Can Foraging for Earthworms Significantly Reduce Global Famine in a Catastrophe?
Abstract
1. Introduction
- How many earthworms are there in accessible land on Earth?
- What is the potential nutritional value of the earthworm population?
- What methods of collecting earthworms are available, and how suitable might they be for the above-mentioned scenario?
- What would be the time and labour costs for producing nutrition from earthworms, and how do they compare to other resilient foods?
- How confident can we be in these calculations, and what limitations contribute to our uncertainty?
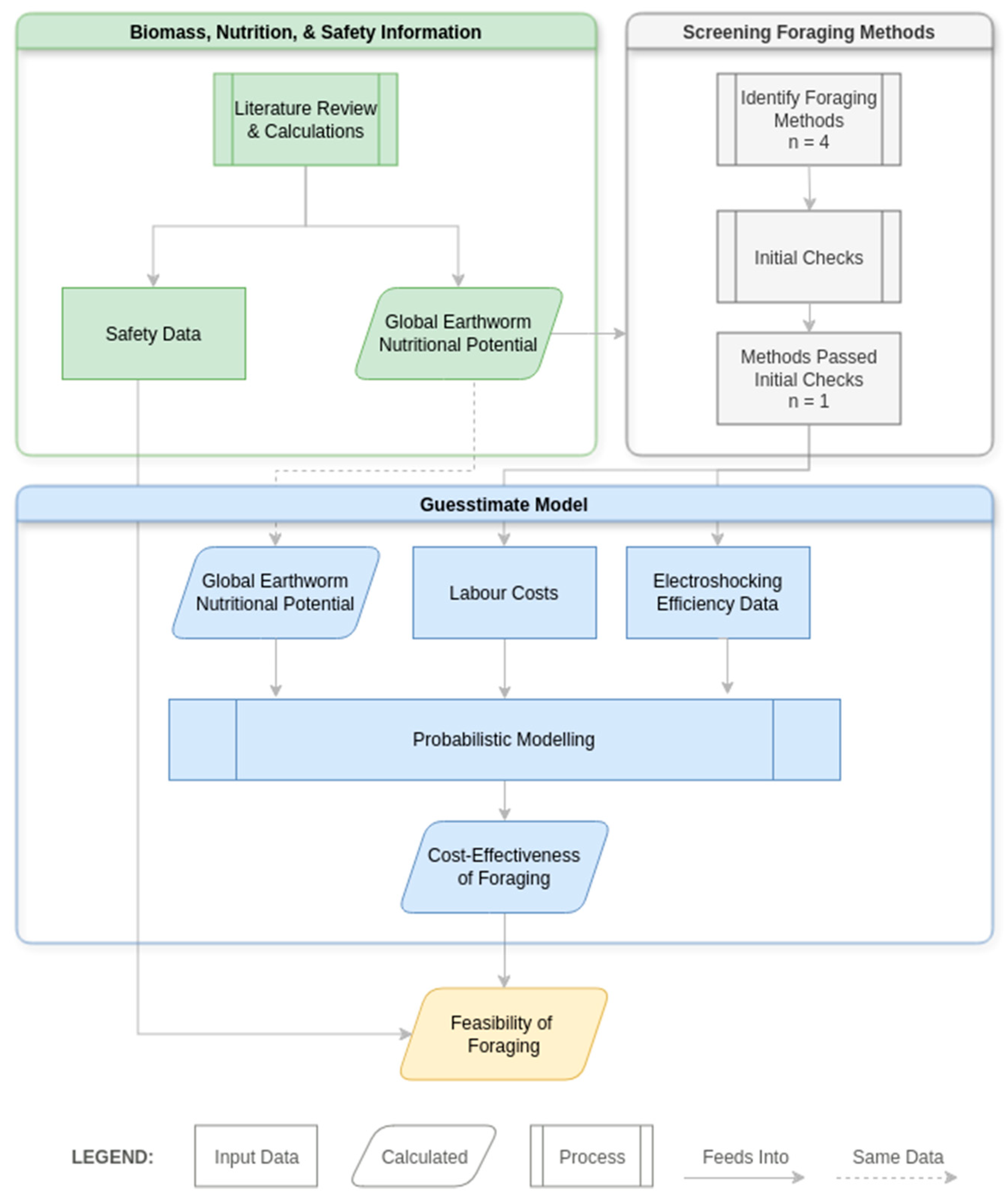
2. Materials and Methods
2.1. Overview of Methods
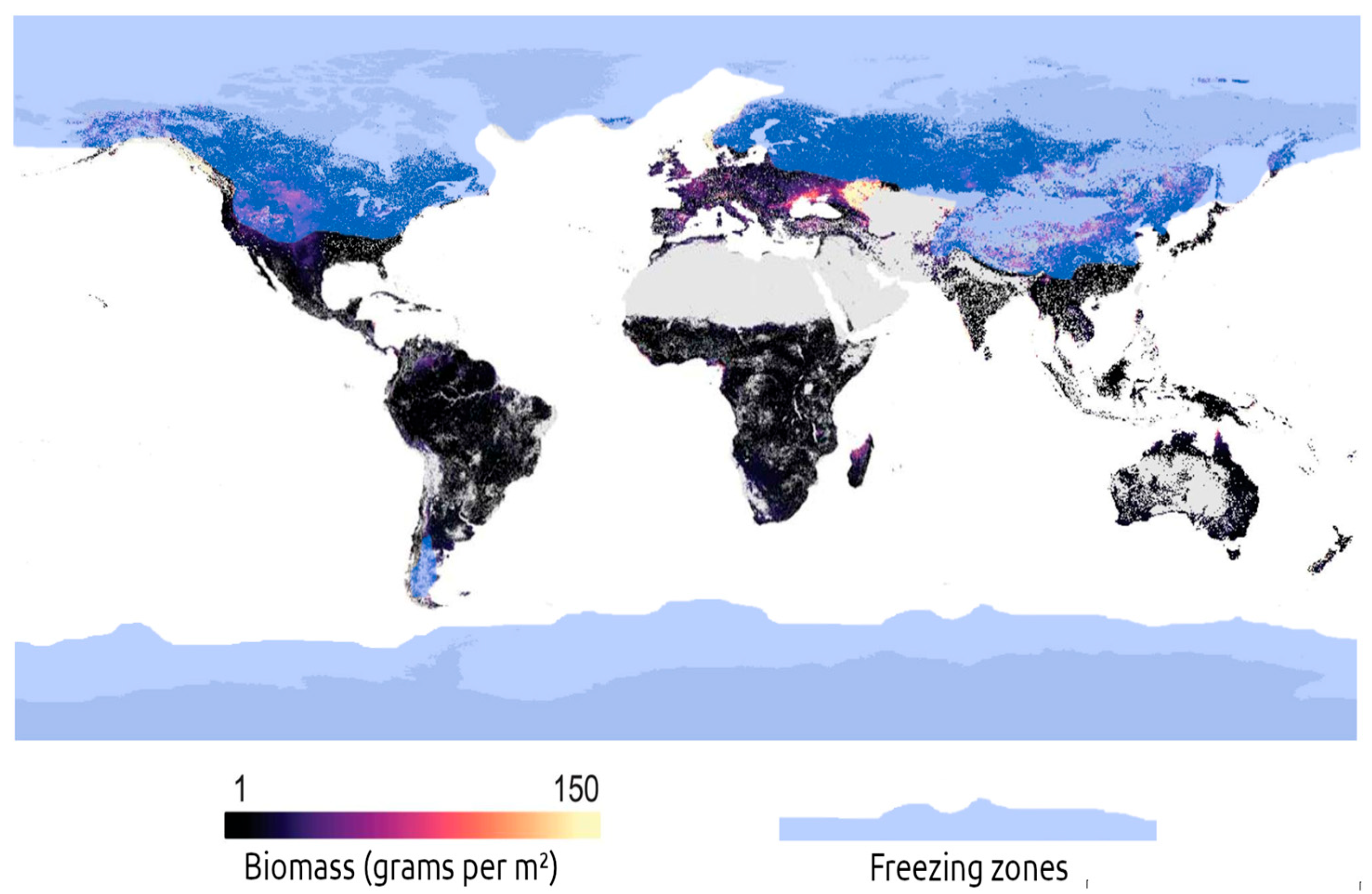
2.2. Estimating the Global Biomass of Wild Earthworms
2.3. Estimating the Nutritional Potential of the Global Wild Earthworm Population
2.4. Methods for Extracting Living Earthworms from the Soil and Efficiency Thereof
2.4.1. Literature Review and Initial Checks
- The percentage of the earthworm population in the soil that can be harvested (extraction percentage);
- The area of land foraged per application of a method (effective area);
- The time taken per application of a method (cycle time).
2.4.2. Guesstimate Model of Electroshocking
3. Results and Discussion
3.1. Global Nutritional Potential of Wild Earthworms
3.2. Feasibility and Cost-Effectiveness of Extracting Earthworms from the Ground
3.2.1. Mechanical Sorting of Soil
3.2.2. Worm Charming or Grunting
3.2.3. Chemical Earthworm Expellents
3.2.4. Electroshocking
3.3. Climate-Related Barriers to Earthworm Foraging
3.3.1. Reduced Temperature May Prevent Earthworm Foraging
3.3.2. Reduced Precipitation May Affect Earthworm Abundance
3.4. Processing Earthworms for Consumption
3.5. Significant Limitations and Uncertainties
3.5.1. Dataset for Global Earthworm Abundance Is Limited and May Underestimate the Resource
3.5.2. Worm Grunting, or Charming, Is Promising, but Reliable Data Are Lacking
3.5.3. Reports of High Yields from Electroshocking Are Unconfirmed by Scientific Literature
3.5.4. Foraged Earthworms May Be Harmful for Humans
3.5.5. Earthworm Foraging Could Reduce the Future Ecological and Agricultural Value of Land
