Potential of Combined Yeast Culture and Enzymatically Hydrolysed Yeast to Improve In Vitro Dry Matter and Nutrient Degradability of Different Feedstuffs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Analysis of Chemical Composition
2.2.1. In Vitro Ruminal DM and Nutrient Digestibility
2.2.2. In Vitro Ruminal CP Degradability
2.3. Experimental Design and Statistical Analysis
3. Results
3.1. Chemical Composition of Substrates
3.2. In Vitro Ruminal Dry Matter and Nutrient Digestibility
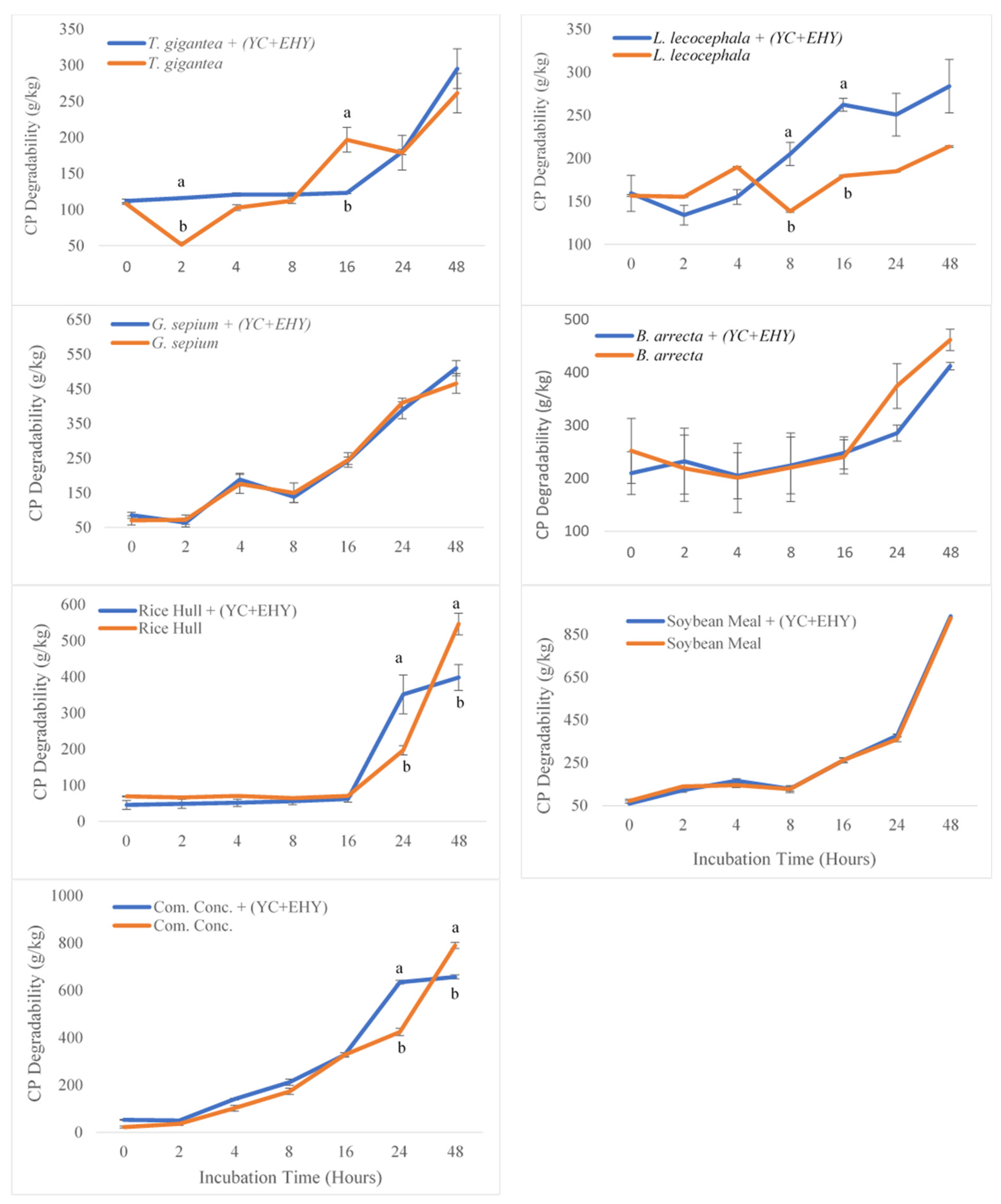
3.3. In Vitro Ruminal Crude Protein Degradability
4. Discussion
4.1. Chemical Composition of Substrates
4.2. In Vitro Ruminal Dry Matter and Nutrient Degradability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, M.; Mlambo, V.; Lallo, O.H.C. Low nitrogen fertilizer rates and stage of maturity influence nitrogen fractionation and in vitro ruminal nitrogen degradability in tropical grasses. Afr. J. Range Forage Sci. 2021, 39, 292–297. [Google Scholar] [CrossRef]
- Smit, H.P.J.; Reinsch, T.; Swanepoel, P.A.; Loges, R.; Kluß, C.; Taube, F. Environmental Impact of Rotationally Grazed Pastures at Different Management Intensities in South Africa. Animals 2021, 11, 1214. [Google Scholar] [CrossRef] [PubMed]
- Leon, E.; Hughes, M.P.; Daley, O. Brachiaria Hybrid and Pennisetum purpureum Supplemented with Pueraria phaseoloides Increased the Concentration of Rumen Undegradable Protein in Forages for Ruminants. Grasses 2023, 2, 207–217. [Google Scholar] [CrossRef]
- Jayasinghe, P.; Ramilan, T.; Donaghy, D.J.; Pembleton, K.G.; Barber, D.G. Comparison of Nutritive Values of Tropical Pasture Species Grown in Different Environments, and Implications for Livestock Methane Production: A Meta-Analysis. Animals 2022, 12, 1806. [Google Scholar] [CrossRef] [PubMed]
- Tadele, Y.; Negassie, A. Use of different non-protein nitrogen sources in ruminant nutrition: A review. Adv. Life Sci. Technol. 2015, 29, 100–105. [Google Scholar]
- Habeeb, A.A.M. Current view of the significance of yeast for ruminants a review 1-role of yeast and modes of action. Am. J. Inf. Sci. Technol. 2017, 1, 8–14. [Google Scholar]
- Ghazanfar, S.; Riaz, A.; Ali, G.M.; Naveed, S.; Arif, I.; Irshad, S.; Riaz, N.; Manzoor, K.N. Common Methods to Understand and Develop Indigenous Probiotics Yeast for Ruminant. In Yeasts in Biotechnology; Basso, T.P., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Denev, S.A.; Peeva, T.; Radulova, P.; Stancheva, N.; Staykova, G.; Todorova, P.B.; Desnoyers, S. Yeast Cultures in Ruminant Nutrition. Bulg. J. Agric. Sci. 2007, 13, 357–374. [Google Scholar]
