Waste Biomass Pretreatments for Biogas Yield Optimization and for the Extraction of Valuable High-Added-Value Products: Possible Combinations of the Two Processes toward a Biorefinery Purpose
Abstract
1. Introduction
2. Pretreatments for Improving the Biogas Production Yield
2.1. Physical Pretreatment
2.2. Chemical Pretreatment
2.3. Biological Pretreatment
3. Recovery of Valuable Products from Biomasses
4. Pretreatments for Recovery of Valuable Products from Residual Biomasses
4.1. Deep Eutectic Solvents
4.2. Acid Pretreatments
4.3. Alkaline Pretreatments
4.4. Organosolvent
4.5. Steam Explosion
4.6. Ultrasound-Assisted Treatments
4.7. Microwaves
4.8. High Pressure Treatments
4.9. Supercritical Fluids
4.10. Non-Thermal Plasma
4.11. Biological Pretreatment
5. Dual Function Pretreatments
Results
- (1)
- Inoculum (¾) and OM (¼);
- (2)
- Inoculum (¾) and OML (¼);
- (3)
- Inoculum (¾) and OMPF (¼).
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hingsamer, M.; Jungmeier, G. Biorefineries. In The Role of Bioenergy in the Bioeconomy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 179–222. [Google Scholar]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.; et al. Resource Recovery and Circular Economy from Organic Solid Waste Using Aerobic and Anaerobic Digestion Technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef]
- Bathia, L.; Bachheti, R.K.; Garlapati, V.K.; Chandel, A.K. Third-generation biorefineries: A sustainable platform for food, clean energy, and nutraceuticals production. Biomass Convers. Biorefin. 2022, 12, 4215–4230. [Google Scholar]
- Liu, Z.; Wang, K.; Chen, Y.; Tan, T.; Nielsen, J. Third-generation biorefineries as the means to produce fuels and chemicals from CO2. Nat. Catal. 2020, 3, 274–288. [Google Scholar] [CrossRef]
- Rabbani, M.; Hosseini, A.; Karim, M.A.; Fahimi, A.; Karimi, K.; Vahidi, E. Environmental impact assessment of a novel third-generation biorefinery approach for astaxanthin and biofuel production. Sci. Total Environ. 2024, 912, 168733. [Google Scholar] [CrossRef] [PubMed]
- Atelge, M.R.; Krisa, D.; Kumar, G.; Eskicioglu, C.; Nguyen, D.D.; Chang, S.W.; Atabani, A.E.; Al-Muhtaseb, A.H.; Unalan, S. Biogas Production from Organic Waste: Recent Progress and Perspectives. Waste Biomass Valorization 2020, 11, 1019–1040. [Google Scholar] [CrossRef]
- Edwiges, T.; Frare, L.; Mayer, B.; Lins, L.; Mi Triolo, J.; Flotats, X.; de Mendonça Costa, M.S.S. Influence of Chemical Composition on Biochemical Methane Potential of Fruit and Vegetable Waste. Waste Manag. 2018, 71, 618–625. [Google Scholar] [CrossRef]
- Carlsson, M.; Lagerkvist, A.; Morgan-Sagastume, F. The Effects of Substrate Pre-Treatment on Anaerobic Digestion Systems: A Review. Waste Manag. 2012, 32, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Benincasa, P.; Balestra, G.M.; Luzi, F.; Mazzaglia, A.; Del Buono, D.; Puglia, D.; Torre, L. Revalorization of Barley Straw and Husk as Precursors for Cellulose Nanocrystals Extraction and Their Effect on PVA_CH Nanocomposites. Ind. Crops Prod. 2016, 92, 201–217. [Google Scholar] [CrossRef]
- Ahring, B.K.; Biswas, R.; Ahamed, A.; Teller, P.J.; Uellendahl, H. Making Lignin Accessible for Anaerobic Digestion by Wet-Explosion Pretreatment. Bioresour. Technol. 2015, 175, 182–188. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of Lignocellulosic Biomass: A Review on Recent Advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Ab Rasid, N.S.; Shamjuddin, A.; Abdul Rahman, A.Z.; Amin, N.A.S. Recent Advances in Green Pre-Treatment Methods of Lignocellulosic Biomass for Enhanced Biofuel Production. J. Clean. Prod. 2021, 321, 129038. [Google Scholar] [CrossRef]
- Gallego-García, M.; Moreno, A.D.; Manzanares, P.; Negro, M.J.; Duque, A. Recent Advances on Physical Technologies for the Pretreatment of Food Waste and Lignocellulosic Residues. Bioresour. Technol. 2023, 369, 128397. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Lv, X.; Qin, N.; Zhang, K.; Ding, X.; Luo, L.; Qu, J.; Sun, Y. Mechanism of Ball Milling Pretreatment to Improve the Anaerobic Digestion Performance and Energy Conversion Efficiency of Corn Straw. Fuel 2024, 366, 131409. [Google Scholar] [CrossRef]
- Arifan, T.F.; Sutaryo, S.; Broto, W.; Ayuningtyas, D.; Sapatra, E.F.; Yudanto, Y.A. Optimization of Biogas Production from Pineapple Peel Waste with Mechanical Pretreatment; AIP Publishing LLC: New York, NY, USA, 2023; p. 030008. [Google Scholar]
- Harmsen, P.F.H.; Huijgen, W.; Bermudez, L.; Bakker, R. Literature Review of Physical and Chemical Pretreatment Processes for Lignocellulosic Biomass; Wageningen UR Food & Biobased Research: Wageningen, The Netherlands, 2010. [Google Scholar]