3.5.6. Earthworms May Suffer during Capture, Processing, and Slaughter
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| Abbreviation | Meaning |
| ASRS | Abrupt sunlight reduction scenario |
| GCIL | Global catastrophic infrastructure loss |
| AITC | Allyl isothiocyanate |
| AC | Alternating current |
| DC | Direct current |
References
- Denkenberger, D.C.; Cole, D.D.; Abdelkhaliq, M.; Griswold, M.; Hundley, A.B.; Pearce, J.M. Feeding Everyone If the Sun Is Obscured and Industry Is Disabled. Int. J. Disaster Risk Reduct. 2017, 21, 284–290. [Google Scholar] [CrossRef]
- Hyder, S.; Chhem, R.K.; Claes, F.; Karlsson, E.A. Pestilence and Famine: Continuing down the Vicious Cycle with COVID-19. PLoS Pathog. 2022, 18, e1010810. [Google Scholar] [CrossRef] [PubMed]
- Tumelty, L.; Fa, J.E.; Coad, L.; Friant, S.; Mbane, J.; Kamogne, C.T.; Tata, C.Y.; Ickowitz, A. A Systematic Mapping Review of Links between Handling Wild Meat and Zoonotic Diseases. One Health 2023, 17, 100637. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.; García Martínez, J.B.; Brynych, V.; Stormbjorne, R.; Pearce, J.M.; Denkenberger, D.C. Nutrition in Abrupt Sunlight Reduction Scenarios: Envisioning Feasible Balanced Diets on Resilient Foods. Nutrients 2022, 14, 492. [Google Scholar] [CrossRef] [PubMed]
- Jehn, F.U.; Dingal, F.J.; Mill, A.; Harrison, C.; Ilin, E.; Roleda, M.Y.; James, S.C.; Denkenberger, D. Seaweed as a Resilient Food Solution after a Nuclear War. Earths Future 2024, 12, e2023EF003710. [Google Scholar] [CrossRef]
- Throup, J.; García Martínez, J.B.; Bals, B.; Cates, J.; Pearce, J.M.; Denkenberger, D.C. Rapid Repurposing of Pulp and Paper Mills, Biorefineries, and Breweries for Lignocellulosic Sugar Production in Global Food Catastrophes. Food Bioprod. Process. 2022, 131, 22–39. [Google Scholar] [CrossRef]
- García Martínez, J.B.; Pearce, J.M.; Throup, J.; Cates, J.; Lackner, M.; Denkenberger, D.C. Methane Single Cell Protein: Potential to Secure a Global Protein Supply Against Catastrophic Food Shocks. Front. Bioeng. Biotechnol. 2022, 10, 906704. [Google Scholar] [CrossRef] [PubMed]
