Abstract
This in vitro study aimed to confirm the effect of the additional features of Kirei Keep Light (KKL), a commercial UV-C irradiation system that was originally created for coating the surface of removable dentures with photoreactive 2-methacryloyloxyethyl phosphorylcholine (MPC), on the antimicrobial efficacy aspect against Candida albicans biofilm on a denture base material (PMMA) and SARS-CoV-2. Materials and Methods: The antimicrobial efficacy on C. albicans biofilm was evaluated through quantitative (CFU) and qualitative (SEM images) analysis of three groups: no treatment (control), KKL, and immersion in a disinfectant solution, MCAE. The quantitative evaluation on SARS-CoV-2 was performed by comparing the untreated (control) group and the KKL group. Results: In comparison with the control group (2.39 × 106 CFU/mL), KKL irradiation resulted in a 91.01% reduction in C. albicans biofilm (2.15 × 105 CFU/mL), whereas for the MCAE group, this reduction was 99.98% (4.64 × 102 CFU/mL). The SEM image results also corroborate the CFU results, which showed that the fewest clean surfaces were found in the control, and this gradually increased with KKL and MCAE. SARS-CoV-2 inhibition, indicated by its TCID50 value, demonstrated that KKL almost completely inhibited SARS-CoV-2 infection and replication (99.99% reduction). Conclusion: KKL possesses antimicrobial efficacy on C. albicans biofilm on PMMA and SARS-CoV-2.
1. Introduction
Biomaterials are essential in medical and dental care, especially in delivering prosthetic treatments for rehabilitation. In dentistry, a wide range of dental materials have been used during treatment to restore missing teeth and oral tissues. The numerous types of artificial dental materials have different properties with broad purposes and benefits [1,2] (Table 1). However, when delivering the materials to patients, the inherent properties of the materials have been reported to attract surrounding microorganisms and harm the surrounding tissue [3].

Table 1.
Examples of artificial dental materials and their applications.
A denture is a typical artificial dental material that is continuously in contact with the oral environment. The dental plaque that exists in the oral environment consists of a diverse microbial composition [4]. Soon after the dentures are placed in the oral cavity, they are exposed to various substances like saliva, microorganisms, and food, which lead to denture plaque formation [5,6]. An insight into biofilm types present in the oral cavity and dentures is provided in Table 2. As the biofilm’s presence is harmful to both the oral tissues and the denture, maintaining denture hygiene is an essential aspect of delivering dental prostheses or restoration.

Table 2.
Biofilm variations in the oral cavity and dentures and their effects.
Past studies have reported that polymethyl methacrylate (PMMA), the most used polymeric denture base material, may present as a plaque or biofilm reservoir [7], which further causes infections either in the oral cavity, such as denture stomatitis, which is mainly caused by C. albicans [8], or even spread to other sites, such as in the case of aspiration pneumonia. Moreover, during the more recent COVID-19 pandemic, SARS-CoV-2 was also reported to exist in the dental biofilm of infected patients [9], and cross-infection in healthcare facility settings must be prevented [10]. The presence of both C. albicans and SARS-CoV-2 in denture wearers might increase morbidity and mortality, especially in older adults, pregnant women, and immunocompromised patients, where the microbiota composition and the host–immune response are altered [11,12,13].
Performing denture hygiene procedures is a mandatory task for both dental health professionals and patients. Various methods have been introduced to the market to remove or inactivate pathogens from dentures. Briefly, the method of regular denture cleaning can be divided into mechanical, chemical, and irradiation techniques and a combination of those techniques [5,14]. Moreover, material properties can be modified during denture fabrication or dental visits. This technique is usually performed by healthcare professionals, like modifying the monomers or polymers with antimicrobial efficacies or modifying surface properties through coating application [15]. Detailed information on each method is provided in Table 3.

Table 3.
Materials and devices utilized in denture hygiene procedures.
Applying a UV-curable coating material is one of the effective measures in maintaining denture hygiene without altering the physical properties of the denture base resins [16]. For example, 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer is a highly biocompatible hydrophilic coating material that is reported to be effective in preventing denture plaque and biofilm formation on polymethyl methacrylate (PMMA) [17]. Recently, a Kirei Keep system has been introduced, offering a straightforward method: spraying pre-mixed photoreactive MPC liquid on the surface, followed by chemical bonding activation inside a portable UV-C light box (Kirei Keep Light®, Sun Medical, Moriyama, Japan), and it has been reported to be effective in preventing mature C. albicans biofilm formation [18].
UV light is a form of electromagnetic radiation with a wavelength between 100 to 400 nm. The detailed UV classification based on wavelength and examples are described in Table 4 [19,20,21]. UV-C light irradiation is commonly known for its antimicrobial properties for surface disinfection through the induction of genomic damage [21,22]. However, its efficacy might differ based on light intensity, radiation time, distance to the object, and microorganism type [23]. Based on these facts, this study was performed to confirm the antimicrobial efficacy of Kirei Keep Light as an additional feature other than for MPC coating activation. In this study, our primary objective was to evaluate the antimicrobial efficacy of the Kirei Keep Light device on its default settings (wavelength: 260–280 nm; intensity: 309 µW/cm2; duration: 3 min). Our evaluation focused on two key areas of interest. First, we aimed to assess the device’s effectiveness against C. albicans biofilm on PMMA and, second, its effectiveness on SARS-CoV-2. We hypothesized that UV-C light irradiation using the Kirei Keep Light device’s default settings has an antimicrobial effect on C. albicans and SARS-CoV-2.

Table 4.
