Light-Emitting Diode Illumination Enhances Biomass, Pigment, and Lipid Production in Halotolerant Cyanobacterium Aphanothece halophytica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cyanobacterial Cultivation
2.2. Effect of Light Source, Quality, and Intensity on Growth, Pigment Content, and Lipid Production
2.3. Growth Determination and Total Cell Concentration Measurements
2.4. Pigment Content Analysis
2.5. Total Lipid Extraction
2.6. Fatty Acid Profile Analysis
2.7. Biodiesel Properties of Fatty Acid Methyl Ester
2.8. Statistical Analysis
3. Results
3.1. Effects of White Fluorescent and White LED Light on Growth, Pigment, and Total Lipid Content
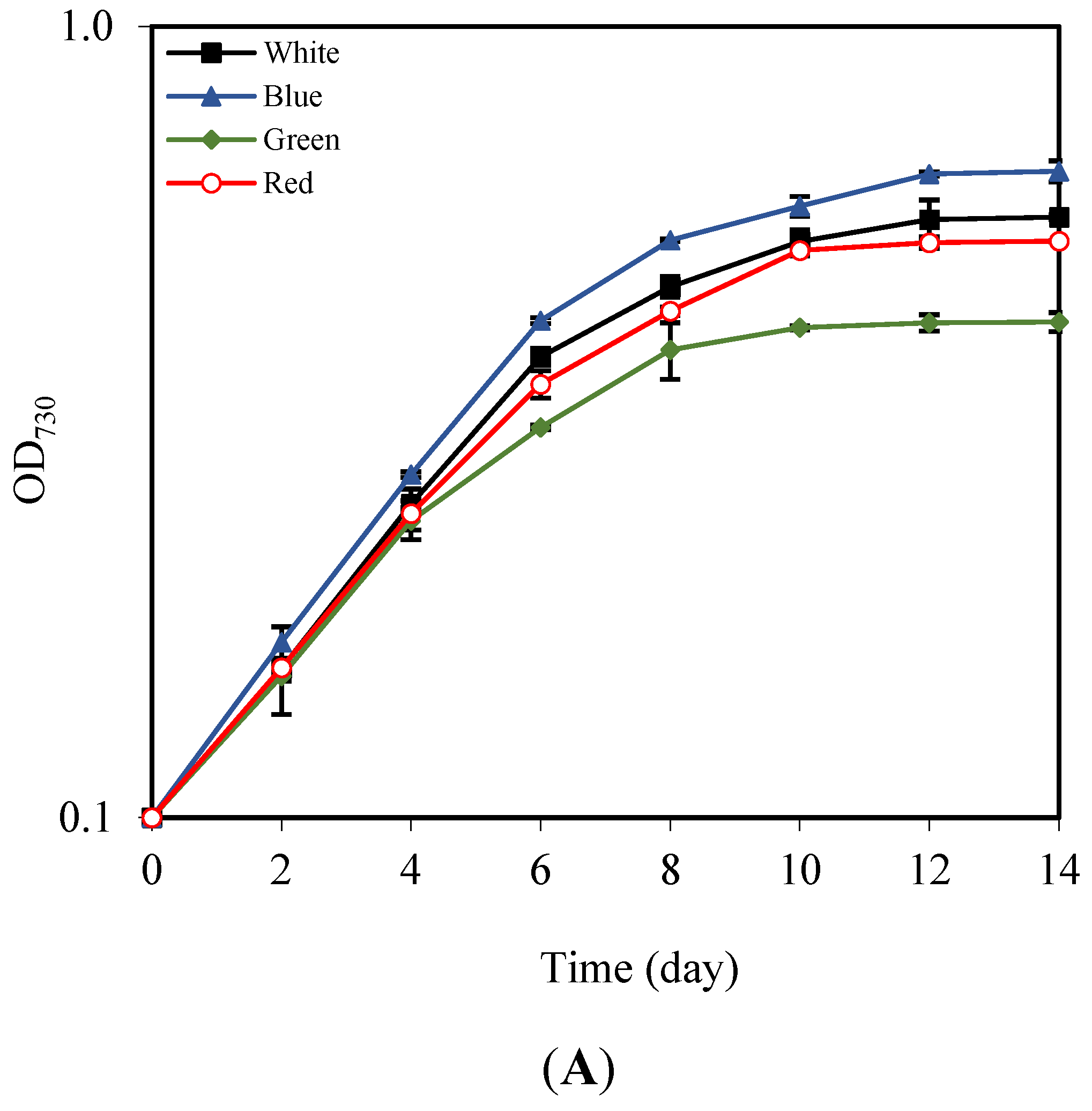
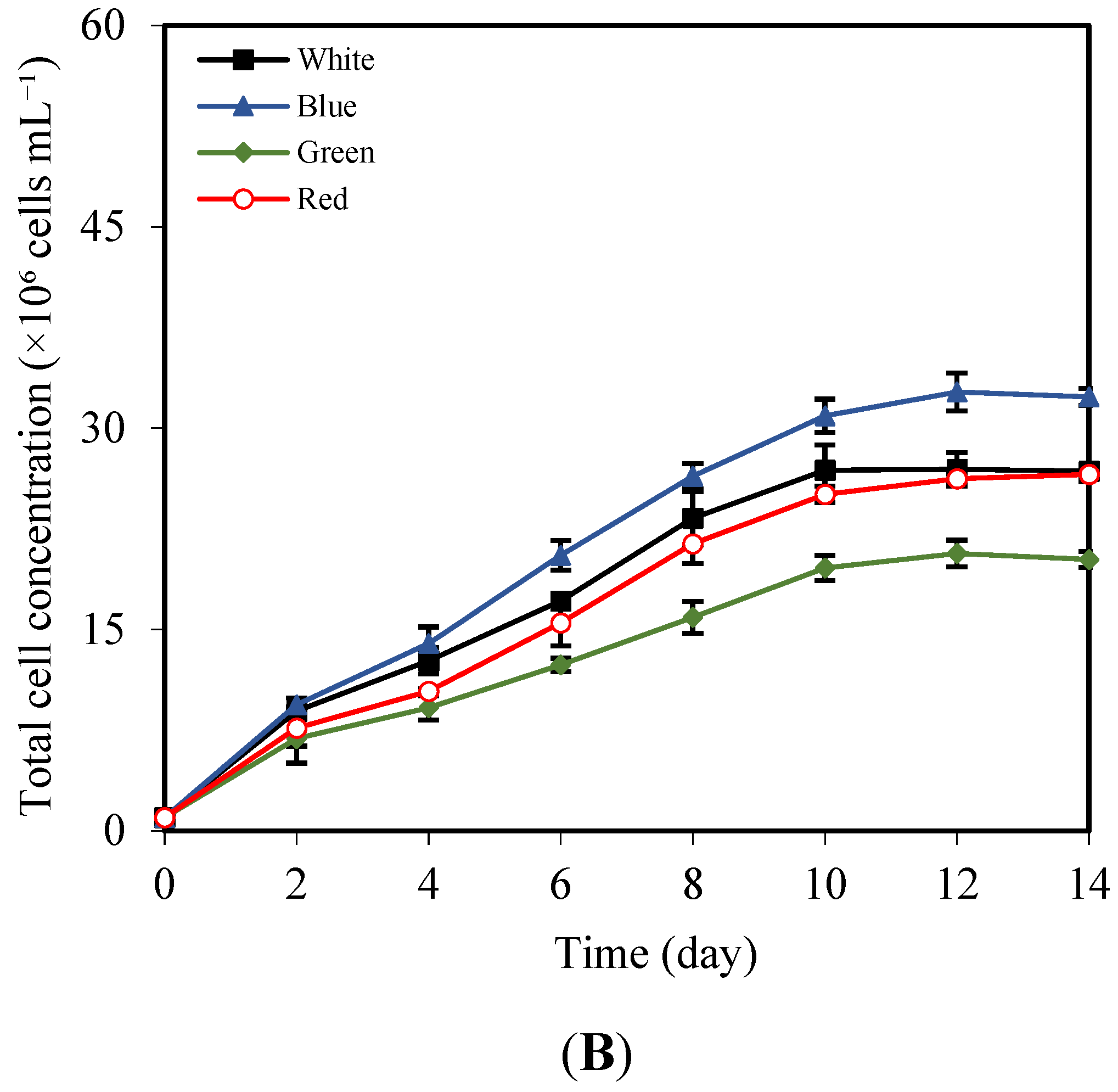
3.2. Effects of LED Light Colors on Growth, Pigment, and Total Lipid Content
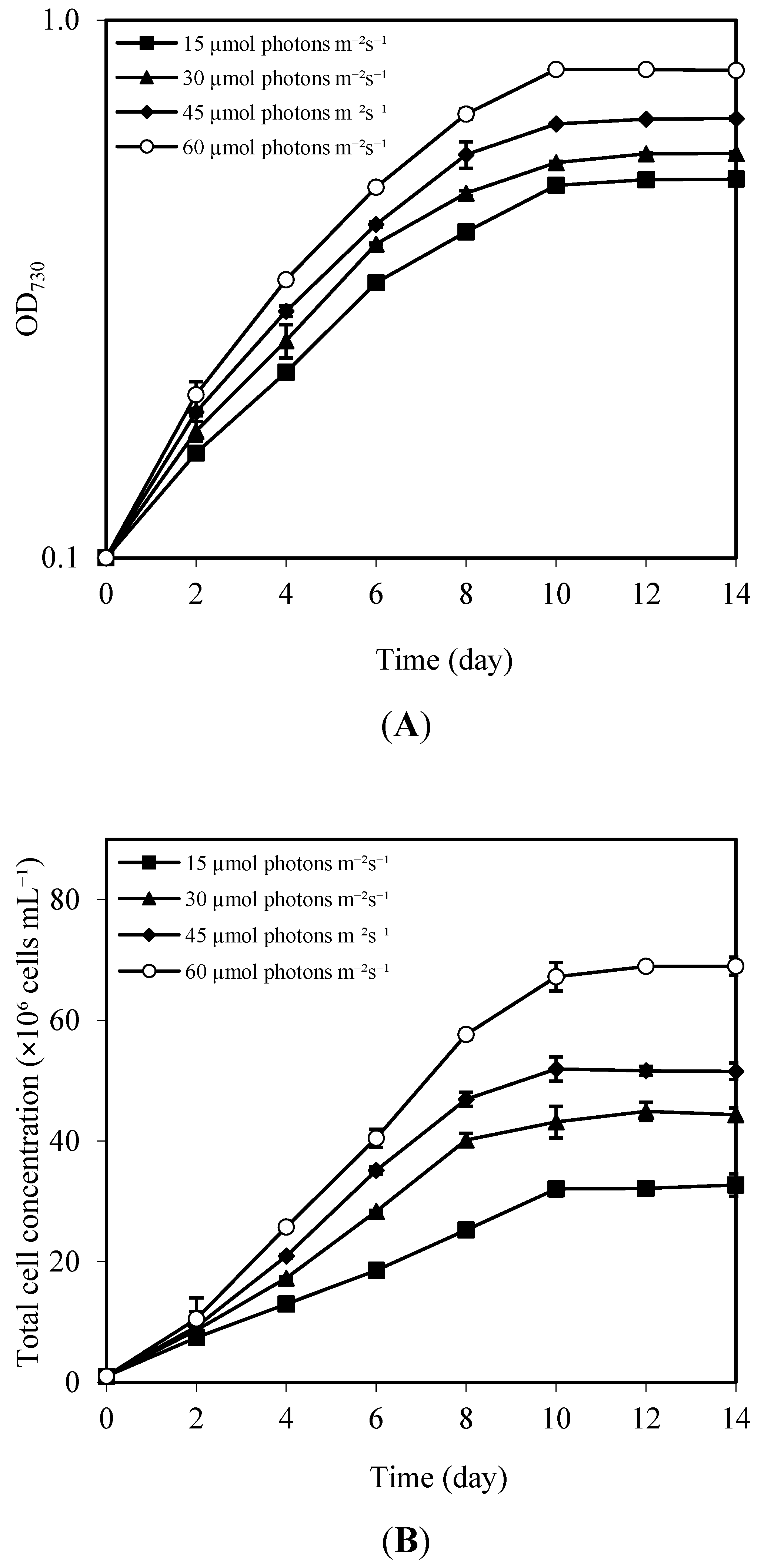
3.3. Effects of Blue LED Intensity on Growth, Pigment, and Lipid Content
3.4. Effects of LED Light Colors on Fatty Acid Profiles and Biodiesel Properties
4. Discussion
4.1. Effect of White Light Sources on Growth and Pigment Content
4.2. Effects of LED Light Colors on Growth and Pigment Content
4.3. Effects of LED Light on Lipid Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trabelsi, L.; Ben Ouada, H.; Bacha, H.; Ghoul, M. Combined Effect of Temperature and Light Intensity on Growth and Extracellular Polymeric Substance Production by the Cyanobacterium Arthrospira platensis. J. Appl. Phycol. 2009, 21, 405–412. [Google Scholar] [CrossRef]
- Rai, S.V.; Rajashekhar, M. Effect of pH, Salinity and Temperature on the Growth of Six Species of Cyanobacteria Isolated from Arabian Sea Coast of Karnataka. Int. J. BioSci. Technol. 2016, 9, 1–6. [Google Scholar]
- Shukla, M.; Tabassum, R.; Singh, R.; Dhar, D.W. Influence of Light Intensity, Temperature and CO2 Concentration on Growth and Lipids in Green Algae and Cyanobacteria. Indian J. Exp. Biol. 2016, 54, 482–487. [Google Scholar]
- Meng, F.; Cui, H.; Wang, Y.; Li, X. Responses of a New Isolated Cyanobacterium aponinum Strain to Temperature, pH, CO2 and Light Quality. J. Appl. Phycol. 2018, 30, 1525–1532. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Abo-Shanab, W.A.; Ende, S.S.W.; Alquraishi, M.; El-Shenody, R.A. Optimizing Phaeodactylum tricornutum Cultivation: Integrated Strategies for Enhancing Biomass, Lipid, and Fucoxanthin Production. Biotechnol. Biofuels Bioprod. 2025, 18, 7. [Google Scholar] [CrossRef]
- Klepacz-Smółka, A.; Pietrzyk, D.; Szeląg, R.; Głuszcz, P.; Daroch, M.; Tang, J.; Ledakowicz, S. Effect of Light Colour and Photoperiod on Biomass Growth and Phycocyanin Production by Synechococcus PCC 6715. Bioresour. Technol. 2020, 313, 123700. [Google Scholar] [CrossRef]
