Monthly Variation and Ultraviolet Stability of Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Samples
2.2. Extraction of Crude MAAs from Dulse
2.3. Spectrophotometric Analysis of MAAs
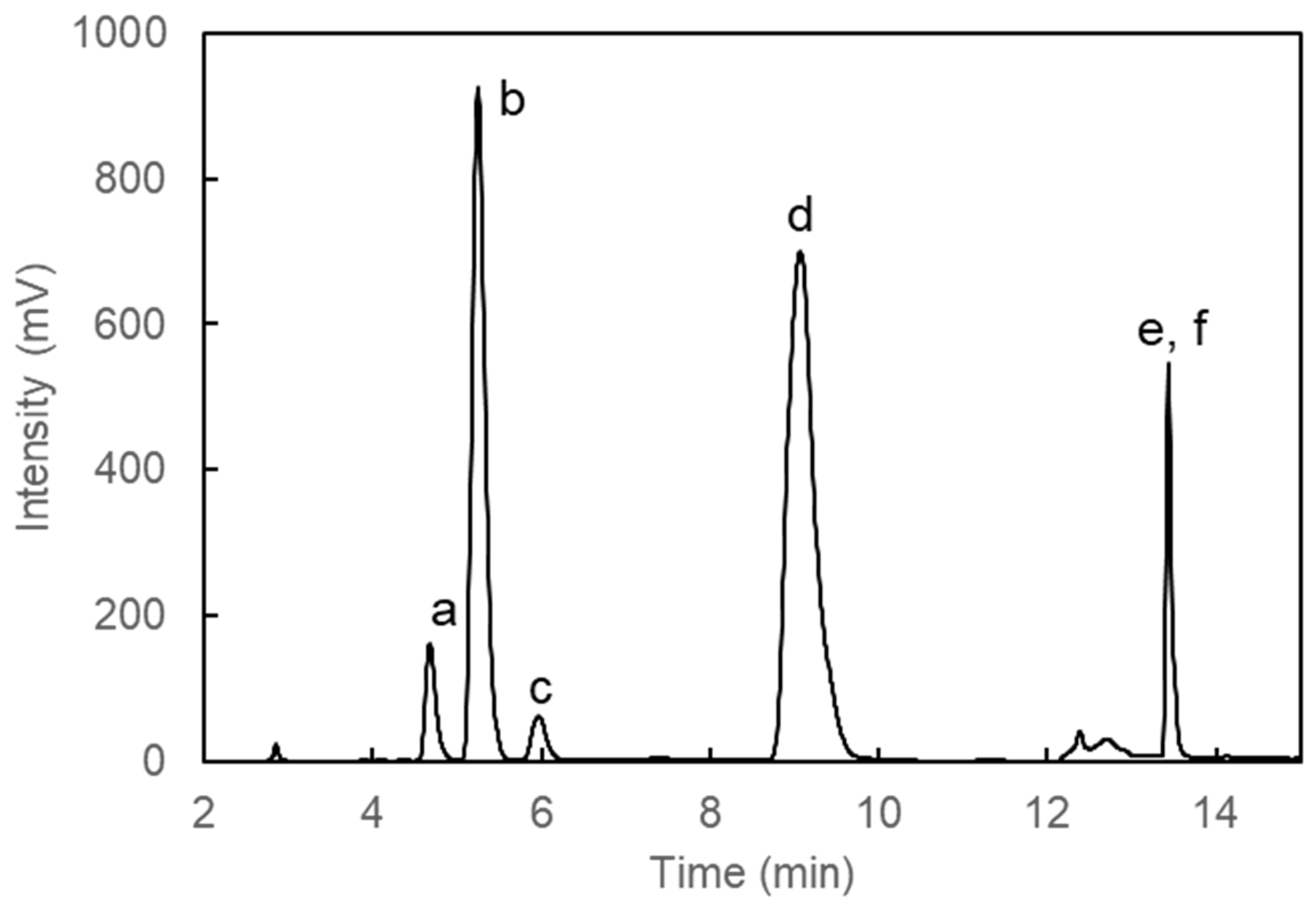
2.4. Separation of MAAs by High Performance Liquid Chromatography (HPLC)
2.5. Ultraviolet Stability
2.6. Phycoerythrin (PE) Content
2.7. Abiotic Data in Hakodate
2.8. Statistical Analysis
3. Results
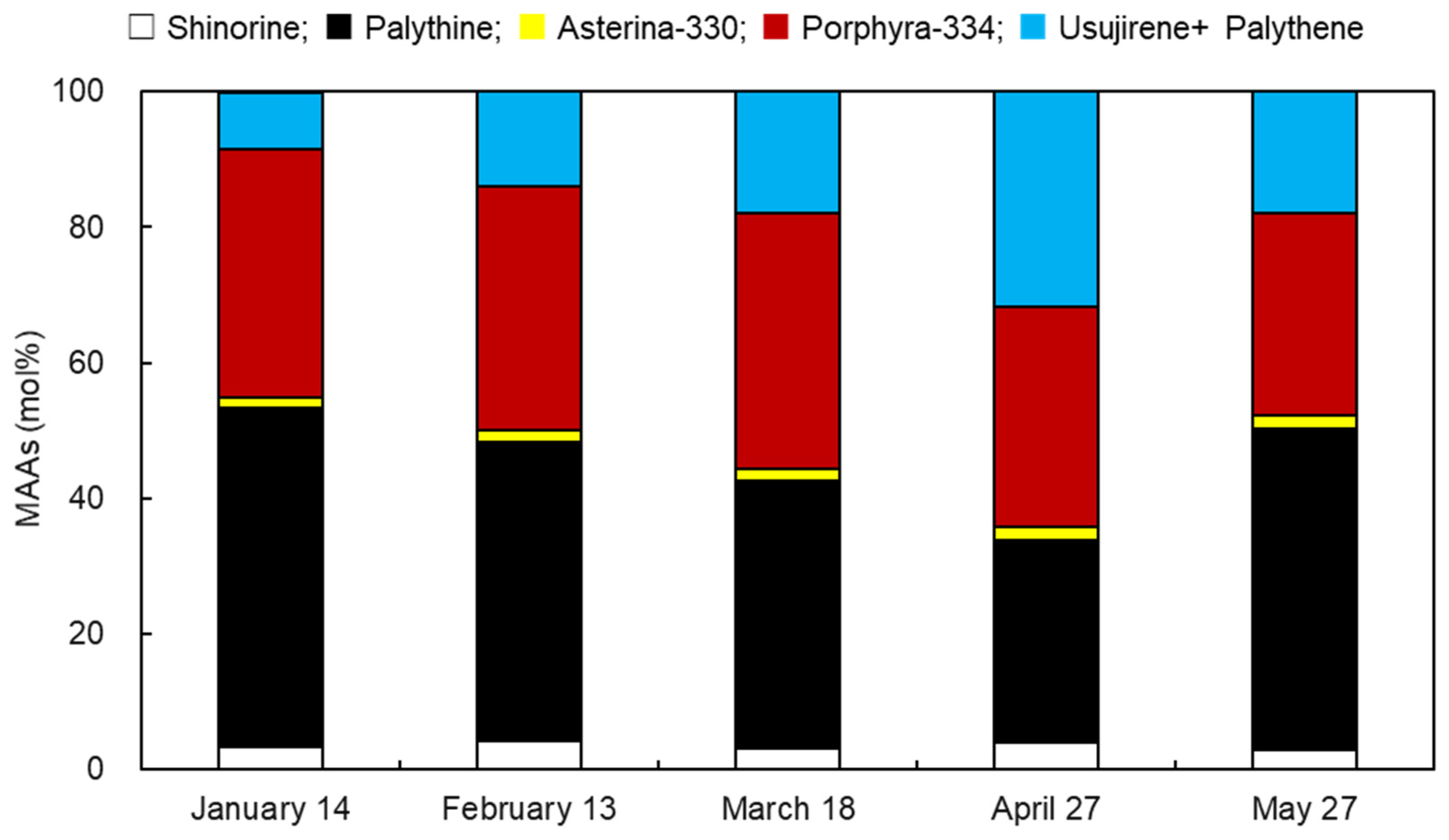
3.1. Monthly Variation of Usujiri Dulse MAAs in 2020
3.2. Monthly Variation of MAA Contents
3.3. Change of MAAs, PE and Erythemal UV Intensity
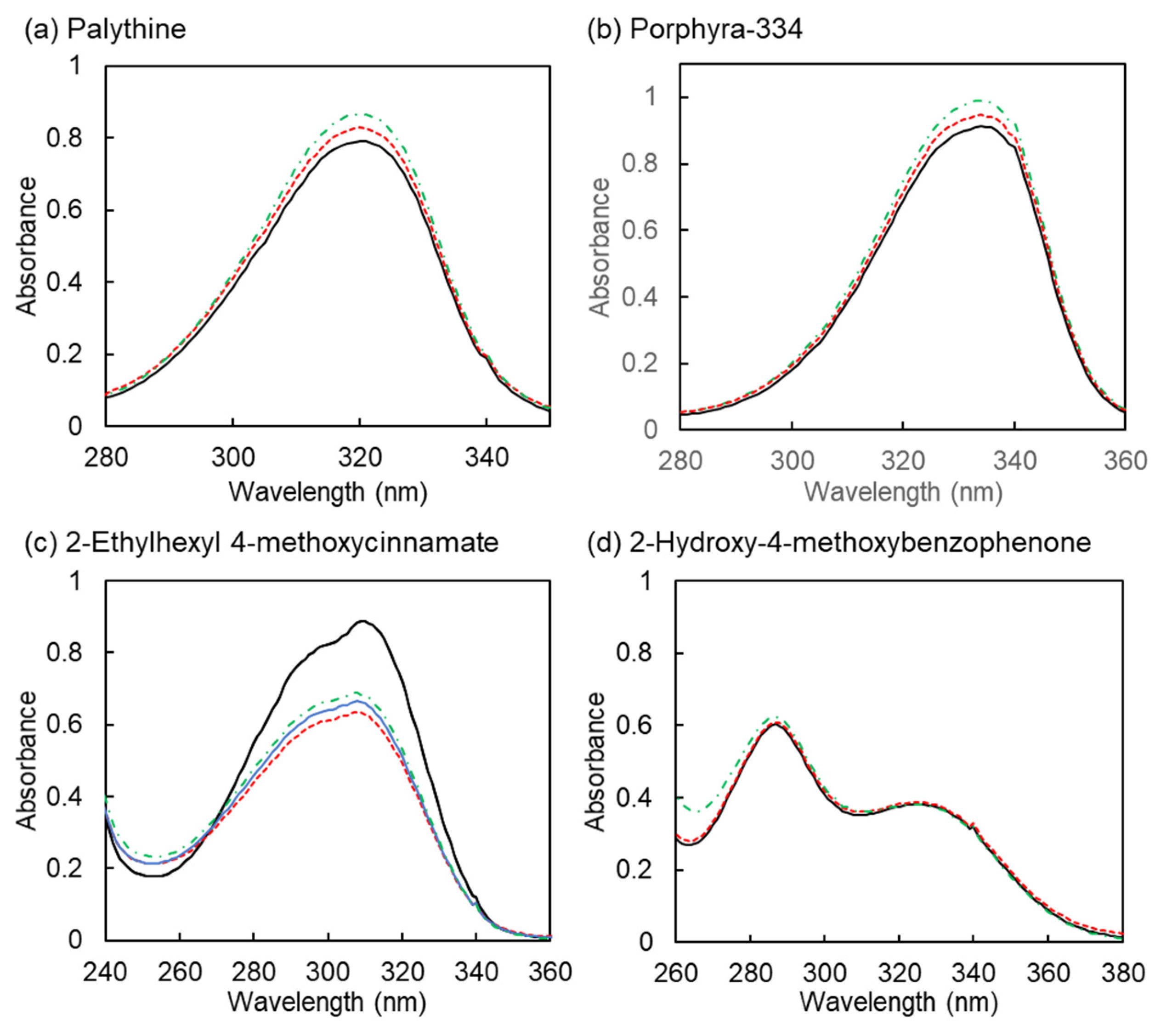
3.4. Stability of Ultraviolet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Rosenstein, B.S.; Wang, Y.; Lebwohl, M.; Wei, H. Identification of Possible Reactive Oxygen Species Involved in Ultraviolet Radiation-Induced Oxidative DNA Damage. Free Radic. Biol. Med. 1997, 23, 980–985. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Neale, R.E.; Khan, S.R.; Lucas, R.M.; Waterhouse, M.; Whiteman, D.C.; Olsen, C.M. The effect of sunscreen on vitamin D: A review. Br. J. Dermatol. 2019, 181, 907–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danno, K.; Horio, T.; Imamura, S. Infrared radiation suppresses ultraviolet B-induced sunburn-cell formation. Arch. Dermatol. Res. 1992, 284, 92–94. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ananthaswamy, H.N. Short-term and long-term cellular and molecular events following UV irradiation of skin: Implications for molecular medicine. Expert Rev. Mol. Med. 2002, 4, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R. Acute effects of UVR on human eyes and skin. Prog. Biophys. Mol. Biol. 2006, 92, 80–85. [Google Scholar] [CrossRef]
- Ma, H.; Brennan, A.; Diamond, S.A. Photocatalytic reactive oxygen species production and phototoxicity of titanium dioxide nanoparticles are dependent on the solar ultraviolet radiation spectrum. Environ. Toxicol. Chem. 2012, 31, 2099–2107. [Google Scholar] [CrossRef]
