Cosmeceutical Significance of Seaweed: A Focus on Carbohydrates and Peptides in Skin Applications
Abstract
1. Introduction
2. Natural Cosmetic Products
Synthetic Cautions: A Road to the Natural Ingredients
3. Introduction to Seaweeds
Seaweeds: Interesting Metabolites
4. Seaweed-Derived Polysaccharides for Skin Benefits
4.1. Agar
4.2. Alginic Acid
4.3. Carrageenan
4.4. Porphyran
4.5. Laminaran
4.6. Fucoidan
4.7. Ulvan
4.8. Remarks
5. Seaweed-Derived Proteins, Peptides, and Amino Acids for Skin Benefits
Peptides Bioactivities
6. Future Roads toward Seaweed-Based Cosmetics
Future Prospects towards Seaweed-Based Cosmetics for Safe Applications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, K.I.; Glaser, D.A. Cosmeceuticals: The new medicine of beauty. Mo. Med. 2011, 108, 60. [Google Scholar] [PubMed]
- Goyal, A.; Sharma, A.; Kaur, J.; Kumari, S.; Garg, M.; Sindhu, R.K.; Rahman, H.; Akhtar, M.F.; Tagde, P.; Najda, A.; et al. Bioactive-based cosmeceuticals: An update on emerging trends. Molecules 2022, 27, 828. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. The cosmeceutical realm. Clin. Dermatol. 2008, 26, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Kligman, D. Cosmeceuticals. Dermatol. Clin. 2000, 18, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Dureja, H.; Kaushik, D.; Gupta, M.; Kumar, V.; Lather, V. Cosmeceuticals: An emerging concept. Indian J. Pharmacol. 2005, 37, 155. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Grumezescu, A.M.; Bejenaru, C.; Bejenaru, L.E. Natural products used for food preservation. In Food Preservation; Academic Press: Cambridge, MA, USA, 2017; pp. 365–411. [Google Scholar]
- Yin, S.N.; Hayes, R.B.; Linet, M.S.; Li, G.L.; Dosemeci, M.; Travis, L.B.; Zhang, Z.N.; Li, D.G.; Chow, W.H.; Wacholder, S.; et al. An expanded cohort study of cancer among benzene-exposed workers in China. Benzene Study Group. Environ. Health Perspect. 1996, 104 (Suppl. S6), 1339–1341. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment. Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Robertson, M.L.; Kolachana, P.; Davison, A.J.; Smith, M.T. Benzene metabolite, 1,2,4-benzenetriol, induces micronuclei and oxidative DNA damage in human lymphocytes and HL60 cells. Environ. Mol. Mutagen. 1993, 21, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Shamasunder, B. The environmental injustice of beauty: Framing chemical exposures from beauty products as a health disparities concern. Am. J. Obstet. Gynecol. 2017, 217, 418.e1–418.e6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.X.; Liu, Y.; Tang, S.; Zhang, W.; Cheong, K.L. Preparation methods, biological activities, and potential applications of marine algae oligosaccharides: A review. Food Sci. Hum. Wellness 2023, 12, 359–370. [Google Scholar] [CrossRef]
- Pereira, J.X.; Pereira, T.C. Cosmetics and its health risks. Glob. J. Med. Res. 2018, 18, 63–70. [Google Scholar] [CrossRef]
- Ridder, M. Market Value for Natural and Organic BeautyWorldwide 2018–2027. Available online: https://www.statista.com/statistics/673641/global-market-value-for-natural-cosmetics/ (accessed on 7 February 2024).
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Yeh, H.Y.; Shih, W.L. Extraction procedure, characteristics, and feasibility of Caulerpa microphysa (Chlorophyta) polysaccharide extract as a cosmetic ingredient. Marine Drugs. 2021, 19, 524. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Pangestuti, R. 15 Biological Properties of Cosmeceuticals Derived from Marine Algae. In Marine Cosmeceuticals: Trends and Prospects; CRC Press: Boca Raton, FL, USA, 2011; p. 191. [Google Scholar]
- Polat, S.; Trif, M.; Rusu, A.; Šimat, V.; Čagalj, M.; Alak, G.; Meral, R.; Özogul, Y.; Polat, A.; Özogul, F. Recent advances in industrial applications of seaweeds. Crit. Rev. Food Sci. Nutr. 2023, 63, 4979–5008. [Google Scholar] [CrossRef] [PubMed]
- Resende, D.I.; Ferreira, M.; Magalhães, C.; Lobo, J.S.; Sousa, E.; Almeida, I.F. Trends in the use of marine ingredients in anti-aging cosmetics. Algal Res. 2021, 55, 102273. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Seaweed Application in Cosmetics. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 423–441. [Google Scholar]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Acosta, N.; Civera-Tejuca, C.; Elorza, B.; Mingo, J.; Castro, C.; Civera-Tejuca, C.; Heras, A. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Imchen, T.; Singh, K.S. Marine algae colorants: Antioxidant, anti-diabetic properties and applications in food industry. Algal Research. 2023, 69, 102898. [Google Scholar] [CrossRef]
- Joshi, S.; Kumari, R.; Upasani, V.N. Applications of algae in cosmetics: An overview. Int. J. Innov. Res. Sci. Eng. Technol. 2018, 7, 1269. [Google Scholar]
- Fonseca, S.; Amaral, M.N.; Reis, C.P.; Custódio, L. Marine natural products as innovative cosmetic ingredients. Marine Drugs 2023, 21, 170. [Google Scholar] [CrossRef] [PubMed]
- Swetman, A.A.; Nicolaides, L.; Wareing, P.W.; New, J.H.; Wood, J.F.; Hammond, L. Food processing and preservation. Crop Post-Harvest. Sci. Technol. Princ. Pract. 2002, 1, 360–422. [Google Scholar]
- Bilal, M.; Iqbal, H.M. An insight into toxicity and human-health-related adverse consequences of cosmeceuticals—A review. Sci. Total Environ. 2019, 670, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Wassie, T.; Niu, K.; Xie, C.; Wang, H.; Xin, W. Extraction techniques, biological activities and health benefits of marine algae Enteromorpha prolifera polysaccharide. Front. Nutr. 2021, 8, 747928. [Google Scholar] [CrossRef] [PubMed]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a functional ingredient for a healthy diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Shin, K.H.; Kim, S.K. Anti-photoaging and potential skin health benefits of seaweeds. Mar. Drugs 2021, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Quitério, E.; Soares, C.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. Marine health-promoting compounds: Recent trends for their characterization and human applications. Foods 2021, 10, 3100. [Google Scholar] [CrossRef] [PubMed]
- Bot, F.; Cossuta, D.; O’Mahony, J.A. Inter-relationships between composition, physicochemical properties and functionality of lecithin ingredients. Trends Food Sci. Technol. 2021, 111, 261–270. [Google Scholar] [CrossRef]
- Guo, Z.; Wei, Y.; Zhang, Y.; Xu, Y.; Zheng, L.; Zhu, B.; Yao, Z. Carrageenan oligosaccharides: A comprehensive review of preparation, isolation, purification, structure, biological activities and applications. Algal Res. 2022, 61, 102593. [Google Scholar] [CrossRef]
- Fernández-Álvarez, M.; Llompart, M.; Sánchez-Prado, L.; García-Jares, C.; Lores, M. Photochemical behavior of UV filter combinations. In Cosmetics: Types, Allergies and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; p. 1. [Google Scholar]
- Knowland, J.; McKenzie, E.A.; McHugh, P.J.; Cridland, N.A. Sunlight-induced mutagenicity of a common sunscreen ingredient. FEBS Lett. 1993, 324, 309–313. [Google Scholar] [CrossRef]
- Kerdudo, A.; Burger, P.; Merck, F.; Dingas, A.; Rolland, Y.; Michel, T.; Fernandez, X. Development of a natural ingredient–Natural preservative: A case study. Comptes Rendus. Chim. 2016, 19, 1077–1089. [Google Scholar] [CrossRef]
- Mowad, C.M. Allergic contact dermatitis caused by parabens: 2 case reports and a review. Am. J. Contact Dermat. 2000, 11, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, F.; Maibach, H. An overview of parabens and allergic contact dermatitis. Skin Ther. Lett. 2013, 18, 5–7. [Google Scholar]
- Barrett, J. Chemical Exposures: The Ugly Side of Beauty Products. Environ. Health Perspect. 2005, 113, A24. [Google Scholar] [CrossRef]
- Khan, A.D.; Alam, M.N. Cosmetics and their associated adverse effects: A review. J. Appl. Pharm. Sci. Res. 2019, 2, 1–6. [Google Scholar] [CrossRef]
- Warbanski, M. The ugly side of the beauty industry. Herizons 2007, 21, 24–28. [Google Scholar]
- Kaličanin, B.; Velimirović, D. A study of the possible harmful effects of cosmetic beauty products on human health. Biol. Trace Elem. Res. 2016, 170, 476–484. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D. Cosmeceuticals derived from bioactive substances found in marine algae. Oceanography 2013, 1, 106. [Google Scholar]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef]
- Pereira, L. Therapeutic and Nutritional Uses of Algae; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781498755382. [Google Scholar]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging role of phenolic compounds as natural food additives in fish and fish products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Napolitano, A. Natural phenol polymers: Recent advances in food and health applications. Antioxidants 2017, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Algae. Litoral of Viana do Castelo; Câmara Municipal de Viana do Castelo: Viana do Castelo, Portugal, 2010; pp. 7–8. ISBN 978-972-588-217-7. [Google Scholar]
- Pereira, L. Guia Ilustrado das Macroalgas—Conhecer e Reconhecer Algumas Espécies da Flora Portuguesa; Universityde Coimbra Press: Coimbra, Portugal, 2009; p. 91. ISBN 978-989-26-0002-4. [Google Scholar]
- Pereira, L. Chapter 4—Cytological and cytochemical aspects in selected carrageenophytes (Gigartinales, Rhodophyta). In Advances in Algal Cell Biology; Heimann, K., Katsaros, C., Eds.; De Gruyter: Berlin, Germany, 2012; pp. 81–104. ISBN 978-3-11-022960-8. [Google Scholar]
- González-Minero, F.J.; Bravo-Díaz, L. The use of plants in skin-care products, cosmetics and fragrances: Past and present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef]
- Fleurence, J.; Morançais, M.; Dumay, J.; Decottignies, P.; Turpin, V.; Munier, M.; Garcia-Bueno, N.; Jaouen, P. What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture? Trends Food Sci. Technol. 2012, 27, 57–61. [Google Scholar] [CrossRef]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M. The evolution road of seaweed aquaculture: Cultivation technologies and the industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef] [PubMed]
- Veluchamy, C.; Palaniswamy, R. A review on marine algae and its applications. Asian J. Pharm. Clin. Res. 2020, 13, 21–27. [Google Scholar] [CrossRef]
- Fu, W.; Nelson, D.R.; Yi, Z.; Xu, M.; Khraiwesh, B.; Jijakli, K.; Chaiboonchoe, A.; Alzahmi, A.; Al-Khairy, D.; Brynjolfsson, S.; et al. Bioactive compounds from microalgae: Current development and prospects. Stud. Nat. Prod. Chem. 2017, 54, 199–225. [Google Scholar]
- Couteau, C.; Coiffard, L. Phycocosmetics and other marine cosmetics, specific cosmetics formulated using marine resources. Mar. Drugs 2020, 18, 322. [Google Scholar] [CrossRef] [PubMed]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, E.; Herrero, M.; Mendiola, J.A.; Castro-Puyana, M. Extraction and characterization of bioactive compounds with health benefits from marine resources: Macro and micro algae, cyanobacteria, and invertebrates. In Marine Bioactive Compounds; Springer: Boston, MA, USA, 2012; pp. 55–98. [Google Scholar]
- Vo, T.S.; Kim, S.K. Fucoidans as a natural bioactive ingredient for functional foods. J. Funct. Foods 2013, 5, 16–27. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K. Osteoporosis treatment: Marine algal compounds. Adv. Food Nutr. Res. 2011, 64, 417–427. [Google Scholar] [PubMed]
- Miguel, S.P.; Ribeiro, M.P.; Otero, A.; Coutinho, P. Application of microalgae and microalgal bioactive compounds in skin regeneration. Algal Res. 2021, 58, 102395. [Google Scholar] [CrossRef]
- Gam, D.H.; Park, J.H.; Hong, J.W.; Jeon, S.J.; Kim, J.H.; Kim, J.W. Effects of Sargassum thunbergii extract on skin whitening and anti-wrinkling through inhibition of TRP-1 and MMPs. Molecules 2021, 26, 7381. [Google Scholar] [CrossRef] [PubMed]
- Querellou, J.; Børresen, T.; Boyen, C.; Dobson, A.; Höfle, M.; Ianora, A.; Jaspars, M.; Kijjoa, A.; Olafsen, J.; Rigos, G. Marine biotechnology: Realising the full potential of Europe. VLIZ Spec. Publ. 2010, 47, 21. [Google Scholar]
- Freitas, R.; Martins, A.; Silva, J.; Alves, C.; Pinteus, S.; Alves, J.; Teodoro, F.; Ribeiro, H.M.; Gonçalves, L.; Petrovski, Ž.; et al. Highlighting the biological potential of the brown seaweed Fucus spiralis for skin applications. Antioxidants 2020, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Yao, Z.; Zhu, B. Ulva (Enteromorpha) polysaccharides and oligosaccharides: A potential functional food source from green-tide-forming macroalgae. Mar. Drugs 2022, 20, 202. [Google Scholar] [CrossRef] [PubMed]
- Pallela, R.; Na-Young, Y.; Kim, S.K. Anti-photoaging and photoprotective compounds derived from marine organisms. Mar. Drugs 2010, 8, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.S.; Kim, M.; Son, K.T.; Jeong, Y.; Jeon, Y.J. Antioxidant activity of marine algal polyphenolic compounds: Amechanistic approach. J. Med. Food 2016, 19, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Indira, K.; Balakrishnan, S.; Srinivasan, M.; Bragadeeswaran, S.; Balasubramanian, T. Evaluation of in vitro antimicrobial property of seaweed (Halimeda tuna) from Tuticorin coast, Tamil Nadu, Southeast coast of India. Afr. J. Biotechnol. 2013, 12, 284–289. [Google Scholar]
- Liu, N.; Fu, X.; Duan, D.; Xu, J.; Gao, X.; Zhao, L. Evaluation of bioactivity of phenolic compounds from the brown seaweed of Sargassum fusiforme and development of their stable emulsion. J. Appl. Phycol. 2018, 30, 1955–1970. [Google Scholar] [CrossRef]
- Brunt, E.G.; Burgess, J.G. The promise of marine molecules as cosmetic active ingredients. Int. J. Cosmet. Sci. 2018, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Percival, E. The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. Br. Phycol. J. 1979, 14, 103–117. [Google Scholar] [CrossRef]
- Fernando, I.S.; Sanjeewa, K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kang, N.; Ranasinghe, P.; Lee, H.S.; Jeon, Y.J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Conde, E.; Soto, M.L.; Pérez-Armada, L.; Domínguez, H. Cosmetics from marine sources. In Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1015–1042. [Google Scholar]
- Wang, Z.J.; Xu, W.; Liang, J.W.; Wang, C.S.; Kang, Y. Effect of fucoidan on B16 murine melanoma cell melanin formation and apoptosis. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Teas, J.; Irhimeh, M.R. Melanoma and brown seaweed: An integrative hypothesis. J. Appl. Phycol. 2017, 29, 941–948. [Google Scholar] [CrossRef]