- Hoi, S.K.; Winayu, B.N.R.; Hsueh, H.T.; Chu, H. Light Factors and Nitrogen Availability to Enhance Biomass and C-Phycocyanin Productivity of Thermosynechococcus sp. CL-1. Biochem. Eng. J. 2021, 167, 107899. [Google Scholar] [CrossRef]
- Han, G.; Shen, G.; Zhao, R.; Bryant, D.A. Additional Families of Orange Carotenoid Proteins in the Photoprotective System of Cyanobacteria. Plant Physiol. Biochem. 2017, 118, 626–636. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Okamoto, A.; Imamura, M.; Tani, K.; Matsumoto, T. The Effect of Using Light Emitting Diodes and Fluorescent Lamps as Different Light Sources in Growth Inhibition Tests of Green Alga, Diatom, and Cyanobacteria. PLoS ONE 2021, 16, e0247426. [Google Scholar] [CrossRef]
- Goldoost, H.; Vahabzadeh, F.; Fallah, N. Lipids Productivity of Cyanobacterium Anabaena vaginicola in an Internally Illuminated Photobioreactor Using LED Bar Lights. Sci. Rep. 2024, 14, 6857. [Google Scholar] [CrossRef]
- Lima, G.M.; Teixeira, P.C.N.; Teixeira, C.M.L.L.; Filócomo, D.; Lage, C.L.S. Influence of Spectral Light Quality on the Pigment Concentrations and Biomass Productivity of Arthrospira platensis. Algal Res. 2018, 31, 157–166. [Google Scholar] [CrossRef]
- Hotos, G.N.; Antoniadis, T.I. The Effect of Colored and White Light on Growth and Phycobiliproteins, Chlorophyll and Carotenoids Content of the Marine Cyanobacteria Phormidium sp. and Cyanothece sp. in Batch Cultures. Life 2022, 12, 837. [Google Scholar] [CrossRef] [PubMed]
- Markou, G. Effect of Various Colors of Light-Emitting Diodes (LEDs) on the Biomass Composition of Arthrospira platensis Cultivated in Semi-Continuous Mode. Appl. Biochem. Biotechnol. 2014, 172, 2758–2768. [Google Scholar] [CrossRef] [PubMed]
- McGee, D.; Archet, L.; Fleming, G.T.A.; Gillespie, E.; Touzet, N. Influence of Spectral Intensity and Quality of LED Lighting on Photoacclimation, Carbon Allocation and High-Value Pigments in Microalgae. Photosynth. Res. 2020, 143, 67–80. [Google Scholar] [CrossRef]
- Luimstra, V.M.; Schuurmans, J.M.; Verschoor, A.M.; Hellingwerf, K.J.; Huisman, J.; Matthijs, H.C.P. Blue Light Reduces Photosynthetic Efficiency of Cyanobacteria through an Imbalance between Photosystems I and II. Photosynth. Res. 2018, 138, 177–189. [Google Scholar] [CrossRef]
- Toyoshima, M.; Toya, Y.; Shimizu, H. Flux Balance Analysis of Cyanobacteria Reveals Selective Use of Photosynthetic Electron Transport Components under Different Spectral Light Conditions. Photosynth. Res. 2020, 143, 31–43. [Google Scholar] [CrossRef]