- Schauder, S.; Ippen, H. Contact and photocontact sensitivity to sunscreens. Contact Dermat. 1997, 37, 221–232. [Google Scholar] [CrossRef]
- Greenspoon, J.; Ahluwalia, R.; Juma, N.; Rosen, C.F. Allergic and Photoallergic Contact Dermatitis: A 10-year Experience. Dermatitis 2013, 24, 29–32. [Google Scholar] [CrossRef]
- de la Coba, F.; Aguilera, J.; Korbee, N.; de Gálvez, M.V.; Herrera-Ceballos, E.; Álvarez-Gómez, F.; Figueroa, F.L. UVA and UVB Photoprotective Capabilities of Topical Formulations Containing Mycosporine-like Amino Acids (MAAs) through Different Biological Effective Protection Factors (BEPFs). Mar. Drugs 2019, 17, 55. [Google Scholar] [CrossRef] [Green Version]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens Cause Coral Bleaching by Promoting Viral Infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rancan, F.; Rosan, S.; Boehm, K.; Fernández, E.; Hidalgo, M.E.; Quihot, W.; Rubio, C.; Boehm, F.; Piazena, H.; Oltmanns, U. Protection against UVB irradiation by natural filters extracted from lichens. J. Photochem. Photobiol. B Biol. 2002, 68, 133–139. [Google Scholar] [CrossRef]
- Nobile, V.; Michelotti, A.; Cestone, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Pérez-Sánchez, A.; Micol, V. Skin photoprotective and antiageing effects of a combination of rosemary (Rosmarinus officinalis) and grapefruit (Citrus paradisi) polyphenols. Food Nutr. Res. 2016, 60, 31871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Alcaraz, M.; Micol, V. Protective effects of citrus and rosemary extracts on UV-induced damage in skin cell model and human volunteers. J. Photochem. Photobiol. B Biol. 2014, 136, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I converting enzyme inhibitory peptides derived from phycobiliproteins of dulse Palmaria palmata. Mar. Drugs 2016, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, N.; Furuta, T.; Takeda, T.; Miyabe, Y.; Ura, K.; Takagi, Y.; Yasui, H.; Kumagai, Y.; Kishimura, H. Antioxidant activity of proteins extracted from red alga dulse harvested in Japan. J. Food Biochem. 