- Desnoyers, M.; Giger-Reverdin, S.; Bertin, S.; Duvaux-Ponter, C.; Sauvant, D. Meta-analysis of the influence of Saccharomyces cerevisiae supplementation on ruminal parameters and milk production of ruminants. J. Dairy Sci. 2009, 92, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Nde, F.F.; Nsahlai, I.V.; Chimonyo, M.; Mawahib, A.A. Effect of Celmanax on feed intake, live weight gain and nematode control in growing sheep. Afr. J. Agric. Res. 2014, 9, 695–700. [Google Scholar]
- Cosme, F.; Inês, A.; Vilela, A. Microbial and Commercial Enzymes Applied in the Beverage Production Process. Fermentation 2023, 9, 385. [Google Scholar] [CrossRef]
- Gunun, N.; Sanjun, I.; Kaewpila, C.; Foiklang, S.; Cherdthong, A.; Wanapat, M.; Polyorach, S. Effect of dietary supplementation of hydrolyzed yeast on growth performance, digestibility, rumen fermentation, and hematology in growing beef cattle. Animals 2022, 12, 2473. [Google Scholar] [CrossRef] [PubMed]
- Papatsiros, V.G.; Panagiotis-Dimitrios, K.; Koutoulis, C.K.; Karatzia, M.; Dedousi, A.; Christodoulopoulos, C. Alternatives to antibiotics for farm animals. CABI Rev. 2013, 8, 1–15. [Google Scholar] [CrossRef]
- Baker, L.M.; Kraft, J.; Karnezos, P.T.; Greenwood, L.S. The effects of dietary yeast and yeast-derived extracts on rumen microbiota and their function. Anim. Feed Sci. Technol. 2022, 294, 115476. [Google Scholar] [CrossRef]
- Froebel, K.L.; Froebel, E.L.; Duong, T. Refined functional carbohydrates reduce adhesion of Salmonella and Campylobacter to poultry epithelial cells in vitro. Poult. Sci. 2020, 99, 7027–7034. [Google Scholar] [CrossRef] [PubMed]
- Sookrali, A.; Hughes, M. Influence of combined yeast culture and enzymatically hydrolyzed yeast on in vitro ruminal fermentation in contrasting feed substrates. J. Sci. Food Agric. 2021, 102, 3628–3635. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Chavira, J.; Montano, M.F.; Torrentera, N.; Zinn, R.A. Influence of feeding enzymatically hydrolysed yeast cell wall + yeast culture on growth performance of calf-fed Holstein steers. J. Appl. Anim. Res. 2018, 46, 327–330. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Analytical Chemists International: Arlington, VA, USA, 2005. [Google Scholar]