- Wong, Y.K.; Ho, Y.H.; Ho, K.C.; Leung, H.M.; Chow, K.P.; Yung, K.K.L. Effect of Different Light Sources on Algal Biomass and Lipid Production in Internal LEDs-Illuminated Photobioreactor. J. Mar. Biol. Aquac. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Atta, M.; Idris, A.; Bukhari, A.; Wahidin, S. Intensity of Blue LED Light: A Potential Stimulus for Biomass and Lipid Content in Freshwater Microalgae Chlorella vulgaris. Bioresour. Technol. 2013, 148, 373–378. [Google Scholar] [CrossRef]
- Ishitani, M.; Takabe, T.; Kojima, K.; Takabe, T. Regulation of Glycinebetaine Accumulation in the Halotolerant Cyanobacterium Aphanothece halophytica. Aust. J. Plant Physiol. 1993, 20, 693–703. [Google Scholar] [CrossRef]
- Hibino, T.; Kaku, N.; Yoshikawa, H.; Takabe, T.; Takabe, T. Molecular Characterization of DnaK from the Halotolerant Cyanobacterium Aphanothece halophytica for ATPase, Protein Folding, and Copper Binding under Various Salinity Conditions. J. Biol. Chem. 1999, 40, 409–418. [Google Scholar] [CrossRef]
- Waditee, R.; Tanaka, Y.; Aoki, K. Isolation and Functional Characterization of N-Methyltransferases that Catalyze Betaine Synthesis from Glycine in a Halotolerant Photosynthetic Organism Aphanothece halophytica. J. Biol. Chem. 2003, 278, 4932–4942. [Google Scholar] [CrossRef] [PubMed]
- Chinchusak, N.; Incharoensakdi, A.; Phunpruch, S. Enhancement of Dark Fermentative Hydrogen Production in Nitrogen-deprived Halotolerant Unicellular Cyanobacterium Aphanothece halophytica by Treatment with Reducing Agents. Biomass Bioenergy 2022, 167, 106624. [Google Scholar] [CrossRef]
- Chinchusak, N.; Incharoensakdi, A.; Phunpruch, S. Dark Fermentative Hydrogen Production and Transcriptional Analysis of Aphanothece halophytica under Nitrogen and Potassium Deprivation. Front. Bioeng. Biotechnol. 2023, 10, 1028151. [Google Scholar] [CrossRef]
- Thongtha, T.; Aryusuk, K.; Kittiwongwattana, C.; Incharoensakdi, A.; Phunpruch, S. Modified Natural Seawater as Growth Medium for the Halotolerant Cyanobacterium Aphanothece halophytica to Increase Lipid Content for Biodiesel Production. J. Appl. Phycol. 2024, 37, 83–95. [Google Scholar] [CrossRef]
- Miriam, L.R.M.; Raj, R.E.; Kings, A.J.; Adhi, V.M. Identification and Characterization of a Novel Biodiesel Producing Halophilic Aphanothece halophytica and Its Growth and Lipid Optimization in Various NET Media. Energy Convers. Manag. 2017, 141, 93–100. [Google Scholar] [CrossRef]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J. CO2 Bio Fixation and Fatty Acid Composition of Scenedesmus obliquus and Chlorella pyrenoidosa in Response to Different CO2 Levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Division Rates. In Handbook of Phycological Methods: Culture Methods and Growth Measurements; Stein, J.R., Ed.; Cambridge University Press: London, UK, 1973; pp. 289–311. [Google Scholar]