Classification of UV light and applications in the hygiene field.
2. Materials and Methods
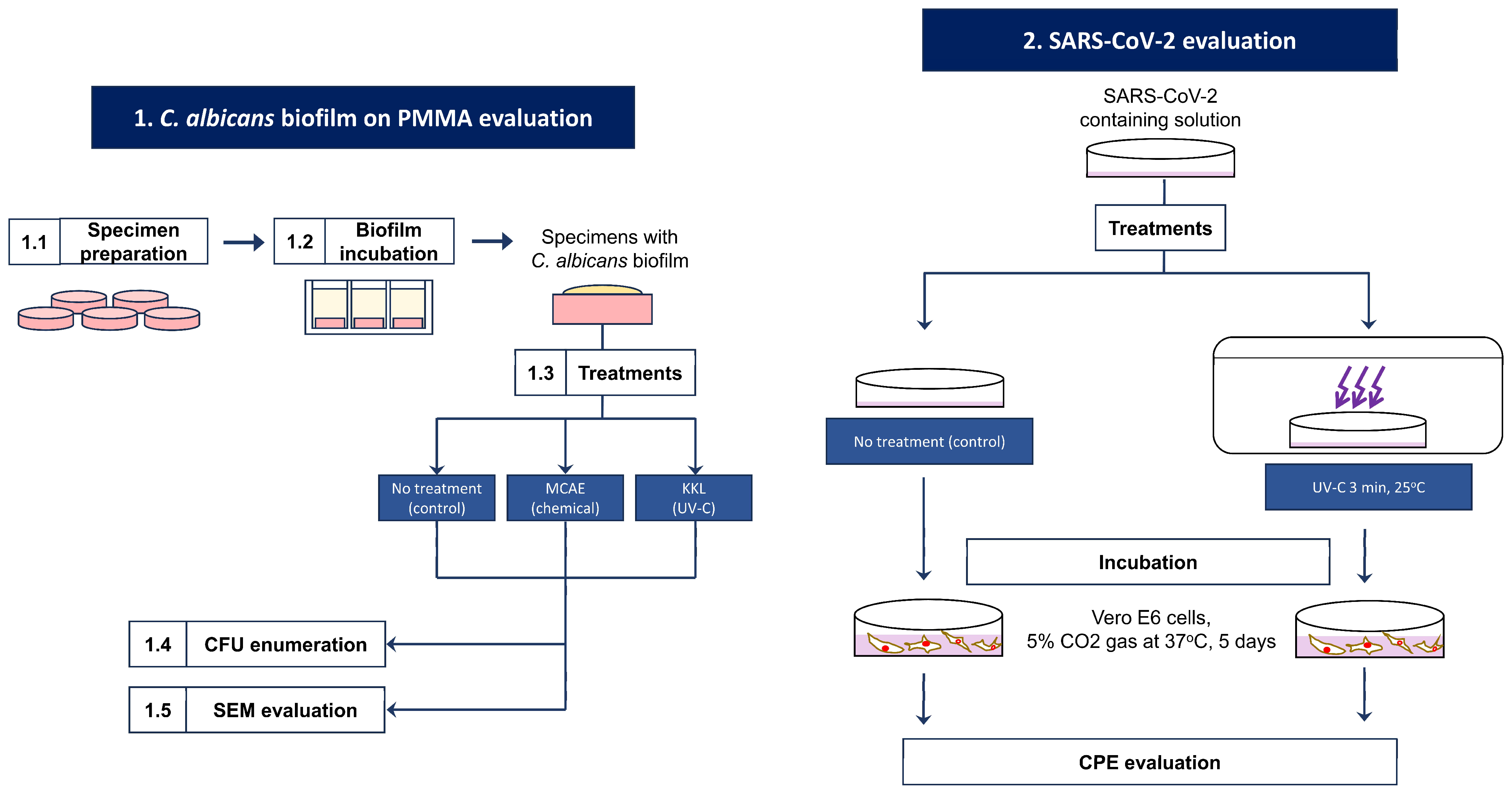
This study is divided into two analyses: KKL’s antifungal properties on C. albicans biofilm on PMMA and KKL’s viral infection and replication inhibition of SARS-CoV-2. The flow diagram of this study is provided in Figure 1. Research flow diagram.
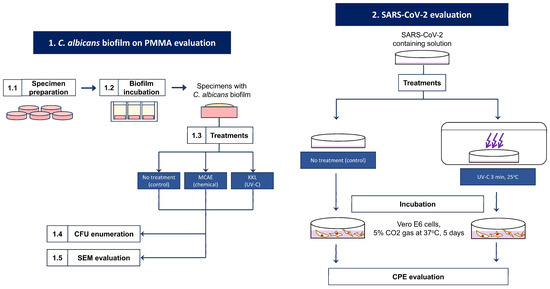
Figure 1.
Research flow diagram.
2.1. C. albicans Biofilm on PMMA Evaluation
2.1.1. Specimen Preparation
Heat-polymerization-type PMMA (Acron®, GC Corporation, Tokyo, Japan) was utilized as a denture base material in this study. It consists of a liquid monomer (methyl methacrylate) and a powder polymer (polymethyl methacrylate). Paraffin wax patterns were prepared to fabricate the specimens to a disc shape with 12 mm diameter and 2 mm thickness. Following the conventional lost-wax technique, the monomer and polymer were mixed according to the manufacturer’s instructions and were then packed into a flask and underwent final heat polymerization inside a water bath at 70 °C for 9 h. The resin discs were then polished using #1200 and #2400 waterproof polishing paper and cleaned using ethanol inside an ultrasonic bath.
