Phycoremediated Microalgae and Cyanobacteria Biomass as Biofertilizer for Sustainable Agriculture: A Holistic Biorefinery Approach to Promote Circular Bioeconomy
Abstract
:1. Introduction
2. Plant Growth Promoting Properties of Microalgae and Cyanobacteria
2.1. Microalgal Biomass as Biofertilizer
2.1.1. Formation of Biological Soil Crust
2.1.2. N-Fixers
2.1.3. P-Solubilizer
2.1.4. Bioavailability of Micronutrients
2.2. Microalgae as Biostimulants
2.2.1. Phytohormones
2.2.2. Hormone-like Compounds as Biostimulants
2.2.3. Microalgal Polysaccharides
2.2.4. Proteins and Amino Acids
2.2.5. Phenolic Compounds
2.2.6. C-Phycocyanin
3. Application Methods of Microalgae and Cyanobacteria-Based Biofertilizers and Biostimulants
3.1. Seed Inoculation
3.2. Foliar Application
3.3. Soil and Root Drenching
4. Microalgae and Cyanobacteria Biomass Production from Wastewater and Its Composition
4.1. Nutrient Removal
4.2. Biomass Composition
5. Challenges Associated with Biomass Cultivation in Wastewater
5.1. Biological Challenges
5.2. Environmental Challenges
5.3. Economic Challenges
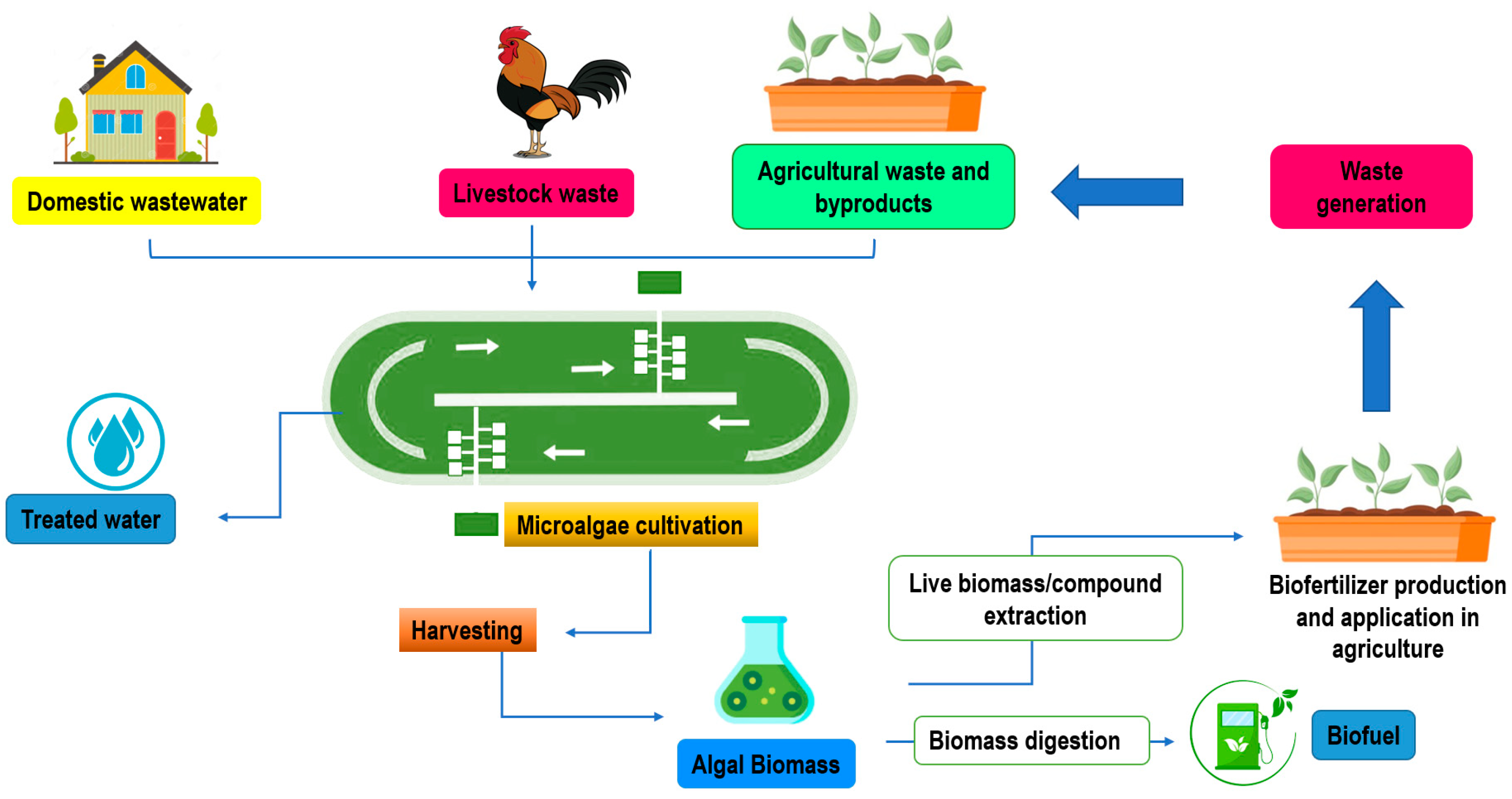
6. Integrated Biorefinery Approaches to Produce Microalgae and Cyanobacteria-Based Biofertilizer toward a Circular Economy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braun, J.C.; Colla, L.M. Use of microalgae for the development of biofertilizers and biostimulants. BioEnergy Res. 2023, 16, 289–310. [Google Scholar] [CrossRef]
- Renganathan, P.; Puente, E.O.R.; Sukhanova, N.V.; Gaysina, L.A. Hydroponics with microalgae and cyanobacteria: Emerging trends and opportunities in modern agriculture. BioTech 2024, 13, 27. [Google Scholar] [CrossRef]
- Parmar, P.; Kumar, R.; Neha, Y.; Srivatsan, V. Microalgae as next generation plant growth additives: Functions, applications, challenges and circular bioeconomy based solutions. Front. Plant Sci. 2023, 14, 1073546. [Google Scholar] [CrossRef]
- Cai, A.; Xu, M.; Wang, B.; Zhang, W.; Liang, G.; Hou, E.; Luo, Y. Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil Tillage Res. 2019, 189, 168–175. [Google Scholar] [CrossRef]
- Woods, J.; Williams, A.; Hughes, J.K.; Black, M.; Murphy, R. Energy and the food system. Philos. Trans. R. Soc. Lond. Ser. B 2010, 365, 2991–3006. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Das, P.; Quadir, M.A.; Thaher, M.I.; Alghasal, G.S.H.S.; Aljabri, H.M.S.J. Microalgal nutrients recycling from the primary effluent of municipal wastewater and use of the produced biomass as bio-fertilizer. Int. J. Environ. Sci. Technol. 2019, 16, 3355–3364. [Google Scholar] [CrossRef]
- Barone, V.; Puglisi, I.; Fragalà, F.; Stevanato, P.; Baglieri, A. Effect of living cells of microalgae or their extracts on soil enzyme activities. Arch. Agron. Soil Sci. 2019, 65, 712–726. [Google Scholar] [CrossRef]
- Lorentz, J.F.; Calijuri, M.L.; Assemany, P.P.; Alves, W.S.; Pereira, O.G. Microalgal biomass as a biofertilizer for pasture cultivation: Plant productivity and chemical composition. J. Clean. Prod. 2020, 276, 124130. [Google Scholar] [CrossRef]
- Sharma, G.K.; Khan, S.A.; Shrivastava, M.; Bhattacharyya, R.; Sharma, A.; Gupta, D.K.; Kishore, P.; Gupta, N. Circular economy fertilization: Phycoremediated algal biomass as biofertilizers for sustainable crop production. J. Environ. Manag. 2021, 287, 112295. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, J. Phytohormones in microalgae: A new opportunity for microalgal biotechnology? Trends Plant Sci. 2015, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Chanda, M.J.; Merghoub, N.; El Arroussi, H. Microalgae polysaccharides: The new sustainable bioactive products for the development of plant bio-stimulants? World J. Microbiol. Biotechnol. 2019, 35, 177. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, E.C.N.; Mógor, Á.F.; Amatussi, J.O.; Mógor, G.; Marques, H.M.C.; de Lara, G.B. Microalga biofertilizer improves potato growth and yield, stimulating amino acid metabolism. J. Appl. Phycol. 2022, 34, 385–394. [Google Scholar] [CrossRef]
- Fugita, Y.; Uesaka, K. Nitrogen fixation in cyanobacteria. In Cyanobacterial Physiology: From Fundamentals to Biotechnology; Kageyama, H., Waditee-Sirisattha, R., Eds.; Academic Press: London, UK, 2022; pp. 29–45, Paperback ISBN 9780323961066, eBook ISBN 9780323993869. [Google Scholar]
- El Arroussi, H.E.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; Mernissi, N.E.; Aafsar, A.; Meftah-Kadmiri, I.; Bendaou, N.; Smouni, A. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J. Appl. Phycol. 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
- Han, X.; Zeng, H.; Bartocci, P.; Fantozzi, F.; Yan, Y. Phytohormones and effects on growth and metabolites of microalgae: A review. Fermentation 2018, 4, 25. [Google Scholar] [CrossRef]
- Mógor, Á.F.; de Oliveira Amatussi, J.; Mógor, G.; de Lara, G.B. Bioactivity of cyanobacterial biomass related to amino acids induces growth and metabolic changes on seedlings and yield gains of organic red beet. Am. J. Plant Sci. 2018, 9, 966–978. [Google Scholar] [CrossRef]
- Priya, H.; Dhar, D.W.; Singh, R.; Kumar, S.; Dhandapani, R.; Pandey, R.; Govindasamy, V.; Kumar, A. Co-cultivation approach to decipher the influence of nitrogen-fixing cyanobacterium on growth and N uptake in rice crop. Curr. Microbiol. 2022, 79, 53. [Google Scholar] [CrossRef]
- Calijuri, M.L.; Silva, T.A.; Magalhães, I.B.; de Paula Pereira, A.S.A.; Marangon, B.B.; de Assis, L.R.; Lorentz, J.F. Bioproducts from microalgae biomass: Technology, sustainability, challenges and opportunities. Chemosphere 2022, 305, 135508. [Google Scholar] [CrossRef]
- Valizadeh, K.; Davarpanah, A. Design and construction of a micro-photo bioreactor in order to dairy wastewater treatment by micro-algae: Parametric study. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 611–624. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Dulta, K.; Kurniawan, S.B.; Omoarukhe, F.O.; Ewuzie, U.; Eshiemogie, S.O.; Ojo, A.U.; Abdullah, S.R.S. Progress in microalgae application for CO2 sequestration. Clean. Chem. Eng. 2022, 3, 100044. [Google Scholar] [CrossRef]
- Liu, X.Y.; Hong, Y. Microalgae-based wastewater treatment and recovery with biomass and value-added products: A brief review. Curr. Pollut. Rep. 2021, 7, 227–245. [Google Scholar] [CrossRef]
- Amaro, H.M.; Salgado, E.M.; Nunes, O.C.; Pires, J.C.; Esteves, A.F. Microalgae systems—Environmental agents for wastewater treatment and further potential biomass valorisation. J. Environ. Manag. 2023, 337, 117678. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.; Swain, D.K.; Sen, R. Strategic valorization of de-oiled microalgal biomass waste as biofertilizer for sustainable and improved agriculture of rice (Oryza sativa L.) crop. Sci. Total Environ. 2019, 682, 475–484. [Google Scholar] [CrossRef]
- Umamaheswari, J.; Shanthakumar, S. Paddy-soaked rice mill wastewater treatment by phycoremediation and feasibility study on use of algal biomass as biofertilizer. J. Chem. Technol. Biotechnol. 2021, 96, 394–403. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Zhou, Z.; Wang, L.; Xue, N.; Liu, B. Growth and nutrient removal characteristics of chlorella cultivated in resource-separated human urine mixed with soap wastewater. Waste Biomass Valorization 2024, 15, 5205–5218. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, S.K.; Shabnam, N.; Oliveira, C.Y.B.; Nema, A.K.; Ansari, F.A.; Bux, F. Role of microalgae in global CO2 sequestration: Physiological mechanism, recent development, challenges, and future prospective. Sustainability 2021, 13, 13061. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Verma, P. Multifaceted role of microalgae for municipal wastewater treatment: A futuristic outlook toward wastewater management. Clean Soil Air Water 2023, 51, 2100286. [Google Scholar] [CrossRef]