- Mackinney, G. Absorption of Light by Chlorophyll Solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Britton, G. General Carotenoid Methods. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1985; pp. 113–149. [Google Scholar] [CrossRef]
- Hsieh, P.; Pedersen, J.Z.; Bruno, L. Photoinhibition of Cyanobacteria and Its Application in Cultural Heritage Conservation. Photochem. Photobiol. 2014, 90, 533–543. [Google Scholar] [CrossRef]
- Axelsson, M.; Gentili, F. A Single-Step Method for Rapid Extraction of Total Lipids from Green Microalgae. PLoS ONE 2014, 9, e89643. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Improved Recovery of Fatty Acid through Direct Transesterification without Prior Extraction or Purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Pojjanapornpun, S.; Nolvachai, Y.; Aryusuk, K.; Kulsing, C.; Krisnangkura, K.; Marriott, P.J. Ionic Liquid Phases with Comprehensive Two-Dimensional Gas Chromatography of Fatty Acid Methyl Esters. Anal. Bioanal. Chem. 2018, 410, 4669–4677. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.F.; Tabatabaei, M.; Chisti, Y. Biodiesel Analyzer: A User-Friendly Software for Predicting the Properties of Prospective Biodiesel. Biofuel Res. J. 2014, 2, 55–57. [Google Scholar] [CrossRef]
- Park, J.; Dinh, T.B. Contrasting Effects of Monochromatic LED Lighting on Growth, Pigments and Photosynthesis in the Commercially Important Cyanobacterium Arthrospira maxima. Bioresour. Technol. 2019, 291, 121846. [Google Scholar] [CrossRef]
- Larkum, A.W.D. Light-Harvesting in Cyanobacteria and Eukaryotic Algae: An Overview. In Advances in Photosynthesis and Respiration; Springer International Publishing: Cham, Switzerland, 2020; pp. 207–260. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Barreira, L.A.; Pereira, H.G.C.; Perales, J.A.; Varela, J.C.S. Light Emitting Diodes (LEDs) Applied to Microalgal Production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef]
- Glemser, M.; Heining, M.; Schmidt, J.; Becker, A.; Garbe, D.; Buchholz, R.; Brück, T. Application of Light-Emitting Diodes (LEDs) in Cultivation of Phototrophic Microalgae: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2016, 100, 1077–1088. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Zhang, H.; Yan, C.; Zhang, Y. Effects of Various LED Light Wavelengths and Intensities on Microalgae-Based Simultaneous Biogas Upgrading and Digestate Nutrient Reduction Process. Bioresour. Technol. 2013, 136, 461–468. [Google Scholar] [CrossRef]