- Rivers, M.; Hinge, M.; García Martínez, J.B.; Tieman, R.; Jaeck, V.; Butt, T.; Jehn, F.; Grillo, V.; Denkenberger, D. Food System Adaptation and Maintaining Trade Greatly Mitigate Global Famine in Abrupt Sunlight Reduction Scenarios; ALLFED: Lafayette, CO, USA, 2022. [Google Scholar]
- Sun, Z.; Jiang, H. Nutritive Evaluation of Earthworms as Human Food. In Future Foods; IntechOpen: London, UK, 2017; ISBN 978-953-51-3552-4. [Google Scholar]
- Conti, C.; Castrica, M.; Balzaretti, C.M.; Tedesco, D.E.A. Edible Earthworms in a Food Safety Perspective: Preliminary Data. Ital. J. Food Saf. 2019, 8, 7695. [Google Scholar] [CrossRef] [PubMed]
- Kavle, R.R.; Nolan, P.J.; Carne, A.; Agyei, D.; Morton, J.D.; Bekhit, A.E.-D.A. Earth Worming—An Evaluation of Earthworm (Eisenia Andrei) as an Alternative Food Source. Foods 2023, 12, 1948. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, M.G.; Buscardo, E.; VanderJagt, D.J.; Pastuszyn, A.; Pizzoferrato, L.; Huang, Y.-S.; Chuang, L.-T.; Millson, M.; Cerda, H.; Torres, F.; et al. Nutrient Content of Earthworms Consumed by Ye’Kuana Amerindians of the Alto Orinoco of Venezuela. Proc. Biol. Sci. 2003, 270, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Byambas, P.; Hornick, J.L.; Marlier, D.; Francis, F. Vermiculture in Animal Farming: A Review on the Biological and Nonbiological Risks Related to Earthworms in Animal Feed. Cogent Environ. Sci. 2019, 5, 1591328. [Google Scholar] [CrossRef]
- Edwards, C.A. Production of Feed Protein from Animal Waste by Earthworms. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1985, 310, 299–307. [Google Scholar]
- Musyoka, S.N.; Liti, D.M.; Ogello, E.; Waidbacher, H. Utilization of the Earthworm, Eisenia Fetida (Savigny, 1826) as an Alternative Protein Source in Fish Feeds Processing: A Review. Aquac. Res. 2019, 50, 2301–2315. [Google Scholar] [CrossRef]
- Catania, K.C. Worm Grunting, Fiddling, and Charming—Humans Unknowingly Mimic a Predator to Harvest Bait. PLoS ONE 2008, 3, e3472. [Google Scholar] [CrossRef] [PubMed]
- Monte Carlo Simulations|Guesstimate. Available online: https://docs.getguesstimate.com/docs/theory/monte_carlo_simulations (accessed on 6 May 2024).
- Miller, H. Electroshocking for Foraging Earthworms as a Food Source from Top 50% of Sites. Available online: https://www.getguesstimate.com/models/23356 (accessed on 4 December 2023).