- Khan, S.A.; Sharma, G.K.; Malla, F.A.; Kumar, A.; Gupta, N. Microalgae based biofertilizers: A biorefinery approach to phycoremediate wastewater and harvest biodiesel and manure. J. Clean. Prod. 2019, 211, 1412–1419. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Sivaramakrishnan, R.; Incharoensakdi, A.; Cornejo, P.; Kamaraj, B.; Chi, N.T.L. Removal of nutrients from domestic wastewater by microalgae coupled to lipid augmentation for biodiesel production and influence of deoiled algal biomass as biofertilizer for Solanum lycopersicum cultivation. Chemosphere 2021, 268, 129323. [Google Scholar] [CrossRef]
- Kumar, M.; Prasanna, R.; Bidyarani, N.; Babu, S.; Mishra, B.K.; Kumar, A.; Adak, A.; Jauhari, S.; Yadav, K.; Singh, R.; et al. Evaluating the plant growth promoting ability of thermotolerant bacteria and cyanobacteria and their interactions with seed spice crops. Sci. Hortic. 2013, 164, 94–101. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.; Prasanna, R.; Kumar, G.; Kumar, A.; Nain, L. Co-cultivation of cyanobacteria for raising nursery of chrysanthemum using a hydroponic system. J. Appl. Phycol. 2019, 31, 3625–3635. [Google Scholar] [CrossRef]
- Kim, M.J.; Shim, C.K.; Kim, Y.K.; Ko, B.G.; Park, J.H.; Hwang, S.G.; Kim, B.H. Effect of biostimulator Chlorella fusca on improving growth and qualities of Chinese chives and spinach in organic farm. Plant Pathol. J. 2018, 34, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Zhou, Q. Co-cultivation of Chlorella spp. and tomato in a hydroponic system. Biomass Bioenergy 2017, 97, 132–138. [Google Scholar] [CrossRef]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Performance evaluation of hydroponic system for co-cultivation of microalgae and tomato plant. J. Clean. Product. 2020, 272, 122823. [Google Scholar] [CrossRef]
- Agwa, O.K.; Ogugbue, C.J.; Williams, E.E. Field evidence of Chlorella vulgaris potentials as a biofertilizer for Hibiscus esculentus. Int. J. Agric. Res. 2017, 12, 181–189. [Google Scholar] [CrossRef]
- Schreiber, C.; Schiedung, H.; Harrison, L.; Briese, C.; Ackermann, B.; Kant, J.; Schrey, S.D.; Hofmann, D.; Singh, D.; Ebenhöh, O.; et al. Evaluating potential of green alga Chlorella vulgaris to accumulate phosphorus and to fertilize nutrient-poor soil substrates for crop plants. J. Appl. Phycol. 2018, 30, 2827–2836. [Google Scholar] [CrossRef]
- Grzesik, M.; Romanowska-duda, Z. Improvements in germination, growth, and metabolic activity of corn seedlings by grain conditioning and root application with cyanobacteria and microalgae. Pol. J. Environ. Stud. 2014, 23, 1147–1153. [Google Scholar]
- Puglisi, I.; La Bella, E.; Rovetto, E.I.; Stevanato, P.; Fascella, G.; Baglieri, A. Morpho-biometric and biochemical responses in lettuce seedlings treated by different application methods of Chlorella vulgaris extract: Foliar spray or root drench? J. Appl. Phycol. 2022, 34, 889–901. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, J.; Gopalsamy, J.; Jayasingam, P.; Arumugam, A.; Kannadasan, S.; Sampathkumar, P. The impact of using microalgae as biofertilizer in maize (Zea mays L.). Waste Biomass Valorization 2019, 10, 1101–1110. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, J.; Arumugam, A.; Ahamed Rasheeq, A.; Sampathkumar, P. Exploring the microalgae biofertilizer effect on onion cultivation by field experiment. Waste Biomass Valorization 2020, 11, 77–87. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Kumaravel, R.; Gopalsamy, J.; Sikder, M.N.A.; Sampathkumar, P. Microalgae as bio-fertilizers for rice growth and seed yield productivity. Waste Biomass Valorization 2018, 9, 793–800. [Google Scholar] [CrossRef]
- Deepika, P.; MubarakAli, D. Production and assessment of microalgal liquid fertilizer for the enhanced growth of four crop plants. Biocatal. Agric. Biotechnol. 2020, 28, 101701. [Google Scholar] [CrossRef]
- Rupawalla, Z.; Shaw, L.; Ross, I.L.; Schmidt, S.; Hankamer, B.; Wolf, J. Germination screen for microalgae-generated plant growth biostimulants. Algal Res. 2022, 66, 102784. [Google Scholar] [CrossRef]
- Coppens, J.; Grunert, O.; Van Den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; De Gelder, L. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2016, 28, 2367–2377. [Google Scholar] [CrossRef]
- Abedi Firoozjaei, M.H.; Hassani, S.B.; Nazifi, E.; Keypour, S. Study the effect of the terrestrial cyanobacterium nostoc commune aqueous extract on seed germination and seedling growth of rice. J. Phycol. Res. 2021, 5, 642–653. [Google Scholar]
- Godlewska, K.; Michalak, I.; Pacyga, P.; Baśladyńska, S.; Chojnacka, K. Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J. Microbiol. Biotechnol. 2019, 35, 80. [Google Scholar] [CrossRef]
- Thinh, N.Q. Influences of seed priming with Spirulina platensis extract on seed quality properties in black gram (Vigna mungo L.). Vietnam J. Sci. Technol. Eng. 2021, 63, 36–41. [Google Scholar] [CrossRef]
- Alshehrei, F.; Al-Enazi, N.M.; Ameen, F. Vermicomposting amended with microalgal biomass and biochar produce phytopathogen-resistant seedbeds for vegetables. Biomass Convers. Biorefinery 2021, 1–8. [Google Scholar] [CrossRef]
- Lababpour, A. Potentials of the microalgae inoculant in restoration of biological soil crusts to combat desertification. Int. J. Environ. Sci. Technol. 2016, 13, 2521–2532. [Google Scholar] [CrossRef]
- Jadhav, R.R.; Panhwar, W.A.; Malik, J.A. Role of microalgae as biofertilizer for sustainable plant and soil health. In Microbial and Biotechnological Interventions in Bioremediation and Phytoremediation; Springer International Publishing: Cham, Switzerland, 2022; pp. 221–236. [Google Scholar]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Soil microalgae and cyanobacteria: The biotechnological potential in the maintenance of soil fertility and health. Crit. Rev. Biotechnol. 2019, 39, 981–998. [Google Scholar] [CrossRef] [PubMed]
- Belnap, J. The potential roles of biological soil crusts in dryland hydrologic cycles. Hydrol. Proc. Int. J. 2006, 20, 3159–3178. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Greene, R.S.B. Microbiotic soil crusts-a review of their roles in soil and ecological processes in the rangelands of Australia. Soil Res. 1994, 32, 389–415. [Google Scholar] [CrossRef]
- Acea, M.J.; Prieto-Fernández, A.; Diz-Cid, N. Cyanobacterial inoculation of heated soils: Effect on microorganisms of C and N cycles and on chemical composition in soil surface. Soil Biol. Biochem. 2003, 35, 513–524. [Google Scholar] [CrossRef]
- Brock, T.D. Primary colonization of Surtsey, with special reference to the blue-green algae. Oikos 1973, 24, 239–243. [Google Scholar] [CrossRef]
- Blank, G.B.; Cameron, R.E. Desert Algae-Soil Crusts and Diaphanous Substrata as Algal Habitats; No. JPL-TR-32-971; National Aeronautics and Space Administration: Washington, DC, USA, 1966.
