Production of Scenedesmus almeriensis Using Pilot-Scale Raceway Reactors Located inside a Greenhouse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalga Used and Culture Media Composition
2.2. Photobioreactors Used
2.3. Analytical Determinations
2.4. Model Validation
2.5. Statistical Analysis
3. Results
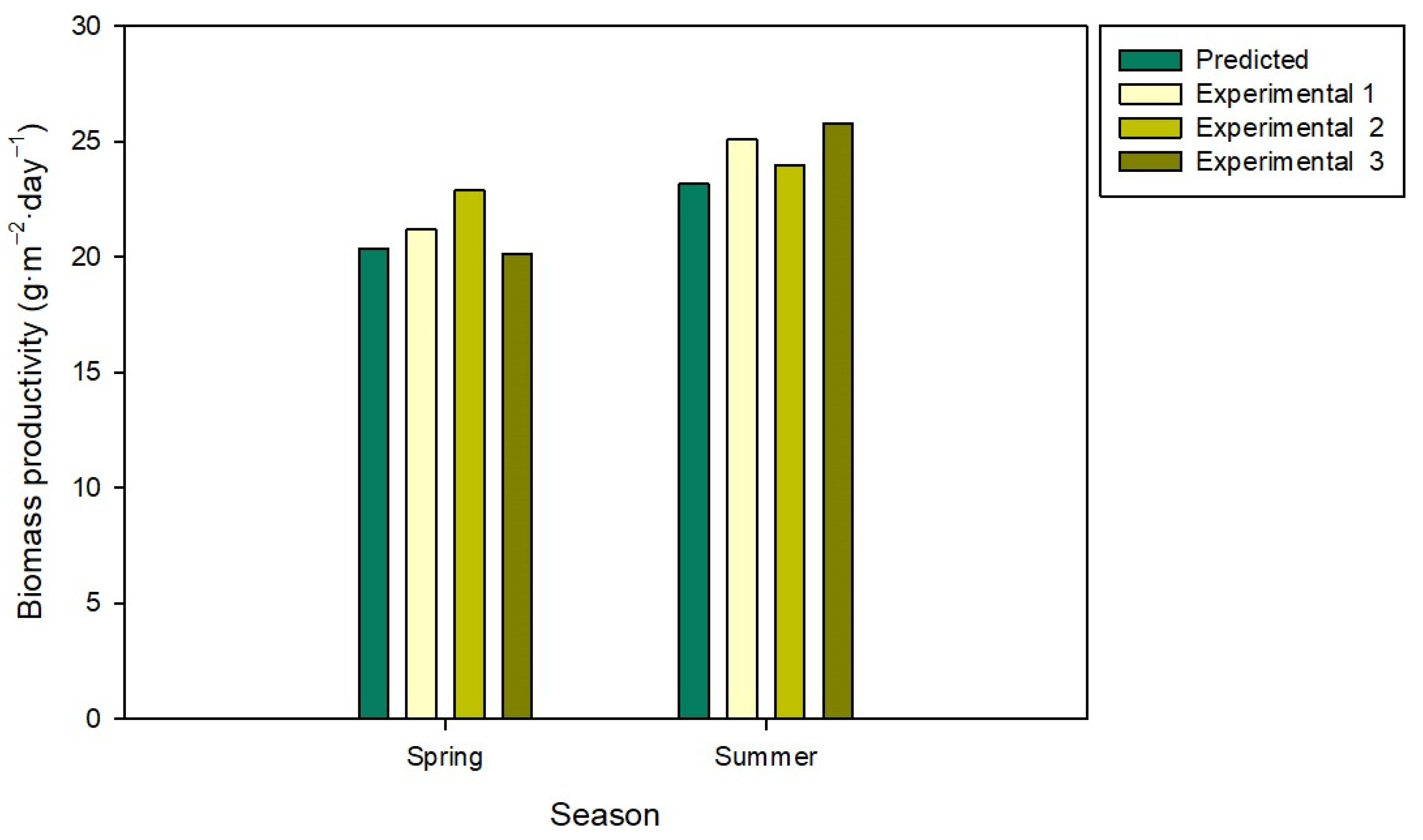
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lafarga, T. Cultured Microalgae and Compounds Derived Thereof for Food Applications: Strain Selection and Cultivation, Drying, and Processing Strategies. Food Rev. Int. 2020, 36, 559–583. [Google Scholar] [CrossRef]
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Harvey, P.J.; Ben-Amotz, A. Towards a sustainable Dunaliella salina microalgal biorefinery for 9-cis β-carotene production. Algal Res. 2020, 50, 102002. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Villaró, S.; Ciardi, M.; Morillas-españa, A.; Sánchez-zurano, A.; Acién-fernández, G.; Lafarga, T. Microalgae Derived Astaxanthin: Research and Consumer Trends and Industrial Use as Food. Foods 2021, 10, 2303. [Google Scholar] [CrossRef]
- AECOSAN. Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition on a request for initial assessment for marketing of the marine microalgae Tetraselmis chuii under Regulation (EC) No 258/97 on novel foods and novel food ingredients. Rev. Del Com. Científico 2017, 25, 1–10. [Google Scholar]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizcaíno, A.J.; López, G.; Sáez, M.I.; Jiménez, J.A.; Barros, A.; Hidalgo, L.; Camacho-Rodríguez, J.; Martínez, T.F.; Cerón-García, M.C.; Alarcón, F.J. Effects of the microalga Scenedesmus almeriensis as fishmeal alternative in diets for gilthead sea bream, Sparus aurata, juveniles. Aquaculture 2014, 431, 34–43. [Google Scholar] [CrossRef]
- Navarro-López, E.; del Carmen Cerón-García, M.; López-Rodríguez, M.; Acién-Fernández, F.G.; Molina-Grima, E. Biostimulants obtained after pilot-scale high-pressure homogenization of Scenedesmus sp. grown in pig manure. Algal Res. 2020, 52, 102123. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, J. Phytohormones in microalgae: A new opportunity for microalgal biotechnology? Plant Physiol. 2015, 20, 273–282. [Google Scholar] [CrossRef]
- Barceló-Villalobos, M.; Guzmán Sánchez, J.L.; Martín Cara, I.; Sánchez Molina, J.A.; Acién Fernández, F.G. Analysis of mass transfer capacity in raceway reactors. Algal Res. 2018, 35, 91–97. [Google Scholar] [CrossRef]
- Banerjee, S.; Ramaswamy, S. Dynamic process model and economic analysis of microalgae cultivation in open raceway ponds. Algal Res. 2017, 26, 330–340. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Herrero-Barbudo, C.; Acién-Fernández, G.; Molina-Grima, E.; Fernández-Sevilla, J.M.; Pérez-Sacristán, B.; Blanco-Navarro, I. In vitro bioaccesibility of lutein and zeaxanthin from the microalgae Scenedesmus almeriensis. Food Chem. 2009, 114, 747–752. [Google Scholar] [CrossRef]