- Phillips, H.R.P.; Guerra, C.A.; Bartz, M.L.C.; Briones, M.J.I.; Brown, G.; Crowther, T.W.; Ferlian, O.; Gongalsky, K.B.; van Den Hoogen, J.; Krebs, J.; et al. Global Distribution of Earthworm Diversity. Science 2019, 366, 480–485, Erratum in Science 2020, 369, eabd9834. [Google Scholar] [CrossRef]
- FAO. Land Use Statistics and Indicators; FAO: Rome, Italy, 2022. [Google Scholar]
- Ritchie, H.; Roser, M. Land Use. Our World Data, September 2019. [Google Scholar]
- Carley, W.W. Water Economy of the Earthworm Lumbricus terrestris L.: Coping with the Terrestrial Environment. J. Exp. Zool. 1978, 205, 71–78. [Google Scholar] [CrossRef]
- Roots, B.I. The Water Relations of Earthworms: II. Resistance to Desiccation and Immersion, and Behaviour When Submerged and When Allowed A Choice of Environment. J. Exp. Biol. 1956, 33, 29–44. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, X.; Sun, L.; Song, C. Earthworm as a Potential Protein Resource. Ecol. Food Nutr. 1997, 36, 221–236. [Google Scholar] [CrossRef]
- Bottinelli, N.; Hedde, M.; Jouquet, P.; Capowiez, Y. An Explicit Definition of Earthworm Ecological Categories—Marcel Bouché’s Triangle Revisited. Geoderma 2020, 372, 114361. [Google Scholar] [CrossRef]
- Fusaro, S.; Gavinelli, F.; Lazzarini, F.; Paoletti, M.G. Soil Biological Quality Index Based on Earthworms (QBS-e). A New Way to Use Earthworms as Bioindicators in Agroecosystems. Ecol. Indic. 2018, 93, 1276–1292. [Google Scholar] [CrossRef]
- Monroy, F.; Aira, M.; Domínguez, J.; Velando, A. Seasonal Population Dynamics of Eisenia fetida (Savigny, 1826) (Oligochaeta, Lumbricidae) in the Field. C. R. Biol. 2006, 329, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Palm, J.; van Schaik, N.L.M.B.; Schröder, B. Modelling Distribution Patterns of Anecic, Epigeic and Endogeic Earthworms at Catchment-Scale in Agro-Ecosystems. Pedobiologia 2013, 56, 23–31. [Google Scholar] [CrossRef]
- De Wandeler, H.; Sousa-Silva, R.; Ampoorter, E.; Bruelheide, H.; Carnol, M.; Dawud, S.M.; Dănilă, G.; Finer, L.; Hättenschwiler, S.; Hermy, M.; et al. Drivers of Earthworm Incidence and Abundance across European Forests. Soil Biol. Biochem. 2016, 99, 167–178. [Google Scholar] [CrossRef]
- Schröder, B. Challenges of Species Distribution Modeling Belowground. J. Plant Nutr. Soil Sci. 2008, 171, 325–337. [Google Scholar] [CrossRef]
- Ding, S.; Lin, X.; He, S. Earthworms: A Source of Protein. J. Food Sci. Eng. 2019, 9, 159–170. [Google Scholar] [CrossRef][Green Version]
- Rhea-Fournier, D.; González, G.; Rhea-Fournier, D.; González, G. Methodological Considerations in the Study of Earthworms in Forest Ecosystems. In Forest Ecology and Conservation; IntechOpen: London, UK, 2017; ISBN 978-953-51-3090-1. [Google Scholar]
- Philips, H.R.P.; Guerra, C.A.; Bartz, M.L.C.; Briones, M.J.I.; Brown, G.G.; Crowther, T.W.; Ferlian, O.; Gongalsky, K.; van den Hoogen, J.; Krebs, J.J.; et al. Global Distribution of Earthworm Diversity (Version 1.1). Dataset Available at iDiv Data Repository. Available online: https://idata.idiv.de/ddm/Data/ShowData/1804?version=7 (accessed on 8 November 2023).
- Satchell, J.E. An Electrical Method of Sampling Earthworm Populations. Soil Zool. 1955, 356, 364. [Google Scholar]
- Thielemann, U.D.B. Electrical Earthworm Trap Using the Octet Method. German Patent No. DE3612464A1, 15 October 1987. [Google Scholar]
- Weyers, S.; Schomberg, H.; Hendrix, P.F.; Spokas, K.; Endale, D. Construction of an Electrical Device for Sampling Earthworm Populations in the Field. Appl. Eng. Agric. 2008, 24, 391–397. [Google Scholar] [CrossRef]
- Greed in Front of Many People Using Electric Shock to Kill Earthworms. Available online: https://www.vietnam.vn/en/ham-loi-truoc-mat-nhieu-nguoi-dan-dung-kich-dien-tan-diet-giun-dat/ (accessed on 8 November 2023).
- Real Minimum Wages. Available online: https://stats.oecd.org/index.aspx?DataSetCode=RMW (accessed on 8 November 2023).
- International Labour Organisation. Convention C030—Hours of Work (Commerce and Offices) Convention, 1930 (No. 30); International Labour Organisation: Geneva, Switzerland, 1930. [Google Scholar]
- Jokkonen, R.; Ghosheh, N. Rest Periods: Definitions and Dimensions; International Labour Organisation: Geneva, Switzerland, 2016. [Google Scholar]
- China: Hourly Minimum Wage by Region 2024. Available online: https://www.statista.com/statistics/233886/minimum-wage-per-hour-in-china-by-city-and-province/ (accessed on 11 May 2024).