- Zhou, J.; Xu, W.; Wong, J.W.C.; Yong, X.; Yan, B.; Zhang, X.; Jia, H. Ultrasonic and Thermal Pretreatments on Anaerobic Digestion of Petrochemical Sludge: Dewaterability and Degradation of PAHs. PLoS ONE 2015, 10, e0136162. [Google Scholar] [CrossRef]
- Saha, B.; Barua, V.B.; Khwairakpam, M.; Haq, I.; Kalamdhad, A.S.; Varjani, S. Thermal Pretreatment of Lantana Camara for Improved Biogas Production: Process Parameter Studies for Energy Evaluation. Environ. Res. 2023, 216, 114661. [Google Scholar] [CrossRef]
- Park, S.; Yoon, Y.-M.; Han, S.K.; Kim, D.; Kim, H. Effect of Hydrothermal Pre-Treatment (HTP) on Poultry Slaughterhouse Waste (PSW) Sludge for the Enhancement of the Solubilization, Physical Properties, and Biogas Production through Anaerobic Digestion. Waste Manag. 2017, 64, 327–332. [Google Scholar] [CrossRef]
- Dwyer, J.; Starrenburg, D.; Tait, S.; Barr, K.; Batstone, D.J.; Lant, P. Decreasing Activated Sludge Thermal Hydrolysis Temperature Reduces Product Colour, without Decreasing Degradability. Water Res. 2008, 42, 4699–4709. [Google Scholar] [CrossRef] [PubMed]
- Apul, O.G.; Sanin, F.D. Ultrasonic Pretreatment and Subsequent Anaerobic Digestion under Different Operational Conditions. Bioresour. Technol. 2010, 101, 8984–8992. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.-Y. Overview of Pretreatment Strategies for Enhancing Sewage Sludge Disintegration and Subsequent Anaerobic Digestion: Current Advances, Full-Scale Application and Future Perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Lehne, G.; Müller, A.; Schwedes, J. Mechanical Disintegration of Sewage Sludge. Water Sci. Technol. 2001, 43, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Buller, L.S.; Sganzerla, W.G.; Lima, M.N.; Muenchow, K.E.; Timko, M.T.; Forster-Carneiro, T. Ultrasonic Pretreatment of Brewers’ Spent Grains for Anaerobic Digestion: Biogas Production for a Sustainable Industrial Development. J. Clean. Prod. 2022, 355, 131802. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.; Dong, Y. Pretreatment for Biogas Production by Anaerobic Fermentation of Mixed Corn Stover and Cow Dung. Energy 2012, 46, 644–648. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A Review on Alkaline Pretreatment Technology for Bioconversion of Lignocellulosic Biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Johansen, K.S.; Meyer, A.S. Low Temperature Lignocellulose Pretreatment: Effects and Interactions of Pretreatment PH Are Critical for Maximizing Enzymatic Monosaccharide Yields from Wheat Straw. Biotechnol. Biofuels 2011, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C.; Iten, L.B.; Cotta, M.A.; Wu, Y.V. Dilute Acid Pretreatment, Enzymatic Saccharification, and Fermentation of Rice Hulls to Ethanol. Biotechnol. Prog. 2008, 21, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H. Pretreatment of Lignocellulosic Biomass. In Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers; Wiley: Hoboken, NJ, USA, 2013; pp. 91–110. [Google Scholar]
- Liew, L.N.; Shi, J.; Li, Y. Enhancing the Solid-State Anaerobic Digestion of Fallen Leaves through Simultaneous Alkaline Treatment. Bioresour. Technol. 2011, 102, 8828–8834. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Guo, X.; Yuan, D.; Xu, J. Comparison of Different Pretreatments of Rice Straw Substrate to Improve Biogas Production. Waste Biomass Valorization 2018, 9, 1503–1512. [Google Scholar] [CrossRef]
- Pan, X.; Gilkes, N.; Kadla, J.; Pye, K.; Saka, S.; Gregg, D.; Ehara, K.; Xie, D.; Lam, D.; Saddler, J. Bioconversion of Hybrid Poplar to Ethanol and Co-Products Using an Organosolv Fractionation Process: Optimization of Process Yields. Biotechnol. Bioeng. 2006, 94, 851–861. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv Pretreatment of Lignocellulosic Biomass for Enzymatic Hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Zamani, A.; Amiri, H.; Horváth, I.S. Enhanced Solid-State Biogas Production from Lignocellulosic Biomass by Organosolv Pretreatment. Biomed Res. Int. 2014, 2014, 350414. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, H.; Luque, R. Advances on Biomass Pretreatment Using Ionic Liquids: An Overview. Energy Environ. Sci. 2011, 4, 3913. [Google Scholar] [CrossRef]
- Kabir, M.M.; Niklasson, C.; Taherzadeh, M.J.; Horváth, I.S. Biogas Production from Lignocelluloses by N-Methylmorpholine-N-Oxide (NMMO) Pretreatment: Effects of Recovery and Reuse of NMMO. Bioresour. Technol. 2014, 161, 446–450. [Google Scholar] [CrossRef]