- Issa, O.M.; Le Bissonnais, Y.; Défarge, C.; Trichet, J. Role of a cyanobacterial cover on structural stability of sandy soils in the Sahelian part of western Niger. Geoderma 2001, 101, 15–30. [Google Scholar] [CrossRef]
- Kheirfam, H.; Sadeghi, S.H.; Homaee, M.; Darki, B.Z. Quality improvement of an erosion-prone soil through microbial enrichment. Soil Tillage Res. 2017, 165, 230–238. [Google Scholar] [CrossRef]
- Stal, L.; Krumbein, W. Isolation and characterization of cyanobacteria from a marine microbial mat. Bot. Mar. 1985, 28, 351–366. [Google Scholar] [CrossRef]
- Prasanna, R.; Kaushik, B.D. Nitrogen fixation and nif gene organization in branched heterocystous cyanobacteria: Variation in the presence of xisA. Folia Microbiol. 1995, 40, 176–180. [Google Scholar] [CrossRef]
- Kalyanasundaram, G.T.; Ramasamy, A.; Rakesh, S.; Subburamu, K. Microalgae and cyanobacteria: Role and applications in agriculture. In Applied Algal Biotechnology; Nova Science Publishers: Hauppauge, NY, USA, 2020. [Google Scholar]
- Kuraganti, G.; Edla, S.; Pallaval, V.B. Cyanobacteria as biofertilizers: Current research, commercial aspects, and future challenges. In Advances in Plant Microbiome and Sustainable Agriculture: Functional Annotation and Future Challenges; Springer: Singapore, 2020; pp. 259–278. [Google Scholar]
- Lee, S.; Ryu, C. Algae as new kids in the beneficial plant microbiome. Front. Plant Sci. 2021, 12, 599742. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Li, D.; Hu, C.; Rao, B. Feasibility of cyanobacterial inoculation for biological soil crusts formation in desert area. Soil Biol. Biochem. 2009, 41, 926–929. [Google Scholar] [CrossRef]
- Nisha, R.; Kaushik, A.; Kaushik, C.P. Effect of indigenous cyanobacterial application on structural stability and productivity of an organically poor semi-arid soil. Geoderma 2007, 138, 49–56. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Yandigeri, M.S.; Yadav, A.K.; Srinivasan, R.; Kashyap, S.; Pabbi, S. Studies on mineral phosphate solubilization by cyanobacteria westiellopsis and anabaena. Microbiology 2011, 80, 558–565. [Google Scholar] [CrossRef]
- Ellwood, N.T.; Di Pippo, F.; Albertano, P. Phosphatase activities of cultured phototrophic biofilms. Water Res. 2012, 46, 378–386. [Google Scholar] [CrossRef]
- Swarnalakshmi, K.; Prasanna, R.; Kumar, A.; Pattnaik, S.; Chakravarty, K.; Shivay, Y.S.; Singh, R.; Saxena, A.K. Evaluating the influence of novel cyanobacterial biofilmed biofertilizers on soil fertility and plant nutrition in wheat. Eur. J. Soil Biol. 2013, 55, 107–116. [Google Scholar] [CrossRef]
- Prasanna, R.; Triveni, S.; Bidyarani, N.; Babu, S.; Yadav, K.; Adak, A.; Khetarpal, S.; Pal, M.; Shivay, Y.S.; Saxena, A.K. Evaluating the efficacy of cyanobacterial formulations and biofilmed inoculants for leguminous crops. Arch. Agron. Soil Sci. 2014, 60, 349–366. [Google Scholar] [CrossRef]
- Prasanna, R.; Adak, A.; Verma, S.; Bidyarani, N.; Babu, S.; Pal, M.; Shivay, Y.S.; Nain, L. Cyanobacterial inoculation in rice grown under flooded and SRI modes of cultivation elicits differential effects on plant growth and nutrient dynamics. Ecol. Eng. 2015, 84, 532–541. [Google Scholar] [CrossRef]
- Liang, G. Iron uptake, signaling, and sensing in plants. Plant Commun. 2022, 3, 100349. [Google Scholar] [CrossRef]
- Kundu, K.; Teta, R.; Esposito, G.; Stornaiuolo, M.; Costantino, V. A four-step platform to optimize growth conditions for high-yield production of siderophores in cyanobacteria. Metabolites 2023, 13, 154. [Google Scholar] [CrossRef]
- Schätzle, H.; Arévalo, S.; Fresenborg, L.; Seitz, H.M.; Flores, E.; Schleiff, E. Functional diversity of TonB-like proteins in the heterocyst-forming Cyanobacterium Anabaena sp. PCC 7120. Msphere 2021, 6, e00214-21. [Google Scholar] [CrossRef] [PubMed]
- Fresenborg, L.S.; Graf, J.; Schätzle, H.; Schleiff, E. Iron homeostasis of cyanobacteria: Advancements in siderophores and metal transporters. In Advances in Cyanobacterial Biology; Academic Press: Cambridge, MA, USA, 2020; pp. 85–117. [Google Scholar]
- Rashmi, V.; ShylajaNaciyar, M.; Rajalakshmi, R.; D’Souza, S.F.; Prabaharan, D.; Uma, L. Siderophore mediated uranium sequestration by marine cyanobacterium Synechococcus elongatus BDU 130911. Bioresour. Technol. 2013, 130, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kaushik, M.S.; Srivastava, M.; Tiwari, D.N.; Mishra, A.K. Siderophore mediated attenuation of cadmium toxicity by paddy field cyanobacterium Anabaena oryzae. Algal Res. 2016, 16, 63–68. [Google Scholar] [CrossRef]
- Renuka, N.; Prasanna, R.; Sood, A.; Bansal, R.; Bidyarani, N.; Singh, R.; Shivay, Y.S.; Nain, L.; Ahluwalia, A.S. Wastewater grown microalgal biomass as inoculants for improving micronutrient availability in wheat. Rhizosphere 2017, 3, 150–159. [Google Scholar] [CrossRef]
- Rana, A.; Kabi, S.R.; Verma, S.; Adak, A.; Pal, M.; Shivay, Y.S.; Prasanna, R.; Nain, L. Prospecting plant growth promoting bacteria and cyanobacteria as options for enrichment of macro-and micronutrients in grains in rice–wheat cropping sequence. Cogent Food Agric. 2015, 1, 1037379. [Google Scholar] [CrossRef]
- Youssef, S.M.S.R.; Ghanem, K.Z.; Elhakem, A.; Abdel Aal, A.A. Foliar spray or soil drench: Microalgae application impacts on soil microbiology, morpho-physiological and biochemical responses, oil and fatty acid profiles of chia plants under alkaline stress. Biology 2022, 11, 1844. [Google Scholar] [CrossRef]
- Vijay, A.K.; Prabha, S.; Thomas, J.; Kurian, J.S.; George, B. Effect of auxin and its synthetic analogues on the biomass production and biochemical composition of freshwater microalga Ankistrodesmus falcatus CMSACR1001. J. Appl. Phycol. 2020, 32, 3787–3797. [Google Scholar] [CrossRef]
- Cruz, C.G.; Vieira Costa, J.A. Identification of the phytohormones indole-3-acetic acid and trans-zeatin in microalgae. J. Chem. Technol. Biotechnol. 2023, 98, 1048–1056. [Google Scholar] [CrossRef]
- Singh, J.; Jain, D.; Agarwal, P.; Singh, R. Auxin and cytokinin synergism augmenting biomass and lipid production in microalgae Desmodesmus spp. JS07. Process Biochem. 2020, 95, 223–234. [Google Scholar] [CrossRef]
- Trinh, C.T.; Tran, T.H.; Bui, T.V. Effects of plant growth regulators on the growth and lipid accumulation of Nanno-chloropsis oculata (droop) Hibberd. In AIP Conference Proceedings; No. 1, 020017; AIP Publishing: Melville, NY, USA, 2017. [Google Scholar]
- Salama, E.S.; Jeon, B.H.; Chang, S.W.; Lee, S.H.; Roh, H.S.; Yang, I.S.; Kurade, M.B.; El-Dalatony, M.M.; Kim, D.H.; Kim, K.H.; et al. Interactive effect of indole-3-acetic acid and diethyl aminoethyl hexanoate on the growth and fatty acid content of some microalgae for biodiesel production. J. Clean. Prod. 2017, 168, 1017–1024. [Google Scholar] [CrossRef]
- Kozlova, T.A.; Hardy, B.P.; Krishna, P.; Levin, D.B. Effect of phytohormones on growth and accumulation of pigments and fatty acids in the microalgae Scenedesmus quadricauda. Algal Res. 2017, 27, 325–334. [Google Scholar] [CrossRef]
- Yu, Z.; Pei, H.; Jiang, L.; Hou, Q.; Nie, C.; Zhang, L. Phytohormone addition coupled with nitrogen depletion almost tripled the lipid productivities in two algae. Bioresour. Technol. 2018, 247, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Renuka, N.; Guldhe, A.; Singh, P.; Ansari, F.A.; Rawat, I.; Bux, F. Evaluating the potential of cytokinins for biomass and lipid enhancement in microalga Acutodesmus obliquus under nitrogen stress. Energy Convers. Manag. 2017, 140, 14–23. [Google Scholar] [CrossRef]
- Parsaeimehr, A.; Mancera-Andrade, E.I.; Robledo-Padilla, F.; Iqbal, H.M.; Parra-Saldivar, R. A chemical approach to manipulate the algal growth, lipid content and high-value alpha-linolenic acid for biodiesel production. Algal Res. 2017, 26, 312–322. [Google Scholar] [CrossRef]
- Ramphal, K.; Lewis, A.; Trzaskalski, N.A.; Kisiala, A.; Morrison, E.N.; Narine, S.S.; Emery, R.J.N. Phytohormonal impacts on fatty acid profiles in Chlorella vulgaris Beijerinck: Endogenous identification and exogenous application of cy-tokinins and abscisic acid. J. Appl. Phycol. 2023, 35, 2205–2218. [Google Scholar] [CrossRef]
- Tiwari, S.; Patel, A.; Prasad, S.M. Kinetin alleviates chromium toxicity on growth and PS II photochemistry in Nostoc muscorum by regulating antioxidant system. Ecotoxicol. Environ. Saf. 2018, 161, 296–304. [Google Scholar] [CrossRef]