- Russia Minimum Wage 2024. Available online: https://www.statista.com/statistics/1023237/russia-monthly-minimum-wage/ (accessed on 11 May 2024).
- Minimum Calculated Indexes. Available online: https://egov.kz/cms/en/articles/article_mci_2012 (accessed on 11 May 2024).
- Minimum Wage Updated in Ukraine by 8.33% from 1 January 2024–27 February 2024. Available online: https://wageindicator.org/salary/minimum-wage/minimum-wages-news/2024/minimum-wage-updated-in-ukraine-by-8-33-from-01-january-2024-february-27-2024 (accessed on 11 May 2024).
- García Martínez, J.B.; Alvarado, K.A.; Denkenberger, D.C. Synthetic Fat from Petroleum as a Resilient Food for Global Catastrophes: Preliminary Techno-Economic Assessment and Technology Roadmap. Chem. Eng. Res. Des. 2022, 177, 255–272. [Google Scholar] [CrossRef]
- WHO. Interim Summary of Conclusions and Dietary Recommendations on Total Fat & Fatty Acids. 2008. Available online: https://www.foodpolitics.com/wp-content/uploads/FFA_summary_rec_conclusion.pdf (accessed on 3 November 2023).
- WHO. Diet, Nutrition and the Prevention of Chronic Diseases; WHO Technical Report Series 916; WHO: Geneva, Switzerland, 2003; ISBN 92-4-120916-X. [Google Scholar]
- World Population Clock: 8.1 Billion People (LIVE, 2023)—Worldometer. Available online: https://www.worldometers.info/world-population/ (accessed on 3 November 2023).
- World Population Prospects—Population Division—United Nations. Available online: https://population.un.org/wpp/ (accessed on 3 November 2023).
- FAO/WHO/UNU. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007; ISBN 978-92-4-120935-9. [Google Scholar]
- Walpole, S.C.; Prieto-Merino, D.; Edwards, P.; Cleland, J.; Stevens, G.; Roberts, I. The Weight of Nations: An Estimation of Adult Human Biomass. BMC Public Health 2012, 12, 439. [Google Scholar] [CrossRef]
- Food and Nutrition Needs in Emergencies. Available online: https://www.unhcr.org/uk/media/food-and-nutrition-needs-emergencies (accessed on 3 November 2023).
- Jiménez, J.J.; Lavelle, P.; Decaëns, T. The Efficiency of Soil Hand-Sorting in Assessing the Abundance and Biomass of Earthworm Communities. Its Usefulness in Population Dynamics and Cohort Analysis Studies. Eur. J. Soil Biol. 2006, 42, S225–S230. [Google Scholar] [CrossRef]
- Lin, J.; Yuan, Q. A Novel Technology for Separating Live Earthworm from Vermicompost: Experiment, Mechanism Analysis, and Simulation. Waste Manag. 2021, 131, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Mitra, O.; Callaham, M.A.; Smith, M.L.; Yack, J.E. Grunting for Worms: Seismic Vibrations Cause Diplocardia Earthworms to Emerge from the Soil. Biol. Lett. 2009, 5, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Singh, S.; Vig, A.P. Extraction of Earthworm from Soil by Different Sampling Methods: A Review. Environ. Dev. Sustain. 2016, 18, 1521–1539. [Google Scholar] [CrossRef]
- Public Health England. Compendium of Chemical Hazards; Public Health England: London, UK, 2019. [Google Scholar]
- Evans, A.C.; Guild, W.J. McL. Studies on the Relationships Between Earthworms and Soil Fertility: I. Biological Studies in the Field. Ann. Appl. Biol. 1947, 34, 307–330. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Singh, S.; Bhat, S.A.; Vig, A.P.; Schädler, M. Eco-Friendly Method for the Extraction of Earthworms: Comparative Account of Formalin, AITC and Allium Cepa as Extractant. Appl. Soil Ecol. 2018, 124, 141–145. [Google Scholar] [CrossRef]
- Steffen, G.P.K.; Antoniolli, Z.I.; Steffen, R.B.; Jacques, R.J.S.; dos Santos, M.L. Earthworm Extraction with Onion Solution. Appl. Soil Ecol. 2013, 69, 28–31. [Google Scholar] [CrossRef]
- Gutiérrez-López, M.; Moreno, G.; Trigo, D.; Juárez, E.; Jesús, J.B.; Díaz Cosín, D.J. The Efficiency of Earthworm Extraction Methods Is Determined by Species and Soil Properties in the Mediterranean Communities of Central-Western Spain. Eur. J. Soil Biol. 2016, 73, 59–68. [Google Scholar] [CrossRef]
- Pelosi, C.; Chiron, F.; Dubs, F.; Hedde, M.; Ponge, J.-F.; Salmon, S.; Cluzeau, D.; Nélieu, S. A New Method to Measure Allyl Isothiocyanate (AITC) Concentrations in Mustard—Comparison of AITC and Commercial Mustard Solutions as Earthworm Extractants. Appl. Soil Ecol. 2014, 80, 1–5. [Google Scholar] [CrossRef]
- Zaborski, E.R. Allyl Isothiocyanate: An Alternative Chemical Expellant for Sampling Earthworms. Appl. Soil Ecol. 2003, 22, 87–95. [Google Scholar] [CrossRef]