2.1.2. Biofilm Incubation
A C. albicans culture isolated from denture plaque (CAD-1) [18,24,25]) was used in this study. An overnight culture was prepared by transferring a single colony of C. albicans into a 15 mL tube containing 3 mL Sabouraud dextrose broth, which was then placed inside a rotating incubator at 37 °C for 24 h with constant shaking (100 rpm). Before incubation, the specimens were immersed in 0.4 mg/mL mucin (Type I Bovine Submaxillary Mucin, Sigma Aldrich, St. Louis, MO, USA) for 10 min on a 70 rpm shaker at 37 °C. Thereafter, 200 µL of the overnight culture was transferred into a 24-well plate containing a specimen and 1.8 mL of yeast nitrogen broth supplemented with n-acetylglucosamine and phosphate buffer (YNBNP media). Finally, biofilm incubation was performed aerobically at 37 °C for 48 h. Prior to the treatment applied to each group, the specimens were rinsed using phosphate-buffered saline (PBS) twice to remove unadhered cells.
2.1.3. Treatments
A UV-C light box for MPC coating activation (Kirei Keep Light®, Sun Medical Company, Moriyama, Japan) was utilized in all experimental groups in this study for UV-light irradiation as a disinfection treatment, hereafter abbreviated as KKL. Treatment application is carried out by putting the specimen in the bottom center of the box (vertical distance to the light source: 38 mm), starting the UV-light irradiation with the default setting for three minutes at 25 °C. As a comparison, a Nα-cocoyl arginine ethyl ester pyloridon carbon-based MCAE disinfection solution (Bee Brand Medico Dental, Osaka, Japan) was also utilized by immersing the specimens within the same period (3 min, 25 °C). The specimens without any applied treatments were assigned as a control.
2.1.4. Colony-Forming Unit (CFU) Enumeration
Following incubation and treatment applied to each group (n = 5), the viable C. albicans cells on the specimen surface were collected using 2.5% trypsin in PBS by pipetting for 1 min, followed by serial dilutions into 15 mL tubes. Subsequently, the diluted solutions were plated using an automatic spiral plater machine (Easyspiral™, Interscience, Osaka, Japan) onto Sabouraud dextrose agar and then incubated aerobically at 37 °C for 48 h. The CFU enumeration was replicated three times on each sample and performed according to ISO4833-2 [26]. Furthermore, the obtained CFU data were processed to a percentage of the reduction rate using the following formula:
2.1.5. Scanning Electron Microscope (SEM) Image Evaluation
Morphological evaluation of C. albicans biofilm was performed by evaluating the SEM images. Following incubation and treatments according to each group, the specimens underwent a fixation procedure using 2.5% glutaraldehyde solution for 15 min. Afterward, serial dehydration was performed by immersion in 40, 75, and 99% ethanol. To facilitate electroconductivity, a gold coating was applied to the specimens with an ion coater (IB-3, Eiko Corporation, Tokyo, Japan) at 3 mA for 5 min. Finally, a low-vacuum SEM (Miniscope TM1000®, Hitachi, Tokyo, Japan) was used to evaluate the biofilm morphology.
2.2. SARS-CoV-2 Evaluation
The SARS-CoV-2 strain used in this study was isolated from Vero cells and confirmed via real-time PCR. Approximately 1 mL of SARS-CoV-2 containing solution was placed onto a Ø30 mm Petri dish. The experimental group samples were subjected to UV-light irradiation inside the KKL box for 3 min at 25 °C and finally collected, whereas the control group samples were collected after 3 min without intervention. Furthermore, those solutions were serially diluted using a cell maintenance medium and then 100 µL was injected into the Vero E6 cells inside a 96-well plate with a growth medium (Minimum Essential Medium Eagle, Sigma Aldrich, St. Louis, MO, USA). Incubation was performed by culturing in a carbon dioxide gas (5%) at 37 °C for 5 days.
The presence or absence of virus replication was confirmed by evaluating the virus-induced cytopathic effect (CPE) that was exhibited in the culture cells under a microscope (Inverted culture microscope CK30, Olympus, Tokyo, Japan), before and after 3 min of each intervention. The concentration was calculated, and finally, the amount of virus per specimen was determined. The efficacy of the KKL on SARS-CoV-2 was confirmed by calculating the percentage of viral virulence in the test area against that of the control area using the following formula:
The SARS-CoV-2 analysis was conducted completely by an accredited laboratory (Laboratory of Food Environment and Hygiene, Gunma, Japan).
2.3. Statistical Analysis
Statistical analysis was performed using SPSS software (version 25, SPSS Japan Inc., Tokyo, Japan). The comparison of data for each group’s results was analyzed using Welch’s analysis of variance (ANOVA), followed by the Games–Howell post hoc test at a p-value of 0.05.