- Morillas-España, A.; Lafarga, T.; Sánchez-Zurano, A.; Acién-Fernández, F.G.; Rodríguez-Miranda, E.; Gómez-Serrano, C.; González-López, C.V. Year-long evaluation of microalgae production in wastewater using pilot-scale raceway photobioreactors: Assessment of biomass productivity and nutrient recovery capacity. Algal Res. 2021, 60, 102500. [Google Scholar] [CrossRef]
- Morillas-España, A.; Lafarga, T.; Acién-Fernández, F.G.; Gómez-Serrano, C.; González-López, C.V. Annual production of microalgae in wastewater using pilot-scale thin-layer cascade photobioreactors. J. Appl. Phycol. 2021, 1, 3861–3871. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F.; Acien-Fernandez, F.G.; Molina-Grima, E. Microalgae research worldwide. Algal Res. 2018, 35, 50–60. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina, E. Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Sánchez, J.F.F.; Fernández-Sevilla, J.M.M.; Acién, F.G.G.; Cerón, M.C.C.; Pérez-Parra, J.; Molina-Grima, E. Biomass and lutein productivity of Scenedesmus almeriensis: Influence of irradiance, dilution rate and temperature. Appl. Microbiol. Biotechnol. 2008, 79, 719–729. [Google Scholar] [CrossRef]
- Morillas-España, A.; Lafarga, T.; Gómez-Serrano, C.; Acién-Fernández, F.G.; González-López, C.V. Year-long production of Scenedesmus almeriensis in pilot-scale raceway and thin-layer cascade photobioreactors. Algal Res. 2020, 51, 102069. [Google Scholar] [CrossRef]
- Perera, E.; Sánchez-Ruiz, D.; Sáez, M.I.; Galafat, A.; Barany, A.; Fernández-Castro, M.; Vizcaíno, A.J.; Fuentes, J.; Martínez, T.F.; Mancera, J.M.; et al. Low dietary inclusion of nutraceuticals from microalgae improves feed efficiency and modifies intermediary metabolisms in gilthead sea bream (Sparus aurata). Sci. Rep. 2020, 10, 18676. [Google Scholar] [CrossRef]
- López, C.V.G.; del Carmen Cerón García, M.; Fernández, F.G.A.; Bustos, C.S.; Chisti, Y.; Sevilla, J.M.F. Protein measurements of microalgal and cyanobacterial biomass. Bioresour. Technol. 2010, 101, 7587–7591. [Google Scholar] [CrossRef]
- García-Márquez, J.; Rico, R.M.; Del Pilar Sánchez-Saavedra, M.; Gómez-Pinchetti, J.L.; Acién, F.G.; Figueroa, F.L.; Alarcón, F.J.; Moriñigo, M.Á.; Abdala-Díaz, R.T. A short pulse of dietary algae boosts immune response and modulates fatty acid composition in juvenile Oreochromis niloticus. Aquac. Res. 2020, 51, 4397–4409. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- BOE Métodos Oficiales de Análisis: Suelos y Aguas. 1982. Available online: https://www.boe.es/diario_boe/txt.php?id=BOE-A-1976-6778 (accessed on 10 January 2022).