- Schmidt, O. Appraisal of the Electrical Octet Method for Estimating Earthworm Populations in Arable Land. Ann. Appl. Biol. 2001, 138, 231–241. [Google Scholar] [CrossRef]
- Applebome, P. Recall Is Ordered for Worm Probes. New York Times, 10 June 1993. [Google Scholar]
- Denkenberger, D.; Pearce, J.; Taylor, A.R.; Black, R. Food without Sun: Price and Life-Saving Potential. Foresight 2019, 21, 118–129. [Google Scholar] [CrossRef]
- Mill, A.; Harrison, C.; James, S.; Shah, S.; Fist, T.; Alvarado, K.; Taylor, A.; Denkenberger, D. Preventing Global Famine in Case of Sun-Blocking Scenarios: Seaweed as an Alternative Food Source; ALLFED: Lafayette, CO, USA, 2019. [Google Scholar]
- Alvarado, K.A.; Mill, A.; Pearce, J.M.; Vocaet, A.; Denkenberger, D. Scaling of Greenhouse Crop Production in Low Sunlight Scenarios. Sci. Total Environ. 2020, 707, 136012. [Google Scholar] [CrossRef] [PubMed]
- García Martínez, J.B.; Brown, M.M.; Christodoulou, X.; Alvarado, K.A.; Denkenberger, D.C. Potential of Microbial Electrosynthesis for Contributing to Food Production Using CO2 during Global Agriculture-Inhibiting Disasters. Clean. Eng. Technol. 2021, 4, 100139. [Google Scholar] [CrossRef]
- VnExpress. Vietnamese Earthworm Hunters Destroying Protective Forest Go Unpunished—VnExpress International. Available online: https://e.vnexpress.net/news/news/environment/vietnamese-earthworm-hunters-destroying-protective-forest-go-unpunished-4653107.html (accessed on 16 May 2024).
- Capturing Earthworms by Electric Shock Poses Environmental Hazards. Available online: https://vietnamnews.vn/environment/1582676/capturing-earthworms-by-electric-shock-poses-environmental-hazards.html (accessed on 8 November 2023).
- Coupe, J.; Bardeen, C.G.; Robock, A.; Toon, O.B. Nuclear Winter Responses to Nuclear War between the United States and Russia in the Whole Atmosphere Community Climate Model Version 4 and the Goddard Institute for Space Studies Model. E. J. Geophys. Res. Atmos. 2019, 124, 8522–8543. [Google Scholar] [CrossRef]
- Reynierse, J.H. Effects of Temperature and Temperature Change on Earthworm Locomotor Behaviour. Anim. Behav. 1968, 16, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Schädler, M.; Demetrio, W.; Brown, G.G.; Eisenhauer, N. Climate Change Effects on Earthworms—A Review. Soil Org. 2019, 91, 114–138. [Google Scholar] [CrossRef] [PubMed]
- Greiner, H.G.; Stonehouse, A.M.T.; Tiegs, S.D. Cold Tolerance among Composting Earthworm Species to Evaluate Invasion Potential. Am. Midl. Nat. 2011, 166, 349–357. [Google Scholar] [CrossRef]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms; Chapman and Hall: London, UK, 1996; p. 426. [Google Scholar]
- Daugbjerg, P. Temperature and Moisture Preferences of Three Earthworm Species (Oligochaeta, Lumbricidae). Pedobiologia 1988, 32, 57–64. [Google Scholar] [CrossRef]
- Holmstrup, M. Overwintering Adaptations in Earthworms: The 7th International Symposium on Earthworm Ecology Cardiff Wales 2002. Pedobiologia 2003, 47, 504–510. [Google Scholar] [CrossRef]
- Patricia, M. Barcelo Production and Utilization of Earthworms as Feeds for Broilers in the Phillipines. Tropicultura 1988, 6, 21–24. [Google Scholar]
- Tedesco, D.E.A.; Castrica, M.; Tava, A.; Panseri, S.; Balzaretti, C.M. From a Food Safety Prospective: The Role of Earthworms as Food and Feed in Assuring Food Security and in Valuing Food Waste. Insects 2020, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- James, S.W.; Csuzdi, C.; Chang, C.-H.; Aspe, N.M.; Jiménez, J.J.; Feijoo, A.; Blouin, M.; Lavelle, P. Comment on “Global Distribution of Earthworm Diversity”. Science 2021, 371, eabe4629. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, M.G.; Gavinelli, F.; Gomiero, T.; Bouché, M.; Concheri, G.; Csuzdi, C.; Dorigo, L.; Dreon, L.A.; Fusaro, S.; James, S.; et al. Mapping the Global Distribution of Earthworm Diversity: A Laudable Initiative Deserving a Better Approach. 2019. Available online: https://www.science.org/doi/10.1126/science.aax4851#elettersSection (accessed on 8 November 2023).
- Blakemore, R.J. Nature Article to Commemorate Charles Darwin’s Birthday on 12th February. VermEcology, 12 February 2017. [Google Scholar]
- Lee, K.E.; Kenneth, E. Earthworms: Their Ecology and Relationships with Soils and Land Use; Academic Press: Sydney, Australia; Orlando, FL, USA, 1985; ISBN 978-0-12-440860-9. [Google Scholar]
- Most Worms Charmed. Available online: https://www.guinnessworldrecords.com/world-records/most-worms-charmed (accessed on 8 November 2023).