- Teo, C.L.; Atta, M.; Bukhari, A.; Taisir, M.; Yusuf, A.M.; Idris, A. Enhancing Growth and Lipid Production of Marine Microalgae for Biodiesel Production via the Use of Different LED Wavelengths. Bioresour. Technol. 2014, 162, 38–44. [Google Scholar] [CrossRef]
- Chang, M.H.; Das, D.; Varde, P.V.; Pecht, M. Light Emitting Diodes Reliability Review. Microelectron. Reliab. 2012, 52, 62–782. [Google Scholar] [CrossRef]
- Ra, C.H.; Sirisuk, P.; Jung, J.H.; Jeong, G.T.; Kim, S.K. Effects of Light-Emitting Diode (LED) with a Mixture of Wavelengths on the Growth and Lipid Content of Microalgae. Bioprocess Biosyst. Eng. 2018, 41, 457–465. [Google Scholar] [CrossRef]
- Sharmila, D.; Suresh, A.; Suresh, A.; Indhumathi, J.; Velmurugan, N. Impact of Various Color Filtered LED Lights on Microalgae Growth, Pigments and Lipid Production. Eur. J. Biotechnol. Biosci. 2018, 6, 1–7. [Google Scholar]
- Asuthkar, M.; Gunti, Y.; Rao, R.; Rao, C.S.; Yadavalli, R. Effect of Different Wavelengths of Light on the Growth of Chlorella pyrenoidosa. Int. J. Pharm. Sci. Res. 2016, 7, 847–851. [Google Scholar] [CrossRef]
- Liu, X.; Hong, Y.; Liu, Y. Cultivation of Chlorella sp. HQ in Inland Saline-Alkaline Water under Different Light Qualities. Front. Environ. Sci. Eng. 2022, 16, 45. [Google Scholar] [CrossRef]
- Das, P.; Aziz, S.S.; Obbard, J.P. Two-Phase Microalgae Growth in the Open System for Enhanced Lipid Productivity. Renew. Energy 2011, 36, 2524–2528. [Google Scholar] [CrossRef]
- Tian, J.-H.; Pourcher, A.-M.; Bize, A.; Wazeri, A.; Peu, P. Impact of Wet Aerobic Pretreatments on Cellulose Accessibility and Bacterial Communities in Rape Straw. Bioresour. Technol. 2017, 237, 31–38. [Google Scholar] [CrossRef]
- Vijaya, V.; Anand, N. Blue Light Enhances the Pigment Synthesis in Cyanobacterium Anabaena ambigua Rao (Nostocales). ARPN J. Agric. Biol. Sci. 2009, 4, 36–43. [Google Scholar]
- Hauschild, C.A.; Heather, M.C.; Murter, H.J.G.; Pick, F.R. Effect of Spectral Quality on Growth and Pigmentation of Picocyanobacteria. J. Phycol. 1991, 27, 698–702. [Google Scholar] [CrossRef]
- Luimstra, V.M.; Schuurmans, J.M.; Hellingwerf, K.J.; Matthijs, H.C.P.; Huisman, J. Blue Light Induces Major Changes in the Gene Expression Profile of the Cyanobacterium Synechocystis sp. PCC 6803. Physiol. Plant. 2020, 170, 10–26. [Google Scholar] [CrossRef]
- Contreras-Ropero, J.E.; Lidueñez-Ballesteros, V.S.; Rodríguez-Bohórquez, A.D.; García-Martínez, J.B.; Urbina-Suarez, N.A.; López-Barrera, G.L.; Barrajas-Solano, A.F.; Bryan, S.J.; Zuorro, A. The Effect of LEDs on Biomass and Phycobiliproteins Production in Thermotolerant Oscillatoria sp. Appl. Sci. 2022, 12, 11664. [Google Scholar] [CrossRef]