- Čater, M.; Zorec, M.; Marinšek Logar, R. Methods for Improving Anaerobic Lignocellulosic Substrates Degradation for Enhanced Biogas Production. Springer Sci. Rev. 2014, 2, 51–61. [Google Scholar] [CrossRef]
- Tolisano, C.; Luzi, F.; Regni, L.; Proietti, P.; Puglia, D.; Gigliotti, G.; Di Michele, A.; Priolo, D.; Del Buono, D. A way to valorize pomace from oil production: Lignin nanoparticles to biostimulate maize plants. Environ. Technol. 2023, 31, 103216. [Google Scholar] [CrossRef]
- Dibble, D.C.; Li, C.; Sun, L.; George, A.; Cheng, A.; Çetinkol, Ö.P.; Benke, P.; Holmes, B.M.; Singh, S.; Simmons, B.A. A facile method for the recovery of ionic liquid and lignin from biomass pretreatment. Green Chem. 2011, 13, 3255–3264. [Google Scholar] [CrossRef]
- Shill, K.; Padmanabhan, S.; Xin, Q.; Prausnitz, J.M.; Clark, D.S.; Blanch, H.W. Ionic liquid pretreatment of cellulosic biomass: Enzymatic hydrolysis and ionic liquid recycle. Biotechnol. Bioeng. 2011, 108, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Weerachanchai, P.; Lee, J.M. Recyclability of an ionic liquid for biomass pretreatment. Bioresour. Technol. 2014, 169, 336–343. [Google Scholar] [CrossRef]
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valorization 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Paranjpe, A.; Saxena, S.; Jain, P. Biogas Yield Using Single and Two Stage Anaerobic Digestion: An Experimental Approach. Energy Sustain. Dev. 2023, 74, 6–19. [Google Scholar] [CrossRef]
- Huiliñir, C.; Pinto-Villegas, P.; Castillo, A.; Montalvo, S.; Guerrero, L. Biochemical Methane Potential from Sewage Sludge: Effect of an Aerobic Pretreatment and Fly Ash Addition as Source of Trace Elements. Waste Manag. 2017, 64, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Weiland, P. Biogas Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Kendir Çakmak, E.; Ugurlu, A. Enhanced Biogas Production of Red Microalgae via Enzymatic Pretreatment and Preliminary Economic Assessment. Algal. Res. 2020, 50, 101979. [Google Scholar] [CrossRef]
- Hosseini Koupaie, E.; Dahadha, S.; Bazyar Lakeh, A.A.; Azizi, A.; Elbeshbishy, E. Enzymatic Pretreatment of Lignocellulosic Biomass for Enhanced Biomethane Production-A Review. J. Environ. Manag. 2019, 233, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, Y. Fungal Pretreatment of Lignocellulosic Biomass. Biotechnol. Adv. 2012, 30, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Pretreatment of Radiata Pine Using Two White Rot Fungal Strains Stereum Hirsutum and Trametes Versicolor. Energy Convers. Manag. 2017, 142, 13–19. [Google Scholar] [CrossRef]
- Alexandropoulou, M.; Antonopoulou, G.; Fragkou, E.; Ntaikou, I.; Lyberatos, G. Fungal Pretreatment of Willow Sawdust and Its Combination with Alkaline Treatment for Enhancing Biogas Production. J. Environ. Manag. 2017, 203, 704–713. [Google Scholar] [CrossRef]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.J. Bioconversion of biomass waste into high value chemical. Biores. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef]
- Montegiove, N.; Leonardi, L.; Cesaretti, A.; Pellegrino, R.M.; Pellegrino, A.; Emiliani, C.; Calzoni, E. Biogenic Amine Content Analysis of Three Chicken-Based Dry Pet Food Formulations. Animals 2023, 13, 1945. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energ. 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Caputo, A.C.; Scacchia, F.; Pelagagge, P.M. Disposal of by-products in olive oil industry: Waste-to-energy solutions. Appl. Therm. Eng. 2003, 23, 197–214. [Google Scholar] [CrossRef]
- Babbar, N.; Dejonghe, W.; Gatti, M.; Sforza, S.; Elst, K. Pectic oligosaccharides from agricultural by-products: Production, characterization and health benefits. Crit. Rev. Biotechnol. 2016, 36, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Tournour, H.H.; Segundo, M.A.; Magalhaes, L.M.; Barreiros, L.; Queiroz, J.; Cunha, L.M. Valorization of grape pomace: Extraction of bioactive phenolics with antioxidant properties. Ind. Crop. Prod. 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Wijaya, Y.P.; Putra, R.D.D.; Widyaya, V.T.; Ha, J.M.; Suh, D.J.; Kim, C.S. Comparative study on two-step concentrated acid hydrolysis for the extraction of sugars from lignocellulosic biomass. Bioresour. Technol. 2014, 164, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Arends, I.; Sheldon, R.; Hanefeld, U. Green Chemistry and Catalysis; John and Wiley and Sons: Hoboken, NJ, USA, 2007; pp. 1–48. [Google Scholar]