- González-Garcinuño, Á.; Sánchez-Álvarez, J.M.; Galán, M.A.; Martin del Valle, E.M. Understanding and optimizing the addition of phytohormones in the culture of microalgae for lipid production. Biotechnol. Prog. 2016, 32, 1203–1211. [Google Scholar] [CrossRef]
- Du, K.; Tao, H.; Wen, X.; Geng, Y.; Li, Y. Enhanced growth and lipid production of Chlorella pyrenoidosa by plant growth regulator GA3. Fresenius Environ. Bull. 2015, 24, 3414–3419. [Google Scholar]
- Madani, N.S.H.; Shamsaie Mehrgan, M.; Hosseini Shekarabi, S.P.; Pourang, N. Regulatory effect of gibberellic acid (GA3) on the biomass productivity and some metabolites of a marine microalga, Isochrysis galbana. J. Appl. Phycol. 2021, 33, 255–262. [Google Scholar]
- Arora, S.; Mishra, G. Effect of gibberellin, methyl jasmonate and myoinositol on biomass and eicosapentaenoic acid productivities in the eustigmatophyte Monodopsis subterranea CCALA 830. J. Appl. Phycol. 2021, 33, 287–299. [Google Scholar]
- Kim, S.H.; Lim, S.R.; Hong, S.J.; Cho, B.K.; Lee, H.; Lee, C.G.; Choi, H.K. Effect of ethephon as an ethylene-releasing compound on the metabolic profile of Chlorella vulgaris. J. Agric. Food Chem. 2016, 64, 4807–4816. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.; Lee, C.; Han, S.I.; Kim, J.Y.; Kim, S.; Choi, Y.E. Effect of the ethylene precursor, 1-aminocyclopropane-1-carboxylic acid on different growth stages of Haematococcus pluvialis. Bioresour. Technol. 2016, 220, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, X.; Zhao, Y.; Gao, H.; Li, L.; Zhang, Y.; Yu, X. Myo-inositol facilitates astaxanthin and lipid coproduction in Haematococcus pluvialis by regulating oxidative stress and ethylene signaling. Bioresour. Technol. 2022, 366, 128222. [Google Scholar] [CrossRef]
- Qiao, T.; Gu, D.; Zhu, L.; Zhao, Y.; Zhong, D.; Yu, X. Coupling of myo-inositol with salinity regulates ethylene-induced microalgal lipid hyperproduction in molasses wastewater. Sci. Total Environ. 2022, 818, 151765. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, W.; Song, Y.; Peng, H.; Zhao, Y. The growth and lipid productivity of Chlorella pyrenoidosa enhanced by plant hormones under ammonium stress. Environ. Prog. Sustain. Energy 2017, 36, 1187–1193. [Google Scholar] [CrossRef]
- Norlina, R.; Norashikin, M.N.; Loh, S.H.; Aziz, A.A.; Cha, T.S. Exogenous abscisic acid supplementation at early stationary growth phase triggers changes in the regulation of fatty acid biosynthesis in Chlorella vulgaris UMT-M1. Appl. Biochem. Biotechnol. 2020, 191, 1653–1669. [Google Scholar] [CrossRef]
- Kozlova, T.A.; Kartashov, A.V.; Zadneprovskaya, E.; Krapivina, A.; Zaytsev, P.; Chivkunova, O.B.; Solovchenko, A.E. Effect of abscisic acid on growth, fatty acid profile, and pigment composition of the Chlorophyte Chlorella (Chromo-chloris) zofingiensis and its co-culture microbiome. Life 2023, 13, 452. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, Q.; Chen, C.; Zhang, Y.; Liu, Y.; Xu, L.; Zhou, Y.; Li, C.; Zhou, D.; Rittmann, B.E. Benzoic and salicylic acid are the signaling molecules of Chlorella cells for improving cell growth. Chemosphere 2021, 265, 129084. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Peng, Y.; Zhang, Y.; Li, Q.; Sun, D. Salicylic acid enhances cell growth, fatty acid and astaxanthin production in heterotrophic Chromochloris zofingiensis without reactive oxygen species elevation. Biotechnol. Biofuels 2024, 17, 1. [Google Scholar] [CrossRef]
- Raman, V.; Ravi, S. Effect of salicylic acid and methyl jasmonate on antioxidant systems of Haematococcus pluvialis. Acta Physiol. Plant 2011, 33, 1043–1049. [Google Scholar] [CrossRef]
- Mc Gee, D.; Archer, L.; Parkes, R.; Fleming, G.T.; Santos, H.M.; Touzet, N. The role of methyl jasmonate in enhancing biomass yields and bioactive metabolites in Stauroneis spp. (Bacillariophyceae) revealed by proteome and biochemical profiling. J. Proteom. 2021, 249, 104381. [Google Scholar] [CrossRef] [PubMed]
- Santner, A.; Calderon-Villalobos, L.I.A.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef]
- El Arroussi, H.; El Mernissi, N.; Benhima, R.; El Kadmiri, I.M.; Bendaou, N.; Smouni, A.; Wahby, I. Microalgae polysaccharides a promising plant growth biostimulant. J. Algal Biomass Util. 2016, 7, 55–63. [Google Scholar]
- Górka, B.; Lipok, J.; Wieczorek, P.P. Biologically active organic compounds, especially plant promoters, in algae extracts and their potential application in plant cultivation. In Marine Algae Extracts: Processes, Products, and Applications; Kim, S.K., Chojnacka, K., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 659–680. [Google Scholar]
- Tan, C.Y.; Dodd, I.C.; Chen, J.E.; Phang, S.M.; Chin, C.F.; Yow, Y.Y.; Ratnayeke, S. Regulation of algal and cyanobacterial auxin production, physiology, and application in agriculture: An overview. J. Appl. Phycol. 2021, 33, 2995–3023. [Google Scholar] [CrossRef]
- Stirk, W.A.; Ördög, V.; Novák, O.; Rolčík, J.; Strnad, M.; Bálint, P.; van Staden, J. Auxin and cytokinin relationships in 24 microalgal strains1. J. Phycol. 2013, 49, 459–467. [Google Scholar] [CrossRef]
- Elakbawy, W.M.; Shanab, S.M.M.; Shalaby, E.A. Enhancement of plant growth regulators production from microalgae cultivated in treated sewage wastewater (TSW). BMC Plant Biol. 2022, 22, 377. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, N.; Prasanna, R.; Sood, A.; Jaiswal, P.; Nayak, S.; Kaushik, B. Physiological characterization and electron microscopic investigation of cyanobacteria associated with wheat rhizosphere. Folia Microbiol. 2009, 54, 43–51. [Google Scholar] [CrossRef]
- Murray, J.D.; Karas, B.J.; Sato, S.; Tabata, S.; Amyot, L.; Szczyglowski, K. A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 2007, 315, 101–104. [Google Scholar] [CrossRef]
- Werner, T.; Motika, V.; Strnad, M.; Schmulling, T. Regulation of plants growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar]
- Mazepa, E.; Malburg, B.V.; Mógor, G.; de Oliveira, A.C.; Amatussi, J.O.; Corrêa, D.O.; Lemos, J.S.; Ducatti, D.R.; Duarte, M.E.R.; Mógor, Á.F.; et al. Plant growth biostimulant activity of the green microalga Desmodesmus subspicatus. Algal Res. 2021, 59, 102434. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Tuna, A.L.; Kaya, C.; Dikilitas, M.; Higgs, D. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 2008, 62, 1–9. [Google Scholar] [CrossRef]
- Stirk, W.A.; Bálint, P.; Tarkowská, D.; Novák, O.; Strnad, M.; Ördög, V.; Van Staden, J. Hormone profiles in microalgae: Gibberellins and brassinosteroids. Plant Physiol. Biochem. 2013, 70, 348–353. [Google Scholar] [CrossRef]
- El-Naggar, A.H.; Osman, M.E.H.E.-S.; Gheda, S.F. Influence of the aqueous extracts of Ulva lactuca and Chlorella kessleri on growth and yield of Vicia faba. Arch. Hydrobiol. Suppl. Algol. Stud. 2005, 116, 213–229. [Google Scholar] [CrossRef]
- Rodríguez, A.; Stella, A.; Storni, M.; Zulpa, G.; Zaccaro, M. Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst. 2006, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Sun, X.; Ma, B.; Zhang, J.; Guo, H. 6—Ethylene. In Hormone Metabolism and Signaling in Plants; Li, J., Li, C., Smith, S.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 203–241. [Google Scholar]
- Plaza, B.M.; Gómez-Serrano, C.; Acién-Fernández, F.G.; Jimenez-Becker, S. Effect of microalgae hydrolysate foliar application (Arthrospira platensis and Scenedesmus spp.) on petunia x hybrida growth. J. Appl. Phycol. 2018, 30, 2359–2365. [Google Scholar] [CrossRef]
- Nambara, E. Abscisic Acid. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Thomas, B., Murray, B.G., Murphy, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 361–366. [Google Scholar]
- Agehara, S.; Leskovar, D.I. Growth suppression by exogenous abscisic acid and uniconazole for prolonged marketability of tomato transplants in commercial conditions. HortScience 2017, 52, 606–611. [Google Scholar] [CrossRef]
- Yamazaki, H.; Nishijima, T.; Koshioka, M. Effects of (+)-s-abscisic acid on the quality of stored cucumber and tomato seedlings. HortScience 1995, 30, 80–82. [Google Scholar] [CrossRef]
- Khasin, M.; Cahoon, R.E.; Alvarez, S.; Beckeris, R.; Eyun, S.; Jia, Q.; Riethoven, J.J.; Nickerson, K.W.; Riekhof, W.R. Synthesis, secretion, and perception of abscisic acid regulates stress responses in Chlorella sorokiniana. bioRxiv 2017, 180547. [Google Scholar] [CrossRef]