2019, 43, e12709. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kishimura, H.; Kinoshita, Y.; Saburi, W.; Kumagai, Y.; Yasui, H.; Ojima, T. Enzymatic production of xylooligosaccharides from red alga dulse (Palmaria sp.) wasted in Japan. Process Biochem. 2019, 82, 117–122. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kumagai, Y.; Yamamoto, Y.; Yasui, H.; Kishimura, H. Identification of a Key Enzyme for the Hydrolysis of β-(1→3)-Xylosyl Linkage in Red Alga Dulse Xylooligosaccharide from Bifidobacterium Adolescentis. Mar. Drugs 2020, 18, 174. [Google Scholar] [CrossRef] [Green Version]
- Miyabe, Y.; Furuta, T.; Takeda, T.; Kanno, G.; Shimizu, T.; Tanaka, Y.; Gai, Z.; Yasui, H.; Kishimura, H. Structural Properties of Phycoerythrin from Dulse Palmaria palmata. J. Food Biochem. 2017, 4, 12301. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, Y.; Tsubouchi, R.; Miyabe, Y.; Takeda, T.; Adachi, K.; Yasui, H.; Kishimura, H. Complete sequence of mitochondrial DNA of red alga dulse Palmaria palmata (Linnaeus) Weber & Mohr in Japan. Mitochondrial DNA Part B 2019, 4, 3177–3178. [Google Scholar]
- Kumagai, Y.; Miyabe, Y.; Takeda, T.; Adachi, K.; Yasui, H.; Kishimura, H. In Silico Analysis of Relationship between Proteins from Plastid Genome of Red Alga Palmaria sp. (Japan) and Angiotensin I Converting Enzyme Inhibitory Peptides. Mar. Drugs 2019, 17, 190. [Google Scholar] [CrossRef] [Green Version]
- Nishida, Y.; Kumagai, Y.; Michiba, S.; Yasui, H.; Kishimura, H. Efficient Extraction and Antioxidant Capacity of Mycosporine-Like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Mar. Drugs 2020, 18, 502. [Google Scholar] [CrossRef] [PubMed]