- Ghorbanzadeh, B.; Mansouri, M.T.; Hemmati, A.A.; Naghizadeh, B.; Mard, S.A.; Rezaie, A. Mechanism underlying the anti-inflammatory effect of sulphated polysaccharide from Padina tetrastromatica against carrageenan induced paw edema in rats. Indian J. Pharmacol. 2015, 47, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Yoon, S.J.; Choi, J.S.; Park, N.G.; Lee, H.H.; Cho, J.Y.; Hong, Y.K. Anti-edema effects of brown seaweed (Undaria pinnatifida) extract on phorbol 12-myristate 13-acetate-induced mouse ear inflammation. Am. J. Chin. Med. 2009, 37, 373–381. [Google Scholar] [CrossRef]
- Vasconcelos, J.B.; de Vasconcelos, E.R.; Urrea-Victoria, V.; Bezerra, P.S.; Reis, T.N.; Cocentino, A.L.; Navarro, D.M.; Chow, F.; Areces, A.J.; Fujii, M.T. Antioxidant activity of three seaweeds from tropical reefs of Brazil: Potential sources for bioprospecting. J. Appl. Phycol. 2019, 31, 835–846. [Google Scholar] [CrossRef]
- Santos, J.P.; Torres, P.B.; dos Santos, D.Y.; Motta, L.B.; Chow, F. Seasonal effects on antioxidant and anti-HIV activities of Brazilian seaweeds. J. Appl. Phycol. 2019, 31, 1333–1341. [Google Scholar] [CrossRef]
- Kim, J.A.; Ahn, B.N.; Kong, C.S.; Kim, S.K. The chromene sargachromanol E inhibits ultraviolet A-induced ageing of skin in human dermal fibroblasts. Br. J. Dermatol. 2013, 168, 968–976. [Google Scholar] [CrossRef]
- Lee, H.Y.; Jang, E.J.; Bae, S.Y.; Jeon, J.E.; Park, H.J.; Shin, J.; Lee, S.K. Anti-melanogenic activity of gagunin D, a highly oxygenated diterpenoid from the marine sponge Phorbas sp., via modulating tyrosinase expression and degradation. Mar. Drugs 2016, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Koh, W.B.; Oh, Y.S.; Kim, I.J. The Anti-melanogenic effects of Petalonia binghamiae extracts in α-melanocyte stimulating hormone-induced B16/F10 murine melanoma cells. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 564–567. [Google Scholar] [CrossRef]
- Wang, H.M.; Li, X.C.; Lee, D.J.; Chang, J.S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Komura, Y.; Nishimura, Y.; Sugawara, T.; Hirata, T. Inhibition of mast cell degranulation by phycoerythrin and its pigment moiety phycoerythrobilin, prepared from Porphyra yezoensis. Food Sci. Technol. Res. 2011, 17, 171–177. [Google Scholar] [CrossRef]
- Hartmann, A.; Gostner, J.; Fuchs, J.E.; Chaita, E.; Aligiannis, N.; Skaltsounis, L.; Ganzera, M. Inhibition of collagenase by mycosporine-like amino acids from marine sources. Planta Med. 2015, 81, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.S.; Choi, J.; Lee, M.S.; Kim, H.R. Hypopigmenting effects of brown algae-derived phytochemicals: A review on molecular mechanisms. Mar. Drugs 2017, 15, 297. [Google Scholar] [CrossRef]
- Li, K.; Li, X.M.; Gloer, J.B.; Wang, B.G. New nitrogen-containing bromophenols from the marine red alga Rhodomela confervoides and their radical scavenging activity. Food Chem. 2012, 135, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.K. Introduction to Seaweed Polysaccharides. In Seaweed Polysaccharides—Isolation, Biological and Biomedical Applications, 1st ed.; Venkatesan, J., Anil, S., Kim, S.-K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Yu, P.; Sun, H. Purification of a fucoidan from kelp polysaccharide and its inhibitory kinetics for tyrosinase. Carbohydr. Polym. 2014, 99, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H.; Dell’Acqua, G.; Gardiner, V.A.; Karpiniec, S.S.; Stringer, D.N.; Davis, E. Topical benefits of two fucoidan-rich extracts from marine macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef]
- Moreira, J.B.; Vaz, B.D.S.; Cardias, B.B.; Cruz, C.G.; Almeida, A.C.A.D.; Costa, J.A.V.; Morais, M.G.D. Microalgae polysaccharides: An alternative source for food production and sustainable agriculture. Polysaccharides 2022, 3, 441–457. [Google Scholar] [CrossRef]
- Ahmed, A.; Taha, R. Marine Phytochemical Compounds and Their Cosmeceutical Applications. In Marine Cosmeceuticals: Trends and Prospects; Kim, S., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 51–61. [Google Scholar]
- Godlewska, K.; Michalak, I.; Tuhy, Ł.; Chojnacka, K. Plant Growth Biostimulants Based on Different Methods of Seaweed Extraction with Water. BioMed. Res. Int. 2016, 2016, 5973760. [Google Scholar] [CrossRef] [PubMed]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Ko, S.C.; Kim, D.; Jeon, Y.J. Screening of marine algae for potential tyrosinase inhibitor: Those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. J. Dermatol. 2011, 38, 354–363. [Google Scholar] [CrossRef]
- Murugan, K.; Iyer, V.V. Differential growth inhibition of cancer cell lines and antioxidant activity of extracts of red, brown, and green marine algae. Vitr. Cell. Dev. Biol.-Anim. 2013, 49, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.; Harrison, S.T.; Smit, M.; Maharajh, D. Major commercial products from micro-and macroalgae. In Algae biotechnology: Products and Processes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 269–300. [Google Scholar]
- Buono, S.; Langellotti, A.L.; Martello, A.; Bimonte, M.; Tito, A.; Carola, A.; Apone, F.; Colucci, G.; Fogliano, V. Biological activities of dermatological interest by the water extract of the microalga Botryococcus braunii. Arch. Dermatol. Res. 2012, 304, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Lee, C.H.; Kim, J.R.; Kwon, J.Y.; Seo, S.G.; Han, J.G.; Kim, B.G.; Kim, J.E.; Lee, K.W. Chlorella vulgaris attenuates Dermatophagoides farinae-induced atopic dermatitis-like symptoms in NC/Nga mice. Int. J. Mol. Sci. 2015, 16, 21021–21034. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; Oliveira, M.B.P.P. Macroalgae-Derived Ingredients for Cosmetic Industry—An Update. Cosmetics 2017, 5, 2. [Google Scholar] [CrossRef]
- Kim, S.; You, D.H.; Han, T.; Choi, E.-M. Modulation of viability and apoptosis of UVB-exposed human keratinocyte HaCaT cells by aqueous methanol extract of laver (Porphyra yezoensis). J. Photochem. Photobiol. B Biol. 2014, 141, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Luthfiyana, N.; Hidayat, T.; Nurilmala, M.; Anwar, E. Utilization of seaweed porridge Sargassum sp. and Eucheuma cottonii as cosmetic in protecting the skin. IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012055. [Google Scholar]
- Heo, S.-J.; Ko, S.-C.; Cha, S.-H.; Kang, D.-H.; Park, H.-S.; Choi, Y.-U.; Kim, D.; Jung, W.-K.; Jeon, Y.-J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. Vitr. 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Quah, C.C.; Kim, K.H.; Lau, M.S.; Kim, W.R.; Cheah, S.H.; Gundamaraju, R. Pigmentation and Dermal Conservative Effects of the Astonishing Algae Sargassum polycystum and Padina tenuis on Guinea Pigs, Human Epidermal Melanocytes (HEM) and Chang Cells. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Maia Campos, P.M.; de Melo, M.O.; de Camargo, F.B., Jr. Effects of polysaccharide-based formulations on human skin. In Polysaccharides; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–8. [Google Scholar]
- Sotelo, C.G.; Blanco, M.; Ramos, P.; Vázquez, J.A.; Perez-Martin, R.I. Sustainable sources from aquatic organisms for cosmeceuticals ingredients. Cosmetics 2021, 8, 48. [Google Scholar] [CrossRef]
- Jutur, P.P.; Nesamma, A.A.; Shaikh, K.M. Algae-Derived Marine Oligosaccharides and Their Biological Applications. Front. Mar. Sci. 2016, 3, 83. [Google Scholar] [CrossRef]
- Li, J.; Chi, Z.; Yu, L.; Jiang, F.; Liu, C. Sulfated modification, characterization, and antioxidant and moisture absorption/retention activities of a soluble neutral polysaccharide from Enteromorpha prolifera. Int. J. Biol. Macromol. 2017, 105, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Tsukahara, K.; Moriwaki, S.; Kitahara, T.; Takema, Y. Effects of natural product extracts on contraction and mechanical properties of fibroblast populated collagen gel. Biol. Pharm. Bull. 2000, 23, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Rasin, A.B.; Kusaykin, M.I.; Malyarenko, O.S.; Shevchenko, N.M.; Zueva, A.O.; Kalinovsky, A.I.; Zvyagintseva, T.N.; Ermakova, S.P. Modification of native fucoidan from Fucus evanescens by recombinant fucoidanase from marine bacteria Formosa algae. Carbohydr. Polym. 2018, 193, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Hanamizu, T.; Sone, T.; Chiba, K.; Kinoshita, T.; Yoshikawa, S. Topical application of Bifidobacterium-fermented soy milk extract containing genistein and daidzein improves rheological and physiological properties of skin. J. Cosmet. Sci. 2004, 55, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.M.; Hellewell, P.G. The effect of the selectin binding polysaccharide fucoidin on eosinophil recruitment in vivo. Br. J. Pharmacol. 1997, 120, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Tu, C.J.; Wu, H.T. Growth-inhibitory effects of the red alga Gelidium amansii on cultured cells. Biol. Pharm. Bull. 2004, 27, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kusaykin, M.I.; Kurilenko, V.V.; Zakharenko, A.M.; Isakov, V.V.; Zaporozhets, T.S.; Gazha, A.K.; Zvyagintseva, T.N. Hydrolysis of fucoidan by fucoidanase isolated from the marine bacterium, Formosa algae. Mar. Drugs 2013, 11, 2413–2430. [Google Scholar] [CrossRef]
- Fujimura, T.; Tsukahara, K.; Moriwaki, S.; Kitahara, T.; Sano, T.; Takema, Y. Treatment of human skin with an extract of Fucus vesiculosus changes its thickness and mechanical properties. J. Cosmet. Sci. 2002, 53, 1–9. [Google Scholar] [PubMed]
- Kakita, H.; Kamishima, H. Some properties of alginate gels derived from algal sodium alginate. In Nineteenth International Seaweed Symposium: Proceedings of the 19th International Seaweed Symposium, Held in Kobe, Japan, 26–31 March 2007; Springer: Dordrecht, The Netherlands, 2009; pp. 93–99. [Google Scholar]
- Park, E.J.; Choi, J.I. Melanogenesis inhibitory effect of low molecular weight fucoidan from Undaria pinnatifida. J. Appl. Phycol. 2017, 29, 2213–2217. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Oh, J.; Cui, Y.; Ryu, B.; Jeon, Y.J. Protective effect of sulfated polysaccharides from celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-induced skin damage by regulating NF-κB, AP-1, and MAPKs signalling pathways in vitro in human dermal fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef]
- Lee, K.Y.; You, H.J.; Jeong, H.G.; Kang, J.S.; Kim, H.M.; Dal Rhee, S.; Jeon, Y.J. Polysaccharide isolated from Poria cocos sclerotium induces NF-κB/Rel activation and iNOS expression through the activation of p38 kinase in murine macrophages. Int. Immunopharmacol. 2004, 4, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Staniforth, V.; Huang, W.C.; Aravindaram, K.; Yang, N.S. Ferulic acid, a phenolic phytochemical, inhibits UVB-induced matrix metalloproteinases in mouse skin via posttranslational mechanisms. J. Nutr. Biochem. 2012, 23, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.C.; Lee, D.-S.; Bae, S.M.; Jung, W.-K.; Chun, J.H.; Urm, S.H.; Lee, D.-Y.; Heo, S.-J.; Park, S.-G.; Seo, S.-K.; et al. The effect of cilostazol on the expression of matrix metalloproteinase-1 and type I procollagen in ultraviolet-irradiated human dermal fibroblasts. Life Sci. 2013, 92, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Wang, P.W.; Yang, S.C.; Chou, W.L.; Fang, J.Y. Cosmetic and therapeutic applications of fish oil’s fatty acids on the skin. Mar. Drugs 2018, 16, 256. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Shao, J.; Han, L.; Lv, R.; Sun, P. Separation, preliminary characterization, and moisture-preserving activity of polysaccharides from Ulva fasciata. Int. J. Biol. Macromol. 2015, 72, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, W.; Hou, Y.; Niu, X.; Zhang, H.; Zhang, Q. Chemical composition and moisture-absorption/retention ability of polysaccharides extracted from five algae. Int. J. Biol. Macromol. 2013, 57, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H.; Jenkins, G. A novel topical ingredient derived from seaweed significantly reduces symptoms of Acne vulgaris: A general literature review. J. Cosmet. Sci. 2013, 64, 219–226. [Google Scholar] [PubMed]
- Sebaaly, C.; Kassem, S.; Grishina, E.; Kanaan, H.; Sweidan, A.; Chmit, M.S.; Kanaan, H.M. Anticoagulant and antibacterial activities of polysaccharides of red algae Corallina collected from Lebanese coast. J. Appl. Pharm. Sci. 2014, 4, 30. [Google Scholar]
- Podkorytova, A.V.; Vafina, L.H.; Kovaleva, E.A.; Mikhailov, V.I. Production of algal gels from the brown alga, Laminaria japonica Aresch., and their biotechnological applications. J. Appl. Phycol. 2007, 19, 827–830. [Google Scholar] [CrossRef]
- Paudel, P.; Wagle, A.; Seong, S.H.; Park, H.J.; Jung, H.A.; Choi, J.S. A New Tyrosinase Inhibitor from the Red Alga Symphyocladia latiuscula (Harvey) Yamada (Rhodomelaceae). Mar. Drugs 2019, 17, 295. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Čopíková, J.; Kim, W.J.; Park, Y.I. Cell wall polysaccharides of marine algae. In Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 543–590. [Google Scholar]
- Sreekumar, K.; Bindhu, B. Alginic acid: A potential biopolymer from brown algae. Mater. Int. 2020, 2, 433–438. [Google Scholar]
- Michalak, I.; Dmytryk, A.; Chojnacka, K. Algae cosmetics. Encycl. Mar. Biotechnol. 2020, 1, 65–85. [Google Scholar]
- Feng, D.; Aldrich, C. Adsorption of heavy metals by biomaterials derived from the marine alga Ecklonia maxima. Hydrometallurgy 2004, 73, 1–10. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Ribeiro-Claro, P.J.A. Identification of selected seaweed polysaccharides (Phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Hempel, M.D.S.S.; Colepicolo, P.; Zambotti-Villela, L. Macroalgae Biorefinery for the Cosmetic Industry: Basic Concept, Green Technology, and Safety Guidelines. Phycology 2023, 3, 211–241. [Google Scholar] [CrossRef]
- Kleinübing, S.J.; Gai, F.; Bertagnolli, C.; Silva, M.G.C.D. Extraction of alginate biopolymer present in marine alga Sargassum filipendula and bioadsorption of metallic ions. Mater. Res. 2013, 16, 481–488. [Google Scholar] [CrossRef]
- Barros, F.C.; da Silva, D.C.; Sombra, V.G.; Maciel, J.S.; Feitosa, J.P.; Freitas, A.L.; de Paula, R.C. Structural characterization of polysaccharide obtained from red seaweed Gracilaria caudata (J Agardh). Carbohydr. Polym. 2013, 92, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Peris-Ortiz, M.; Álvarez-García, J. Health and Wellness Tourism: Emergency of New Market Segment; Springer: Madrid, Spain, 2014. [Google Scholar]
- Gutiérrez, G. Compositions of Padina Algae or Their Extracts, and Their Pharmaceutical, Food Compositions, or Use for the Culture of Molluscs or Arthropods. European Patent EP 0655250 Al, 31 May 1995. Available online: https://patents.google.com/patent/EP0655250A1/en (accessed on 1 October 2018).
