Biogas Production Using the Taguchi Design of Experiment Method. Biomass. 2024; 4(3):687-703. https://doi.org/10.3390/biomass4030038
Chicago/Turabian StyleSidi Habib, Sidahmed, Shuichi Torii, Kavitha Mol S., and Ajimon Charivuparampil Achuthan Nair. 2024. "Optimization of the Factors Affecting Biogas Production Using the Taguchi Design of Experiment Method" Biomass 4, no. 3: 687-703. https://doi.org/10.3390/biomass4030038
APA StyleSidi Habib, S., Torii, S., S., K. M., & Charivuparampil Achuthan Nair, A. (2024). Optimization of the Factors Affecting Biogas Production Using the Taguchi Design of Experiment Method. Biomass, 4(3), 687-703. https://doi.org/10.3390/biomass4030038