- Balskus, E.P.; Walsh, C.T. The Genetic and Molecular Basis for Sunscreen Biosynthesis in Cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shick, J.M.; Dunlap, W.C. Mycosporine-Like Amino Acids and Related Gadusols: Biosynthesis, Accumulation, and UV-Protective Functions in Aquatic Organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karentz, D.; McEuen, F.S.; Land, M.C.; Dunlap, W.C. Survey of mycosporine-like amino acid compounds in Antarctic marine organisms: Potential protection from ultraviolet exposure. Mar. Biol. 1991, 108, 157–166. [Google Scholar] [CrossRef]
- Banaszak, A.T.; Trench, R.K. Effects of ultraviolet (UV) radiation on marine microalgal-invertebrate symbioses. II. The synthesis of mycosporine-like amino acids in response to exposure to UV in Anthopleura elegantissima and Cassiopeia xamachana. J. Exp. Mar. Biol. Ecol. 1995, 194, 233–250. [Google Scholar] [CrossRef]
- Chrapusta, E.; Kaminski, A.; Zabaglo, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-like amino acids: Potential health and beauty ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karsten, U.; Sawall, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res. 1998, 46, 271–279. [Google Scholar]
- Chuang, L.F.; Chou, H.N.; Sung, P.J. Porphyra-334 isolated from the marine algae Bangia atropurpurea: Conformational performance for energy conversion. Mar. Drugs 2014, 12, 4732–4740. [Google Scholar] [CrossRef] [Green Version]
- Conde, F.R.; Carignan, M.O.; Churio, M.S.; Carreto, J.I. In Vitro cis–trans photoisomerization of palythene and usujirene. Implications on the in vivo transformation of mycosporine-like amino acids. Photochem. Photobiol. 2003, 77, 146–150. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B Biol. 2000, 56, 139–144. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.; Chow, F.; Ferreira, M.J.P.; Santos, D. Comparative analysis of in vitro antioxidant capacities of mycosporine-like amino acids (MAAs). Algal Res. 2018, 34, 57–67. [Google Scholar] [CrossRef]
- Whittock, A.L.; Auckloo, N.; Cowden, A.M.; Turner, M.A.P.; Woolley, J.M.; Wills, M.; Corre, C.; Stavros, V.G. Exploring the blueprint of photoprotection in mycosporine-like amino acids. J. Phys. Chem. Lett. 2021, 12, 3641–3646. [Google Scholar] [CrossRef]