- Teleky, B.E.; Vodnar, D.C. Biomass-derived production of itaconic acid as a building block in specialty polymers. Polymers 2019, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Bagley, D.M.; Leung, K.T.; Liss, S.N.; Liao, B.Q. Recent advances in membrane technologies for biorefining and bioenergy production. Biotechnol. Adv. 2012, 33, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.D.; Banerjee, J.; Arora, A. Prebiotic potential of oligosaccharides: A focus on xylan derived oligosaccharides. Bioact. Carbohydr. Diet. Fibre 2015, 5, 19–30. [Google Scholar] [CrossRef]
- Carvalho, A.F.A.; Neto, P.D.; Da Silva, D.F.; Pastore, G.M. Xylo-oligosaccharides from lignocellulosic materials: Chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res. Int. 2013, 51, 75–85. [Google Scholar] [CrossRef]
- Yamabhai, M.; Sak-Ubol, S.; Srila, W.; Haltrich, D. Mannan biotechnology: From biofuels to health. Crit. Rev. Biotechnol. 2016, 36, 32–42. [Google Scholar] [CrossRef]
- Montegiove, N.; Calzoni, E.; Pelosi, D.; Gammaitoni, L.; Barelli, L.; Emiliani, C.; Di Michele, A.; Cesaretti, A. Optimizing covalent immobilization of glucose oxidase and laccase on PV15 fluoropolymer-based bioelectrodes. J. Funct. Biomater. 2022, 13, 270. [Google Scholar] [CrossRef]
- Pelosi, D.; Barelli, L.; Montegiove, N.; Calzoni, E.; Cesaretti, A.; Di Michele, A.; Emiliani, C.; Gammaitoni, L. Immobilizing Enzymes on a Commercial Polymer: Performance Analysis of a GOx-Laccase Based Enzymatic Biofuel Cell Assembly. Energies 2022, 15, 2182. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Garcia, I.L.; Kookos, I.K.; Papanikolaous, S.; Kwan, T.H.; Lin, C.S. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, J.P. Sustainable bio-ethanol production from agro-residues: A review. Sustain. Energ. Rew. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Baral, N.R.; Slutzky, L.; Shah, A.; Ezeji, T.C.; Cornish, K.; Christy, A. Acetone-butanol-ethanol fermentation of corn stover: Current production methods, economic viability and commercial use. FEMS Microbiol. Lett. 2016, 363, fnw033. [Google Scholar] [CrossRef] [PubMed]
- Santana-Meridas, O.; Gonzalez-Coloma, A.; Sanchez-Vioque, R. Agricultural residues as a source of bioactive natural products. Phytochem. Rev. 2012, 11, 447–466. [Google Scholar] [CrossRef]
- Chen, X.M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food. Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.A.; Rebecchi, S.; Decorti, D.; Domingos, J.M.B.; Natolino, A.; Rio, D.D.; Bertin, L.; Porto, C.D.; Fava, F. Towards multi-purpose biorefinery platforms for the valorisation of red grape pomace: Production of polyphenols, volatile fatty acids, polyhydroxyalkanoates and biogas. Green Chem. 2016, 18, 261–270. [Google Scholar] [CrossRef]
- Garcia, A.; Gandini, A.; Labidi, J.; Belgacem, N.; Bras, J. Industrial and crop wastes: A new source for nanocellulose biorefinery. Ind. Crop. Prod. 2016, 93, 26–38. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: Chemistry, kinetics, and substrate structures. In Biofuels. Bioprod. Biorefining 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and sustainable pretreatment methods for cellulose extraction from lignocellulosic biomass and its applications: A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Pinto, E.; Aggrey, W.N.; Boakye, P.; Amenuvor, G.; Sokama-Neuyam, Y.A.; Fokuo, M.K.; Karimaie, H.; Sarkodie, K.; Adenutsi, C.D.; Erzuah, S.; et al. Cellulose processing from biomass and its derivatization into carboxymethylcellulose: A review. Sci. Afr. 2022, 15, 01078. [Google Scholar] [CrossRef]
- Pienkos, P.T.; Zhang, M. Role of pretreatment and conditioning processes on toxicity of lignocellulosic biomass hydrolysates. Cellulose 2009, 16, 743–762. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, X.; Tao, S.; Hu, L.; Zhang, X.; Lin, X. Process optimization for deep eutectic solvent pretreatment and enzymatic hydrolysis of sugar cane bagasse for cellulosic ethanol fermentation. Renew. Energy 2021, 177, 259–267. [Google Scholar]
- Toma, F.S.; Jemaat, Z.; Beg, M.D.H.; Khan, M.R.; Yunus, M.R. Comparison between lignin extraction by alkaline and ultrasound-assisted alkaline treatment from oil palm empty fruit bunch. IOP Conf. Ser. Mat. Sci. Eng. 2021, 1092, 012027. [Google Scholar] [CrossRef]
- Kham, L.; Le Bigot, Y.; Delmas, M.; Avignon, G. Delignification of wheat straw using a mixture of carboxylic acids and peroxoacids. Ind. Crops Prod. 2005, 21, 9–15. [Google Scholar] [CrossRef]