- Pan, S.; Jeevanandam, J.; Danquah, M.K. Benefits of algal extracts in sustainable agriculture. In Grand Challenges in Algae Biotechnology; Springer: Cham, Switzerland, 2019; pp. 501–534. [Google Scholar]
- McAdam, S.A.; Brodribb, T.J.; Ross, J.J. Shoot-derived abscisic acid promotes root growth. Plant Cell Environ. 2016, 39, 652–659. [Google Scholar] [CrossRef]
- Ghorbel, M.; Brini, F. Role of brassinosteroids in regulating physiological and molecular aspects of plants under abiotic stress. In The Role of Growth Regulators and Phytohormones in Overcoming Environmental Stress; Sharma, A., Pandey, S., Bhardwaj, R., Zheng, B., Tripathi, D.K., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 197–233. [Google Scholar]
- Zhu, B.C.; Su, J.; Cham, M.C.; Verma, D.P.S.; Fan, Y.L.; Wu, R. Overexpression of pyrroline-5-carboxylate synthetase gene and analysis of tolerance to water stress and salt stress in transgenic rice. Plant Sci. 1998, 139, 41–48. [Google Scholar] [CrossRef]
- Singh, I.; Shono, M. Physiological and molecular effects of 24-epibrassinolide, a brassinosteroid, on thermotolerance of tomato. Plant Growth Regul. 2005, 47, 111–119. [Google Scholar] [CrossRef]
- El-Bassiony, A.M.; Ghoname, A.A.; El-Awadi, M.E.; Fawzy, Z.F.; Gruda, N. Ameliorative effects of brassinosteroids on growth and productivity of snap beans grown under high temperature. Gesunde Pflanz. 2012, 64, 175–182. [Google Scholar] [CrossRef]
- Bajguz, A. Isolation and characterization of brassinosteroids from algal cultures of Chlorella vulgaris Beijerinck (Trebouxiophyceae). J. Plant Physiol. 2009, 166, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Xu, L. The effect of polyamine on flower bud differentiation and bud germination of chrysanthemum. Shandong Agric. Univ. 2015, 2, 31–36. [Google Scholar]
- Xu, L.; Xing, S.; Sun, X. Effects of polyamines on hormones contents and the relationship with the flower bud differentiation in chrysanthemum. Plant Physiol. J. 2014, 50, 1195–1202. [Google Scholar]
- Mustafavi, S.H.; Naghdi Badi, H.; Sekara, A.; Mehrafarin, A.; Janda, T.; Ghorbanpour, M.; Rafiee, H. Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol. Plant 2018, 40, 102. [Google Scholar] [CrossRef]
- Wang, W.; Paschalidis, K.; Feng, J.C.; Song, J.; Liu, J.H. Polyamine catabolism in plants: A universal process with diverse functions. Front. Plant Sci. 2019, 10, 561. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lin, H.J. Polyamines in microalgae: Something borrowed, something new. Mar. Drugs 2019, 17, 1. [Google Scholar] [CrossRef]
- Tamaoki, D.; Seo, S.; Yamada, S.; Kano, A.; Miyamoto, A.; Shishido, H.; Miyoshi, S.; Taniguchi, S.; Akimitsu, K.; Gomi, K. Jasmonic acid and salicylic acid activate a common defense system in rice. Plant Signal. Behav. 2013, 8, e24260. [Google Scholar] [CrossRef]
- Hou, S.; Tsuda, K. Salicylic acid and jasmonic acid crosstalk in plant immunity. Essays Biochem. 2022, 66, 647–656. [Google Scholar] [PubMed]
- Synytsya, A.; Sushytskyi, L.; Saloň, I.; Babayeva, T.; Čopíková, J. Intracellular and extracellular carbohydrates in microalgae. In Handbook of Food and Feed from Microalgae; Jacob-Lopes, E., Queiroz, M.I., Maroneze, M.M., Zepka, L.Q., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 87–102. [Google Scholar]
- Rachidi, F.; Benhima, R.; Sbabou, L.; El Arroussi, H. Microalgae polysaccharides bio-stimulating effect on tomato plants: Growth and metabolic distribution. Biotechnol. Rep. 2020, 25, e00426. [Google Scholar] [CrossRef] [PubMed]
- Matos, Â.P.; da Silva, T.; Sant’Anna, E.S. The feasibility of using inland desalination concentrate (DC) as an alternative substrate for Spirulina platensis mass cultivation. Waste Biomass Valorization 2021, 12, 3193–3203. [Google Scholar] [CrossRef]
- Yahia, E.M.; Gardea-Béjar, A.; Ornelas-Paz, J.D.J.; Maya-Meraz, I.O.; Rodríguez-Roque, M.J.; Rios-Velasco, C.; Ornelas-Paz, J.; Salas-Marina, M.A. Postharvest Technology of Perishable Horticultural Commodities. In Preharvest Factors Affecting Postharvest Quality; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 99–128. [Google Scholar]
- Adamuchio-Oliveira, L.G.; Mazaro, S.M.; Mógor, G.; Sant’Anna-Santos, B.F.; Mógor, Á.F. Chitosan associated with chelated copper applied on tomatoes: Enzymatic and anatomical changes related to plant defense responses. Sci. Hortic. 2020, 271, 109431. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals from algae and cyanobacteria. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–89. [Google Scholar]
- Zainan, N.H.; Sapardi, M.A.M.; Ho, B.C.H.; Siajam, S.I.; Kamal, S.M.M.; Danquah, M.K.; Harun, R. Kinetic and thermodynamic characterization of amino acids generation via subcritical water reaction of microalgae Nannochloropsis spp. biomass. Biomass Convers. Biorefinery 2022, 12, 2001–2014. [Google Scholar] [CrossRef]
- Bulut, O.; Akın, D.; Sönmez, Ç.; Öktem, A.; Yücel, M.; Öktem, H.A. Phenolic compounds, carotenoids, and antioxidant capacities of a thermo-tolerant Scenedesmus spp. (Chlorophyta) extracted with different solvents. J. Appl. Phycol. 2019, 31, 1675–1683. [Google Scholar] [CrossRef]
- Haoujar, I.; Cacciola, F.; Abrini, J.; Mangraviti, D.; Giuffrida, D.; El Majdoub, Y.O.; Kounnoun, A.; Miceli, N.; Taviano, M.F.; Mondello, L.; et al. The contribution of carotenoids, phenolic compounds, and flavonoids to the antioxidative properties of marine microalgae isolated from Mediterranean Morocco. Molecules 2019, 24, 4037. [Google Scholar] [CrossRef]
- Varia, J.; Kamaleson, C.; Lerer, L. Biostimulation with phycocyanin-rich Spirulina extract in hydroponic vertical farming. Sci. Hortic. 2022, 299, 111042. [Google Scholar] [CrossRef]
- Metwally, R.A.; Abdelhameed, R.E.; Soliman, S.A.; Al-Badwy, A.H. Potential use of beneficial fungal microorganisms and C-phycocyanin extract for enhancing seed germination, seedling growth and biochemical traits of Solanum lycopersicum L. BMC Microbiol. 2022, 22, 108. [Google Scholar] [CrossRef]
- Gupta, S.; Doležal, K.; Kulkarni, M.G.; Balázs, E.; Van Staden, J. Role of non-microbial biostimulants in regulation of seed germination and seedling establishment. Plant Growth Regul. 2022, 97, 271–313. [Google Scholar] [CrossRef]
- Sharma, H.; Fleming, C.; Selby, C.; Rao, J.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Arahou, F.; Lijassi, I.; Wahby, A.; Rhazi, L.; Arahou, M.; Wahby, I. Spirulina-based biostimulants for sustainable agriculture: Yield improvement and market trends. BioEnergy Res. 2022, 15, 1401–1416. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multi-functional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- Fernández, V.; Sotiropoulos, T.; Brown, P.H. Foliar fertilization: Scientific principles and field pratices. In International Fertilizer Industry Association; IFA: Paris, France, 2013. [Google Scholar]
- Pandey, R.; Krishnapriya, V.; Bindraban, P.S. Biochemical nutrient pathways in plants applied as foliar spray: Phosphorus and iron. VFRC Rep. 2013, 1, 6–60. [Google Scholar]
- Zou, Y.; Zeng, Q.; Li, H.; Liu, H.; Lu, Q. Emerging technologies of algae-based wastewater remediation for bio-fertilizer production: A promising pathway to sustainable agriculture. J. Chem. Technol. Biotechnol. 2021, 96, 551–563. [Google Scholar] [CrossRef]
- Pandey, A.; Srivastava, S.; Kumar, S. Development and cost-benefit analysis of a novel process for biofuel production from microalgae using pre-treated high-strength fresh cheese whey wastewater. Environ. Sci. Pollut. Res. 2020, 27, 23963–23980. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Koutra, E.; Kornaros, M.; Farhadian, O.; Bhatnagar, A. Sequential cultivation of microalgae in raw and recycled dairy wastewater: Microalgal growth, wastewater treatment and biochemical composition. Bioresour. Technol. 2019, 273, 556–564. [Google Scholar] [CrossRef]
- Sankaran, K.; Premalatha, M.; Vijayasekaran, M.; Somasundaram, V.T. DEPHY project: Distillery wastewater treatment through anaerobic digestion and phycoremediation—A green industrial approach. Renew. Sustain. Energy Rev. 2014, 37, 634–643. [Google Scholar] [CrossRef]