- Morrice, L.M.; McLean, M.W.; Long, W.F.; Williamson, F.B. Porphyran primary structure. In Eleventh International Seaweed Symposium: Proceedings of the Eleventh International Seaweed Symposium, Held in Qingdao, People’s Republic of China, 19–25 June 1983; Springer: Dordrecht, The Netherlands, 1983; pp. 572–575. [Google Scholar]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Simal- Gandara, J. Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Kim, M.-S.; Oh, G.-H.; Kim, M.-J.; Hwang, J.-K. Fucosterol Inhibits Matrix Metalloproteinase Expression and Promotes Type-1 Procollagen Production in UVB-induced HaCaT Cells. Photochem. Photobiol. 2013, 89, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Lorbeer, A.J.; Tham, R.; Zhang, W. Potential products from the highly diverse and endemic macroalgae of Southern Australia and pathways for their sustainable production. Environ. Boil. Fishes 2013, 25, 717–732. [Google Scholar] [CrossRef]
- Drozd, N.N.; Tolstenkov, A.S.; Makarov, V.A.; Kuznetsova, T.A.; Besednova, N.N.; Shevchenko, N.M.; Zvyagintseva, T.N. Pharmacodynamic parameters of anticoagulants based on sulfated polysaccharides from marine algae. Bull. Exp. Biol. Med. 2006, 142, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Jang, J.; Njamen, D.; Mbafor, J.T.; Fomum, Z.T.; Kim, B.Y.; Oh, W.K.; Ahn, J.S. Protein tyrosine phosphatase-1B inhibitory activity of isoprenylated flavonoids isolated from Erythrina mildbraedii. J. Nat. Prod. 2006, 69, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
- Blondin, C.; de Agostiniz, A. Biological activities of polysaccharides from marine algae. Drugs Fut. 1995, 20, 1237–1249. [Google Scholar]
- Kim, K.B.; Jeong, S.M.; Kim, M.J.; Ahn, D.H. Tyrosinase Inhibitory Effects of Sargachromanol G, Sargachromanol I and Mojabanchromanol b isolated from Myagropsis myagroides. Indian J. Pharm. Sci. 2020, 82, 170–173. [Google Scholar] [CrossRef]
- Mensah, E.O.; Kanwugu, O.N.; Panda, P.K.; Adadi, P. Marine fucoidans: Structural, extraction, biological activities and their applications in the food industry. In Food Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2023; p. 108784.
- Carvalho, L.G.; Pereira, L. Review of marine algae as source of bioactive metabolites. In Marine Algae—Biodiversity, Taxonomy, Environmental Assessment and Biotechnology, 1st ed.; Pereira, L., Neto, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781466581678. [Google Scholar]
- Ukai, K.; Mizutani, Y.; Hisada, K.; Yokoyama, M.; Futaki, S.; Toya, H. Fuel Electrode Material, a Fuel Electrode, and a Solid Oxide Fuel Cell. U.S. Patent 20060110633 A1, 25 May 2006. [Google Scholar]
- Thanh, T.T.T.; Quach, T.M.T.; Nguyen, T.N.; Luong, D.V.; Bui, M.L.; Van Tran, T.T. Structure and cytotoxic activity of ulvan extracted from green seaweed Ulva lactuca. Int. J. Biol. Macromol. 2016, 93, 695–702. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar]
- Bodin, J.; Adrien, A.; Bodet, P.E.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Ulva intestinalis protein extracts promote in vitro collagen and hyaluronic acid production by human dermal fibroblasts. Molecules 2020, 25, 2091. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Li, B.; Wang, X.; Xu, X.; Liu, X.; Li, W.; Chang, X.; Li, H.; Qi, H. The antioxidant and antihyperlipidemic activities of phosphorylated polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 145, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from microalgae for cosmetic applications. Cosmetics 2021, 8, 52. [Google Scholar] [CrossRef]
- Ayoub, A.; Pereira, J.M.; Rioux, L.E.; Turgeon, S.L.; Beaulieu, M.; Moulin, V.J. Role of seaweed laminaran from Saccharina longicruris on matrix deposition during dermal tissue-engineered production. Int. J. Biol. Macromol. 2015, 75, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ozanne, H.; Toumi, H.; Roubinet, B.; Landemarre, L.; Lespessailles, E.; Daniellou, R.; Cesaro, A. Laminarin effects, a β-(1, 3)-glucan, on skin cell inflammation and oxidation. Cosmetics 2020, 7, 66. [Google Scholar] [CrossRef]
- Susano, P.; Silva, J.; Alves, C.; Martins, A.; Gaspar, H.; Pinteus, S.; Mouga, T.; Goettert, M.I.; Petrovski, Ž.; Branco, L.B.; et al. Unravelling the dermatological potential of the brown seaweed Carpomitra costata. Marine Drugs 2021, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.; Barqawi, A.A.; Mansour, A.T. Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Morya, V.K.; Kim, J.; Kim, E.K. Algal fucoidan: Structural and size-dependent bioactivities and their perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.M.; Helley, D.; Colliec-Jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.A.; Kang, N.; Ahn, G.; Jee, Y.; Kim, Y.T.; Jeon, Y.J. Bioactive potentials of sulfated polysaccharides isolated from brown seaweed Sargassum spp. in related to human health applications: A review. Food Hydrocoll. 2018, 81, 200–208. [Google Scholar] [CrossRef]
- Nagaoka, M.; Shibata, H.; Kimura-Takagi, I.; Hashimoto, S.; Aiyama, R.; Ueyama, S.; Yokokura, T. Anti-ulcer effects and biological activities of polysaccharides from marine algae. Biofactors 2000, 12, 267. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Lee, S.H.; Ku, M.J.; Yu, B.C.; Jeon, M.J.; Jeong, S.H.; Stonik, V.A.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. Eur. J. Dermatol. 2009, 19, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oh, J.Y.; Lee, W.; Jeon, Y.J. Fucoidan isolated from Hizikia fusiforme suppresses ultraviolet B-induced photodamage by down-regulating the expressions of matrix metalloproteinases and pro-inflammatory cytokines via inhibiting NF-κB, AP-1, and MAPK signaling pathways. Int. J. Biol. Macromol. 2021, 166, 751–759. [Google Scholar] [CrossRef]
- Su, W.; Wang, L.; Fu, X.; Ni, L.; Duan, D.; Xu, J.; Gao, X. Protective effect of a fucose-rich fucoidan isolated from Saccharina japonica against ultraviolet B-induced photodamage in vitro in human keratinocytes and in vivo in zebrafish. Mar. Drugs 2020, 18, 316. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jayawardena, T.U.; Yang, H.W.; Lee, H.G.; Jeon, Y.J. The potential of sulfated polysaccharides isolated from the brown seaweed Ecklonia maxima in cosmetics: Antioxidant, anti-melanogenesis, and photoprotective activities. Antioxidants 2020, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy—Cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Fernando, P.S.; Kim, K.N.; Kim, D.; Jeon, Y.J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2018, 1–15. [Google Scholar]
- Xue, C.; Yu, G.; Hirata, T.; Terao, J.; Lin, H. Antioxidative activities of several marine polysaccharides evaluated in a phosphatidylcholine-liposomal suspension and organic solvents. Biosci. Biotechnol. Biochem. 1998, 62, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Perde-Schrepler, M.; Chereches, G.; Brie, I.; Tatomir, C.; Postescu, I.D.; Soran, L.; Filip, A. Grape seed extract as photochemopreventive agent against UVB-induced skin cancer. J. Photochem. Photobiol. B Biol. 2013, 118, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Mohamed, M.A.A.; Park, S.Y.; Yi, T.H. Fucosterol protects cobalt chloride induced inflammation by the inhibition of hypoxia-inducible factor through PI3K/Akt pathway. Int. Immunopharmacol. 2015, 29, 642–647. [Google Scholar] [CrossRef]
- Verdin, A.; Cazier, F.; Fitoussi, R.; Blanchet, N.; Vié, K.; Courcot, D.; Momas, I.; Seta, N.; Achard, S. An in vitro model to evaluate the impact of environmental fine particles (PM 0.3–2.5) on skin damage. Toxicol. Lett. 2019, 305, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive polysaccharides from seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef] [PubMed]
- Sachan, N.K.; Pushkar, S.; Jha, A.; Bhattcharya, A.J.J.P.R. Sodium alginate: The wonder polymer for controlled drug delivery. J. Pharm. Res. 2009, 2, 1191–1199. [Google Scholar]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae–A review. J. Algal Biomass Util. 2012, 3, 89–100. [Google Scholar]
- Xu, S.Y.; Kan, J.; Hu, Z.; Liu, Y.; Du, H.; Pang, G.C.; Cheong, K.L. Quantification of neoagaro-oligosaccharide production through enzymatic hydrolysis and its anti-oxidant activities. Molecules 2018, 23, 1354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jin, W.; Duan, D.; Zhang, Q. Structural analysis and anti-complement activity of polysaccharides extracted from Grateloupia livida (Harv.) Yamada. J. Oceanol. Limnol. 2019, 37, 806–814. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Song, X.N.; Lin, Y.; Xiao, Q.; Du, X.P.; Chen, Y.H.; Xiao, A.F. Antioxidant capacity and prebiotic effects of Gracilaria neoagaro oligosaccharides prepared by agarase hydrolysis. Int. J. Biol. Macromol. 2019, 137, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Leandro, A.; Pereira, L.; Gonçalves, A.M. Diverse applications of marine macroalgae. Mar. Drugs 2019, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Ktari, L.; Chebil Ajjabi, L.; De Clerck, O.; Gómez Pinchetti, J.L.; Rebours, C. Seaweeds as a promising resource for blue economy development in Tunisia: Current state, opportunities, and challenges. J. Appl. Phycol. 2022, 34, 489–505. [Google Scholar] [CrossRef]
- Nussinovitch, A. Hydrocolloid Applications; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 1997; ISBN 978-1-4613-7933-1. [Google Scholar]
- Pereira, L.; Correia, F. Algas Marinhas da Costa Portuguesa-Ecologia, Biodiversidade e Utilizações; Nota de Rodapé Editores: Paris, France, 2015; ISBN 978-989-20-5754-5. [Google Scholar]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A promising source of valuable bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Probst, Y. A review of the nutrient composition of selected Rubus berries. Nutr. Food Sci. 2015, 45, 242–254. [Google Scholar] [CrossRef]
- Rasmussen, R.S.; Morrissey, M.T. Marine biotechnology for production of food ingredients. Adv. Food Nutr. Res. 2007, 52, 237–292. [Google Scholar] [PubMed]
- Ouyang, Q.-Q.; Hu, Z.; Li, S.-D.; Quan, W.-Y.; Wen, L.-L.; Yang, Z.-M.; Li, P. Thermal degradation of agar: Mechanism and toxicity of products. Food Chem. 2018, 264, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, V.A.; Navarro, D.A.; Ponce, N.M.; Stortz, C.A. Seaweed polysaccharides: Structure and applications. In Industrial Applications of Renewable Biomass Products: Past, Present and Future; Springer: Berlin/Heidelberg, Germany, 2017; pp. 75–116. [Google Scholar]
- Alp Arıcı, T.; Özcan, A.S.; Özcan, A. Biosorption characteristics of Cu (II) and Cd (II) ions by modified alginate. J. Polym. Environ. 2020, 28, 3221–3234. [Google Scholar] [CrossRef]
- Chen, X.; Fu, X.; Huang, L.; Xu, J.; Gao, X. Agar oligosaccharides: A review of preparation, structures, bioactivities and application. Carbohydr. Polym. 2021, 265, 118076. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.; Batool, R.; Khan, M.U.; Rauf, A.; Akhtar, W.; Heydary, M.; Rehman, S.; Shahzad, T.; Malik, A.; Mosavat, S.-H.; et al. An overview on red algae bioactive compounds and their pharmaceutical applications. J. Altern. Complement. Med. 2021, 17, 20190203. [Google Scholar] [CrossRef] [PubMed]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. Antioxidant and immunostimulating activities in vitro of sulfated polysaccharides isolated from Gracilaria rubra. J. Funct. Foods 2017, 28, 64–75. [Google Scholar] [CrossRef]
- Isaka, S.; Cho, K.; Nakazono, S.; Abu, R.; Ueno, M.; Kim, D.; Oda, T. Antioxidant and anti-inflammatory activities of porphyrin isolated from discolored nori (Porphyra yezoensis). Int. J. Biol. Macromol. 2015, 74, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, M.; Golmoradizadeh, A.; Homaei, A. Carrageenans and carrageenases: Versatile polysaccharides and promising marine enzymes. Phytochem. Rev. 2018, 17, 535–571. [Google Scholar] [CrossRef]
- Kwon, M.; Nam, T. Porphyran induces apoptosis related signal pathway in AGS gastric cancer cell lines. Life Sci. 2006, 79, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Farahani, M.; Sedighi, M.; Rabiee, N.; Savoji, H. Carrageenans for tissue engineering and regenerative medicine applications: A review. Carbohydr. Polym. 2022, 281, 119045. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.B.; Brocksom, T.J.; de Oliveira, K.T. Tetrabenzoporphyrins: Synthetic developments and applications. Chem. Soc. Rev. 2013, 42, 3302–3317. [Google Scholar] [CrossRef] [PubMed]