- Amendola, D.; De Faveri, D.M.; Egues, I.; Serrano, L.; Labidi, J.; Spigno, G. Autohydrolysis and organosolv process for recovery of hemicellulose, phenolic compounds and lignin from grape stalks. Bioresour. Technol. 2012, 107, 267–274. [Google Scholar] [CrossRef]
- Ouyang, X.; Chen, L.; Zhang, S.; Yuan, Q.; Wang, W.; Linhardt, R.J. Effect of simultaneous steam explosion and alkaline depolymerization on corncob lignin and cellulose structure. Chem. Biochem. Eng. Q. 2018, 32, 177–189. [Google Scholar] [CrossRef]
- Dinh Vu, N.; Tran, H.T.; Bui, N.D.; Duc Vu, C.; Nguyen, H.V. Lignin and cellulose extraction from Vietnam’s rice straw ultrasound-assisted alkaline treatment method. Int. J. Polym. Sci. 2017, 2017, 1063695. [Google Scholar] [CrossRef]
- Ferreira, A.R.F.C.; Figueiredo, A.B.; Evtuguin, D.V.; Saraiva, J.A. High pressure pre-treatments promote higher rate and degree of enzymatic hydrolysis of cellulose. Green Chem. 2011, 13, 2764–2767. [Google Scholar] [CrossRef]
- Ndruru, S.T.C.L.; Wahyuningrum, D.; Bundjali, B.; Arcana, I.M. Green simple microwave-assisted extraction (MAE) of cellulose from Theobroma cacao L. (TCL) husk. IOP Conf. Ser. Mat. Sci. Eng. 2019, 541, 012017. [Google Scholar] [CrossRef]
- Brand, S.; Susanti, R.F.; Kim, S.K.; Lee, H.S.; Kim, J.; Sang, B.I. Supercritical ethanol as an enhanced medium for lignocellulosic biomass liquefaction: Influence of physical process parameters. Energy 2013, 59, 173–182. [Google Scholar] [CrossRef]
- Ravindran, R.; Sarangapani, C.; Jaiswal, S.; Lu, P.; Cullen, P.J.; Bourke, P.; Jaiswal, A.K. Improving enzymatic hydrolysis of brewer spent grain with nonthermal plasma. Bioresour. Technol. 2019, 282, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Li, Y.; Zhang, Y.; Mei, Y.; Tabassum, S. Biological treatment of refractory organic compounds in coal gasification wastewater: A review. J. Water Process Eng. 2024, 60, 105255. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Raj, T.; Morya, R.; Chandrasekhar, K.; Kumar, D.; Soam, S.; Kumar, R.; Patel, A.K.; Kim, S.H. Microalgae biomass deconstruction using green solvents: Challenges and future opportunities. Bioresour. Technol. 2023, 369, 128429. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Kalhor, P.; Ghandi, K. Deep eutectic solvents for pretreatment, extraction, and catalysis of biomass and food waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef]
- Tang, B.; Row, K.H. Recent developments in deep eutectic solvents in chemical sciences. Monatsh. Chem. 2013, 144, 1427–1454. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.Y.; Xu, P.; Yang, F.X.; Wu, H.; Zong, M.H.; Lou, W.Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of rutin from Sophora japonica. Engineering 2015, 11, 2746–2755. [Google Scholar] [CrossRef]
- Vigier, K.D.O.; Chatel, G.; Jerome, F.J.C. Contribution of deep eutectic solvents for biomass processing: Opportunities, challenges, and limitations. ChemCatChem 2015, 7, 1250–1260. [Google Scholar] [CrossRef]
- Loow, Y.L.; New, E.K.; Yang, G.H.; Ang, L.Y.; Foo, L.Y.W.; Wu, T.Y.J.C. Potential use of deep eutectic solvents to facilitate lignocellulosic biomass utilization and conversion. Cellulose 2017, 24, 3591–3618. [Google Scholar] [CrossRef]
- Pan, Y.; Alam, M.A.; Wang, Z.; Huang, D.; Hu, K.; Chen, H.; Yuan, Z. One-step production of biodiesel from wet and unbroken microalgae biomass using deep eutectic solvents. Bioresour. Technol. 2017, 238, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, G.; Wang, J.; Xiong, W.; Lv, Y.; Zhang, S.; Zhao, A.; Janhanbakhsh-Bonab, P.; Solovchenko, A.; Xu, J.; Alam, M.A. Polyol based deep eutectic solvent-assisted pretreatment for enhanced lutein extraction from Chlorella pyrenoidosa. J. Mol. Liq. 2022, 368, 120775. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Neto, C.J.D.; de Souza Vandenberghe, L.P.; de Carvalho Neto, D.P.; Sydney, A.C.N.; Letti, L.A.; Karp, S.G.; Torres, L.A.Z.; Socco, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Ummalyma, S.B.; Okram, A.K.; Pandey, A.; Sankar, M.; Sukumaran, R.K. Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from Loktak Lake for enzymatic hydrolysis. Bioresour. Technol. 2018, 253, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Solarte-Toro, J.C.; Romero-Garcia, J.M.; Martinez-Patino, J.C.; Ruiz-Ramos, E.; Castro-Galiano, E.; Cardona-Alzate, C.A. Acid pretreatment of lignocellulosic biomass for energy vectors production: A review focused on operational conditions and techno-economic assessment for bioethanol production. Renew. Sustain. Energ. Rev. 2019, 107, 587–601. [Google Scholar] [CrossRef]
- Hsu, T.; Guo, G.; Chen, W.; Hwang, W. Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol. 2010, 101, 4907–4913. [Google Scholar] [CrossRef]