3. Results
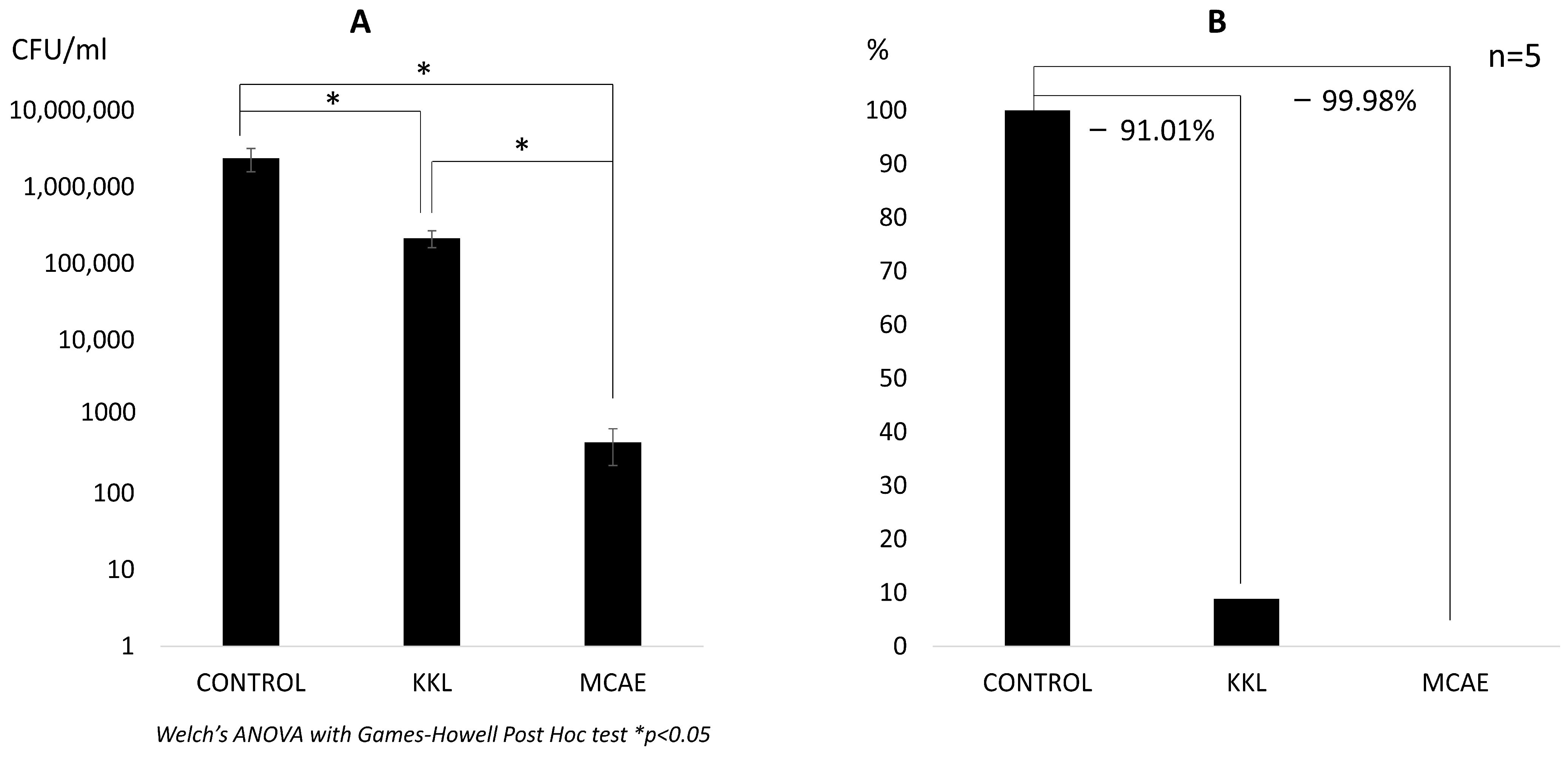
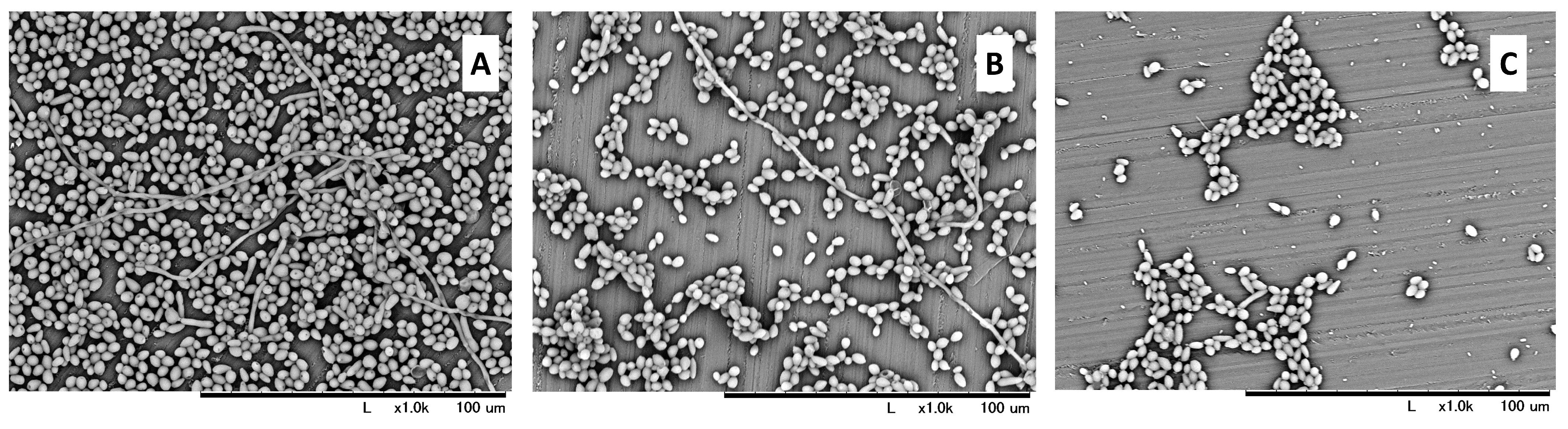
The highest amount of viable C. albicans was observed in the control group (2.39 × 106 CFU/mL). A significantly lower value was observed both in the KKL group (2.15 × 105 CFU/mL) and the MCAE group (4.64 × 102 CFU/mL). Nevertheless, a statistically significant difference (p < 0.05) was also found between the KKL and MCAE groups (Figure 2A). Based on these CFU data, the calculated biofilm reduction rate was 91.01% for the KKL group, whereas it was 99.98% for the MCAE group (Figure 2B). Based on the morphological evaluation results (Figure 3), C. albicans biofilm consisting of hyphae and yeast cell types was observed covering most of the specimen surface in the control group. Less PMMA area covered with biofilm was found in the KKL group, and the lowest coverage was found in the MCAE group. These findings corroborate with the quantitative results.