- Earthworm Electrocution Is Destroying the Environment for China Trade. VNExpress, 7 August 2023.
- FAO. Introduction and Control of Food Hazards—Section 1; FAO Good Hygiene Practices (GHP) and Hazard Analysis and Critical Control Point (HACCP) Toolbox for Food Safety; FAO: Rome, Italy, 2023. [Google Scholar]
- Richardson, J.B.; Görres, J.H.; Sizmur, T. Synthesis of Earthworm Trace Metal Uptake and Bioaccumulation Data: Role of Soil Concentration, Earthworm Ecophysiology, and Experimental Design. Environ. Pollut. 2020, 262, 114126. [Google Scholar] [CrossRef] [PubMed]
- Nfor, B.; Fai, P.B.A.; Tamungang, S.A.; Fobil, J.N.; Basu, N. Soil Contamination and Bioaccumulation of Heavy Metals by a Tropical Earthworm Species (Alma Nilotica) at Informal E-Waste Recycling Sites in Douala, Cameroon. Environ. Toxicol. Chem. 2022, 41, 356–368. [Google Scholar] [CrossRef] [PubMed]
- FAO; UNEP. Global Assessment of Soil Pollution: Report Rome; FAO: Rome, Italy, 2021; ISBN 978-92-5-134469-9. [Google Scholar]
- Scutarașu, E.C.; Trincă, L.C. Heavy Metals in Foods and Beverages: Global Situation, Health Risks and Reduction Methods. Foods 2023, 12, 3340. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. General Standard for Contaminants and Toxins in Food and Feed; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Li, X.; Farid, M. A Review on Recent Development in Non-Conventional Food Sterilization Technologies. J. Food Eng. 2016, 182, 33–45. [Google Scholar] [CrossRef]
- Shapiro, C.; Harvey, T. Radioactive Fallout. In The Medical Implications of Nuclear War; National Academies Press (US): Washington, DC, USA, 1986. [Google Scholar]
- Krivolutzkii, D.A.; Pokarzhevskii, A.D.; Viktorov, A.G. Earthworm Populations in Soils Contaminated by the Chernobyl Atomic Power Station Accident, 1986–1988. Soil Biol. Biochem. 1992, 24, 1729–1731. [Google Scholar] [CrossRef]
- Rybak, A.V.; Belykh, E.S.; Maystrenko, T.A.; Shadrin, D.M.; Pylina, Y.I.; Chadin, I.F.; Velegzhaninov, I.O. Genetic Analysis in Earthworm Population from Area Contaminated with Radionuclides and Heavy Metals. Sci. Total Environ. 2020, 723, 137920. [Google Scholar] [CrossRef] [PubMed]
- Sutou, S. Black Rain in Hiroshima: A Critique to the Life Span Study of A-Bomb Survivors, Basis of the Linear No-Threshold Model. Genes Environ. 2020, 42, 1. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Ito, M.T.; Kaneko, S.; Kiyono, Y.; Ikeda, S.; Makino, S. Radiocesium Concentrations in Epigeic Earthworms at Various Distances from the Fukushima Nuclear Power Plant 6 Months after the 2011 Accident. J. Environ. Radioact. 2013, 126, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Maystrenko, T.; Rybak, A. Radium Uptake by Earthworms E. Fetida after Exposure to Contaminated Soil. J. Environ. Radioact. 2023, 257, 107085. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Takahashi, T.; Nguyen, P.; Kubota, Y.; Gamou, S.; Sakurai, S.; Takahashi, S. Uptake and Retention of Radio-Caesium in Earthworms Cultured in Soil Contaminated by the Fukushima Nuclear Power Plant Accident. J. Environ. Radioact. 2015, 139, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.R.; Shapiro, C.S. Internal Dose Following a Major Nuclear War. Health Phys. 1992, 62, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.E. Anesthesia, Analgesia, and Euthanasia of Invertebrates. ILAR J. 2011, 52, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, J. Earthworms and Vermicomposting. In Earthworms—The Ecological Engineers of Soil; Ray, S., Ed.; IntechOpen: London, UK, 2018; ISBN 978-1-78923-397-1. [Google Scholar]
- Bundy, J.G.; Ramløv, H.; Holmstrup, M. Multivariate Metabolic Profiling Using 1H Nuclear Magnetic Resonance Spectroscopy of Freeze-Tolerant and Freeze-Intolerant Earthworms Exposed to Frost. CryoLetters 2003, 24, 347–358. [Google Scholar] [PubMed]
- Bhorgin Lourdumary, A.J.; Uma, K. Nutritional Evaluation of Earthworm Powder (Lampito mauritii). J. Appl. Pharm. Sci. 2012, 3, 82–84. [Google Scholar] [CrossRef]
- Jones, R.C. Science, Sentience, and Animal Welfare. Biol. Philos. 2013, 28, 1–30. [Google Scholar] [CrossRef]
- Opinion: Estimating Invertebrate Sentience. Available online: https://rethinkpriorities.org/publications/opinion-estimating-invertebrate-sentience (accessed on 28 November 2023).