- McMillan, J.D. Pretreatment of lignocellulosic biomass. In Enzymatic Conversion of Biomass for Fuels Production; Himmel, M.E., Baker, J.O., Overend, R.P., Eds.; ACS Publications: Washington, DC, USA, 1994; pp. 292–324. [Google Scholar]
- Bravo, C.; Garces, D.; Faba, L.; Sastre, H.; Ordonez, S. Selective arabinose extraction from Pinus sp. Sawdust by two-step soft acid hydrolysis. Ind. Crops Prod. 2017, 104, 229–236. [Google Scholar] [CrossRef]
- Tu, W.C.; Hallett, J.P. Recent advances in the pretreatment of lignocellulosic biomass. Curr. Opin. Green Sustain. Chem. 2019, 20, 11–17. [Google Scholar] [CrossRef]
- Kundu, C.; Lee, J.W. Bioethanol production from detoxified hydrolysate and the characterization of oxalic acid pretreatment Eucalyptus (Eucalyptus globulus) biomass. Ind. Crops Prod. 2016, 83, 322–328. [Google Scholar] [CrossRef]
- Ragauskas, A.J. Biotechnology in the Pulp and Paper industry. A challenge for change. In Biotechnology in the Pulp and Paper Industry–Progress in Biotechnology; Viikari, L., Lantto, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 21, pp. 7–12. [Google Scholar]
- Kim, S.; Kim, C.H. Bioethanol production using the sequential acid/alkali-pretreated empty palm fruit bunch fiber. Renew. Energy 2012, 54, 150–155. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Wei Kit Chin, D.; Lim, S.; Pang, Y.L.; Lam, M.K. Fundamental review of organosolv pretreatment and its challenges in emerging consolidated bioprocessing. Biofuels Bioprod. Biorefining 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Zhao, H.; Jones, C.J.; Baker, G.A.; Xia, S.; Olubajo, O.; Person, V.N. Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J. Biotech. 2009, 139, 47–54. [Google Scholar] [CrossRef]
- Shah, A.A.; Seehar, T.H.; Sharma, K.; Toor, S.S. Biomass pretreatment technologies. In Hydrocarbon Biorefinery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–228. [Google Scholar]
- Simangunsong, E.; Ziegler-Devin, I.; Chrusciel, L.; Girods, P.; Wistara, N.J.; Brosse, N. Steam explosion of beech wood: Effect of the particle size on the xylans recovery. Waste Biomass Valoriz. 2020, 11, 625–633. [Google Scholar] [CrossRef]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131732. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nguyen, X.P.; Duong, X.Q.; Agbulut, U.; Len, C.; Nguyen, P.Q.P.; Kchaou, M.; Chen, W.H. Steam explosion as sustainable biomass pretreatment technique for biofuel production: Characteristics and challenges. Biores. Technol. 2023, 385, 129398. [Google Scholar] [CrossRef]
- Li, W.; He, X.; Chen, Y.; Lei, L.; Li, F.; Zhao, J.; Zeng, K.; Ming, J. Improving antioxidant activity and modifying Tartary buckwheat bran by steam explosion treatment. LWT 2022, 170, 114106. [Google Scholar] [CrossRef]
- Baral, N.R.; Shah, A. Comparative techno-economic analysis of steam explosion, dilute sulfuric acid, ammonia fiber explosion and biological pretreatments of corn stover. Bioresour. Technol. 2017, 232, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Moukagni, E.M.; Ziegler-Devin, I.; Safou-Tchima, R.; Aymes, A.; Kapel, R.; Brosse, N. Steam explosion of Aucoumea klaineana sapwood: Membrane separation of acetylated hemicelluloses. Carbohydr. Res. 2022, 519, 108622. [Google Scholar] [CrossRef] [PubMed]
- Brethauer, S.; Antczak, A.; Balan, R.; Zielenkiewicz, T.; Studer, M.H. Steam explosion pretreatment of beechwood. Part 2: Quantification of cellulase inhibitors and their effect on avicel hydrolysis. Energies 2020, 13, 3638. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Li, C.; Du, X.; Liu, Z.H.; Li, B.Z.; Meng, X.; Zhao, J.; Zhao, Z.M.; Ragauskas, A.J. Steam explosion pretreatment coupling high-temperature short-time sterilization facilitating cellulose degradation and sporulation-regulatory gene expression in high-solid fermentation. Int. J. Biol. Macromol. 2023, 232, 123475. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Negi, S.; Binod, P.; Larroche, C. Pretreatment of Biomass: Processes and Technologies; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of ultrasound in food science and technology: A perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Leonelli, C.; Mason, T.J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Process. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Chen, D.; Sharma, S.K.; Mudhoo, A. Handbook on Applications of Ultrasound: Sonochemistry for Sustainability, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Mason, T.; Peters, D. Practical Sonochemistry: Power Ultrasound Uses and Applications, 2nd ed.; Woodhead: Cambridge, UK, 2003. [Google Scholar]