- Hariz, H.B.; Takriff, M.S.; Mohd Yasin, N.H.; Ba-Abbad, M.M.; Mohd Hakimi, N.I.N. Potential of the microalgae-based integrated wastewater treatment and CO2 fixation system to treat Palm Oil Mill Effluent (POME) by indigenous microalgae; Scenedesmus sp.; Chlorella sp. J. Water Proc. Eng. 2019, 32, 100907. [Google Scholar] [CrossRef]
- García, D.; de Godos, I.; Domínguez, C.; Turiel, S.; Bolado, S.; Muñoz, R. A systematic comparison of the potential of microalgae-bacteria and purple phototrophic bacteria consortia for the treatment of piggery wastewater. Bioresour. Technol. 2019, 276, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Ummalyma, S.B.; Chiang, A.; Herojit, N.; Arumugam, M. Sustainable microalgal cultivation in poultry slaughterhouse wastewater for biorefinery products and pollutant removal. Bioresour. Technol. 2023, 374, 128790. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tan, X.; Li, D.; Chu, H.; Zhou, X.; Zhang, Y.; Yu, H. Nutrients removal and lipids production by Chlorella pyrenoidosa cultivation using anaerobic digested starch wastewater and alcohol wastewater. Bioresour. Technol. 2015, 181, 54–61. [Google Scholar] [CrossRef]
- Singh, R.; Birru, R.; Sibi, G. Nutrient removal efficiencies of Chlorella vulgaris from urban wastewater for reduced eutrophication. J. Environ. Prot. 2017, 8, 1. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Hu, B.; Min, M.; Chen, P.; Ruan, R.R. Integration of algae cultivation as biodiesel production feedstock with municipal wastewater treatment: Strains screening and significance evaluation of environmental factors. Bioresour. Technol. 2011, 102, 10861–10867. [Google Scholar] [CrossRef]
- Dhandwal, A.; Bashir, O.; Malik, T.; Salve, R.V.; Dash, K.K.; Amin, T.; Wani, A.W.; Shah, Y.A. Sustainable microalgal biomass as a potential functional food and its applications in food industry: A comprehensive review. Environ. Sci. Pollut. Res. 2024, 1–19. [Google Scholar] [CrossRef]
- Renuka, N.; Prasanna, R.; Sood, A.; Ahluwalia, A.S.; Bansal, R.; Babu, S.; Singh, R.; Shivay, Y.S.; Nain, L. Exploring the efficacy of wastewater-grown microalgal biomass as a biofertilizer for wheat. Environ. Sci. Pollut. Res. Int. 2016, 23, 6608–6620. [Google Scholar] [CrossRef]
- Sasi, P.K.C.; Viswanathan, A.; Mechery, J.; Thomas, D.M.; Jacob, J.P.; Paulose, S.V. Phycoremediation of paper and pulp mill effluent using plankto Chlorella nurekis and Chlamydomonas reinhardtii—A comparative study. J. Environ. Treat. Tech. 2020, 8, 809–817. [Google Scholar]
- Sisman-Aydin, G. Comparative study on phycoremediation performance of three native microalgae for primary-treated municipal wastewater. Environ. Technol. Innov. 2022, 28, 102932. [Google Scholar]
- El-Moustaqim, K.; El Bakraoui, H.; Mabrouki, J.; Fouad, Y.; Slaoui, M.; Hmouni, D.; Benyeogor, M.S.; Igbigbi, T.L. Combination of microalgae method, decantation, and filtration for domestic wastewater treatment. Sustainability 2023, 15, 16110. [Google Scholar] [CrossRef]
- Singh, D.V.; Bhat, R.A.; Upadhyay, A.K.; Singh, R.; Singh, D.P. Microalgae in aquatic environs: A sustainable approach for remediation of heavy metals and emerging contaminants. Environ. Technol. Innov. 2021, 21, 101340. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Verma, P. An exploration of natural synergy using microalgae for the remediation of pharmaceuticals and xenobiotics in wastewater. Algal Res. 2022, 64, 102703. [Google Scholar] [CrossRef]
- Khakimova, N.; Maravić, N.; Davidović, P.; Blagojević, D.; Simeunović, J.; Pešić, V.; Šereš, Z.; Mandić, A.; Pojić, M.; Mišan, A. Sugar beet processing wastewater treatment by microalgae through biosorption. Water 2021, 14, 860. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kuo, E.W.; Nagarajan, D.; Ho, S.H.; Dong, C.D.; Lee, D.J.; Chang, J.S. Cultivating Chlorella sorokiniana AK-1 with swine wastewater for simultaneous wastewater treatment and algal biomass production. Bioresour. Technol. 2020, 302, 122814. [Google Scholar] [CrossRef]
- Javed, F.; Rehman, F.; Khan, A.U.; Fazal, T.; Hafeez, A.; Rashid, N. Real textile industrial wastewater treatment and biodiesel production using microalgae. Biomass Bioenergy 2022, 165, 106559. [Google Scholar] [CrossRef]
- Spennati, E.; Casazza, A.A.; Converti, A. Winery wastewater treatment by microalgae to produce low-cost biomass for energy production purposes. Energies 2019, 13, 2490. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Asraful Alam, M.; Mehmood, M.A. Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef]
- Madadi, R.; Zahed, M.A.; Pourbabaee, A.A.; Tabatabaei, M.; Naghavi, M.R. Simultaneous phycoremediation of petrochemical wastewater and lipid production by Chlorella vulgaris. SN Appl. Sci. 2021, 3, 505. [Google Scholar] [CrossRef]
- Han, W.; Jin, W.; Li, Z.; Wei, Y.; He, Z.; Chen, C.; Tu, R.; Zhou, X. Cultivation of microalgae for lipid production using municipal wastewater. Process Saf. Environ. Prot. 2021, 155, 155–165. [Google Scholar] [CrossRef]
- Yuan, S.; Ye, S.; Yang, S.; Luo, G. Purification of potato wastewater and production of byproducts using microalgae Scenedesmus and Desmodesmus. J. Water Proc. Eng. 2021, 43, 102237. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef]
- Khalid, A.A.H.; Yaakob, Z.; Abdullah, S.R.S.; Takriff, M.S. Assessing the feasibility of microalgae cultivation in agricultural wastewater: The nutrient characteristics. Environ. Technol. Innov. 2019, 15, 100402. [Google Scholar] [CrossRef]
- Pathak, V.V.; Kothari, R.; Chopra, A.K.; Singh, D.P. Experimental and kinetic studies for phycoremediation and dye removal by Chlorella pyrenoidosa from textile wastewater. J. Environ. Manag. 2015, 163, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Ravindran, B.; Gupta, S.K.; Nasr, M.; Rawat, I.; Bux, F. Techno-economic estimation of wastewater phycoremediation and environmental benefits using Scenedesmus obliquus microalgae. J. Environ. Manag. 2019, 240, 293–302. [Google Scholar] [CrossRef]
- Guo, G.; Cao, W.; Sun, S.; Zhao, Y.; Hu, C. Nutrient removal and biogas upgrading by integrating fungal–microalgal cultivation with anaerobically digested swine wastewater treatment. J. Appl. Phycol. 2017, 29, 2857–2866. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef]
- Leite, L.D.S.; Hoffmann, M.T.; Daniel, L.A. Microalgae cultivation for municipal and piggery wastewater treatment in Brazil. J. Water Process Eng. 2019, 31, 100821. [Google Scholar] [CrossRef]
- Hu, X.; Meneses, Y.E.; Hassan, A.A. Integration of sodium hypochlorite pretreatment with co-immobilized microalgae/bacteria treatment of meat processing wastewater. Bioresour. Technol. 2020, 304, 122953. [Google Scholar] [CrossRef]
- Zhang, F.; Yue, Q.; Gao, Y.; Gao, B.; Xu, X.; Ren, Z.; Jin, Y. Application for oxytetracycline wastewater pretreatment by Fenton iron mud based cathodic-anodic-electrolysis ceramic granular fillers. Chemosphere 2017, 182, 483–490. [Google Scholar] [CrossRef]
- Fang, Y.; Lin, G.; Liu, Y.; Zhang, J. Contaminant removal performance and lipid productivity of a cyanobacteria-bacteria consortium containing exogenous phytohormones during the treatment of antibiotic-polluted wastewater. Chemosphere 2024, 361, 142473. [Google Scholar] [CrossRef] [PubMed]
- Aditya, L.; Mahlia, T.I.; Nguyen, L.N.; Vu, H.P.; Nghiem, L.D. Microalgae-bacteria consortium for wastewater treatment and biomass production. Sci. Total Environ. 2022, 838, 155871. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.L.; Pires, J.C.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lei, Z. Microalgal-bacterial aggregates for wastewater treatment: A mini-review. Bioresour. Technol. Rep. 2019, 8, 100199. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Binh, Q.A.; Bui, X.T.; Ngo, H.H.; Vo, H.N.P.; Lin, K.Y.A.; Vo, T.D.H.; Guo, W.; Lin, C.; Breider, F. Co-culture of microalgae-activated sludge for wastewater treatment and biomass production: Exploring their role under different inoculation ratios. Bioresour. Technol. 2020, 314, 123754. [Google Scholar] [CrossRef] [PubMed]
- Kok, Y.Y.; Chu, W.L.; Phang, S.M.; Mohamed, S.M.; Naidu, R.; Lai, P.J.; Khoo, A.S.B. Inhibitory activities of microalgal extracts against Epstein-Barr virus DNA release from lymphoblastoid cells. J. Zhejiang Univ. Sci. B 2011, 12, 335–345. [Google Scholar] [CrossRef]