- Butcher, K.S.; Crown, L.D.; Gentry, E.J. The International System of Units (SI)—Conversion Factors for General Use; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2006; p. 12. [Google Scholar]
- Slavin, J.; Carlson, J. Carbohydrates1. Adv. Nutr. 2014, 5, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.M.; Holub, B.J. Nutritional Aspects of Fats and Oils. In Technological Advances in Improved and Alternative Sources of Lipids; Kamel, B.S., Kakuda, Y., Eds.; Springer US: Boston, MA, USA, 1994; pp. 16–49. ISBN 978-1-4615-2109-9. [Google Scholar]
- Dirt and Mud—Densities. Available online: https://www.engineeringtoolbox.com/dirt-mud-densities-d_1727.html (accessed on 3 November 2023).
- Ritchie, H.; Rosado, P.; Roser, M. Electricity Mix. Our World in Data, July 2020. [Google Scholar]
| Parameter | 90th Percentile Range | Reasoning |
|---|---|---|
| Extraction percentage | 10–88% | Satchell [34] did not give an extraction percentage for the total earthworm population, but the data show electroshocking to yield 10% of the number of worms extracted using potassium permanganate. A patent filed by Thiellemann [35] for an electrode octet earthworm extractor reports 87.7% of earthworms were extracted with the equipment. |
| Effective area | 0.22–2.6 m2 | Weyers et al. [36] built an electroshocking device that covered an area of at least 0.22 m2. Satchell [34] refers to earthworms surfacing up to 3 feet from the electrode. A circle of this radius has an area of 2.6 m2. |
| Cycle time | 20–40 min | Thiellemann [35] reports a cycle taking approximately 20 min. Satchell [34] gives figures for earthworms collected after 40 min. |
| Electrical supply voltage | 30–480 V | Thiellemann [35] states a minimum voltage required of 30 V. Weyers et al. [36] applied an effective maximum of 480 V. |
| Electrical supply current | 0.2–4 A | Rhea Fourier and González [32] state 0.2 A as the lower effective current for extraction. Satchell [34] maintained an upper limit current of 4 A. |
| Number of electrodes managed in series | 3–10 | Estimate based on unpublished reports of earthworm electroshocking in the media [37]. |
| Hourly wage for labour | USD 1.8–13.8 per hour | The OECD.Stat data show the range of hourly minimum wages in member nations was USD 1.8 to 13.8 per hour in 2022 when expressed with purchasing power parity [38]. |
| Length of a working day | 6–12 h | Daily rest requirements of 12 h were assumed, as informed by International Labour Organization data [39,40]. |
| Metrics for Calculating Global Requirements | Value | ||
|---|---|---|---|
| World human population | 8.07 billion [48,49] | ||
| Daily protein requirement per person | 51 g * [50,51] | ||
| Daily energy requirement per person | 2100 kcal [52] | ||
| Daily fat requirement per person | 315 kcal * [45,52] | ||
| Daily global requirement | Potential earthworm resource | Potential days of nutrient from earthworms | |
| Protein | 4.15 × 1011 g * | 5.44 × 1014 g * | 1310 * |
| Energy | 1.69 × 1013 kcal * | 3.65 × 1015 kcal * | 220 * |
| Fat | 2.54 × 1012 kcal * | 1.10 × 1015 kcal * | 440 * |
| Resilient Food | Affordability (USD/Person/Day) |
|---|---|
| Lignocellulosic sugar (repurposed paper factory) [6] | 0.50 |
| Methane single cell protein [7] | 1.60 |
| Lignocellulosic sugar (new construction) [6] | 1.70 |
| Synthetic fat from petroleum [45] | 1.70 |
| Seaweed in tropical areas (Southeast Asia prices) [67] | 2.20 |
| Low-tech greenhouses in tropical areas (crops) [68] | 3.00 |
| Acetic acid from microbial electrosynthesis [69] | 5.40 |
| Farmed mealworms [66] | 9.45 |
| Earthworm foraging (targeted) | 31.50 |
| White button mushrooms [66] | 35.18 |
| Artificial light algae [66] | 57.75 |
| Earthworm foraging (median) | 185.33 |
| Artificial light vegetables [66] | 315.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, H.; Mulhall, J.; Pfau, L.A.; Palm, R.; Denkenberger, D.C. Can Foraging for Earthworms Significantly Reduce Global Famine in a Catastrophe? Biomass 2024, 4, 765-783. https://doi.org/10.3390/biomass4030043
Miller H, Mulhall J, Pfau LA, Palm R, Denkenberger DC. Can Foraging for Earthworms Significantly Reduce Global Famine in a Catastrophe? Biomass. 2024; 4(3):765-783. https://doi.org/10.3390/biomass4030043
Chicago/Turabian StyleMiller, Henry, James Mulhall, Lou Aino Pfau, Rachel Palm, and David C. Denkenberger. 2024. "Can Foraging for Earthworms Significantly Reduce Global Famine in a Catastrophe?" Biomass 4, no. 3: 765-783. https://doi.org/10.3390/biomass4030043
APA StyleMiller, H., Mulhall, J., Pfau, L. A., Palm, R., & Denkenberger, D. C. (2024). Can Foraging for Earthworms Significantly Reduce Global Famine in a Catastrophe? Biomass, 4(3), 765-783. https://doi.org/10.3390/biomass4030043