- Araujo, I.W.F.; Vanderlei, E.S.O.; Rodrigues, J.A.G.; Coura, C.O.; Quindere, A.L.G.; Fontes, B.P.; Queiroz, I.N.L.; Jorge, R.J.B.; Bezerra, M.M.; Silva, A.A.R.; et al. Effects of a sulfated polysaccharide isolated from the red seaweed Solieria filiformis on models of nociception and inflammation. Carbohydr. Polym. 2011, 86, 1207–1215. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, R.; Wu, H.; Li, X.; Li, S.; Bian, L. Evaluation of acute and sub-chronic toxicity of Lithothamnion sp. in mice and rats. Toxicol. Rep. 2020, 7, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Cho, J.G.; Hwang, E.S.; Yang, J.E.; Gao, W.; Fang, M.Z.; Zheng, S.D.; Yi, T.H. Enhancement of protective effects of radix scutellariae on UVB-induced photo damage in human HaCaT keratinocytes. Appl. Biochem. Biotechnol. 2018, 184, 1073–1093. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-T.; Zhang, X.; Liu, Y.; Chen, X.-Q.; Cheong, K.-L. Quantification of 3,6-anhydro-galactose in red seaweed polysaccharides and their potential skin-whitening activity. 3 Biotech 2020, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.; Pereira, L.; Sousa-Pinto, I. The seaweed resources of Portugal. Bot. Mar. 2019, 62, 499–525. [Google Scholar] [CrossRef]
- Armisén, R. Agar and agarose biotechnological applications. In International Workshop on Gelidium: Proceedings of the International Workshop on Gelidium Held in Santander, Spain, 3–8 September 1991; Springer: Dordrecht, The Netherlands, 1991; pp. 157–166. [Google Scholar]
- Yalçın, S. The mechanism of heavy metal biosorption on green marine macroalga Enteromorpha linza. Clean–Soil Air Water. 2014, 42, 251–259. [Google Scholar] [CrossRef]
- Araki, C. Some recent studies on the polysaccharides of agarophytes. In Proceedings of the Fifth International Seaweed Symposium, Halifax, Nova Scotia, 25–28 August 1966; pp. 3–17. [Google Scholar]
- Li, S.S.; Song, Y.L.; Yang, H.R.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R. Modifying alginate beads using polycarboxyl component for enhanced metal ions removal. Int. J. Biol. Macromol. 2020, 158, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, E.; Cifuentes, A. Benefits of using algae as natural sources of functional ingredients. J. Sci. Food Agric. 2013, 93, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Bi, D.; Yao, L.; Li, T.; Gu, L.; Xu, H.; Li, X.; Li, H.; Hu, Z.; Xu, X. The inhibitory activity of alginate against allergic reactions in an ovalbumin-induced mouse model. Food Funct. 2020, 11, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Sosnowska, K.; Tomczykowa, M.; Winnicka, K.; Kasacka, I.; Tomczyk, M. In vivo anti-inflammatory and antiallergic activities of cynaroside evaluated by using hydrogel formulations. Biomed. Pharmacother. 2020, 121, 109681. [Google Scholar] [CrossRef] [PubMed]
- Mišurcová, L. Chemical composition of seaweeds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 171–192. [Google Scholar]
- Burtin, P. Nutritional value of seaweeds. Electron. J. Environ. Agric. Food Chem. 2003, 2, 498–503. [Google Scholar]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tian, Y.; Pei, J.; Zhang, Y.; Hao, D.; Han, T.; Wang, X.; Song, S.; Huang, L.; Wang, Z. Sea cucumber chondroitin sulfate polysaccharides attenuate OVA-induced food allergy in BALB/c mice associated with gut microbiota metabolism and Treg cell differentiation. Food Funct. 2023, 14, 7375–7386. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Kim, D.; Banerjee, I.; Pal, K. Carrageenan: A wonder polymer from marine algae for potential drug delivery applications. Curr. Pharm. Des. 2019, 25, 1172–1186. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Matsuhiro, B. Vibrational spectroscopy of seaweed galactans. In Fifteenth International Seaweed Symposium: Proceedings of the Fifteenth International Seaweed Symposium Held in Valdivia, Chile, in January 1995; Springer: Dordrecht, The Netherlands, 1995; pp. 481–489. [Google Scholar]
- Rinaudo, M. Seaweed Polysaccharides. In Comprehensive Glycoscience; Kamerling, J.P., Ed.; Elsevier: Oxford, UK, 2007; pp. 691–735. ISBN 978-0-444-51967-2. [Google Scholar]
- Usov, A.I.; Zelinsky, N.D. Chemical structures of algal polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing: Cambridge, UK, 2013; pp. 23–86. [Google Scholar]
- Charlier, R.H.; Chaineux, M.-C.P. The Healing Sea: A Sustainable Coastal Ocean Resource: Thalassotherapy. J. Coast. Res. 2009, 254, 838–856. [Google Scholar] [CrossRef]
- Fernando, I.S.; Sanjeewa, K.A.; Kim, S.-Y.; Lee, J.-S.; Jeon, Y.-J. Reduction of heavy metal (Pb2+) biosorption in zebrafish model using alginic acid purified from Ecklonia cava and two of its synthetic derivatives. Int. J. Biol. Macromol. 2018, 106, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhan, X.; Wan, J.; Wang, Y.; Wang, C. Review for carrageenan-based pharmaceutical biomaterials: Favourable physical features versus adverse biological effects. Carbohydr. Polym. 2015, 121, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Villarroel, L.H.; Zanlungo, A.B. Structural studies on the porphyran from Porphyra columbina (Montagne). Carbohydr. Res. 1981, 88, 139–145. [Google Scholar] [CrossRef]
- Bhatia, S.; Sharma, A.; Sharma, K.; Kavale, M.; Chaugule, B.; Dhalwal, K.; Namdeo, A.; Mahadik, K. Novel algal polysaccharides from marine source: Porphyran. Pharmacogn. Rev. 2008, 2, 271. [Google Scholar]
- Bhatia, S.; Sharma, K.; Sharma, A.; Nagpal, K.; Bera, T. Anti-inflammatory, Analgesic and Antiulcer properties of Porphyra vietnamensis. Avicenna J. Phytomed. 2015, 5, 69. [Google Scholar] [PubMed]
- Mondal, S.; Pain, T.; Sahu, K.; Kar, S. Large-scale Green synthesis of porphyrins. ACS Omega 2021, 6, 22922–22936. [Google Scholar] [CrossRef]
- Usman, A.; Khalid, S.; Usman, A.; Hussain, Z.; Wang, Y. Algal polysaccharides, novel application, and outlook. In Algae Based Polymers, Blends, and Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 115–153. [Google Scholar]
- Šimat, V.; Elabed, N.; Kulawik, P.; Ceylan, Z.; Jamroz, E.; Yazgan, H.; Čagalj, M.; Regenstein, J.M.; Özogul, F. Recent advances in marine-based nutraceuticals and their health benefits. Mar. Drugs 2020, 18, 627. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Mannan, M.A.U. The role of algae in nutraceutical and pharmaceutical production. In Bioactive Natural Products in Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2020; pp. 665–685. [Google Scholar]
- Ughy, B.; Nagy, C.I.; Kós, P.B. Biomedical potential of cyanobacteria and algae. Acta Biol. Szeged. 2015, 59 (Suppl. S2), 203–224. [Google Scholar]
- Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.C.; Pereira, L.; Gonçalves, A.M. Environmental impact on seaweed phenolic production and activity: An important step for compound exploitation. Mar. Drugs 2021, 19, 245. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Costa, A.M.S.; Rodrigues, J.M.M.; Pérez-Madrigal, M.M.; Dove, A.P.; Mano, J.F. Modular Functionalization of Laminarin to Create Value-Added Naturally Derived Macromolecules. J. Am. Chem. Soc. 2020, 142, 19689–19697. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, C.; Yang, C.; Li, J.; Sun, T.; Yu, G. Recent advances in pharmaceutical potential of brown algal polysaccharides and their derivatives. Curr. Pharm. Des. 2019, 25, 1290–1311. [Google Scholar] [CrossRef] [PubMed]
- Tümen Erden, S.; Ekentok Atici, C.; Cömez, B.; Sezer, A.D. Preparation and in vitro characterization of laminarin based hydrogels. J. Res. Pharm. 2021, 25, 164–172. [Google Scholar]
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef] [PubMed]
- Murapa, P.; Dai, J.; Chung, M.; Mumper, R.J.; D’Orazio, J. Anthocyanin-rich fractions of blackberry extracts reduce UV-induced free radicals and oxidative damage in keratinocytes. Phytother. Res. 2012, 26, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lee, S.G.; Lim, K.T.; Kim, H.R. Potential anti-aging substances derived from seaweeds. Marine Drugs 2020, 18, 564. [Google Scholar] [CrossRef] [PubMed]
- Chevolot, L.; Mulloy, B.; Ratiskol, J.; Foucault, A.; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr. Res. 2001, 330, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Cho, Y.-N.; Patil, M.P.; Cho, Y.-J.; Kim, G.-D.; Park, Y.B.; Woo, H.-C.; Chun, B.-S. Hydrothermal degradation of seaweed polysaccharide: Characterization and biological activities. Food Chem. 2018, 268, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Deepika, C.; Ravishankar, G.A.; Rao, A.R. Potential products from macroalgae: An overview. In Sustainable Global Resources of Seaweeds Volume 1: Bioresources, Cultivation, Trade and Multifarious Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 17–44. [Google Scholar]
- Kuznetsova, T.A.; Besednova, N.N.; Mamaev, A.; Momot, A.P.; Shevchenko, N.M.; Zvyagintseva, T.N. Anticoagulant Activity of Fucoidan from Brown Algae Fucus evanescens of the Okhotsk Sea. Bull. Exp. Biol. Med. 2003, 136, 471–473. [Google Scholar] [CrossRef]
- Obluchinsksya, E.D.; Makarova, M.N.; Pozharitskaya, O.N.; Shikov, A.N. Effects of Ultrasound Treatment on the Chemical Composition and Anticoagulant Properties of Dry Fucus Extract. Pharm. Chem. J. 2015, 49, 183–186. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Holtkamp, A.D.; Kelly, S.; Ulber, R.; Lang, S. Fucoidans and fucoidanases-focus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl. Microbiol. Biotechnol. 2009, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Rupérez, P.; Gómez-Ordóñez, E.; Jiménez-Escrig, A. Biological activity of algal sulfated and nonsulfated polysaccharides. In Bioactive Compounds from Marine Foods: Plant and Animal Sources; John Wiley & Sons: New York, NY, USA, 2013; pp. 219–247. [Google Scholar]
- Moon, H.E.; Islam, N.; Ahn, B.R.; Chowdhury, S.S.; Sohn, H.S.; Jung, H.A.; Choi, J.S. Protein Tyrosine Phosphatase 1B and α-Glucosidase Inhibitory Phlorotannins from Edible Brown Algae, Ecklonia stolonifera and Eisenia bicyclis. Biosci. Biotechnol. Biochem. 2011, 75, 1472–1480. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.-K. Beneficial Effects of Marine Algal Compounds in Cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Freag, M.S.; Elkhodairy, K.A. Biopolymeric Nanoparticles for Targeted Drug Delivery to Brain Tumors; Kesharwani, P., Gupta, U., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 169–190. ISBN 978-0-12-812218-1. [Google Scholar]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Liu, X.; Hao, J.; Cai, C.; Fan, F.; Dun, Y.; Zhao, X.; Liu, X.; Li, C.; Yu, G. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int. J. Biol. Macromol. 2016, 82, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.; Ganesan, P.; Suresh, P.V.; Bhaskar, N. Seaweeds as a source of nutritionally beneficial compounds-a review. J. Food Sci. Technol. 2008, 45, 1. [Google Scholar]
- Narayanaswamy, R.; Jo, B.W.; Choi, S.K.; Ismail, I.S. Fucoidan: Versatile cosmetic ingredient. An overview. J. Appl. Cosmetol. 2013, 31, 131–138. [Google Scholar]
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Biology of Seaweeds in Seaweed. In Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Nikki Levy Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 41–106. [Google Scholar]
- Li, N.; Zhang, Q.; Song, J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem. Toxicol. 2005, 43, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef] [PubMed]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarasaiyar, K.; Goh, B.H.; Jeon, Y.J.; Yow, Y.Y. Algae metabolites in cosmeceutical: An overview of current applications and challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.S.; Vaas, A.; De Silva, H.I.C.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, W.; Ahn, G.; Lee, D.S.; et al. A keratinocyte and integrated fibroblast culture model for studying particulate matter-induced skin lesions and therapeutic intervention of fucosterol. Life Sci. 2019, 233, 116714. [Google Scholar] [CrossRef]