- Kahn, A.; Oliveira, P.; Cuau, M.; Leão, P.N. Incorporation, Fate, and Turnover of Free Fatty Acids in Cyanobacteria. FEMS Microbiol. Rev. 2023, 47, fuad015. [Google Scholar] [CrossRef]
- Mori-Moriyama, N.; Yoshitomi, T.; Sato, N. Acyl Plastoquinol Is a Major Cyanobacterial Substance That Co-Migrates with Triacylglycerol in Thin-Layer Chromatography. bioRxiv 2023, 641, 8–26. [Google Scholar] [CrossRef]
- Yuan, X.; Iannetta, A.A.; Kim, M.; Sadecki, P.W.; Arend, M. Phototropin Connects Blue Light Perception to Starch Metabolism in Green Algae. bioRxiv 2024, 170, 1216. [Google Scholar] [CrossRef]
- Jungandreas, A.; Schellenberger Costa, B.; Jakob, T.; Von Bergen, M.; Baumann, S.; Wilhelm, C. The Acclimation of Phaeodactylum tricornutum to Blue and Red Light Does Not Influence the Photosynthetic Light Reaction but Strongly Disturbs the Carbon Allocation Pattern. PLoS ONE 2014, 9, e99727. [Google Scholar] [CrossRef]
- Raqiba, H.; Sibi, G. Light-Emitting Diode (LED) Illumination for Enhanced Growth and Cellular Composition in Three Microalgae. Adv. Microb. Res. 2019, 3, 007. [Google Scholar] [CrossRef]
- Ra, C.-H.; Kang, C.-H.; Jung, J.-H.; Jeong, G.-T.; Kim, S.-K. Effects of Light-Emitting Diodes (LEDs) on the Accumulation of Lipid Content Using a Two-phase Culture Process with Three Microalgae. Bioresour. Technol. 2016, 212, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Fleurent, G.; Awwad, F.; Cheng, M.; Meddeb-Mouelhi, F.; Budge, S.M.; Germain, H.; Desgagné-Penix, I. Red Light Variation as an Effective Alternative to Regulate Biomass and Lipid Profiles in Phaeodactylum tricornutum. Appl. Sci. 2020, 10, 2531. [Google Scholar] [CrossRef]
- Kim, S.H.; Sunwoo, L.Y.; Hong, H.J.; Awah, C.C.; Jeong, G.T.; Kim, S.K. Lipid and Unsaturated Fatty Acid Productions from Three Microalgae Using Nitrate and Light-Emitting Diodes with Complementary LED Wavelength in a Two-Phase Culture System. Bioprocess Biosyst. Eng. 2019, 42, 1517–1526. [Google Scholar] [CrossRef]
- Zhong, Y.; Jin, P.; Cheng, J.J. A Comprehensive Comparable Study of the Physiological Properties of Four Microalgal Species under Different Light Wavelength Conditions. Planta 2018, 248, 489–498. [Google Scholar] [CrossRef]



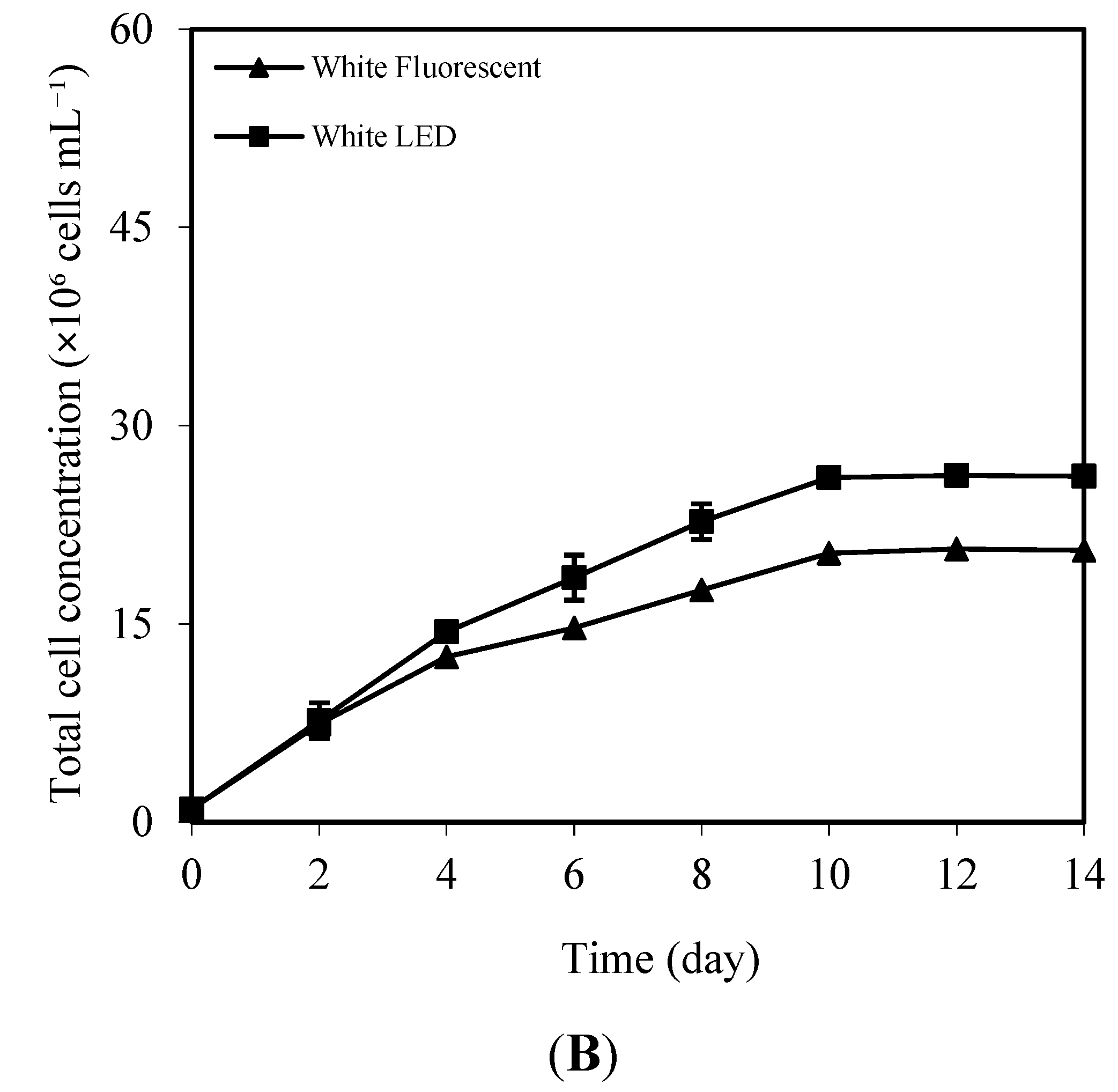
| Type of Light Source | Cell Density (×106 Cells mL−1) | Specific Growth Rate (Day−1) | Doubling Time (Days) | Lipid Content (%) | Lipid Productivity (mg L−1 day−1) |
|---|---|---|---|---|---|