- Van Soest, J.P.; Robertson, B.J.; Lewis, A.B. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- National Research Institute. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. Available online: http://oacu.od.nih.gov/regs/guide (accessed on 24 May 2011).
- Kalogianni, A.I.; Moschovas, M.; Chrysanthakopoulou, F.; Lazou, T.; Theodorou, G.; Politis, I.; Bossis, I.; Gelasakis, A.I. The Effects of Replacing Soybean Meal with Rapeseed Meal, Cottonseed Cake, and Fava Beans on the Milk Yield and Quality Traits in Milking Ewes. Animals 2022, 12, 274. [Google Scholar] [CrossRef]
- Castro, S.I.B.; Phillip, E.L.; Lapierre, H.; Jardon, W.P.; Berthiaume, R. Ruminal degradability and intestinal digestibility of protein and amino acids in treated soybean meal products. J. Dairy Sci. 2007, 90, 810–822. [Google Scholar] [CrossRef]
- Parrini, S.; Aquilani, C.; Pugliese, C.; Bozzi, R.; Sirtori, F. Soybean Replacement by Alternative Protein Sources in Pig Nutrition and Its Effect on Meat Quality. Animals 2023, 13, 494. [Google Scholar] [CrossRef]
- De Angelis, A.; Gasco, L.; Parisi, G.; Danieli, P.P. A Multipurpose Leguminous Plant for the Mediterranean Countries: Leucaena leucocephala as an Alternative Protein Source: A Review. Animals 2021, 11, 2230. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Abd El-Hack, E.M.; Ragni, M. The Efficacy of High-Protein Tropical Forages as Alternative Protein Sources for Chickens: A Review. Agriculture 2018, 8, 86. [Google Scholar] [CrossRef]
- Tefera, S.; Mlambo, V.; Dlamini, J.B.; Dlamini, M.A.; Koralagama, N.D.K.; Mould, L.F. Chemical composition and in vitro ruminal fermentation of common tree forages in the semi-arid rangelands of Swaziland. Anim. Feed Sci. Technol. 2008, 142, 99–110. [Google Scholar] [CrossRef]
- Nunes, H.P.B.; Teixeira, S.; Maduro Dias, C.S.A.M.; Borba, A.E.S. Alternative Forages as Roughage for Ruminant: Nutritional Characteristics and Digestibility of Six Exotic Plants in Azores Archipelago. Animals 2022, 12, 3587. [Google Scholar] [CrossRef]
- Neubauer, V.; Petri, R.; Humer, E.; Kröger, I.; Mann, E.; Reisinger, N.; Wagner, M.; Zebeli, Q. High-grain diets supplemented with phytogenic compounds or autolyzed yeast modulate ruminal bacterial community and fermentation in dry cows. J. Dairy Sci. 2018, 101, 2335–2349. [Google Scholar] [CrossRef]
- Yusiati, M.L.; Kurniawati, A.; Hanium, C.; Anas, A.M. Protein Binding Capacity of Different Forages Tannin. Conf. Ser. Earth Environ. Sci. 2018, 119, 012007. [Google Scholar] [CrossRef]