- Diehl, N.; Michalik, D.; Zuccarello, G.C.; Karsten, U. Stress metabolite pattern in the eulittoral red alga Pyropia plicata (Bangiales) in New Zealand—Mycosporine-like amino acids and heterosides. J. Exp. Mar. Boil. Ecol. 2019, 510, 23–30. [Google Scholar] [CrossRef]
- Jofre, J.; Celis-Plá, P.S.M.; Figueroa, F.L.; Navarro, N. Seasonal variation of mycosporine-like amino acids in three subantarctic red seaweeds. Mar. Drugs 2020, 18, 75. [Google Scholar] [CrossRef] [Green Version]
- Beer, S.; Eshel, A. Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Mar. Freshw. Res. 1985, 36, 785–792. [Google Scholar] [CrossRef]
- Karsten, U.; Franklin, L.A.; Lüning, K.; Wiencke, C. Natural ultraviolet radiation and photosynthetically active radiation induce formation of mycosporine-like amino acids in the marine macroalga Chondrus crispus (Rhodophyta). Planta 1998, 205, 257–262. [Google Scholar] [CrossRef]
- Bhatia, S.; Sharma, K.; Namdeo, A.G.; Chaugule, B.B.; Kavale, M.; Nanda, S. Broad-spectrum sun-protective action of Porphyra-334 derived from Porphyra vietnamensis. Pharmacogn. Res. 2010, 2, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, R.P.; Incharoensakdi, A. UV radiation-induced biosynthesis, stability and antioxidant activity of mycosporine-like amino acids (MAAs) in a unicellular cyanobacterium Gloeocapsa sp. CU2556. J. Photochem. Photobiol. B Biol. 2014, 130, 287–292. [Google Scholar] [CrossRef]
- Herzog, B.; Amorós-Galicia, L.; Sohn, M.; Hofer, M.; Quass, K.; Giesinger, J. Analysis of photokinetics of 2′-ethylhexyl-4-methoxycinnamate in sunscreens. Photochem. Photobiol. Sci. 2019, 18, 1773–1781. [Google Scholar] [CrossRef]