- Leong, T.; Ashokkumar, M.; Kentish, S. The fundamentals of power ultrasound—A review. Acoust. Aust. 2011, 39, 54–63. [Google Scholar]
- Rooze, J.; Rebrov, E.V.; Schouten, J.C.; Keurentjes, J.T.F. Dissolved gas and ultrasonic cavitation—A review. Ultrason. Sonochem. 2013, 20, 1–11. [Google Scholar] [CrossRef]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Bussemaker, M.J.; Zhang, D. Effect of ultrasound on lignocellulosic biomass as a pretreatment for biorefinery and biofuel applications. Ind. Eng. Chem. Res. 2013, 52, 3563–3580. [Google Scholar] [CrossRef]
- Mussatto, S.I. Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Flores, E.M.M.; Cravotto, G.; Bizzi, C.A.; Santos, D.; Iop, G.D. Ultrasound-assisted biomass valorization to industrial interesting products: State-of-the-art, perspectives and challenges. Ultrason. Sonochem. 2021, 72, 105455. [Google Scholar] [CrossRef] [PubMed]
- Fia, A.Z.; Amorim, J. Microwave pretreatment of biomass for conversion of lignocellulosic materials into renewable biofuels. J. Energy Inst. 2023, 106, 101146. [Google Scholar] [CrossRef]
- Motasemi, F.; Azfal, M.T. A review on the microwave-assisted pyrolysis technique. Renew. Sustain. Energy Rew. 2013, 28, 317–330. [Google Scholar] [CrossRef]
- Tsubaki, S.; Hiraoka, M.; Hadano, S.; Nishimura, H.; Kashimura, K.; Mitani, T. Functional group dependent dielectric properties of sulphated hydrocolloids extracted from green macroalgal biomass. Carbohydr. Polym. 2014, 107, 192–197. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romanì, A.; Rodriguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Alvarez-OrdonezOrd, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; Silano, V. The efficacy and safety of high-pressure processing of food EFSA Panel on Biological Hazards (BIOHAZ Panel), Panel members. EFSA J. 2022, 20, 7128. [Google Scholar]
- Castanos-Rodriguez, J.F.; Welti-Chanes, J.; Palacios, A.J.; Torrestiana-Sanchez, B.; Ramirez de Leon, J.A.; Velazquez, G.; Aguilar-Uscanga, M.G. Influence of high pressure processing and alkaline treatment on sugarcane bagasse hydrolysis. CyTA—J. Food 2015, 13, 613–620. [Google Scholar]
- Jin, K.; Tang, Y.; Liu, J.; Wang, J.; Ye, C. Nanofibrillated cellulose as coating agent for food packaging paper. Int. J. Biol. Macromol. 2021, 168, 331–338. [Google Scholar] [CrossRef]
- Daza Serna, L.V.; Orrego Alzate, C.E.; Cardona Alzate, C.A. Supercritical fluids as a green technology for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Reyes, T.; Bandyopadhyay, S.S.; McCoy, B.J. Extraction of lignin from wood with supercritical alcohols. J. Supercrit. Fluids 1989, 2, 80–84. [Google Scholar] [CrossRef]
- Gao, M.; Xu, F.; Li, S.; Ji, X.; Chen, S.; Zhang, D. Effect of SC-CO2 pretreatment in increasing rice straw biomass conversion. Biosyst. Eng. 2010, 106, 470–475. [Google Scholar] [CrossRef]
- Narayanaswamy, N.; Faik, A.; Goetz, D.J.; Gu, T. Supercritical carbon dioxide pretreatment of corn stover and switchgrass for lignocellulosic ethanol production. Bioresour. Technol. 2011, 102, 6995–7000. [Google Scholar] [CrossRef] [PubMed]
- Samanta, K.K.; Joshi, A.G.; Jassal, M.; Agrawal, A.K. Hydrophobic functionalization of cellulosic substrate by tetrafluoroethane dielectric barrier discharge plasma at atmospheric pressure. Carbohydr. Polym. 2021, 253, 117272. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Ye, Z.; Sun, J.; Liu, C.; Yan, J.; Liu, T.; Li, X.; Zhang, H.; Xiao, R. A review on the application of non-thermal plasma (NTP) in the conversion of biomass: Catalyst preparation, thermal utilization and catalyst regeneration. Fuel 2022, 330, 125420. [Google Scholar] [CrossRef]
- Hooshmand, N.; Rahimpour, M.R.; Jahanmiri, A.; Taghvaei, H.; Mohamadzadeh, S.M. Hexadecane cracking in a hybrid pulsed dielectric barrier discharge plasma reactor. Ind. Eng. Chem. Res. 2013, 52, 4443–4449. [Google Scholar] [CrossRef]
- Wang, B.; Chen, B.; Sun, Y.; Xiao, H.; Xu, X.; Fu, M.; Wu, J.; Chen, L.; Ye, D. Effects of dielectric barrier discharge plasma on the catalytic activity of Pt/CeO2 catalysts. Appl. Catal. B Environ. 2018, 238, 328–338. [Google Scholar] [CrossRef]
- Taghvaei, H.; Kheirollahivash, M.; Ghasemi, M.; Rostami, P.; Rahimpour, M.R. Noncatalytic upgrading of anisole in an atmospheric DBD plasma reactor: Effect of carrier gas type, voltage, and frequency. Energy Fuels 2014, 28, 2535–2543. [Google Scholar] [CrossRef]