- Wang, H.; Deng, L.; Qi, Z.; Wang, W. Constructed microalgal-bacterial symbiotic (MBS) system: Classification, performance, partnerships and perspectives. Sci. Total Environ. 2022, 803, 150082. [Google Scholar] [CrossRef] [PubMed]
- Larsdotter, K. Wastewater treatment with microalgae—A literature review. Vatten 2006, 62, 31. [Google Scholar]
- Gonçalves, A.L.; Pires, J.C.; Simões, M. The effects of light and temperature on microalgal growth and nutrient removal: An experimental and mathematical approach. RSC Adv. 2016, 6, 22896–22907. [Google Scholar] [CrossRef]
- Lee, K.; Lee, C.G. Effect of light/dark cycles on wastewater treatments by microalgae. Biotechnol. Bioprocess Eng. 2001, 6, 194–199. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, Y.; Han, S.H.; Hwang, S.J. The effects of wavelength and wavelength mixing ratios on microalgae growth and nitrogen, phosphorus removal using Scenedesmus sp. for wastewater treatment. Bioresour. Technol. 2013, 130, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Bermudez, S.P.; Aleman-Nava, G.S.; Chandra, R.; Garcia-Perez, J.S.; Contreras-Angulo, J.R.; Markou, G.; Muylaert, K.; Rittmann, B.E.; Parra-Saldivar, R. Nutrients utilization and contaminants removal. A review of two approaches of algae and cyanobacteria in wastewater. Algal Res. 2017, 24, 438–449. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater. Bioprocess Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef]
- Su, Y. Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Ganeshkumar, V.; Subashchandrabose, S.R.; Dharmarajan, R.; Venkateswarlu, K.; Naidu, R.; Megharaj, M. Use of mixed wastewaters from piggery and winery for nutrient removal and lipid production by Chlorella sp. MM3. Bioresour. Technol. 2018, 256, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Q. Microalgae-based nitrogen bioremediation. Algal Res. 2020, 46, 101775. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Perez, C.; Perales, J.A. Capability of different microalgae species for phytoremediation processes: Wastewater tertiary treatment, CO2 bio-fixation and low cost biofuels production. Water Res. 2014, 49, 465–474. [Google Scholar] [CrossRef]
- Osorio-Reyes, J.G.; Valenzuela-Amaro, H.M.; Pizaña-Aranda, J.J.P.; Ramírez-Gamboa, D.; Meléndez-Sánchez, E.R.; López-Arellanes, M.E.; Castañeda-Antonio, M.D.; Coronado-Apodaca, K.G.; Gomes Araújo, R.; Sosa-Hernández, J.E.; et al. Microalgae-Based Biotechnology as Alternative Biofertilizers for Soil Enhancement and Carbon Footprint Reduction: Advantages and Implications. Mar. Drugs 2023, 21, 93. [Google Scholar] [CrossRef]
- Stephenson, A.L.; Kazamia, E.; Dennis, J.S.; Howe, C.J.; Scott, S.A.; Smith, A.G. Life-cycle assessment of potential algal biodiesel production in the United Kingdom: A comparison of raceways and air-lift tubular bioreactors. Energy Fuels 2010, 24, 4062–4077. [Google Scholar] [CrossRef]
- Lundquist, T.J.; Woertz, I.C.; Quinn, N.W.T.; Benemann, J.R. A realistic technology and engineering assessment of algae biofuel production. Energy Biosci. Inst. 2010, 1, 1–178. [Google Scholar]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef]
- Amenorfenyo, D.K.; Huang, X.; Zhang, Y.; Zeng, Q.; Zhang, N.; Ren, J.; Huang, Q. Microalgae brewery wastewater treatment: Potentials, benefits and the challenges. Int. J. Environ. Res. Public Health 2019, 16, 1910. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Parisa, T.A.; Islam, N.; Kusumo, F.; Inayat, A.; Le, V.G.; Badruddin, I.A.; Khan, T.Y.; Ong, H.C. Progress and challenges of contaminate removal from wastewater using microalgae biomass. Chemosphere 2021, 286, 131656. [Google Scholar] [CrossRef] [PubMed]
- Banu, J.R.; Kavitha, S.; Gunasekaran, M.; Kumar, G. Microalgae based biorefinery promoting circular bioeconomy-techno economic and life-cycle analysis. Bioresour. Technol. 2020, 302, 122822. [Google Scholar]
- Ferreira, A.; Ribeiro, B.; Ferreira, A.F.; Tavares, M.L.; Vladic, J.; Vidović, S.; Cvetkovic, D.; Melkonyan, L.; Avetisova, G.; Goginyan, V.; et al. Scenedesmus obliquus microalga-based biorefinery–from brewery effluent to bioactive compounds, biofuels and biofertilizers–aiming at a circular bioeconomy. Biofuels Bioprod. Biorefining 2019, 13, 1169–1186. [Google Scholar] [CrossRef]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The role of microalgae in the bioeconomy. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef]
- Juárez, J.M.; Pastor, E.R.; Sevilla, J.M.F.; Torre, R.M.; García-Encina, P.A.; Rodríguez, S.B. Effect of pretreatments on biogas production from microalgae biomass grown in pig manure treatment plants. Bioresour. Technol. 2018, 257, 30–38. [Google Scholar] [CrossRef]
| Microalgae | Mode of Application | Plants | Outcomes | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Germination | Shoot/Root Length | Plant Biomass | Nutrient Content | Other Results | ||||
| Live cell suspensions or fresh biomass | ||||||||
| Anabaena laxa, Calothrix elenkinii | Seed treatment | Coriandrum sativum, Cuminum cyminum, Foeniculum vulgare | + | + | + | Increased peroxidase activity in shoots and roots and antifungal activities against Macrophomina phaseolina and Fusarium moniliforme | [31] | |
| Anabaena torulosa, Trichormus doliolum, A. laxa | Soil/root drench | Chrysanthemum morifolium | + | + | Enhanced leaf pigments, indole-3-acetic acid (IAA) production, and phosphoenolpyruvate (PEP) carboxylase activity | [33] | ||
| Chlorella fusca | Foliar spray | Spinacia oleracea | + | + | Increased plant yield, leaf width, thickness and number, and resistance to gray mold disease | [34] | ||
| Chlorella infusionum | Soil/root drench | Solanum lycopersicum | + | + | + | [35] | ||
| Chlorella sp.; Scenedesmus sp.; Synechocystis sp.; L. platensis | Soil/root drench | S. lycopersicum | + | + | + | + | Enhanced chlorophyll pigments and dissolved oxygen | [36] |
| Chlorella vulgaris | Soil/root drench | Hibiscus esculentus | + | + | Increased number of flower buds | [37] | ||
| C. vulgaris | Soil/root drench | Triticum aestivum L. | + | + | Increased plant growth, leaf area, and root hair production | [38] | ||
| Microcystis aeruginosa, Anabaena sp.; Chlorella sp. | Soil/root drench | Zea mays | + | + | Inhibited the growth of pathogenic bacteria and fungi | [39] | ||
| Dry biomass, cell extracts, or hydrolysates | ||||||||
| Asterarcys quadricellulare | Foliar spray | Solanum tuberosum | + | + | Increased yield, chlorophyll, amino acid, sugar, and nitrate reductase enzyme activity. | [13] | ||
| Tetradesmus dimorphus | Seed inoculation and foliar spray | S. lycopersicum | + | + | + | + | Increased number of flowers and branches | [32] |
| C. vulgaris | Foliar spray and root drench | Lactuca sativa L. | + | + | + | + | Increased leaf chlorophyll, carotenoid, and protein content | [40] |
| C. vulgaris, Limnospira platensis | Soil/root drench | Z. mays L. | + | Enhanced early seedling growth and improved yield characteristics | [41] | |||
| C. vulgaris and L. platensis | Soil/root drench | Allium cepa L. | + | + | + | Increased leaf numbers, area, and weight, neck thickness, bulb length, bulb diameters and bulb weight, and pigment contents Enhanced biochemical composition such as total soluble sugars, total phenols free amino acids, and total indoles | [42] | |
| C. vulgaris and L. platensis | Soil/root drench | Oryza sativa | + | + | Enhanced leaf number and area, number of seed/pod, weight of/100 seeds, and yield/pod Stimulated soil biological activity (dehydrogenase and nitrogenase) and increased chemical properties of soil (pH, EC, and available-NPK) | [43] | ||
| Chorococcum sp. | Seed treatment | Cucumis sativus, Solanum lycopersicum, Capsicum annuum, and Vigna radiata | + | + | Increased total protein, lipids, carbohydrate; phenolic compounds | [44] | ||
| Chlorococcum sp.; Micractinium sp.; Scenedesmus sp.; Chlorella sp. | Seed treatment | S. oleracea L. | + | + | + | Synthesis of cytokinins (trans-zeatin, DHZR, tZMP, iP, iPA, and iPAMP), gibberellins (GA1, GA3, GA4, GA20, and GA29), auxin (IAA), and abscisic acid (ABA) | [45] | |
| Nannochloropsis oculata | S. lycopersicum cv. Maxifort | + | + | + | Improved the fruit quality through an increase in sugar and carotenoid contents | [46] | ||
| Nostoc commune | Oryza sativa cv. Shiroodi L. | + | + | + | [47] | |||
| L. platensis | Raphanus sativus | + | + | + | Enhanced leaf pigments | [48] | ||