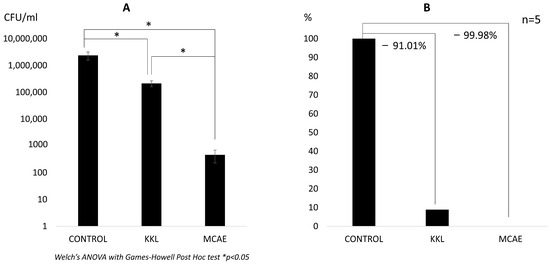
Figure 2.
(A) The number of viable C. albicans in each group in CFU/mL. (B) C. albicans cells comparison in each group in percentage, where the control group is referred to as 100%. The values below the connecting line between bars represent the reduction rate (KKL: Kirei Keep Light; MCAE: commercial surface disinfection solution).
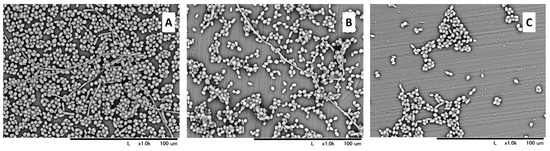
Figure 3.
SEM images at 1000× magnification of C. albicans biofilm morphology on the PMMA surface in the control (A), UV-C irradiation using KKL (B), and chemical treatment using MCAE (C).
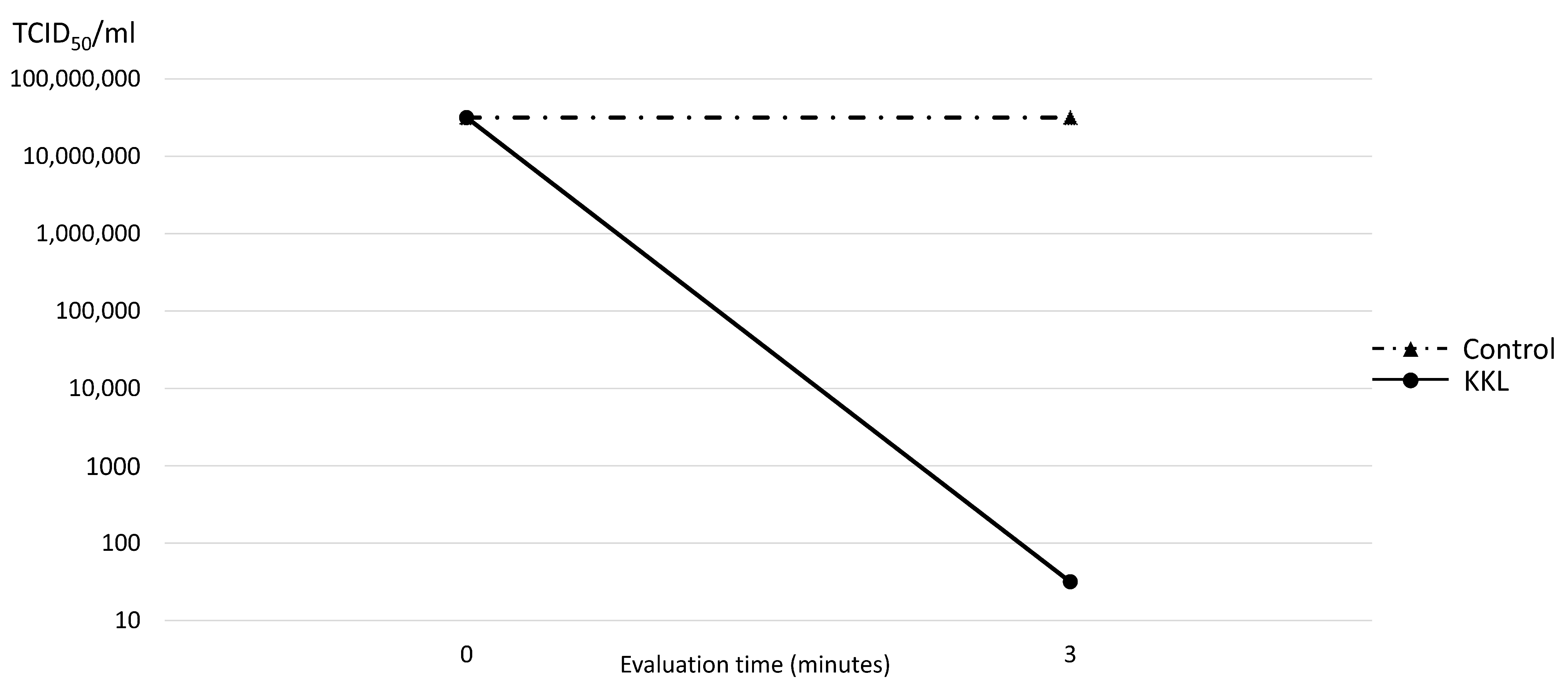
Three minutes of UV-C irradiation using KKL resulted in almost complete inhibition of SARS-CoV-2 to 101.5 TCID50/mL, compared to that of the control group (107.5 TCID50/mL). Thus, the reduction rate of KKL on SARS-CoV-2 was 99.99%, as demonstrated in Figure 4.