- Boontiam, W.; Bunchasak, C.; Kim, Y.Y.; Kitipongpysan, S.; Hong, J. Hydrolyzed Yeast Supplementation to Newly Weaned Piglets: Growth Performance, Gut Health, and Microbial Fermentation. Animals 2022, 12, 350. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Wang, H.; Shi, H.; Li, Q.; Zhang, Y.; Li, T.; Ma, Y. Yeast Products Mediated Ruminal Sub-Environmental Microbiota, and Abnormal Metabolites and Digestive Enzymes Regulated Rumen Fermentation Function in Sheep. Animals 2022, 12, 3221. [Google Scholar] [CrossRef] [PubMed]
- Promkot, C.; Wanapat, M. Ruminal degradation and intestinal digestion of crude protein of tropical protein resources using nylon bag technique and three-step in vitro procedure in dairy cattle. Livest. Res. Rural Dev. 2003, 15, 1–12. Available online: http://www.lrrd.org/lrrd15/11/prom1511.htm (accessed on 1 March 2024).
- Edwards, A.; Mlambo, V.; Lallo, O.H.C.; Garcia, W.G.; Diptee, M. In Vitro Ruminal Protein Degradability of Leaves from Three Tree Species Harvested at Two Cutting Intervals. Online J. Anim. Feed Res. 2012, 2, 224–230. [Google Scholar]
| Substrates | DM (g/kg) | Chemical Composition (g/kg DM) | ||||
|---|---|---|---|---|---|---|
| Ash | CP | NDF | ADF | Lignin | ||
| Com. Concentrate | 906 a | 93.0 de | 209 b | 378 d | 222 d | 40.0 de |
| G. sepium | 221 c | 101 cd | 204 b | 694 bc | 599 b | 139 c |
| L. leucocephala | 280 b | 64.0 f | 208 b | 771 ab | 644 b | 293 a |
| Rice hull | 898 a | 126 b | 67.9 e | 687 bc | 626 b | 179 b |
| Soybean meal | 890 a | 75.0 ef | 483 a | 210 e | 125 e | 10.0 e |
| B. arrecta | 158 d | 115 bc | 123 d | 837 a | 715 a | 71.0 d |
| T. gigantea | 160 d | 242 a | 153 c | 628 c | 542 c | 174 b |
| SEM | 3.70 | 3.50 | 1.30 | 9.90 | 11.5 | 5.40 |
| Significance (p-value) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Substrate | YC+EHY (+/−) | DM (g/kg) | 24 h In Vitro Digestibility (g/kg DM) | ||
|---|---|---|---|---|---|
| CP | NDF | ADF | |||
| Com. Concentrate | (+) | 571 b | 109 b | 129 fg | 27.0 fg |
| (−) | 607 b | 97.0 b | 130 fg | 25.0 fg | |
| G. sepium | (+) | 557 b | 59.0 c | 398 ab | 315 a |
| (−) | 560 b | 96.0 b | 402 a | 312 a | |
| L. lecocephala | (+) | 239 ef | 59.0 c | 302 cd | 162 cd |
| (−) | 233 ef | 54.0 c | 291 cd | 164 cd | |
| Rice hull | (+) | 215 ef | 17.0 ef | 120 fg | 79.0 ef |
| (−) | 271 de | 4.00 f | 162 ef | 103 de | |
| Soybean meal | (+) | 625 b | 199 a | 72 g | 50.0 g |
| (−) | 746 a | 217 a | 101 fg | 50.0 efg | |
| B. arrecta | (+) | 361 c | 47.0 cd | 315 bc | 297 a |
| (−) | 344 cd | 35.0 cde | 275 cd | 225 b | |