- Sánchez-Zurano, A.; Morillas-España, A.; Gómez-Serrano, C.; Ciardi, M.; Acién, G.; Lafarga, T. Annual assessment of the wastewater treatment capacity of the microalga Scenedesmus almeriensis and optimisation of operational conditions. Sci. Rep. 2021, 11, 21651. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.C.; Mendoza Martin, J.L.; Heaven, S.; Banks, C.J.; Acien Fernandez, F.G.; Molina Grima, E. Cultivation and anaerobic digestion of Scenedesmus spp. grown in a pilot-scale open raceway. Algal Res. 2014, 5, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Del Mar Morales-Amaral, M.; Gómez-Serrano, C.; Acién, F.G.; Fernández-Sevilla, J.M.; Molina-Grima, E. Outdoor production of Scenedesmus sp. in thin-layer and raceway reactors using centrate from anaerobic digestion as the sole nutrient source. Algal Res. 2015, 12, 99–108. [Google Scholar] [CrossRef]
- Maroušek, J.; Maroušková, A. Economic Considerations on Nutrient Utilization in Wastewater Management. Energies 2021, 14, 3468. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.; Yang, H.; Wu, L.; Hu, C. Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour. Technol. 2015, 191, 219–228. [Google Scholar] [CrossRef]
- Tan, L.; Xu, W.; He, X.; Wang, J. The feasibility of Fv/Fm on judging nutrient limitation of marine algae through indoor simulation and in situ experiment. Estuar. Coast. Shelf Sci. 2019, 229, 106411. [Google Scholar] [CrossRef]
- Gan, T.; Zhao, N.; Yin, G.; Chen, M.; Wang, X.; Liu, J.; Liu, W. Optimal chlorophyll fluorescence parameter selection for rapid and sensitive detection of lead toxicity to marine microalgae Nitzschia closterium based on chlorophyll fluorescence technology. J. Photochem. Photobiol. B Biol. 2019, 197, 111551. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Hall, D.O.; Cañizares Guerrero, E.; Krishna Rao, K.; Molina Grima, E. Outdoor production of Phaeodactylum tricornutum biomass in a helical reactor. J. Biotechnol. 2003, 103, 137–152. [Google Scholar] [CrossRef]
- Sánchez-Zurano, A.; Lafarga, T.; del Mar Morales-Amaral, M.; Gómez-Serrano, C.; Fernández-Sevilla, J.M.; Acién-Fernández, F.G.; Molina-Grima, E. Wastewater treatment using Scenedesmus almeriensis: Effect of operational conditions on the composition of the microalgae-bacteria consortia. J. Appl. Phycol. 2021, 1, 3885–3897. [Google Scholar] [CrossRef]
- Sánchez-Zurano, A.; Rodríguez-Miranda, E.; Guzmán, J.L.; Acien-Fernandez, F.G.; Fernandez-Sevilla, J.M.; Molina-Grima, E. ABACO: A new model of microalgae-bacteria consortia for biological treatment of wastewater. Appl. Sci. 2021, 11, 998. [Google Scholar] [CrossRef]