- Manlusoc, J.K.T.; Hsieh, C.L.; Hsieh, C.Y.; Salac, E.S.N.; Lee, Y.T.; Tsai, P.W. Pharmacologic application potentials of sulfated polysaccharide from marine algae. Polymers 2019, 11, 1163. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.; Jeon, Y. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Kartik, A.; Akhil, D.; Lakshmi, D.; Panchamoorthy Gopinath, K.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A critical review on production of biopolymers from algae biomass and their applications. Bioresour. Technol. 2021, 329, 124868. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.-H.; Fang, Y.; Lin, H.; Chen, L.; Li, Z.-J.; Deng, D.; Lu, C.-X. Chemical characters and antioxidative properties of sulphated polysaccharides from Laminaria japonica. J. Appl. Phycol. 2001, 13, 67–70. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Ahmed, T.A.; Rjabovs, V.; Hammami, R.; Critchley, A.T.; Tuvikene, R.; Hincke, M.T. Immunomodulation by xylan and carrageenan-type polysaccharides from red seaweeds: Anti-inflammatory, wound healing, cytoprotective, and anticoagulant activities. Int. J. Biol. Macromol. 2024, 260, 129433. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kang, K.; Jeon, J.S.; Jho, E.H.; Kim, C.Y.; Nho, C.W.; Um, B.H. Isolation of phlorotannins from Eisenia bicyclis and their hepatoprotective effect against oxidative stress induced by tert-butyl hyperoxide. Appl. Biochem. Biotechnol. 2011, 165, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Jiang, Y.; Wang, J.; Hwang, H.; Yang, X.; Wang, P. Structure characterization of low molecular weight sulfate Ulva polysaccharide and the effect of its derivative on iron deficiency anemia. Int. J. Biol. Macromol. 2019, 126, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Ushakova, N.A.; Usov, A.I.; Kiselevskiy, M.V.; Nifantiev, N.E. Fucoidans: Pro-or antiangiogenic agents? Glycobiology 2014, 24, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Ali Karami, M.; Sharif Makhmalzadeh, B.; Pooranian, M.; Rezai, A. Preparation and optimization of silibinin-loaded chitosan–fucoidan hydrogel: An in vivo evaluation of skin protection against UVB. Pharm. Dev. Technol. 2021, 26, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.L.; Jiang, Y.F.; Lu, Y.A.; Kang, M.C.; Jeon, Y.J. Fucoidan from acid-processed Hizikia fusiforme attenuates oxidative damage and regulate apoptosis. Int. J. Biol. Macromol. 2020, 160, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K. A review of the usability of fucoidan extracted from brown seaweed as a functional ingredient of cosmetics. Kor J Aesthet Cosmetol. 2014, 12, 447–452. [Google Scholar]
- Ji, D.; You, L.; Ren, Y.; Wen, L.; Zheng, G.; Li, C. Protective effect of polysaccharides from Sargassum fusiforme against UVB-induced oxidative stress in HaCaT human keratinocytes. J. Funct. Foods 2017, 36, 332–340. [Google Scholar] [CrossRef]
- Yarkent, Ç.; Gürlek, C.; Oncel, S.S. Potential of microalgal compounds in trending natural cosmetics: A review. Sustainable Chemistry and Pharmacy. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Vo, T.; Ngo, D.; Kang, K.; Jung, W.; Kim, S. The beneficial properties of marine polysaccharides in alleviation of allergic responses. Mol. Nutr. Food Res. 2015, 59, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.-E.; Kim, K.H.; Kang, N.J. Beneficial effects of marine algae-derived carbohydrates for skin health. Mar. Drugs 2018, 16, 459. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Lahaye, M. Cell-wall polysaccharides from the marine green alga Ulva “rigida” (Ulvales, Chlorophyta). Chemical structure of ulvan. Carbohydr. Res. 1995, 274, 313–318. [Google Scholar] [CrossRef]
- Yaich, H.; Ben Amira, A.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int. J. Biol. Macromol. 2017, 105, 1430–1439. [Google Scholar] [CrossRef]
- Pereira, L. Biological and therapeutic properties of the seaweed polysaccharides. Int. Biol. Rev. 2018, 2, 1–50. [Google Scholar] [CrossRef]
- Lahaye, M.; Rochas, C. Chemical structure and physico-chemical properties of agar. Hydrobiology 1991, 221, 137–148. [Google Scholar] [CrossRef]
- Zanella, L.; Alam, M.A. Extracts and bioactives from microalgae (sensu stricto): Opportunities and challenges for a new generation of cosmetics. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Berlin/Heidelberg, Germany, 2020; pp. 295–349. [Google Scholar]
- Robic, A.; Rondeau-Mouro, C.; Sassi, J.-F.; Lerat, Y.; Lahaye, M. Structure and interactions of ulvan in the cell wall of the marine green algae Ulva rotundata (Ulvales, Chlorophyceae). Carbohydr. Polym. 2009, 77, 206–216. [Google Scholar] [CrossRef]
- Basti, D.; Bricknell, I.; Beane, D.; Bouchard, D. Recovery from a near-lethal exposure to ultraviolet-C radiation in a scleractinian coral. J. Invertebr. Pathol. 2009, 101, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Massironi, A.; Puppi, D.; Creti, D.; Martinez, E.D.; Bonistalli, C.; Fabroni, C.; Morgenni, F.; Chiellini, F. Development of ulvan-based emulsions containing flavour and fragrances for food and cosmetic applications. Flavour Fragr. J. 2019, 34, 411–425. [Google Scholar] [CrossRef]
- Duraikkannu, S.L.; Sankaranarayanan, S.; Gajaria, T.K.; Li, G.; Kujawski, W.; Kujawa, J.; Navia, R. A Short Review on the Valorization of Green Seaweeds and Ulvan: FEEDSTOCK for Chemicals and Biomaterials. Biomolecules 2020, 10, 991. [Google Scholar]
- Bai, L.; Xu, D.; Zhou, Y.M.; Zhang, Y.B.; Zhang, H.; Chen, Y.B.; Cui, Y.L. Antioxidant activities of natural polysaccharides and their derivatives for biomedical and medicinal applications. Antioxidants 2022, 11, 2491. [Google Scholar] [CrossRef]
- Oucif, H.; Benaissa, M.; Ali Mehidi, S.; Prego, R.; Aubourg, S.P.; Abi-Ayad, S.M.E.A. Chemical composition and nutritional value of different seaweeds from the west Algerian coast. J. Aquat. Food Prod. Technol. 2020, 29, 90–104. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and function properties of Ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, T.; Du, Y.; Jiang, L.; Yao, Z.; Ning, L.; Zhu, B. Ulvan and Ulva oligosaccharides: A systematic review of structure, preparation, biological activities and applications. Bioresour. Bioprocess. 2023, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Carnachan, S.M.; Magnusson, M.; Lawton, R.J.; Sims, I.M.; Hinkley, S.F.R.; de Nys, R.; Glasson, C.R.K. Are all ulvans equal? A comparative assessment of the chemical and gelling properties of ulvan from blade and filamentous Ulva. Carbohyd. Polym. 2021, 264, 118010. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, H.; Wang, X.; Wang, Y.; Jiang, N.; Qi, H.; Liu, X. Antioxidant and antihyperlipidemic activities of high sulfate content purified polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 146, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.L.; Yu, G.L.; Zhang, J.Z.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Fournière, M.; Bedoux, G.; Lebonvallet, N.; Lescchiera, R.; Goff-Pain, C.L.; Bourgougnon, N.; Latire, T. Poly-and oligosaccharide ulva sp. Fractions from enzyme-assisted extraction modulate the metabolism of extracellular matrix in human skin fibroblasts: Potential in anti-aging dermo-cosmetic applications. Mar. Drugs 2021, 19, 156. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Zhang, Q.; Zhang, Z.; Shi, X.; Li, P. Synthesized different derivatives of low molecular fucoidan extracted from Laminaria japonica and their potential antioxidant activity in vitro. Int. J. Biol. Macromol. 2009, 44, 379–384. [Google Scholar] [CrossRef]
- Marudhupandi, T.; Kumar, T.T.; Senthil, S.L.; Devi, K.N. In vitro antioxidant properties of fucoidan fractions from Sargassum tenerrimum. Pak. J. Biol. Sci. 2014, 17, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Lee, S.R.; Shim, S.N.; Jeong, S.H.; Stonik, V.A.; Rasskazov, V.A.; Zvyagintseva, T.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 expression in human skin fibroblasts. Biol. Pharm. Bull. 2008, 31, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Leirós, G.J.; Kusinsky, A.G.; Balañá, M.E.; Hagelin, K. Triolein reduces MMP-1 upregulation in dermal fibroblasts generated by ROS production in UVB-irradiated keratinocytes. J. Dermatol. Sci. 2017, 85, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H. Topical application of fucoidan improves atopic dermatitis symptoms in NC/Nga mice. Phytother. Res. 2012, 26, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Park, K.S.; Ku, M.J.; Lee, M.S.; Jeong, S.H.; Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Effect of Costaria costata fucoidan on expression of matrix metalloproteinase-1 promoter, mRNA, and protein. J. Nat. Prod. 2009, 72, 1731–1734. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Balcos, M.C.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Kim, M.K.; Kim, D.S. ERK activation by fucoidan leads to inhibition of melanogenesis in Mel-Ab cells. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2015, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tamauchi, H.; Kawakami, F.; Yoshinaga, K.; Nakano, T. Suppressive effect of dietary fucoidan on proinflammatory immune response and MMP-1 expression in UVB-irradiated mouse skin. Planta Medica 2015, 81, 1370–1374. [Google Scholar] [PubMed]
- Rocha, H.A.; Franco, C.R.; Trindade, E.S.; Veiga, S.S.; Leite, E.L.; Nader, H.B.; Dietrich, C.P. Fucan inhibits Chinese hamster ovary cell (CHO) adhesion to fibronectin by binding to the extracellular matrix. Planta Medica 2005, 71, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Chang, H.; He, K.; Ni, Y.; Li, C.; Hou, M.; Chen, L.; Xu, Z.; Chen, B.; Ji, M. Fucoidan from seaweed Fucus vesiculosus inhibits 2, 4-dinitrochlorobenzene-induced atopic dermatitis. Int. Immunopharmacol. 2019, 75, 105823. [Google Scholar] [CrossRef]
- Oomizu, S.; Yanase, Y.; Suzuki, H.; Kameyoshi, Y.; Hide, M. Fucoidan prevents Cε germline transcription and NFκB p52 translocation for IgE production in B cells. Biochem. Biophys. Res. Commun. 2006, 350, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Hiragun, T.; Takahagi, S.; Yanase, Y.; Morioke, S.; Mihara, S.; Kameyoshi, Y.; Hide, M. Fucoidan suppresses IgE production in peripheral blood mononuclear cells from patients with atopic dermatitis. Arch. Dermatol. Res. 2011, 303, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Zvyagintseva, T.N.; Shevchenko, N.M.; Popivnich, I.B.; Isakov, V.V.; Scobun, A.S.; Sundukova, E.V.; Elyakova, L.A. A new procedure for the separation of water-soluble polysaccharides from brown seaweeds. Carbohydr. Res. 1999, 322, 32–39. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ermakova, S.P.; Choi, H.K.; Kusaykin, M.I.; Shevchenko, N.M.; Zvyagintseva, T.N.; Choi, H.S. Fucoidan from Laminaria cichorioides inhibits AP-1 transactivation and cell transformation in the mouse epidermal JB6 cells. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Anderson Cancer Cent. 2008, 47, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, Q.; Zhao, T.; Hu, R.; Zhang, K.; Li, Z. In vitro antioxidant activity of acetylated and benzoylated derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta). Bioorganic Med. Chem. Lett. 2006, 16, 2441–2445. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, L.; Qin, Y.; Liang, W.H.; Mo, M.Q.; Liu, S.L.; Liang, F.; Wang, Y.; Tan, W.; Liang, Y. Effect of laminarin polysaccharide on activity of matrix metalloproteinase in photoaging skin. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 2013, 38, 2370–2373. [Google Scholar]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Pilot production of ulvans from Ulva sp. and their effects on hyaluronan and collagen production in cultured dermal fibroblasts. Carbohydr. Polym. 2017, 157, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Thevanayagam, H.; Mohamed, S.M.; Chu, W.L. Assessment of UVB-photoprotective and antioxidative activities of carrageenan in keratinocytes. J. Appl. Phycol. 2014, 26, 1813–1821. [Google Scholar] [CrossRef]
- Kuda, T.; Tsunekawa, M.; Goto, H.; Araki, Y. Antioxidant properties of four edible algae harvested in the Noto Peninsula, Japan. J. Food Compos. Anal. 2005, 18, 625–633. [Google Scholar] [CrossRef]