| L. platensis | Vigna mungo L. | + | + | + | + | [49] | ||
| Ulothrix sp.; Pinnularia sp.; and Oscillatoria sp. | S. lycopersicum, Capsicum annuum, Solanum melongena | + | + | + | Improved disease resistance | [50] | ||
| Microalgal Species | Metabolites | Targets Promoted | Reference |
|---|---|---|---|
| Auxin | |||
| Ankistrodesmus falcatus | Indole-3-acetic acid (IAA), | Biomass, carbohydrates, carotenoids, lipids, and protein content | [83] |
| C. fusca, C. vulgaris, Scenedesmus obliquus, Synechococcus nidulans, L. platensis LEB 18 | IAA | Protein and carbohydrates | [84] |
| Desmodesmus sp. | IAA, indole-3-butyric acid (IBA), indole-3-pyruvic acid (IPA) | Biomass, lipids, and fatty acids | [85] |
| N. oculata | IAA | Cell division and chlorophyll a | [86] |
| S. obliquusi, Pilidiocystis multispora, C. vulgaris | IAA | Growth and polyunsaturated fatty acids (PUFAs) | [87] |
| Scenedesmus quadricauda | Auxins | Cell divisions, growth, biomass, chlorophyll, carotenoids, fatty acids | [88] |
| Scenedesmus sp.; Chlorella sorokiniana | IBA | Lipids | [89] |
| Cytokinin | |||
| Tetradesmus obliquus | Kinetin, zeatin | Biomass, carbohydrates, and lipids | [90] |
| C. fusca, C. vulgaris, S. obliquus, S. nidulans, L. platensis LEB 18 | Trans-zeatin | Protein and carbohydrates | [84] |
| Auxenochlorella protothecoides | Cytokinin | Biomass and lipids | [91] |
| C. vulgaris | Benzyladenine, trans-zeatin, 2-methylthio-trans-zeatin | α-Linolenic, linoleic, palmitic, oleic, and stearic acids | [92] |
| Desmodesmus sp. | 6-benzylaminopurine, Thidiazuron | Biomass, lipids, and fatty acids | [85] |
| Nostoc muscorum | Kinetin | Biomass and carotenoids | [93] |
| Gibberellic acid | |||
| Chlorella ellipsoidea | Gibberellic acid (GA) | Growth and lipids | [94] |
| Auxenochlorella pyrenoidosa | GA3 | Growth and lipids | [95] |
| Isochrysis galbana | GA3 | Biomass, chlorophyll a, protein, lipid, and PUFAs | [96] |
| Monodopsis subterranea | GA | Biomass, total fatty acids, and eicosapentaenoic acid | [97] |
| N. oculata | GA | Cell diameter and lipids | [86] |
| Ethylene | |||
| C. vulgaris | Ethephon | Saturated fatty acids (SFAs), a-tocopherol, c-aminobutyric acid, asparagine, and proline | [98] |
| Haematococcus lacustris | 1-Aminocyclopropane-1-carboxylic acid (ACC) | Astaxanthin | [99] |
| H. lacustris | Ethylene | Astaxanthin and lipid | [100] |
| Monoraphidium sp. | Ethylene | Lipids | [101] |
| Abscisic acid | |||
| A. pyrenoidosa | Abscisic acid (ABA) | Lipids | [102] |
| C. vulgaris | ABA | Biomass and total fatty acids | [103] |
| C. vulgaris | ABA | Fatty acids | [92] |
| Chromochloris zofingiensis | ABA | Growth, fatty acids, pigmentation | [104] |
| Salicylic acid | |||
| Chlorella sp. | Salicylic acid (SA) | Cell growth | [105] |
| C. zofingiensis | SA | Cell growth, total fatty acids, and astaxanthin | [106] |
| H. lacustris | SA | Biomass and astaxanthin | [107] |
| Jasmonic acid | |||
| H. lacustris | Methyl jasmonate (MJ) | β-Carotene and lutein | [107] |
| M. subterranea | MJ | Biomass, total fatty acids, and eicosapentaenoic acid | [97] |
| Stauroneis sp. | MJ | Lipids and pigments | [108] |
| Microalgal Species | Wastewater | Biomass | Promoted Plant Growth Parameters and Soil Properties | Reference | |||
|---|---|---|---|---|---|---|---|
| Culture Medium | Characteristics | Removal Efficiency | Production | Composition | |||
| Chlorella minutissima, Nostoc muscorum, Scendesmus sp., and Scendesmus consortium | Domestic wastewater | EC: 3.14 dS m−1, NH4+: 39.5 mg L−1, NO3−: 2.38 mg L−1, P: 3.68 mg L−1, COD: 149.75 mg L−1, BOD5: 99.5 mg L−1, TDS: 2196 mg L−1 | NH4: 92%, NO3: 87%, PO43−: 85%, COD: 81%, BOD5: 90%, TDS: 96% | 0.14–0.45 g L−1 | N: 2–6%, P: 0.5–1%, K: <0.5%, LP: 11.33–81.23 mg L−1 | - | [29] |
| C. minutissima | Domestic wastewater | EC: 3.52 dS m−1, NH4+: 5.60 mg L−1, NO3−: 3.06 mg L−1, P: 3.54 mg L−1, K: 5.50 mg L−1, COD: 157 mg L−1, BOD5: 114 mg L−1, DO: 3.50 mg L−1, TDS: 2416 mg L−1 | EC: 92.9%, NH4+: 48.2%, NO3−: 88.9%, P: 67.5%, K: 66.4%, COD: 80.5%, BOD5: 93.2%, TDS: 94.4% | 1.26 ± 0.07 g L−1 FW and 0.44 ± 0.04 g L−1 DW | N: 6.0%, P: 1.0%, K: 0.48% | Leaf length, leaf and root biomass in spinach. Yields with and without husk and cob length in baby. Soil organic carbon, nitrogen and phosphorous. Dehydrogenase, urease, and nitrate reductase activity. | [10] |
| Chlorella sp., Scendesmus sp., and Scendesmus consortium | Domestic wastewater | TN: 61.47 mg L−1, NH4+: 37.64 mg L−1, NO3−: 16.58 mg L−1, P: 7.42 mg L−1, COD: 446.25 mg L−1, TOC: 208.15 mg L−1 | TN: 85–94%, NH4+: 95–98%, NO3−: 84–96%, P: 89–95%, COD: 78–88% TOC: 81–86% | 1.78 g L−1 | N: 7.21–7.81%, P: 1.55–1.72%, K: 0.75–1.06%, Ca: 0.21–0.28%, Na: 1.08–1.18%, Mg: 0.11–0.17%, S: 0.21–0.27% Fe: 0.30–0.36% Chlorophyll: 27.03 μg mL−1 Protein: 175 μg mL−1 Lipid: 34.83% dry cell weight | Shoot and root length, fresh and dry weight, and yields of tomato. Increased macro (N, P, K, Ca) and micro-nutrients (Mg, Fe) of tomato. | [30] |
| Scenedesmus sp. | Domestic wastewater | NH4+: 38.6 mg L−1, NO3−: 17.1 mg L−1, PO43−: 9.24 mg L−1, COD: 142.2 mg L−1 | - | 0.68 g L−1 | N: 7.45%, P: 1.6%, K: 0.7%, S: 0.3%, Na: 1.41%, Ca: 0.14%, Mg: 0.12%, Fe: 0.3%, Mn: 210 ppm, Cu: 6.8 ppm, Zn: 34 ppm, Lipid: 24.1% | Plant height, root weight, and yields in rice. Increased NPK content in grain and straw of rice. | [24] |
| Chlorella sp., Scenedesmus sp., Chlorococcum sp., Chroococcus sp. | Domestic wastewater | - | - | - | - | Plant fresh and dry weight, root length, spike and grain weight, and nutrient contents (NPK) of wheat. Increased soil nutrients NPK Higher acetylene-reducing activity. | [174] |
| Phormidium sp., Anabaena sp., Westiellopsis sp., Fischerella sp., Spirogyra sp. | |||||||
| Scenedesmus sp.; Chlorella vulgaris | Dairy cattle wastewater | TOC: 623.3 mg L−1, DOC: 361.7 mg L−1, NH3: 141.8 mg L−1, TKN: 174 mg L−1, TP: 1144.1 mg L−1, SP: 629.4 mg L−1, VSS: 623.9 mg L−1, TSS: 729.9 mg L−1, tCOD: 3106.3 mg L−1, sCOD: 1015 mg L−1 | TOC: 83.9%, DOC: 82.4%, NH3−: 99.8%, TKN: 78.4%, TP: 53.2%, SP: 66.9%, VSS: 57.4%, TSS: 55.7%, tCOD: 35.4%, sCOD: 55.7% | 7.1 gm−2 day−1 | TP: 1992 mg L−1, TKN: 1657.4 mg L−1, Cu: 0.53 mg L−1, B: 0.55 mg L−1, Mo: <0.05 mg L−1, Zn: 0.005 mg L−1 | Pasture yield, dry matter, ash, nutrients (P, Ca, Zn, Mn, B). | [9] |
| Chlorella pyrenoidosa | Paddy-soaked rice mill wastewater | NH4+: 147- 154 mg L−1, PO4: 67–70 mg L−1, S:30–38 mg L−1, C: 640–760 mg L−1, TS: 4554–4640 mg L−1, SS: 106–160 mg L−1, DS: 4448–4480 mg L−1, COD: 960–1280 mg L−1, BOD: 680–851 mg L−1 | NH4+: 69.39%, PO4: 64.76% | 0.11 g L−1 d−1 | Carbohydrate: 8.84%, lipid: 32.12%, protein: 34.15% | Seed germination, chlorophyll, fresh shoot and root weight, dry shoot and root weight, and root-to-shoot biomass ratio of okra. | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renganathan, P.; Gaysina, L.A.; Holguín-Peña, R.J.; Sainz-Hernández, J.C.; Ortega-García, J.; Rueda-Puente, E.O. Phycoremediated Microalgae and Cyanobacteria Biomass as Biofertilizer for Sustainable Agriculture: A Holistic Biorefinery Approach to Promote Circular Bioeconomy. Biomass 2024, 4, 1047-1077. https://doi.org/10.3390/biomass4040059
Renganathan P, Gaysina LA, Holguín-Peña RJ, Sainz-Hernández JC, Ortega-García J, Rueda-Puente EO. Phycoremediated Microalgae and Cyanobacteria Biomass as Biofertilizer for Sustainable Agriculture: A Holistic Biorefinery Approach to Promote Circular Bioeconomy. Biomass. 2024; 4(4):1047-1077. https://doi.org/10.3390/biomass4040059
Chicago/Turabian StyleRenganathan, Prabhaharan, Lira A. Gaysina, Ramón Jaime Holguín-Peña, Juan Carlos Sainz-Hernández, Jesus Ortega-García, and Edgar Omar Rueda-Puente. 2024. "Phycoremediated Microalgae and Cyanobacteria Biomass as Biofertilizer for Sustainable Agriculture: A Holistic Biorefinery Approach to Promote Circular Bioeconomy" Biomass 4, no. 4: 1047-1077. https://doi.org/10.3390/biomass4040059
APA StyleRenganathan, P., Gaysina, L. A., Holguín-Peña, R. J., Sainz-Hernández, J. C., Ortega-García, J., & Rueda-Puente, E. O. (2024). Phycoremediated Microalgae and Cyanobacteria Biomass as Biofertilizer for Sustainable Agriculture: A Holistic Biorefinery Approach to Promote Circular Bioeconomy. Biomass, 4(4), 1047-1077. https://doi.org/10.3390/biomass4040059