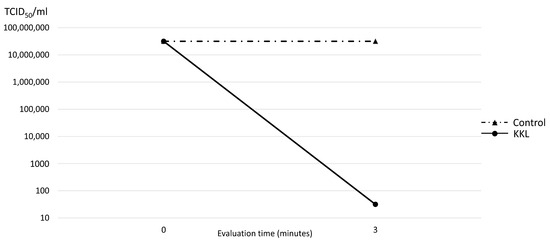
Figure 4.
Median tissue culture infection dose of SARS-CoV-2 (TCID50/mL) comparison between the control and UV-C irradiation using KKL at 0 and 3 min.
4. Discussion
The antimicrobial efficacy of disinfection and sterilization relies on factors that come from each microorganism’s intrinsic qualities (number, location, and innate resistance mechanism) and other extrinsic chemical or physical factors (concentration, temperature, pH, exposure duration, and organic and inorganic matter) [27]. Despite the increased popularity of UV sterilization during the COVID-19 pandemic, wherein a lot of portable UV sterilization boxes were sold commercially, Harada et al. (2023) reported that not all possess true antimicrobial efficacy [28]. Some devices in dental healthcare facilities that utilize UV-C, such as Kirei Keep Light, might also become helpful for disinfection if the configuration (wavelength, irradiation dose, and time) is proven effective. The results of this study can provide evidence of the antimicrobial efficacy of KKL UV-C irradiation (260–280 nm wavelength) when using its default setting for three minutes of exposure time. We selected C. albicans and SARS-CoV-2 as evaluation targets considering the evidence that the first one is the major pathogen reported in denture-related stomatitis, and the latter is a virus that is responsible for the latest COVID-19 pandemic, which was reported to be found in a patient’s oral cavity as well [9].
The microbial reduction rate of KKL treatment varies between C. albicans biofilm on PMMA (91.01%) and SARS-CoV-2 (99.9%). This finding can be explained based on the theory of morphological difference and sensitivity to UV radiation. C. albicans is an eucaryotic microorganism, where the nucleic acid material inside a nucleus is protected by a complex structure like a cell wall on the outermost layer, plasma membrane, and cytoplasm [29]. In contrast, SARS-CoV-2 possesses a simpler structure, where the nucleic acid materials inside a nucleocapsid are only protected by an envelope [30]. More importantly, C. albicans was evaluated in the form of a biofilm on the PMMA surface to represent the clinical situation, whereas SARS-CoV-2 was in the form of a solution. The adhesion and structure of C. albicans cells in the form of biofilm are stronger, and the presence of hyphae structures elevates their resistance further [31] so that the cells located on the base layer of the biofilm may remain unexposed to the UV-C photons. In accordance with a previous study, SARS-CoV-2 was reported to be highly susceptible to UV-C light irradiation, resulting in complete inhibition [32]. The exposure dose applied in this study was 55.62 mJ/cm2 (309 µW/cm2 × 180 s) and is considered more than enough for the complete inhibition of SARS-CoV-2, which can be achieved at16.9 mJ/cm2 [33].
In C. albicans biofilm evaluation, despite the reduction in viable cell numbers above 90%, there was a significant difference between KKL (UV-C irradiation) and MCAE (an amino acid-derived compound with a high ethanol concentration). In accordance with Theraud et al.’s findings, UV light irradiation has less efficacy than ethanol on C. albicans biofilm [34]. Another limitation of UV-light irradiation is that its efficacy might not be optimized in areas with high microbial loads or concentrations [35]. In contrast, ethanol treatment showed more efficacy even in highly concentrated areas with hyphae structures [33,36]. It was reported that ethanol treatment reduced >99% of C. albicans mature biofilm’s metabolic activity and prevented its regrowth [37]. MCAE solution is an ethanol-based bactericidal cleaning solution developed for dental use and is effective against biofilm.
UV-C irradiation using the default settings of KKL for three minutes demonstrated an effective antimicrobial effect with a reduction rate above 90% for C. albicans and SARS-CoV-2; thus, our hypothesis was accepted. This indicates that the 260–280 nm UV-C irradiation of Kirei Keep Light can be useful for surface disinfection, as an additional feature of its main function as an MPC coating activation apparatus. Nevertheless, for a surface with a high microbial load like a thick denture plaque, it is better to use this measure as an addition to conventional cleansing (mechanical and chemical) to ensure the complete disinfection of small undercuts and gaps and to remove thick biofilms which cannot be irradiated thoroughly by light.
There are some limitations of this study. The C. albicans biofilm evaluation in this study was performed within an in vitro setting with a single species, whereas in the clinical situation, a more complex biofilm consisting of many other microorganism species coexists. The topography and surface area of the specimen were in the form of a simple and flat disc shape, and we only evaluated the typical denture base material: PMMA, whereas, currently, there are plenty of material options available that possess various material characteristics. The UV-C irradiation time was only performed using one variation, based on the default setting of the light box Kirei Keep Light, as well as the chemical treatment used as a comparison in this study also only utilizing an ethanol-based cleansing agent. Further studies confirming more in vitro variables or in a clinical setting using prostheses or other dental materials that are currently installed in the patient’s mouth are recommended.