- Takematsu, H.; Seiji, M. Effect of macrophages on elimination of dermal melanin from the dermis. Arch. Dermatol. Res. 1984, 276, 96–98. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Zhou, G.; Lu, X.; Xu, Z.; Li, Z. In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodephyta) in aging mice. Pharmacol. Res. 2003, 48, 151–155. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Liu, X.; Zhao, Z.; Li, Z.; Xu, Z. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr. Res. 2004, 339, 105–111. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Q.; Qi, H.; Zhang, H.; Niu, X.; Xu, Z.; Li, Z. Degradation of porphyran from Porphyra haitanensis and the antioxidant activities of the degraded porphyrans with different molecular weight. Int. J. Biol. Macromol. 2006, 38, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Hama, Y.; Yamaguchi, K.; Oda, T. Inhibitory effect of sulphated polysaccharide porphyran on nitric oxide production in lipopolysaccharide-stimulated RAW264. 7 macrophages. J. Biochem. 2012, 151, 65–74. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.; Sharma-Shivappa, R.; van Zanten, J. Antioxidant activity of phlorotannins from brown algae. International J. Agric. Biol. Eng. 2017, 10, 184–191. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, J.; Zhang, H.; Niu, X.; Li, P. Preparation of the different derivatives of the low-molecular-weight porphyran from Porphyra haitanensis and their antioxidant activities in vitro. Int. J. Biol. Macromol. 2009, 45, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Shanura Fernando, I.P.; Asanka Sanjeewa, K.K.; Samarakoon, K.W.; Kim, H.S.; Gunasekara, U.K.D.S.S.; Park, Y.J.; Abeytungaa, D.T.U.; Lee, W.W.; Jeon, Y.-J. The potential of fucoidans from Chnoospora minima and Sargassum polycystum in cosmetics: Antioxidant, anti-inflammatory, skin-whitening, and antiwrinkle activities. J. Appl. Phycol. 2018, 30, 3223–3232. [Google Scholar] [CrossRef]
- Han, J.; Liu, B.; Liu, Q.-M.; Zhang, Y.-F.; Liu, Y.-X.; Liu, H.; Cao, M.-J.; Liu, G.-M. Red algae sulfated polysaccharides effervescent tablets attenuated ovalbumin-induced anaphylaxis by upregulating regulatory T cells in mouse models. J. Agric. Food Chem. 2019, 67, 11911–11921. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Patra, S.; Nayak, R.; Behera, C.; Dash, S.R.; Nayak, S.; Sahu, B.B.; Bhutia, S.K.; Jena, M. Multifunctional role of fucoidan, sulfated polysaccharides in human health and disease: A journey under the sea in pursuit of potent therapeutic agents. Int. J. Biol. Macromol. 2020, 164, 4263–4278. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.P.; Rodríguez-Hermida, V.; Pérez-Larrán, P.; Conde, E.; Liveri, M.T.; Ribeiro, D.; Fernandes, E.; Domínguez, H. In vitro bioactive properties of phlorotannins recovered from hydrothermal treatment of Sargassum muticum. Sep. Purif. Technol. 2016, 167, 117–126. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-E.; Kim, H.; Seo, C.; Park, T.; Lee, K.B.; Yoo, S.-Y.; Hong, S.-C.; Kim, J.T.; Lee, J. Marine polysaccharides: Therapeutic efficacy and biomedical applications. Arch. Pharm. Res. 2017, 40, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Chen, X.; Sun, P. In vitro antioxidant and antitumor activities of different sulfated polysaccharides isolated from three algae. Int. J. Biol. Macromol. 2013, 62, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Cikoš, A.M.; Jerković, I.; Molnar, M.; Šubarić, D.; Jokić, S. New trends for macroalgal natural products applications. Nat. Prod. Res. 2021, 35, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Sousa, R.A.; Reis, R.L. A practical perspective on ulvan extracted from green algae. J. Appl. Phycol. 2013, 25, 407–424. [Google Scholar] [CrossRef]
- Wong, T.; Brault, L.; Gasparotto, E.; Vallée, R.; Morvan, P.Y.; Ferrières, V.; Nugier-Chauvin, C. Formation of Amphiphilic Molecules from the Most Common Marine Polysaccharides, toward a Sustainable Alternative? Molecules 2021, 26, 4445. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Telles, C.B.S.; Dantas-Santos, N.; Camara, R.B.G.; Cordeiro, S.L.; Costa, M.S.; Almetida-Lima, J.; Oliveira, R.M.; Alburquerque, I.R.; et al. Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar. Drugs 2011, 9, 952–966. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Li, Y.; Chi, Y.; Ji, Y.; Gao, Y.; Hwang, H.; Aker, W.G.; Wang, P. Rheological properties, gelling behavior and texture characteristics of polysaccharide from Enteromorpha prolifera. Carbohydr. Polym. 2016, 136, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.-P.; Wu, D.-S.; Xiao, X.-W.; Huang, Q.-Y.; Chen, H.-B.; Liu, D.; Fu, H.; Chen, X.-H.; Zhao, C. Structural characterization and antioxidant effect of green alga Enteromorpha prolifera polysaccharide in Caenorhabditis elegans via modulation of microRNAs. Int. J. Biol. Macromol. 2020, 150, 1084–1092. [Google Scholar] [CrossRef]
- Cindana Mo’o, F.R.; Wilar, G.; Devkota, H.P.; Wathoni, N. Ulvan, a polysaccharide from macroalga Ulva sp.: A review of chemistry, biological activities and potential for food and biomedical applications. Appl. Sci. 2020, 10, 5488. [Google Scholar] [CrossRef]
- Qiu, S.M.; Aweya, J.J.; Liu, X.; Liu, Y.; Tang, S.; Zhang, W.; Cheong, K.L. Bioactive polysaccharides from red seaweed as potent food supplements: A systematic review of their extraction, purification, and biological activities. Carbohydr. Polym. 2022, 275, 118696. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.B.A.; Adel, M.; Karimi, P.; Peidayesh, M. Pharmaceutical, cosmeceutical, and traditional applications of marine carbohydrates. Adv. Food Nutr. Res. 2014, 73, 197–220. [Google Scholar] [PubMed]
- Schuster, S.; Fell, D.A.; Dandekar, T. A general definition of metabolic pathways useful for systematic organization and analysis of complex metabolic networks. Nat. Biotechnol. 2000, 18, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, K.; Jeon, Y.J. Bio-functionalities of proteins derived from marine algae—A review. Food Res. Int. 2012, 48, 948–960. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae-A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Fabrowska, J.; Łęska, B.; Schroeder, G.; Messyasz, B.; Pikosz, M. Biomass and Extracts of Algae as Material for Cosmetics. In Marine Algae Extracts; Kim, S., Chojnacka, K., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 681–706. ISBN 9783527679577. [Google Scholar]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. Nutrition and nutraceutical supplements in the treatment of hypertension. Expert Rev. Cardiovasc. Ther. 2010, 8, 821–833. [Google Scholar] [CrossRef]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive Components from Seaweeds: Cosmetic Applications and Future Development. In Advances in Botanical Research; Bourgougnon, N., Ed.; Academic Press Inc.: Cambridge, MA, USA, 2014; pp. 345–378. [Google Scholar]
- Brown, B.E.; Dunne, R.P. Solar radiation modulates bleaching and damage protection in a shallow water coral. Mar. Ecol. Prog. Ser. 2008, 362, 99–107. [Google Scholar] [CrossRef]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds compounds: An ecosustainable source of cosmetic ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Echave, J.; Otero, P.; Garcia-Oliveira, P.; Munekata, P.E.; Pateiro, M.; Lorenzo, J.M.; Simal-Gandara, J.; Prieto, M.A. Seaweed-derived proteins and peptides: Promising marine bioactives. Antioxidants 2022, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Samarathunga, J.; Wijesekara, I.; Jayasinghe, M. Seaweed proteins as a novel protein alternative: Types, extractions, and functional food applications. Food Rev. Int. 2023, 39, 4236–4261. [Google Scholar] [CrossRef]
- Gressler, V.; Fujii, M.T.; Martins, A.P.; Colepicolo, P.; Mancini-Filho, J.; Pinto, E. Biochemical composition of two red seaweed species grown on the Brazilian coast. J. Sci. Food Agric. 2011, 91, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Qasim, R. Amino acids composition of some common seaweeds. Pak. J. Pharmac. Sci. 1991, 4, 49–54. [Google Scholar]
- Wong, K.H.; Cheung, P.C.K. Nutritional evaluation of some subtropical red and green seaweeds. Part II. In vitro protein digestibility and amino acid profiles of protein concentrates. Food Chem. 2001, 72, 11–17. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottoni, Caulerpa lentillifera and Sargassum polycysum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Aondona, M.M.; Ikya, J.K.; Ukeyima, M.T.; Gborigo TW, J.; Aluko, R.E.; Girgih, A.T. In vitro antioxidant and antihypertensive properties of sesame seed enzymatic protein hydrolysate and ultrafiltration peptide fractions. J. Food Biochem. 2021, 45, e13587. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H. Multifunctional peptides encrypted in milk proteins. Biofactors 2004, 21, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.A.; FitzGerald, R.J. Angiotensin converting enzyme inhibitory peptides derived from food proteins: Biochemistry, bioactivity and production. Curr. Pharmac. Des. 2007, 13, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Orfanoudaki, M.; Hartmann, A.; Karsten, U.; Ganzera, M. Chemical profiling of mycosporine-like amino acids in twenty-three red algal species. J. Phycol. 2019, 55, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, D.G.; Wagemaker, T.A.L.; Alves, V.M.; Benevenuto, C.G.; Gaspar, L.R.; Campos, P.M. In vivo photoprotective effects of cosmetic formulations containing UV filters, vitamins, Ginkgo biloba and red algae extracts. J. Photochem. Photobiol. B: Biol. 2015, 153, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Rangel, K.C.; Villela, L.Z.; de Castro Pereira, K.; Colepicolo, P.; Debonsi, H.M.; Gaspar, L.R. Assessment of the photoprotective potential and toxicity of Antarctic red macroalgae extracts from Curdiea racovitzae and Iridaea cordata for cosmetic use. Algal Res. 2020, 50, 101984. [Google Scholar] [CrossRef]
- Barceló-Villalobos, M.; Figueroa, F.L.; Korbee, N.; Álvarez-Gómez, F.; Abreu, M.H. Production of mycosporine-like amino acids from Gracilaria vermiculophylla (Rhodophyta) cultured through one year in an integrated multi-trophic aquaculture (IMTA) system. Mar. Biotechnol. 2017, 19, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y.V. Antiproliferative and antioxidant activities and mycosporine-like amino acid profiles of wild-harvested and cultivated edible Canadian marine red macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Pliego-Cortés, H.; Bedoux, G.; Boulho, R.; Taupin, L.; Freile-Pelegrín, Y.; Bourgougnon, N.; Robledo, D. Stress tolerance and photoadaptation to solar radiation in Rhodymenia pseudopalmata (Rhodophyta) through mycosporine-like amino acids, phenolic compounds, and pigments in an Integrated Multi-Trophic Aquaculture system. Algal Res. 2019, 41, 101542. [Google Scholar] [CrossRef]
- Leelapornpisid, P.; Mungmai, L.; Sirithunyalug, B.; Jiranusornkul, S.; Peerapornpisal, Y. A novel moisturizer extracted from freshwater macroalga [Rhizoclonium hieroglyphicum (C. Agardh) Kützing] for skin care cosmetic. Chiang Mai J. Sci. 2014, 41, 1195–1207. [Google Scholar]
- Lee, H.A.; Kim, I.H.; Nam, T.J. Bioactive peptide from Pyropia yezoensis and its anti-inflammatory activities. Int. J. Mol. Med. 2015, 36, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Gianeti, M.D.; Maia Campos, P.M. Efficacy evaluation of a multifunctional cosmetic formulation: The benefits of a combination of active antioxidant substances. Molecules. 2014, 19, 18268–18282. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Bai, L.; Mao, X.; Zhang, X. Novel peptides with anti-proliferation activity from the Porphyra haitanesis hydrolysate. Process Biochem. 2017, 60, 98–107. [Google Scholar] [CrossRef]
- Verdy, C.; Branka, J.E.; Mekideche, N. Quantitative assessment of lactate and progerin production in normal human cutaneous cells during normal ageing: Effect of an Alaria esculenta extract. Int. J. Cosmet. Sci. 2011, 33, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Indumathi, P.; Mehta, A. A novel anticoagulant peptide from the nori hydrolysate. J. Funct. Foods 2016, 20, 606–617. [Google Scholar] [CrossRef]
- Jo, C.; Khan, F.F.; Khan, M.I.; Iqbal, J. Marine bioactive peptides: Types, structures, and physiological functions. Food Rev. Int. 2016, 33, 44–61. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién-Fernández, F.G.; Garcia-Vaquero, M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Res. 2020, 48, 101909. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 2021, 47, 110468. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.K. Seaweed Proteins, Peptides, and Amino Acids. In Seaweed Sustainability: Food and Non-Food Applications; Tiwari, B.K., Toy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 125–140. [Google Scholar]
- Yanshin, N.; Kushnareva, A.; Lemesheva, V.; Birkemeyer, C.; Tarakhovskaya, E. Chemical composition and potential practical application of 15 red algal species from the White Sea Coast (the Arctic Ocean). Molecules 2021, 26, 2489. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, S.A.; Begum, N.; Kim, S.J.; Choi, Y.H.; Nam, T.J. Biopeptides of Pyropia yezoensis and their potential health benefits: A review. Asian Pac. J. Trop. Biomed. 2021, 11, 375–384. [Google Scholar]