- Dupré, C.; Guary, J.-C.; Grizeau, D. Effect of photon fluence rate, nitrogen limitation and nitrogen recovery on the level of phycoerythrin in the unicellular alga, Rhodosorus marinus (Rhodophyceae). Physiol. Plant. 1994, 92, 521–527. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 521–527. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Rodas-Zuluaga, L.I.; Castillo-Zacarías, C.; Rostro-Alanís, M.; de la Cruz, R.; Carrillo-Nieves, D.; Salinas-Salazar, C.; Fuentes Grunewald, C.; Llewellyn, C.A.; Olguín, E.J.; et al. Light intensity and nitrogen concentration impact on the biomass and phycoerythrin production by Porphyridium purpureum. Mar. Drugs 2019, 17, 460. [Google Scholar] [CrossRef] [Green Version]
- Hoyer, K.; Karsten, U.; Sawall, T.; Wiencke, C. Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and developmental stages. Mar. Ecol. Prog. Ser. 2001, 211, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Korbee, N.; Huovinen, P.; Figueroa, F.L.; Aguilera, J.; Karsten, U. Availability of ammonium influences photosynthesis and the accumulation of mycosporine-like amino acids in two Porphyra species (Bangiales, Rhodophyta). Mar. Biol. 2005, 146, 645–654. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, K.; Helbling, E.W. UV-absorbing compounds in Porphyra haitanensis (Rhodophyta) with special reference to effects of desiccation. Environ. Boil. Fishes 2007, 20, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef]
- Briani, B.; Sissini, M.N.; Lucena, L.A.; Batista, M.B.; Costa, I.O.; Nunes, J.M.C.; Schmitz, C.; Ramlov, F.; Maraschin, M.; Korbee, N.; et al. The influence of environmental features in the content of mycosporine-like amino acids in red marine algae along the Brazilian coast. J. Phycol. 2018, 54, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.S.; Schafer, F.; Shick, J.M.; Dunlap, W.C. Ultraviolet radiation-absorbing mycosporine-like amino acids (MAAs) are acquired from their diet by medaka fish (Oryzias latipes) but not by SKH-1 hairless mice. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 120, 587–598. [Google Scholar] [CrossRef]