- Prieto, G.; Okumoto, M.; Shimano, K.; Takashima, K.; Katsura, S.; Mizuno, A. Reforming of heavy oil using nonthermal plasma. IEEE Trans. Ind. Appl. 2001, 37, 1464–1467. [Google Scholar] [CrossRef]
- Deng, J.; Xiong, T.; Wang, H.; Zheng, A.; Wang, Y. Effects of cellulose, hemicellulose, and lignin on the structure and morphology of porous carbons. ACS Sustain. Chem. Eng. 2016, 4, 3750–3756. [Google Scholar] [CrossRef]
- Schmitt, N.; Apfelbacher, A.; Jager, N.; Daschner, R.; Stenzel, F.; Hornung, A. Thermochemical conversion of biomass and upgrading to biofuel: The thermos-catalytic reforming process-a review. Biofuels Bioprod. Biorefin. 2019, 13, 822–837. [Google Scholar] [CrossRef]
- Montegiove, N.; Gambelli, A.M.; Calzoni, E.; Bertoldi, A.; Emiliani, C.; Gigliotti, G. Olive pomace protein hydrolysate waste valorization through biogas production: Evaluation of energy produced and process efficiency. Chem. Eng. Trans. 2024, 109, 319–324. [Google Scholar]
- Tocco, D.; Carucci, C.; Monduzzi, M.; Salis, A.; Sanjust, E. Recent developments in the delignification and exploiting of grass lignocellulosic biomass. ACS Sustain. Chem. Eng. 2021, 9, 2412–2432. [Google Scholar] [CrossRef]
- Sumardioni, S.; Matin, H.H.A.; Sulistianingtias, I.; Nugroho, T.Y.; Budiyono, B. Effect of physical and biological pretreatment on sugarcane bagasse waste-based biogas production. Mat. Today Proceed. 2023, 87, 41–44. [Google Scholar] [CrossRef]
- Meng, F.; Wang, D. Effects of vacuum freeze drying pretreatment on biomass and biochar properties. Renew. Energy 2020, 155, 1–9. [Google Scholar] [CrossRef]
- Montegiove, N.; Gambelli, A.M.; Calzoni, E.; Bertoldi, A.; Puglia, D.; Zadra, C.; Emiliani, C.; Gigliotti, G. Biogas production with residuals deriving from olive mill wastewater and olive pomace wastes: Quantification of produced energy, spent energy, and process efficiency. Agronomy 2024, 14, 531. [Google Scholar] [CrossRef]
- Dunbabin, J.S.; Bowmer, K.H. Potential use of constructed wetlands for treatment of industrial wastewaters containing metals. Sci. Total Environ. 1992, 111, 151–168. [Google Scholar] [CrossRef]
- Paritosh, K.; Yadav, M.; Mathur, S.; Balan, V.; Liao, W.; Pareek, N.; Vivekanand, V. Organic fraction of municipal solid waste: Overview of treatment methodologies to enhance anaerobic biodegradability. Front. Energy Res. 2018, 6, 75. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]

| Parameter | Inoculum |
|---|---|
| Moisture [%] | 88.08 |
| pH | 8.76 |
| TOC [% on DM] | 56.2 |
| TKN [% on DM] | 5.8 |
| Total P [g/kg of DM] | 3.3 |
| Total K [g/kg of DM] | 80.11 |
| WEOC [g/kg of DM] | 117.06 |
| WEN [g/kg of DM] | 71.89 |
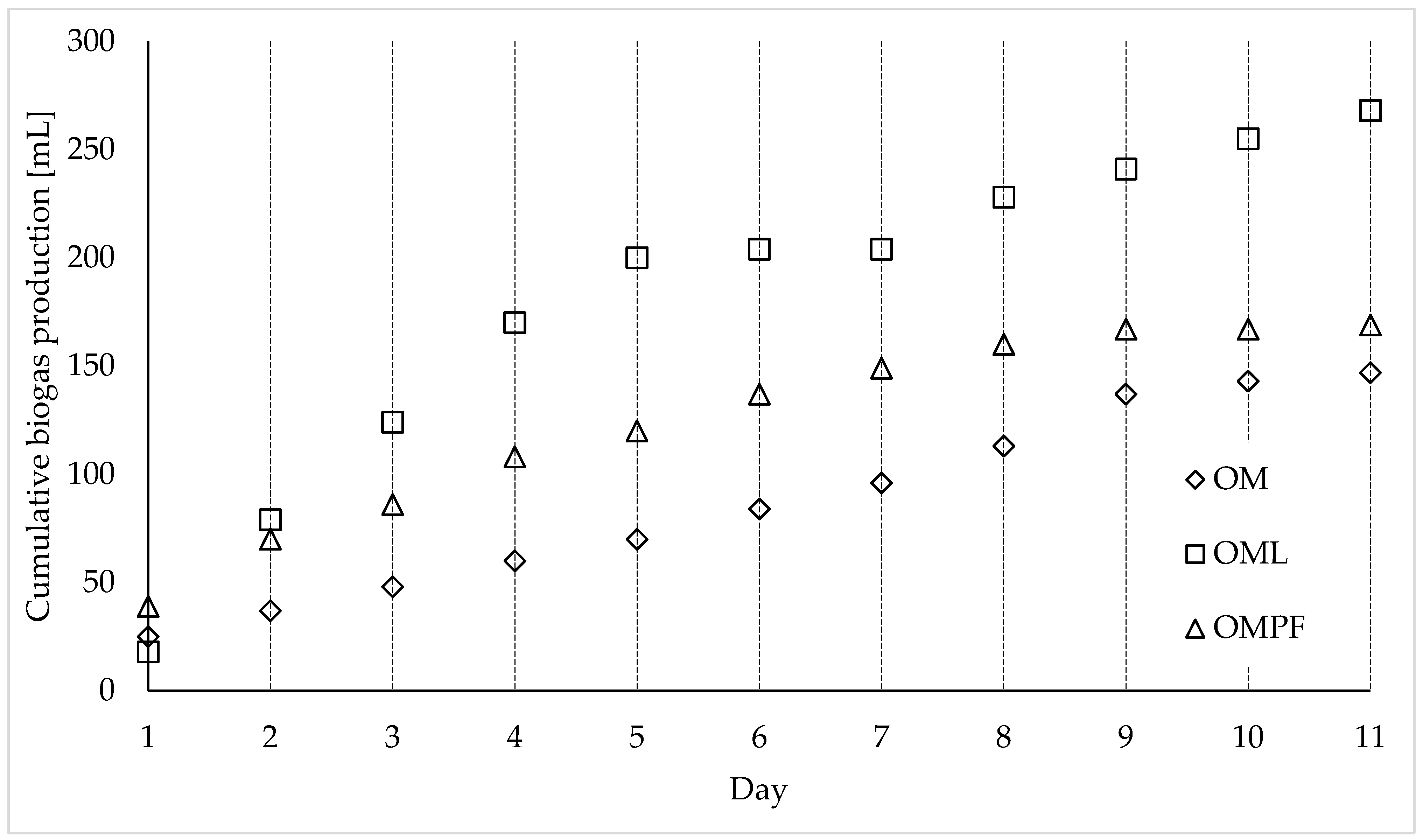
| Day n° | OM [mL] | OML [mL] | OMPF [mL] |
|---|---|---|---|
| 1 | 25 | 18 | 39 |
| 2 | 12 | 61 | 31 |
| 3 | 11 | 45 | 16 |
| 4 | 12 | 46 | 22 |
| 5 | 10 | 30 | 12 |
| 6 | 14 | 4 | 17 |
| 7 | 12 | 0 | 12 |
| 8 | 17 | 24 | 11 |
| 9 | 24 | 13 | 7 |
| 10 | 6 | 14 | 0 |
| 11 | 4 | 13 | 2 |
| OM | OML | OMPF | |
|---|---|---|---|
| TS [%] | 14 | 98 | 98.5 |
| VS [mg/L] | 162 | 298 | 188 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mario, J.; Montegiove, N.; Gambelli, A.M.; Brienza, M.; Zadra, C.; Gigliotti, G. Waste Biomass Pretreatments for Biogas Yield Optimization and for the Extraction of Valuable High-Added-Value Products: Possible Combinations of the Two Processes toward a Biorefinery Purpose. Biomass 2024, 4, 865-885. https://doi.org/10.3390/biomass4030048
Di Mario J, Montegiove N, Gambelli AM, Brienza M, Zadra C, Gigliotti G. Waste Biomass Pretreatments for Biogas Yield Optimization and for the Extraction of Valuable High-Added-Value Products: Possible Combinations of the Two Processes toward a Biorefinery Purpose. Biomass. 2024; 4(3):865-885. https://doi.org/10.3390/biomass4030048
Chicago/Turabian StyleDi Mario, Jessica, Nicolò Montegiove, Alberto Maria Gambelli, Monica Brienza, Claudia Zadra, and Giovanni Gigliotti. 2024. "Waste Biomass Pretreatments for Biogas Yield Optimization and for the Extraction of Valuable High-Added-Value Products: Possible Combinations of the Two Processes toward a Biorefinery Purpose" Biomass 4, no. 3: 865-885. https://doi.org/10.3390/biomass4030048
APA StyleDi Mario, J., Montegiove, N., Gambelli, A. M., Brienza, M., Zadra, C., & Gigliotti, G. (2024). Waste Biomass Pretreatments for Biogas Yield Optimization and for the Extraction of Valuable High-Added-Value Products: Possible Combinations of the Two Processes toward a Biorefinery Purpose. Biomass, 4(3), 865-885. https://doi.org/10.3390/biomass4030048