5. Conclusions
UV-C light irradiation using the default setting of Kirei Keep Light for three minutes effectively reduced C. albicans biofilm on PMMA and inhibited SARS-CoV-2 infection and replication. This indicates that it can also be used as an effective surface disinfection device, as an additional function other than as a portable MPC coating activation box.
Author Contributions
Conceptualization, T.I.; literature collection, A.Y.P.W. and M.W.; C. albicans experimental analysis, A.Y.P.W., M.W. and T.I.; SARS-CoV-2 study design and outsourcing to a third-party laboratory, T.O.; review and editing, A.Y.P.W., M.W., Y.I., T.G., T.O. and T.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank Miura from Sun Medical Corporation for providing the Kirei Keep Light apparatus, Akikazu Murakami for his support with the microbiological incubation, and Kazumitsu Sekine for his support with the SEM apparatus. This study was supported by the Support Center for Advanced Medical Sciences, Tokushima University Graduate School of Biomedical Sciences.
Conflicts of Interest
The authors, except T.O., declare no conflicts of interest. T.O. works for Sun Medical Company, which developed the UV-C light box and devised the SARS-CoV-2 study design, which was outsourced the test to a trusted third-party organization.
References
- Saeed, F.; Muhammad, N.; Khan, A.S.; Sharif, F.; Rahim, A.; Ahmad, P.; Irfan, M. Prosthodontics dental materials: From conventional to unconventional. Mater. Sci. Eng. C. 2020, 106, 110167. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D.; Reichl, F.; Hickel, R. Wear of dental materials: Clinical significance and laboratory wear simulation methods—A review. Dent. Mater. J. 2019, 38, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial biofilm formation on biomaterials and approaches to its treatment and prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. Dental Biofilm and Laboratory Microbial Culture Models for Cariology Research. Dent. J. 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, P.; Milward, P.; McAndrew, R. Denture cleanliness and hygiene: An overview. Br. Dent. J. 2022, 233, 20–26. [Google Scholar] [CrossRef] [PubMed]
- O’donnell, L.E.; Robertson, D.; Nile, C.J.; Cross, L.J.; Riggio, M.; Sherriff, A.; Bradshaw, D.; Lambert, M.; Malcolm, J.; Buijs, M.J.; et al. The oral microbiome of denture wearers is influenced by levels of natural dentition. PLoS ONE 2015, 10, 0137717. [Google Scholar] [CrossRef]
- Yoshijima, Y.; Murakami, K.; Kayama, S.; Liu, D.; Hirota, K.; Ichikawa, T.; Miyake, Y. Effect of substrate surface hydrophobicity on the adherence of yeast and hyphal Candida. Mycoses 2010, 53, 221–226. [Google Scholar] [CrossRef]
- Le Bars, P.; Kouadio, A.A.; Bandiaky, O.N.; Le Guéhennec, L.; de La Cochetière, M.-F. Host’s Immunity and Candida Species Associated with Denture Stomatitis: A Narrative Review. Microorganisms 2022, 10, 1437. [Google Scholar] [CrossRef]
- Gomes, S.C.; Fachin, S.; da Fonseca, J.G.; Angst, P.D.M.; Lamers, M.L.; da Silva, I.S.B.; Nunes, L.N. Dental biofilm of symptomatic COVID-19 patients harbours SARS-CoV-2. J. Clin. Periodontol. 2021, 48, 880–885. [Google Scholar] [CrossRef]
- Riad, A.; Gad, A.; Boccuzzi, M.; Cosola, S. Are ‘family bubbles’ safe? Br. Dent. J. 2020, 229, 147. [Google Scholar] [CrossRef]
- Jerônimo, L.S.; Esteves Lima, R.P.; Suzuki, T.Y.U.; Discacciati, J.A.C.; Bhering, C.L.B. Oral Candidiasis and COVID-19 in Users of Removable Dentures: Is Special Oral Care Needed? Gerontology 2022, 68, 80–85. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Colnaghi, A.; Morittu, S.; Barbieri, S.; Ricci, M.; Guerrisi, G.; Piloni, D.; et al. Assessment of Oral Microbiome Changes in Healthy and COVID-19-Affected Pregnant Women: A Narrative Review. Microorganisms 2021, 9, 2385. [Google Scholar] [CrossRef]
- Dioguardi, M.; Cantore, S.; Scacco, S.; Quarta, C.; Sovereto, D.; Spirito, F.; Alovisi, M.; Troiano, G.; Aiuto, R.; Garcovich, D.; et al. From Bench to Bedside in Precision Medicine: Diabetes Mellitus and Peri-Implantitis Clinical Indices with a Short-Term Follow-Up: A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 235. [Google Scholar] [CrossRef]
- Schmutzler, A.; Rauch, A.; Nitschke, I.; Lethaus, B.; Hahnel, S. Cleaning of removable dental prostheses—A systematic review. J. Evid. Based Dent. Pract. 2021, 21, 101644. [Google Scholar] [CrossRef]
- Bajunaid, S.O. How Effective Are Antimicrobial Agents on Preventing the Adhesion of Candida albicans to Denture Base Acrylic Resin Materials? A Systematic Review. Polymers 2022, 14, 908. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, Y.; Fukuyama, T.; Hamano, N.; Iwashita, H.; Watanabe, M.; Ino, S. The stain resistant effect of an ultraviolet curable coating material on denture base resin. Dent. Mater. J. 2023, 42, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Ikeya, K.; Fukunishi, M.; Iwasa, F.; Inoue, Y.; Ishihara, K.; Baba, K. 2-methacryloyloxyethyl phosphorylcholine polymer treatment of complete dentures to inhibit denture plaque deposition. J. Vis. Exp. 2016, 118, e54965. [Google Scholar] [CrossRef]
- Putra Wigianto, A.Y.; Ishida, Y.; Iwawaki, Y.; Goto, T.; Watanabe, M.; Sekine, K.; Hamada, K.; Murakami, K.; Fujii, H.; Ichikawa, T. 2-methacryloyloxyethyl phosphorylcholine polymer treatment prevents Candida albicans biofilm formation on acrylic resin. J. Prosthodont. Res. 2023, 67, 384–391. [Google Scholar] [CrossRef] [PubMed]
- International Ultraviolet Association. What is UV? Available online: https://www.iuva.org/What-is-UV (accessed on 26 January 2024).