| White Fluorescent | 20.56 ± 0.37 b | 0.216 ± 0.001 b | 3.21 ± 0.02 b | 43.12 ± 0.14 b | 39.37 ± 0.87 b |
| White LED | 26.16 ± 0.36 a | 0.233 ± 0.001 a | 2.97 ± 0.01 a | 50.63 ± 1.06 a | 47.74 ± 0.97 a |
| Type of Light Source | Chlorophyll a (µg mL−1) | Carotenoid (µg mL−1) | Phycocyanin (µg mL−1) | Allophycocyanin (µg mL−1) | Phycoerythrin (µg mL−1) |
|---|---|---|---|---|---|
| White Fluorescent | 2.57 ± 0.05 a | 0.74 ± 0.01 a | 4.52 ± 0.13 b | 5.45 ± 0.11 b | 1.66 ± 0.40 b |
| White LED | 2.68 ± 0.09 a | 0.66 ± 0.01 b | 5.21 ± 0.80 a | 7.78 ± 0.09 a | 2.76 ± 0.31 a |
| LED Color | Cell Density (×106 Cells mL−1) | Specific Growth Rate (Day−1) | Doubling Time (Days) | Lipid Content (%) | Lipid Productivity (mg L−1 day−1) |
|---|---|---|---|---|---|
| White | 26.82 ± 0.22 b | 0.235 ± 0.001 b | 2.95 ± 0.01 b | 51.51 ± 0.81 b | 46.38 ± 2.15 b |
| Blue | 32.33 ± 0.63 a | 0.248 ± 0.001 a | 2.79 ± 0.02 a | 53.10 ± 0.73 a | 50.53 ± 0.59 a |
| Green | 20.23 ± 0.59 c | 0.214 ± 0.002 c | 3.23 ± 0.03 c | 47.87 ± 0.42 c | 43.05 ± 0.24 c |
| Red | 26.56 ± 0.54 b | 0.234 ± 0.002 b | 2.96 ± 0.02 b | 50.38 ± 0.66 b | 47.33 ± 0.78 b |
| LED Color | Chlorophyll a (µg mL−1) | Carotenoid (µg mL−1) | Phycocyanin (µg mL−1) | Allophycocyanin (µg mL−1) | Phycoerythrin (µg mL−1) |
|---|---|---|---|---|---|
| White | 2.68 ± 0.09 ab | 0.66 ± 0.01 e | 5.21 ± 0.80 c | 7.78 ± 0.09 c | 2.76 ± 0.31 c |
| Blue | 2.82 ± 0.07 a | 1.26 ± 0.02 a | 13.02 ± 0.21 a | 15.63 ± 0.36 a | 5.89 ± 0.06 a |
| Green | 2.60 ± 0.08 b | 0.93 ± 0.01 c | 7.12 ± 0.46 b | 10.90 ± 0.46 b | 4.39 ± 0.24 b |
| Red | 2.57 ± 0.10 b | 1.02 ± 0.01 b | 13.57 ± 1.44 a | 16.16 ± 1.04 a | 6.23 ± 0.40 a |
| Blue LED Intensity (µmol Photons m−2 s−1) | Cell Density (×106 Cells mL−1) | Specific Growth Rate (µ) (Day−1) | Doubling Time (Days) | Lipid Content (%) | Lipid Productivity (mg L−1 day−1) |
|---|---|---|---|---|---|
| 15 | 32.70 ± 1.86 d | 0.249 ± 0.004 d | 2.78 ± 0.04 d | 52.97 ± 0.23 d | 52.37 ± 0.77 d |
| 30 | 44.33 ± 1.15 c | 0.270 ± 0.002 c | 2.56 ± 0.02 c | 53.76 ± 0.34 c | 53.82 ± 0.40 c |
| 45 | 51.53 ± 1.37 b | 0.281 ± 0.002 b | 2.46 ± 0.02 b | 54.41 ± 0.08 b | 55.23 ± 0.45 b |
| 60 | 68.96 ± 1.52 a | 0.302 ± 0.002 a | 2.29 ± 0.01 a | 55.16 ± 0.10 a | 56.81 ± 0.75 a |
| Blue LED Intensity (µmol Photons m−2 s−1) | Chlorophyll a (µg mL−1) | Carotenoid (µg mL−1) | Phycocyanin (µg mL−1) | Allophycocyanin (µg mL−1) | Phycoerythrin (µg mL−1) |
|---|---|---|---|---|---|
| 15 | 3.41 ± 0.07 d | 1.26 ± 0.02 d | 12.71 ± 0.57 d | 18.95 ± 0.21 d | 6.73 ± 0.33 d |
| 30 | 4.57 ± 0.05 c | 1.74 ± 0.01 c | 14.86 ± 0.34 c | 21.79 ± 0.32 c | 7.04 ± 0.64 c |