- De la Coba, F.; Aguilera, J.; De Galvez, M.V.; Alvarez, M.; Gallego, E.; Figueroa, F.L.; Herrera, E. Prevention of the ultraviolet effects on clinical and histopathological changes, as well as the heat shock protein-70 expression in mouse skin by topical application of algal UV-absorbing compounds. J. Dermatol. Sci. 2009, 55, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Romarís-Hortas, V.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Ultrasound-assisted enzymatic hydrolysis for iodinated amino acid extraction from edible seaweed before reversed-phase high performance liquid chromatography-inductively coupled plasma-mass spectrometry. J. Chromatogr. A 2013, 1309, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Mora, L.; Hayes, M. In vitro and in silico approaches to generating and identifying angiotensin-converting enzyme I inhibitory peptides from green macroalga Ulva lactuca. Mar. Drugs 2019, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Wang, J.; Zheng, B.D.; Pang, J.; Chen, L.J.; Lin, H.T.; Guo, X. Simultaneous Determination of 8 Small Antihypertensive Peptides with Tyrosine at the C-Terminal in Laminaria japonica Hydrolysates by RP-HPLC Method. J. Food Process. Preserv. 2016, 40, 492–501. [Google Scholar] [CrossRef]
- Pan, S.; Wang, S.; Jing, L.; Yao, D. Purification and characterisation of a novel angiotensin-I converting enzyme (ACE)-inhibitory peptide derived from the enzymatic hydrolysate of Enteromorpha clathrata protein. Food Chem. 2016, 211, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.; Bondu, S.; Doiron, K.; Rioux, L.E.; Turgeon, S.L. Characterization of antibacterial activity from protein hydrolysates of the macroalga Saccharina longicruris and identification of peptides implied in bioactivity. J. Funct. Foods 2015, 17, 685–697. [Google Scholar] [CrossRef]
- Cermeño, M.; Stack, J.; Tobin, P.R.; O’Keeffe, M.B.; Harnedy, P.A.; Stengel, D.B.; FitzGerald, R.J. Peptide identification from a Porphyra dioica protein hydrolysate with antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities. Food Funct. 2019, 10, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, Y.; San, S. Efficacy of a novel ACE-inhibitory peptide from Sargassum maclurei in hypertension and reduction of intracellular endothelin-1. Nutrients 2020, 12, 653. [Google Scholar] [CrossRef] [PubMed]
- Joel, C.H.; Sutopo, C.C.; Prajitno, A.; Su, J.H.; Hsu, J.L. Screening of angiotensin-I converting enzyme inhibitory peptides derived from Caulerpa lentillifera. Molecules 2018, 23, 3005. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, D.; Sun, X.; Sun, S.; Xu, N. Preparation and identification of antioxidant peptides from protein hydrolysate of marine alga Gracilariopsis lemaneiformis. J. Appl. Phycol. 2019, 31, 2585–2596. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Machado, M.; Cermeño, M.; Kleekayai, T.; Machado, S.; Rego, A.M.; Abreu, M.H.; Alves, R.C.; Oliveira, M.B.P.; FitzGerald, R.J. Enzyme-Assisted Release of Antioxidant Peptides from Porphyra dioica Conchocelis. Antioxidants 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ryu, J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Activation of the mTOR signaling pathway in breast cancer MCF-7 cells by a peptide derived from Porphyra yezoensis. Oncol. Rep. 2015, 33, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Martínez-Augustin, O.; Drago, S.R. Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Res. Int. 2012, 49, 364–372. [Google Scholar] [CrossRef]
- Beaulieu, L.; Sirois, M.; Tamigneaux, É. Evaluation of the in vitro biological activity of protein hydrolysates of the edible red alga, Palmaria palmata (dulse) harvested from the Gaspe coast and cultivated in tanks. J. Appl. Phycol. 2016, 28, 3101–3115. [Google Scholar] [CrossRef]
- Cian, R.E.; López-Posadas, R.; Drago, S.R.; De Medina, F.S.; Martínez-Augustin, O.A. Porphyra columbina hydrolysate upregulates IL-10 production in rat macrophages and lymphocytes through an NF-κB, and p38 and JNK dependent mechanism. Food Chem. 2012, 134, 1982–1990. [Google Scholar] [CrossRef]
- Song, J.; Li, T.; Cheng, X.; Ji, X.; Gao, D.; Du, M.; Jiang, N.; Liu, X.; Mao, X. Sea cucumber peptides exert anti-inflammatory activity through suppressing NF-κB and MAPK and inducing HO-1 in RAW264. 7 macrophages. Food Funct. 2016, 7, 2773–2779. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Cai, Q.F.; Yoshida, A.; Sun, L.C.; Liu, Y.X.; Liu, G.M.; Su, W.J.; Cao, M.J. Purification and characterization of two novel angiotensin I-converting enzyme inhibitory peptides derived from R-phycoerythrin of red algae (Bangia fusco-purpurea). Eur. Food Res. Technol. 2017, 243, 779–789. [Google Scholar] [CrossRef]
- Rosic, N.N. Mycosporine-like amino acids: Making the foundation for organic personalised sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef] [PubMed]
- Bedoux, G.; Hardouin, K.; Marty, C.; Taupin, L.; Vandanjon, L.; Bourgougnon, N. Chemical characterization and photoprotective activity measurement of extracts from the red macroalga Solieria chordalis. Bot. Mar. 2014, 57, 291–301. [Google Scholar] [CrossRef]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free. Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Shick, J.M.; Dunlap, W.C.; Buettner, G.R. Ultraviolet (UV) Protection in Marine Organisms II, Biosynthesis, Accumulation, and Sunscreening Function of Mycosporine-Like Amino Acids. In Free Radicals in Chemistry, Biology and Medicine; Yoshikawa, S., Toyokuni, S., Yamamoto, Y., Naito, Y., Eds.; OICA International: London, UK, 2000; pp. 215–228. [Google Scholar]
- Dunlap, W.C.; Yamamoto, Y. Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine. Compar. Biochem. Physiol. 1995, 112, 105–114. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Mycosporines: Are they nature’s sunscreens? Nat. Prod. Rep. 1998, 15, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B Biol. 2000, 56, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Gröniger, A.; Sinha, R.P.; Klisch, M.; Häder, D.P. Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae—A database. J. Photochem. Photobiol. B Biol. 2000, 58, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Adams, N.L.; Shick, J.M. Mycosporine-like amino acids prevent UVB-induced abnormalities during early development of the green sea urchin Strongylocentrotus droebachiensis. Mar. Biol. 2001, 138, 267–280. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J. Experim. Biol. 2008, 46, 7–17. [Google Scholar]
- Karsten, U.; Sawall, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res. 2006, 46, 271–279. [Google Scholar]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. UV Photoprotectants from Algae—Synthesis and Bio-Functionalities. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 17–38. [Google Scholar]
- Sinha, R.P.; Klisch, M.; Gröniger, A.; Häder, D.P. Mycosporine-like amino acids in the marine red alga Gracilaria cornea—effects of UV and heat. Environ. Exp. Bot. 2000, 43, 33–43. [Google Scholar] [CrossRef]
- Murphy, C.; Hotchkiss, S.; Worthington, J.; McKeown, S.R. The potential of seaweed as a source of drugs for use in cancer chemotherapy. J. Appl. Phycol. 2014, 26, 2211–2264. [Google Scholar] [CrossRef]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-like amino acids: Potential health and beauty ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [PubMed]
- Arad, S.; Yaron, A. Natural pigments from red microalgae for use in foods and cosmetics. Trends Food Sci. Technol. 1992, 3, 92–97. [Google Scholar] [CrossRef]
- Bermejo, R.; Talavera, E.M.; del Valle, C.; Alvarez-Pez, J.M. C-phycocyanin incorporated into reverse micelles: A fluorescence study. Colloids Surf. B Biointerfaces 2000, 18, 51–59. [Google Scholar] [CrossRef]
- Chronakis, I.S. Biosolar proteins from aquatic algae. Dev. Food Sci. 2000, 41, 39–75. [Google Scholar]
- Rossano, R.; Ungaro, N.; D’Ambrosio, A.; Liuzzi, G.M.; Riccio, P. Extracting and purifying R-phycoeythrin from Mediterranean red algae Corallina elongata Ellis and Solander. J. Biotecnol. 2003, 101, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Fleurence, J. Seaweed Proteins. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2004; pp. 197–213. [Google Scholar]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Viskari, P.J.; Colyer, C.L. Rapid extraction of phycobiliproteins from cultures cyanobacteria samples. Anal. Biochem. 2003, 319, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Vieira, F.A.; Correia, I.; Ferreira, R.A.S.; Abreu, H.; Coutinho, J.A.P.; Ventura, S.P.M. Recovery of phycobiliproteins from the red macroalga Gracilaria sp. using ionic liquid aqueous solutions. Green Chem. 2016, 18, 4287–4296. [Google Scholar] [CrossRef]
- Capillo, G.; Savoca, S.; Costa, R.; Sanfilippo, M.; Rizzo, C.; Lo Giudice, A.; Albergamo, A.; Rando, R.; Bartolomeo, G.; Spanò, N.; et al. New insights into the culture method and antibacterial potential of Gracilaria gracilis. Marine Drugs 2018, 16, 492. [Google Scholar] [CrossRef] [PubMed]
- Saluri, M.; Kaldmäe, M.; Rospu, M.; Sirkel, H.; Paalme, T.; Landreh, M.; Tuvikene, R. Spatial variation and structural characteristics of phycobiliproteins from the red algae Furcellaria lumbricalis and Coccotylus truncatus. Algal Res. 2020, 52, 102058. [Google Scholar] [CrossRef]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments content (chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Tovar-Pérez, E.G.; Guerrero-Legarreta, I.; Farrés-González, A.; Soriano-Santos, J. Angiotensin I-converting enzyme-inhibitory peptide fractions from albumin 1 and globulin as obtained of amaranth grain. Food Chem. 2009, 116, 437–444. [Google Scholar] [CrossRef]
- Balti, R.; Bougatef, A.; Sila, A.; Guillochon, D.; Dhulster, P.; Nedjar-Arroume, N. Nine novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepia officinalis) muscle protein hydrolysates and antihypertensive effect of the potent active peptide in spontaneously hypertensive rats. Food Chem. 2015, 170, 519–525. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Tsioufis, K.; Agabiti-Rosei, E.; Burnier, M.; Cicero, A.F.G.; Clement, D.; Coca, A.; Desideri, G.; Grassi, G.; Lovic, D.; et al. Nutraceuticals and blood pressure control: A European Society of Hypertension position document. J. Hypertens. 2020, 38, 799–812. [Google Scholar] [PubMed]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Marine Drugs. 2017, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.K. Photoprotective substances derived from marine algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kwon, M.J.; Kim, I.H.; Kim, Y.M.; Lee, M.K.; Nam, T.J. Pyropia yezoensis glycoprotein promotes the M1 to M2 macrophage phenotypic switch via the STAT3 and STAT6 transcription factors. Int. J. Mol. Med. 2016, 38, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, K.H.; Marques, L.G.; Carvalho, V.M.; Carignan, M.O.; Pinto, E.; Marinho-Soriano, E.; Colepicolo, P. Analyses of photoprotective compounds in red algae from the Brazilian coast. Rev. Bras. Farmacogn. 2011, 21, 202–208. [Google Scholar] [CrossRef]
- Hoyer, K.; Karsten, U.; Sawall, T.; Wiencke, C. Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and developmental stages. Mar. Ecol. Prog. Ser. 2001, 211, 117–129. [Google Scholar] [CrossRef]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oresajo, C.; Yatskayer, M.; Galdi, A.; Foltis, P.; Pillai, S. Complementary effects of antioxidants and sunscreens in reducing UV-induced skin damage as demonstrated by skin biomarker expression. J. Cosmet. Laser Ther. 2010, 12, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.S.; Lee, J.H.; Moh, S.H.; Lee, T.K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, C.J.; de Andrade, L.M. An overview on the application of genus Chlorella in biotechnological processes. J. Adv. Res. Biotechnol. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Kang, K.A.; Lee, K.H.; Chae, S.; Koh, Y.S.; Yoo, B.S.; Kim, J.H.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Triphlorethol-A from Ecklonia cava protects V79-4 lung fibroblast against hydrogen peroxide induced cell damage. Free Radic. Res. 2005, 39, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Sharma, S.A. Toxicological effects of marine seaweeds: A cautious insight for human consumption. Crit. Rev. Food Sci. Nutr. 2021, 61, 500–521. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.R.; Kim, Y.M.; Lee, M.K.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Pyropia yezoensis peptide promotes collagen synthesis by activating the TGF-_/Smad signaling pathway in the human dermal fibroblast cell line Hs27. Int. J. Mol. Med. 2017, 39, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Bjarnadóttir, M.; Aðalbjörnsson, B.V.; Nilsson, A.; Slizyte, R.; Roleda, M.Y.; Hreggviðsson, G.; Friðjónsson, H.; Jónsdóttir, R. Palmaria palmata as an alternative protein source: Enzymatic protein extraction, amino acid composition, and nitrogen-to-protein conversion factor. J. Appl. Phycol. 2018, 30, 2061–2070. [Google Scholar] [CrossRef]