- Huesemann, M.; Crowe, B.; Waller, P.; Chavis, A.; Hobbs, S.; Edmundson, S.; Wigmosta, M. A validated model to predict microalgae growth in outdoor pond cultures subjected to fluctuating light intensities and water temperatures. Algal Res. 2016, 13, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Collao, J.; del Mar Morales-Amaral, M.; Acién-Fernández, F.G.; Bolado-Rodríguez, S.; Fernandez-Gonzalez, N. Effect of operational parameters, environmental conditions, and biotic interactions on bacterial communities present in urban wastewater treatment photobioreactors. Chemosphere 2021, 284, 131271. [Google Scholar] [CrossRef] [PubMed]
- Finkel, Z.V.; Follows, M.J.; Liefer, J.D.; Brown, C.M.; Benner, I.; Irwin, A.J. Phylogenetic diversity in the macromolecular composition of microalgae. PLoS ONE 2016, 11, e0155977. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, K.E.; Whitney, C.G.; McGinn, P.J. Nutrient remediation rates in municipal wastewater and their effect on biochemical composition of the microalga Scenedesmus sp. AMDD. Algal Res. 2013, 2, 127–134. [Google Scholar] [CrossRef]
- Gómez, C.; Guzmán-Carrasco, A.; Lafarga, T.; Acién-Fernández, F.G. Optimization of a new culture medium for the large-scale production of protein-rich Arthrospira platensis (oscillatoriales, cyanophyceae). J. Phycol. 2020, 27, 636–644. [Google Scholar] [CrossRef]
- Stringham, J.M.; Bovier, E.R.; Wong, J.C.; Hammond, B.R. The influence of dietary lutein and zeaxanthin on visual performance. J. Food Sci. 2010, 75, R24–R29. [Google Scholar] [CrossRef] [PubMed]
- Limón, P.; Malheiro, R.; Casal, S.; Acién-Fernández, F.G.; Fernández-Sevilla, J.M.; Rodrigues, N.; Cruz, R.; Bermejo, R.; Pereira, J.A. Improvement of stability and carotenoids fraction of virgin olive oils by addition of microalgae Scenedesmus almeriensis extracts. Food Chem. 2015, 175, 203–211. [Google Scholar] [CrossRef] [PubMed]



| Season | Protein (g·100 g−1) | Lipid (g·100 g−1) | Ash (g·100 g−1) | Carbohydrate (g·100 g−1) |
|---|---|---|---|---|
| Spring | 38.0 ± 0.2 | 4.6 ± 0.3 | 9.9 ± 0.3 | 47.5 ± 0.7 |
| Summer | 37.8 ± 0.4 | 4.6 ± 0.5 | 11.6 ± 2.4 | 45.9 ± 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morillas-España, A.; Villaró, S.; Ciardi, M.; Acién, G.; Lafarga, T. Production of Scenedesmus almeriensis Using Pilot-Scale Raceway Reactors Located inside a Greenhouse. Phycology 2022, 2, 76-85. https://doi.org/10.3390/phycology2010005
Morillas-España A, Villaró S, Ciardi M, Acién G, Lafarga T. Production of Scenedesmus almeriensis Using Pilot-Scale Raceway Reactors Located inside a Greenhouse. Phycology. 2022; 2(1):76-85. https://doi.org/10.3390/phycology2010005
Chicago/Turabian StyleMorillas-España, Ainoa, Silvia Villaró, Martina Ciardi, Gabriel Acién, and Tomás Lafarga. 2022. "Production of Scenedesmus almeriensis Using Pilot-Scale Raceway Reactors Located inside a Greenhouse" Phycology 2, no. 1: 76-85. https://doi.org/10.3390/phycology2010005