- Food and Drug Administration. Ultraviolet (UV) Radiation. Available online: https://www.fda.gov/radiation-emitting-products/tanning/ultraviolet-uv-radiation (accessed on 26 January 2024).
- Bono, N.; Ponti, F.; Punta, C.; Candiani, G. Effect of UV irradiation and TiO2-Photocatalysis on airborne bacteria and viruses: An overview. Materials 2021, 14, 1075. [Google Scholar] [CrossRef] [PubMed]
- Raeiszadeh, M.; Adeli, B. A critical review on ultraviolet disinfection systems against COVID-19 outbreak: Applicability, validation, and safety considerations. ACS Photonics 2020, 7, 2941–2951. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W.; Matsuura, R.; Iimura, K.; Wada, S.; Shinjo, A.; Benno, Y.; Nakagawa, M.; Takei, M.; Aida, Y. UVC disinfects SARS-CoV-2 by induction of viral genome damage without apparent effects on viral morphology and proteins. Sci. Rep. 2021, 11, 13804. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hirota, K.; Yumoto, H.; Matsuo, T.; Miyake, Y.; Ichikawa, T. Enhanced germicidal effects of pulsed UV-LED irradiation on biofilms. J. Appl. Microbiol. 2010, 109, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Murakami, K.; Yoshida, K.; Sakurai, S.; Kudo, Y.; Ozaki, K.; Hirota, K.; Fujii, H.; Suzuki, M.; Miyake, Y.; et al. Suppressive effects of 2-methacryloyloxyethyl phosphorylcholine (MPC)-polymer on the adherence of Candida species and MRSA to acrylic denture resin. Heliyon 2020, 6, e04211. [Google Scholar] [CrossRef] [PubMed]
- ISO 4833-2:2013; Microbiology of the Food Chain, Horizontal Method for the Enumeration of Microorganisms Part 2: Colony Count at 30 °C by the Surface Plating Technique 4833-2. International Organization for Standardization: Geneva, Switzerland, 2013.
- Factors Affecting the Efficacy of Disinfection and Sterilization. Centers for Disease Control and Prevention, Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/efficacy.html (accessed on 3 July 2023).
- Harada, K.; Horinouchi, N.; Yamashita, Y.; Murakami, M.; Nishi, Y.; Nishimura, M. Sterilization effect of ultraviolet irradiation system against Candida albicans and its application to dentures. In Proceedings of the 15th Scientific Meeting of Japan Denture Care Society, Iwate University, Iwate (Online Conference), Online, 22 January 2023. [Google Scholar]
- De Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.O.; Cartagenes, M.S.S.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida infections and therapeutic strategies: Mechanisms of action for traditional and alternative agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, R.; Gao, L.; Gao, X.; Wang, D.; Cao, J. SARS-CoV-2: Structure, biology, and structure-based therapeutics development. Front. Cell. Infect. Microbiol. 2020, 10, 3389. [Google Scholar] [CrossRef]
- Atriwal, T.; Azeem, K.; Husain, F.M.; Hussain, A.; Khan, M.N.; Alajmi, M.; Abid, M. Mechanistic understanding of Candida albicans biofilm formation and approaches for its inhibition. Front. Microbiol. 2021, 12, 638609. [Google Scholar] [CrossRef]
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control. 2020, 48, 1273–1275. [Google Scholar] [CrossRef]
- Biasin, M.; Bianco, A.; Pareschi, G.; Cavalleri, A.; Cavatorta, C.; Fenizia, C.; Galli, P.; Lessio, L.; Lualdi, M.; Tombetti, E.; et al. UV-C irradiation is highly effective in inactivating SARS-CoV-2 replication. Sci. Rep. 2021, 11, 6260. [Google Scholar] [CrossRef]
- Théraud, M.; Bédouin, Y.; Guiguen, C.; Gangneux, J.P. Efficacy of antiseptics and disinfectants on clinical and environmental yeast isolates in planktonic and biofilm conditions. J. Med. Microbiol. 2004, 53, 1013–1018. [Google Scholar] [CrossRef]
- Duering, H.; Westerhoff, T.; Kipp, F.; Stein, C. Short-Wave Ultraviolet-Light-Based Disinfection of Surface Environment Using Light-Emitting Diodes: A New Approach to Prevent Health-Care-Associated Infections. Microorganisms 2023, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Asano, K.; Naito, K.; Ohashi, H.; Sasaki, M.; Morimoto, Y.; Igarashi, T.; Nakane, A. Ultraviolet C light with wavelength of 222 nm inactivates a wide spectrum of microbial pathogens. J. Hosp. Infect. 2020, 105, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Rane, H.S.; Bernardo, S.M.; Walraven, C.J.; Lee, S.A. In vitro analyses of ethanol activity against Candida albicans biofilms. Antimicrob. Agents. Chemother. 2012, 56, 4487–4489. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).