- Houston, M.C. Nutraceuticals, Vitamins, Antioxidants, and Minerals in the Prevention and Treatment of Hypertension. Prog. Cardiovasc. Dis. 2005, 47, 396–449. [Google Scholar] [CrossRef] [PubMed]
- Galland-Irmouli, A.-V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.-P.; Villaume, C.; Guéant, J.-L. Nutritional value of proteins from edible seaweed Palmaria palmata (dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Khairy, H.M.; El-Shafay, S.M. Seasonal variations in the biochemical composition of some common seaweed species from the coast of Abu Qir Bay, Alexandria, Egypt. Oceanologia. 2013, 55, 435–452. [Google Scholar] [CrossRef]
- Mohammed, H.O.; O’Grady, M.N.; O’Sullivan, M.G.; Hamill, R.M.; Kilcawley, K.N.; Kerry, J.P. An Assessment of Selected Nutritional, Bioactive, Thermal and Technological Properties of Brown and Red Irish Seaweed Species. Foods 2021, 10, 2784. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-like amino acids from red macroalgae: UV-photoprotectors with potential cosmeceutical applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Araújo, R.G.; Alcantar-Rivera, B.; Meléndez-Sánchez, E.R.; Martínez-Prado, M.A.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Parra-Saldivar, R.; Martínez-Ruiz, M. Effects of UV and UV-vis Irradiation on the Production of Microalgae and Macroalgae: New Alternatives to Produce Photobioprotectors and Biomedical Compounds. Molecules 2022, 27, 5334. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.M.; Lin, T.J.; Chiu, C.Y.; Chang, C.W.; Hsu, K.C.; Fan, P.C.; Wen, K.C. Coffea arabica extract and its constituents prevent photoaging by suppressing MMPs expression and MAP kinase pathway. Food Chem. Toxicol. 2011, 49, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-T.; Chen, P.-Y.; Wu, J.-J.; Lee, T.-M.; Hsu, S.-L.; Chang, C.-M.J.; Young, C.-C.; Shieh, C.-J. Purification of algal antityrosinase zeaxanthin from Nannochloropsis oculata using supercritical anti-solvent precipitation. J. Supercrit. Fluids 2011, 55, 955–9625. [Google Scholar] [CrossRef]


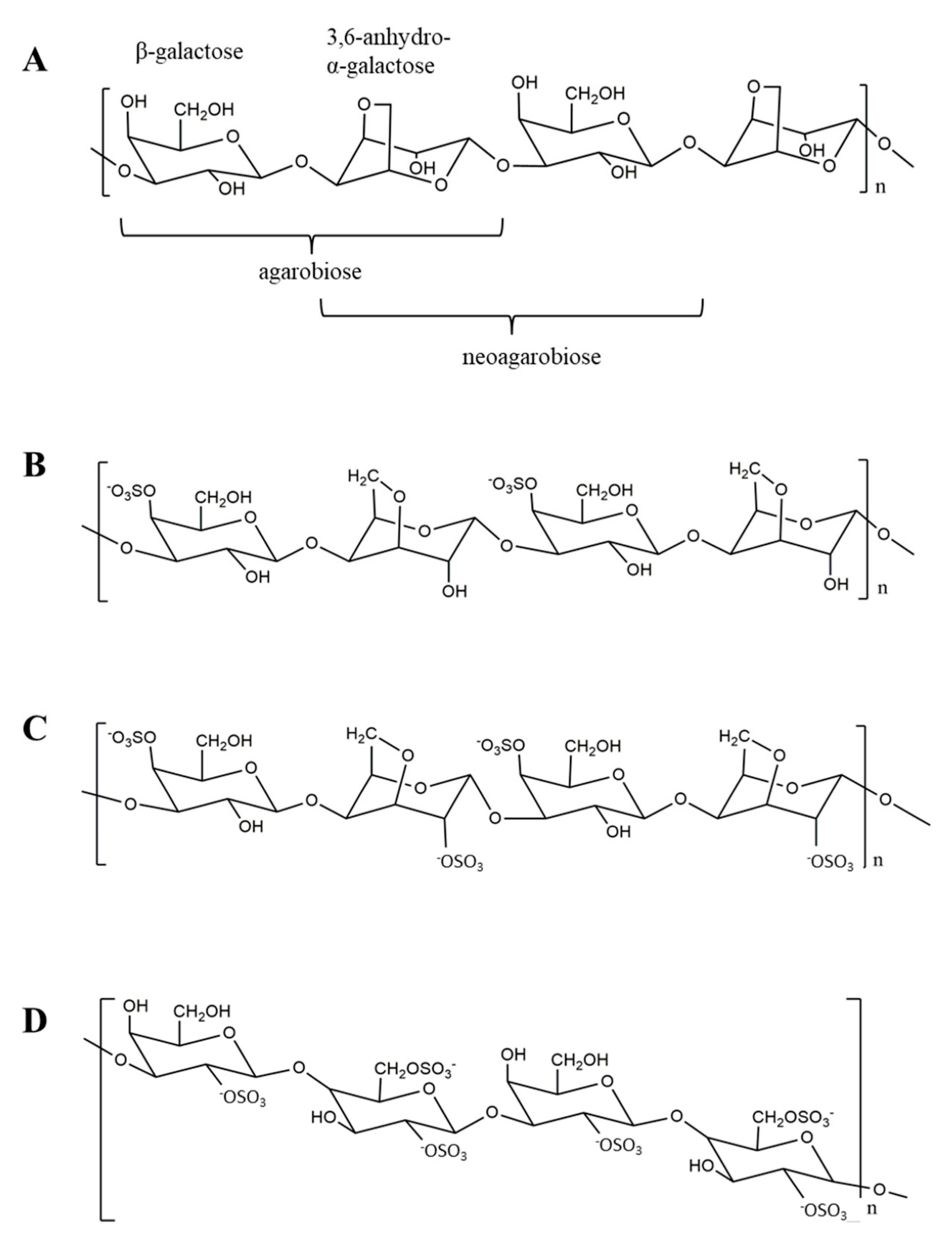
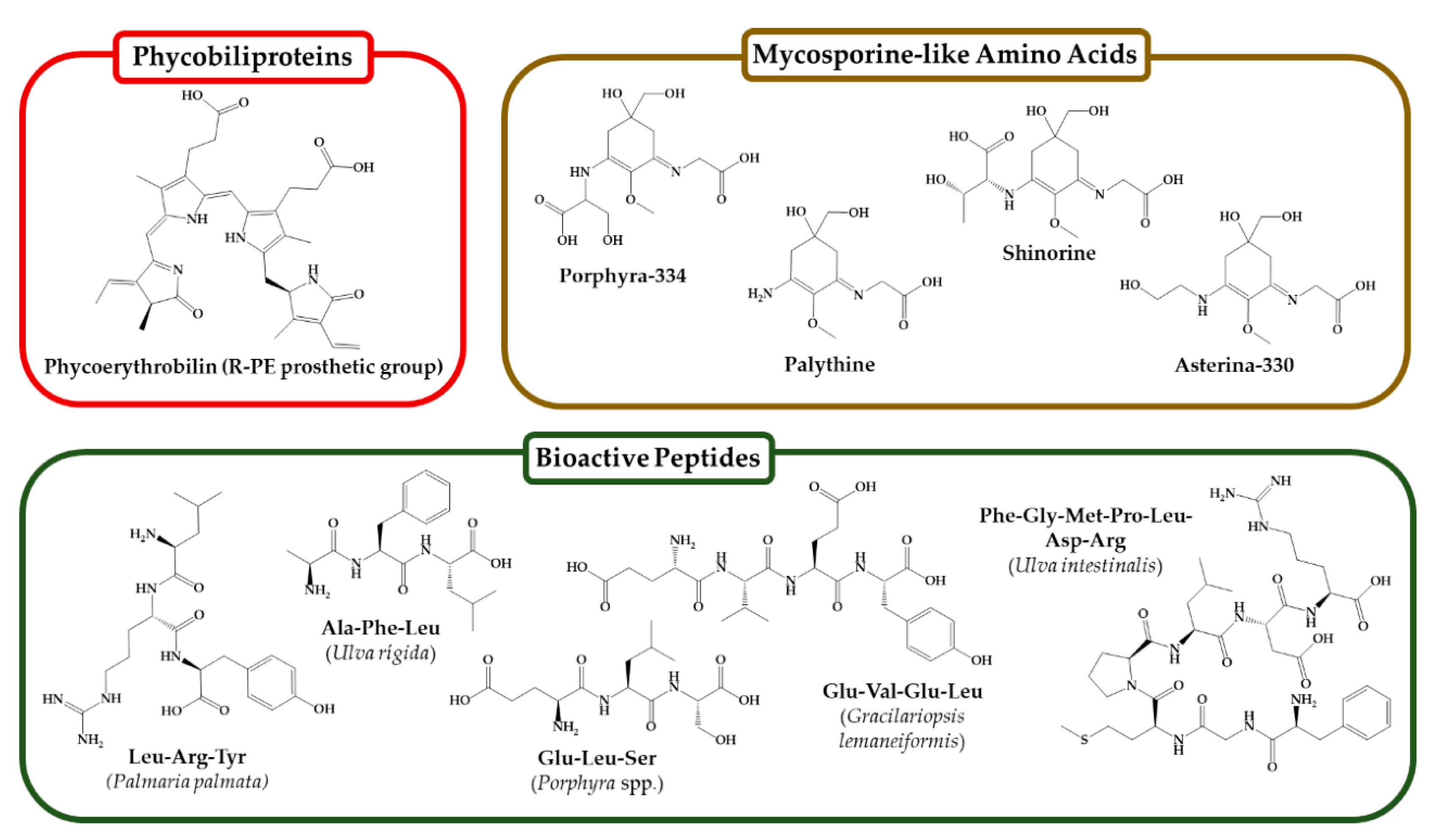
| Seaweed/s | Compound | Properties/Activities | References |
|---|---|---|---|
| Ulva pertusa, Ulva sp. | Ulvan | Antiaging, antiherpetic | [153,154] |
| Saccharina japonica, Chondrus crispus, Codium tomentosum | Polysaccharides | Hydration | [155] |
| Saccharina longicruris, Laminarin (Sigma) | Laminaran | Reconstructed dermis; skin cell anti-inflammation; antioxidant | [156,157] |
| Ascophyllum nodosum, Chnoospora minima, Ecklonia maxima, Hizikia fusiforme, Saccharina japonica, Sargassum hemiphyllum, Sargassum horneri, Sargassum polycystum, Sargassum vachellianum | Fucoidans | Photoaging inhibition; minimized elastase activity; antioxidant, anti-inflammatory; collagenase and elastase inhibition; skin-whitening | [158,159,160,161,162,163,164,165,166,167,168] |
| Red seaweeds, Porphyra haitanensis, Gracilaria chouae, Gracilaria blodgettii | Carrageenans | Antioxidant, antitumor, antiaging, thickening properties, radiation protection | [169,170,171,172,173,174,175] |
| Brown seaweeds | Alginate | High stability, thickening agent, gelling agent | [176,177,178] |
| Pterocladia, Pterocladiella, Gelidium amansii, Gracilaria | Agar | Thickener; antioxidant | [179,180,181] |
| Seaweed/s | Compound | Properties/Activities | References |
|---|---|---|---|
| Sargassum tenerrimum | Fucoidan | Antioxidant: Decrease in DPPH radical and superoxide radical, high total antioxidant and FRAP ability | [297,298,299,300] |
| Costaria costata | Fucoidan | Skin anti-aging: Decrease in UVB-induced mRNA and protein expression of MMP-1, increase in type 1 pro-collagen, and decrease in activation of ERK and JNK | [301,302] |
| Laminaria cichorioides | Fucoidan | Anti-atopic dermatitis: Reduction in DNCB-induced atopic dermatitis symptoms, including clinical severity scores, scratching counts, and serum histamine levels | [303] |
| Costaria costata | Fucoidan | Skin anti-aging: Decrease in expression of MMP-1 and increase in type 1 pro-collagen | [276,304] |
| Sargassum tenerrimum | Fucoidan | Anti-melanogenesis: Activation of the ERK pathway leading to a decrease in melanin content | [305] |
| Mekab | Fucoidan | Skin anti-aging: Decrease in UVB-induced edema, thickness of prickle cell layer, and MMP-1 activity and expression | [306] |
| Ascophyllum nodosum | Fucoidan (16 kDa) | Skin elasticity: Decrease in elastic fiber degradation and leukocyte elastase activity | [307] |
| Ascophyllum nodosum | Fucoidan (16 kDa) | Inflammatory response: Reduction in IL-1β-induced MMP-9 and MMP-3 expression/secretion, increase in TIMP-1 | [308] |
| Laminaria cichorioides | Fucoidan | Atopic dermatitis: Reduction in AD-associated chemokines, including TARC, MDC, and RANTES | [309] |
| Laminaria cichorioides | Fucoidan | Atopic dermatitis: Decrease in IgE production in PBMC from patients with AD and immunoglobulin germline transcripts of B cells | [310] |
| Saccharina longicruris | Laminaran | Skin tissue engineering: Increase in deposition of matrix | [311] |
| Saccharina japonica | Fucoidan | Moisturizing: Higher moisture absorption and retention ability compared to HA | [312] |
| Laminaria cichorioides | Fucoidan (water soluble) | Skin cancer prevention: Decrease in EGF or TPA-induced neoplastic cell transformation and binding of EGF and EGFR | [313] |
| Ulva pertusa | Ulvans | Antioxidant: Reduction in superoxide and hydroxyl radicals, increase in reducing power and metal chelating ability | [314,315] |
| Saccharina longicruris | Laminaran | Skin anti-aging: Decrease in UVA+UVB-induced skin dermal thickness, increase in Hyp content, decrease in MMP-1 expression, and increase in TIMP-1 | [316] |
| Ulva sp. | Crude ulvans (57 kDa) | Skin anti-aging: Increase in hyaluronan production and decrease in collagen release | [317] |
| Eucheuma spinosum (Eucheuma denticulatum) Eucheuma cottonii (Kappaphycus alvarezii) | Carrageenan | Antioxidant, Photoprotective: Decrease in UVB-induced cell death, intracellular ROS, and DPPH radical | [318] |
| Porphyra sp. | Porphyran | Antioxidant: Ferrous ion chelating, increase in reducing power, decrease in DPPH radical and superoxide | [319] |
| Porphyridium sp. | Carrageenan | Anti-melanogenesis: Decrease in level of melanosome | [320] |
| Porphyra haitanensis | Porphyran | Antioxidant: Increase in antioxidant enzyme activity, decrease in lipid peroxidation, increase in total antioxidant capacity | [321,322] |
| Porphyra haitanensis | Porphyran with different MW | Antioxidant: Decrease in DPPH radicals, increase in reducing power | [323] |
| Porphyra yezoensis | Porphyran | | Anti-inflammation: Decrease in LPS-induced inflammatory markers including NO, iNOS, NF-κB activation, and TNF-α production | [324,325] |
| Porphyra haitanensis | LMW Porphyran SD, AD, PD, BD | Antioxidant: Decrease in DPPH radicals, hydroxyl radicals, and superoxide | [326] |
| Extract/Compound | Activity | Seaweed | References |
|---|---|---|---|
| Eleven mycosporine-like amino acids | UV-protective effect, antioxidant | Agarophyton chilense, Pyropia plicata, Champia novaezelandiae | [366] |
| Mycosporine-like amino acids extract | Anti-aging | Phorphyra umbilicalis | [367] |
| Mycosporine-like amino acids extract | Antioxidant, UV-protective effect, anti-aging | Curdieara covitzae, Iridaea cordata | [368] |
| Mycosporine-like amino acids extract | Antioxidant; UV-protective effect | Gracilaria vermiculophylla | [369] |
| Mycosporine-like amino acids extract | Antioxidant, antiproliferative | Chondrus crispus, Mastocarpus stellatus, Palmaria palmata | [370] |
| Mycosporine-like amino acids extract | Antioxidant | Rhodymenia pseudopalmata | [371] |
| Aqueous extract from freshwater macroalga | Skin moisturizing effect | Rhizoclonium hieroglyphicum | [372] |
| Peptide PPY1 | Anti-inflammatory | Pyropia yezoensis | [373] |
| Peptides PYP1-5 and porphyra 334 | Increase in the production of elastin and collagen | Porphyra yezoensis f. coreana Ueda | [374] |
| Methanol extract rich in proteins, vitamins, minerals, porphyra-334 and shinorine | Hydration, skin protective, anti-wrinkle, anti-roughness | Phorphyra umbilicalis | [375] |
| Phycobiliproteins (R-phycoerythrin allophycocyanin and phycocyanin) | Antioxidant | Gracilaria gracilis | [376] |
| Hydrolyzed extract | Antitumor | Porphyra haitanensis | [377] |
| Algae extract | Decrease in progerin production, anti-elastase, and anti-collagenase | Alaria esculenta | [378] |
| Species | Hydrolysis | Bioactivity | Results | Ref. |
|---|---|---|---|---|
| Ulva lactuca | Papain | In vitro, antihypertensive | ACE inhibition (93%) in the >1 kDa hydrolysate fraction | [388] |
| Laminaria japonica | Alcalase, papain, trypsin, and pepsin | In vitro, antihypertensive | ACE inhibition. The hydrolysate of all combined proteases achieved an IC50 = 0.6 mg/mL | [389] |
| Enteromorpha clathrata | Alcalase | In vitro, antihypertensive | ACE inhibition. IC50 = 0.014 mg/mL | [390] |
| Saccharina longicruris | Trypsin | In vitro, antimicrobial | A mix of all peptides (2.50 mg/mL) inhibited 40% of Staphylococcus aureus growth | [391] |
| Porphyra dioica | Alcalase and flavoenzyme | In vitro, antioxidant | Most antioxidants on the ORAC assay: IC50 (AFIT) = 0.4 μg/mL, IC50 (MKTPITE) = 0.007 mg/mL | [392] |
| Sargassum maclurei | Pepsin | In vivo and in vitro, antihypertensive | Systolic blood pressure reduction from 170 to 150 mm Hg at 100 mg/kg bw; 25% endothelin-1 inhibition at 1.5 mg/mL | [393] |
| Caulerpa lentillifera | Thermolysin | In vitro, antihypertensive | ACE inhibition. IC50 (FDGIP) = 0.03 mg/mL, IC50 (AIDPVRA) = 0.04 mg/mL | [394] |
| Gracilariopsis lemaneiformis | α-Chymotrypsin | In vitro, antioxidant | DPPH radical scavenging, EC50 = 1.51 mg/mL | [395] |
| Porphyra dioica | Prolyve | In vitro, antioxidant | ORAC (IC50 = 2.7 mmol TE/g), DPPH (IC50 = 0.2 mmol TE/g), FRAP (IC50 = 0.4 mmol TE/g) | [396] |
| Porphyra yezoensis | Chemical synthesis | In vitro, antitumor | Doses ≥ 125 ng/mL induced autophagy and apoptosis in MCF-7 cells via the mTOR pathway | [397] |
| Pyropia columbina | Trypsin | In vitro, antioxidant and anti-inflammatory | DPPH (IC50 = 2.8 mg/mL), ABTS (IC50 = 2.4 mg/mL); upregulation of IL10 at 0.1 mg/mL | [398] |
| Palmaria palmata | Chymotrypsin | In vitro, antioxidant and antihypertensive | A < 10 kDa fraction was the most bioactive at ≥0.75 mg/mL | [399] |
| Pyropia columbina | Fungal protease | In vitro, anti-inflammatory | Upregulation of IL-10 in murine spenocytes, macrophages, and lymphocytes at ≥0.01 mg/mL | [400] |
| Neopyropia yezoensis | Chemical synthesis | In vitro, anti-inflammatory | Doses ≥ 250 ng/mL inhibited the expression of inflammatory cytokines in murine macrophages | [401] |
| Bangia fusco-purpurea | Pepsin | In vitro, antihypertensive | ACE inhibition. IC50(ALLAGDPSVLEDR) = 57.2 mg/mL, IC50(VVGGTGPVDEWGIAGAR) = 66.2 μg/mL | [402] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalasariya, H.S.; Maya-Ramírez, C.E.; Cotas, J.; Pereira, L. Cosmeceutical Significance of Seaweed: A Focus on Carbohydrates and Peptides in Skin Applications. Phycology 2024, 4, 276-313. https://doi.org/10.3390/phycology4020015
Kalasariya HS, Maya-Ramírez CE, Cotas J, Pereira L. Cosmeceutical Significance of Seaweed: A Focus on Carbohydrates and Peptides in Skin Applications. Phycology. 2024; 4(2):276-313. https://doi.org/10.3390/phycology4020015
Chicago/Turabian StyleKalasariya, Haresh S., Carlos Eliel Maya-Ramírez, João Cotas, and Leonel Pereira. 2024. "Cosmeceutical Significance of Seaweed: A Focus on Carbohydrates and Peptides in Skin Applications" Phycology 4, no. 2: 276-313. https://doi.org/10.3390/phycology4020015
APA StyleKalasariya, H. S., Maya-Ramírez, C. E., Cotas, J., & Pereira, L. (2024). Cosmeceutical Significance of Seaweed: A Focus on Carbohydrates and Peptides in Skin Applications. Phycology, 4(2), 276-313. https://doi.org/10.3390/phycology4020015