- Hartmann, A.; Holzinger, A.; Ganzera, M.; Karsten, U. Prasiolin, a new UV-sunscreen compound in the terrestrial green macroalga Prasiola calophylla (Carmichael ex Greville) Kützing (Trebouxiophyceae, Chlorophyta). Planta 2016, 243, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| MAAs | Collection Date (2020) | ||||
|---|---|---|---|---|---|
| 14 January | 13 February | 18 March | 27 April | 27 May | |
| Shinorine | 0.167 ± 0.002 c | 0.226 ± 0.001 a | 0.215 ± 0.001 b | 0.079 ± 0.003 d | 0.031 ± 0.001 e |
| Palythine | 2.444 ± 0.021 b | 2.367 ± 0.019 b | 2.625 ± 0.006 a | 0.588 ± 0.019 c | 0.493 ± 0.019 d |
| Asterina-330 | 0.080 ± 0.001 c | 0.098 ± 0.001 b | 0.108 ± 0.001 a | 0.039 ± 0.001 d | 0.019 ± 0.001 e |
| Porphyra-334 | 1.795 ± 0.015 c | 1.940 ± 0.019 b | 2.526 ± 0.024 a | 0.640 ± 0.021 d | 0.311 ± 0.015 e |
| Usujirene + Palythene | 0.402 ± 0.003 c | 0.750 ± 0.006 b | 1.203 ± 0.006 a | 0.623 ± 0.014 d | 0.187 ± 0.008 e |
| Total | 4.888 ± 0.035 c | 5.380 ± 0.043 b | 6.696 ± 0.002 a | 1.968 ± 0.057 d | 1.041 ± 0.044 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, Y.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Monthly Variation and Ultraviolet Stability of Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Phycology 2021, 1, 119-128. https://doi.org/10.3390/phycology1020009
Nishida Y, Miyabe Y, Kishimura H, Kumagai Y. Monthly Variation and Ultraviolet Stability of Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Phycology. 2021; 1(2):119-128. https://doi.org/10.3390/phycology1020009
Chicago/Turabian StyleNishida, Yuki, Yoshikatsu Miyabe, Hideki Kishimura, and Yuya Kumagai. 2021. "Monthly Variation and Ultraviolet Stability of Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan" Phycology 1, no. 2: 119-128. https://doi.org/10.3390/phycology1020009
APA StyleNishida, Y., Miyabe, Y., Kishimura, H., & Kumagai, Y. (2021). Monthly Variation and Ultraviolet Stability of Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Phycology, 1(2), 119-128. https://doi.org/10.3390/phycology1020009