| 45 | 5.15 ± 0.05 b | 2.31 ± 0.01 b | 15.99 ± 0.44 b | 23.09 ± 0.34 b | 7.61 ± 0.46 a |
| 60 | 6.03± 0.05 a | 2.81 ± 0.08 a | 17.11 ± 0.73 a | 24.01 ± 0.40 a | 8.02 ± 0.49 a |
| Fatty Acids | Fatty Acid Composition (%) | |||
|---|---|---|---|---|
| White LED | Blue LED | Green LED | Red LED | |
| Myristic Acid (C14:0) | 1.87 | 2.12 | 0.90 | 2.05 |
| Palmitic Acid (C16:0) | 52.51 | 35.62 | 37.02 | 28.62 |
| Palmitoleic Acid (C16:1) | 2.57 | 8.84 | 4.03 | 6.03 |
| Stearic Acid (C18:0) | 10.97 | 5.19 | 7.35 | 4.35 |
| Oleic acid (C18:1) | 8.12 | 3.82 | 4.81 | 3.10 |
| Linoleic Acid (C18:2) | 9.98 | 16.30 | 16.20 | 19.52 |
| α-Linolenic acid (C18:3) | 13.98 | 28.10 | 29.69 | 36.32 |
| Saturated fatty acid (SFA) | 65.35 | 42.93 | 45.27 | 35.02 |
| Monounsaturated fatty acid (MUFA) | 10.69 | 12.66 | 8.84 | 9.13 |
| Polyunsaturated fatty acid (PUFA) | 23.96 | 44.40 | 45.89 | 55.84 |
| Biodiesel Properties | White LED | Blue LED | Green LED | Red LED |
|---|---|---|---|---|
| DU | 58.6 | 101.4 | 100.6 | 120.8 |
| SV | 210.6 | 209.4 | 207.9 | 207.5 |
| IV | 66.2 | 118.6 | 118.9 | 143.5 |
| CN | 57.3 | 45.6 | 45.7 | 40.3 |
| LCSF | 10.7 | 6.1 | 7.3 | 5.0 |
| CFPP | 17.2 | 2.8 | 6.7 | −0.6 |
| CP | 22.6 | 13.7 | 14.4 | 10.0 |
| APE | 56.0 | 92.6 | 96.5 | 114.7 |
| BAPE | 37.9 | 72.5 | 75.5 | 92.1 |
| OS | 7.5 | 5.2 | 5.1 | 4.7 |
| HHV | 39.2 | 39.2 | 39.2 | 39.2 |
| υ | 1.3 | 1.2 | 1.2 | 1.1 |
| Ρ | 870 | 870 | 870 | 880 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongtha, S.; Kittiwongwattana, C.; Incharoensakdi, A.; Phunpruch, S. Light-Emitting Diode Illumination Enhances Biomass, Pigment, and Lipid Production in Halotolerant Cyanobacterium Aphanothece halophytica. Phycology 2025, 5, 12. https://doi.org/10.3390/phycology5020012
Thongtha S, Kittiwongwattana C, Incharoensakdi A, Phunpruch S. Light-Emitting Diode Illumination Enhances Biomass, Pigment, and Lipid Production in Halotolerant Cyanobacterium Aphanothece halophytica. Phycology. 2025; 5(2):12. https://doi.org/10.3390/phycology5020012
Chicago/Turabian StyleThongtha, Sitthichai, Chokchai Kittiwongwattana, Aran Incharoensakdi, and Saranya Phunpruch. 2025. "Light-Emitting Diode Illumination Enhances Biomass, Pigment, and Lipid Production in Halotolerant Cyanobacterium Aphanothece halophytica" Phycology 5, no. 2: 12. https://doi.org/10.3390/phycology5020012
APA StyleThongtha, S., Kittiwongwattana, C., Incharoensakdi, A., & Phunpruch, S. (2025). Light-Emitting Diode Illumination Enhances Biomass, Pigment, and Lipid Production in Halotolerant Cyanobacterium Aphanothece halophytica. Phycology, 5(2), 12. https://doi.org/10.3390/phycology5020012