| T. gigantea | (+) | 239 ef | 34.0 cde | 271 cd | 191 bc |
| (−) | 172 f | 27.0 def | 229 de | 177 bc | |
| SEM | 14.6 | 4.40 | 14.9 | 9.80 | |
| Significance (p-value) | |||||
| Substrate | 0.000 | 0.000 | 0.000 | 0.000 | |
| YC+EHY | 0.043 | 0.728 | 0.797 | 0.666 | |
| Substrate × YC+EHY | 0.000 | 0.000 | 0.135 | 0.001 | |
| Substrate | YC+EHY | DM (g/kg) | 48 h In Vitro Digestibility (g/kg DM) | ||
|---|---|---|---|---|---|
| (+/−) | CP | NDF | ADF | ||
| Com. Concentrate | (+) | 719 b | 157 b | 201 b | 71.0 d |
| (−) | 714 b | 147 b | 192 b | 77.0 d | |
| G. sepium | (+) | 746 b | 166 b | 515 a | 419 a |
| (−) | 739 b | 162 b | 516 a | 408 ab | |
| L. lecocephala | (+) | 378 d | 71.0 c | 383 a | 237 c |
| (−) | 378 d | 63.0 cd | 391 a | 242 c | |
| Rice hull | (+) | 306 d | 38.0 d | 150 b | 108 d |
| (−) | 326 d | 34.0 d | 167 b | 126 d | |
| Soybean meal | (+) | 972 a | 450 a | 204 b | 123 d |
| (−) | 970 a | 446 a | 201 b | 112 d | |
| B. arrecta | (+) | 551 c | 83.0 c | 457 a | 386 ab |
| (−) | 531 c | 74.0 c | 444 a | 370 ab | |
| T. gigantea | (+) | 513 c | 57.0 cd | 412 a | 327 bc |
| (−) | 539 c | 62.0 cd | 401 a | 358 ab | |
| SEM | 19.6 | 5.9 | 24.9 | 15 | |
| Significance (p-value) | |||||
| Substrates | 0.000 | 0.000 | 0.000 | 0.000 | |
| YC+EHY | 0.872 | 0.171 | 0.936 | 0.740 | |
| Substrate × YC+EHY | 0.954 | 0.914 | 0.999 | 0.827 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sookrali, A.A.; Hughes, M.P. Potential of Combined Yeast Culture and Enzymatically Hydrolysed Yeast to Improve In Vitro Dry Matter and Nutrient Degradability of Different Feedstuffs. Ruminants 2024, 4, 352-361. https://doi.org/10.3390/ruminants4030025
Sookrali AA, Hughes MP. Potential of Combined Yeast Culture and Enzymatically Hydrolysed Yeast to Improve In Vitro Dry Matter and Nutrient Degradability of Different Feedstuffs. Ruminants. 2024; 4(3):352-361. https://doi.org/10.3390/ruminants4030025
Chicago/Turabian StyleSookrali, Alisha A., and Martin P. Hughes. 2024. "Potential of Combined Yeast Culture and Enzymatically Hydrolysed Yeast to Improve In Vitro Dry Matter and Nutrient Degradability of Different Feedstuffs" Ruminants 4, no. 3: 352-361. https://doi.org/10.3390/ruminants4030025
APA StyleSookrali, A. A., & Hughes, M. P. (2024). Potential of Combined Yeast Culture and Enzymatically Hydrolysed Yeast to Improve In Vitro Dry Matter and Nutrient Degradability of Different Feedstuffs. Ruminants, 4(3), 352-361. https://doi.org/10.3390/ruminants4030025






