Behavioral Response of Multiple Rainbow Trout to Vertically Suspended Environmental Enrichment
Abstract
:1. Introduction
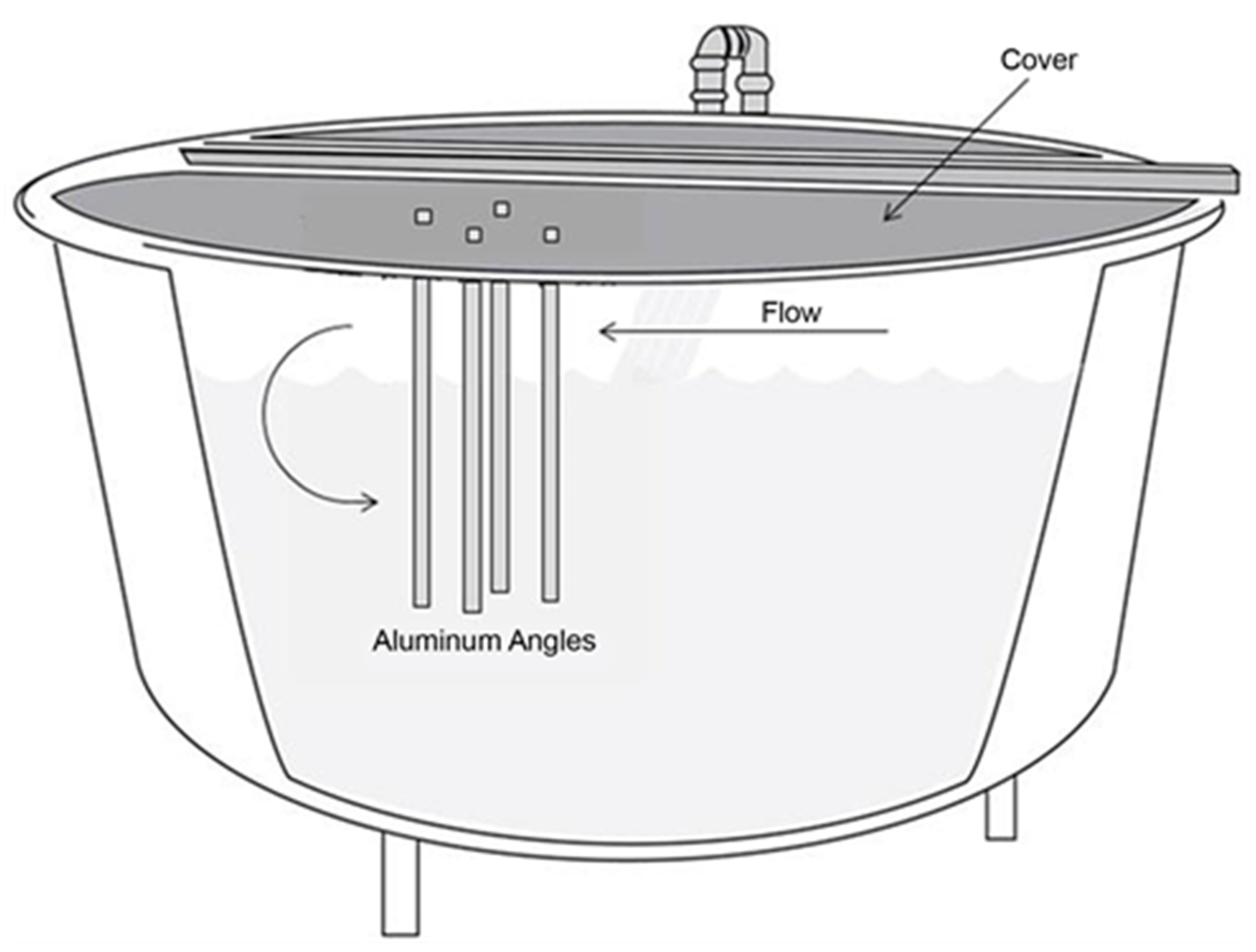
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, C.; Davidson, T.; Laland, K. Environmental enrichment and prior experience of live prey improve foraging behaviour in hatchery-reared Atlantic salmon. J. Fish Biol. 2003, 63, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Batzina, A.; Karakatsouli, N. The presence of substrate as a means of environmental enrichment in intensively reared gilthead seabream Sparus aurata: Growth and behavioral effects. Aquaculture 2012, 370-371, 54–60. [Google Scholar] [CrossRef]
- Näslund, J.; Rosengren, M.; Del Villar, D.; Gansel, L.; Norrgård, J.R.; Persson, L.; Winkowski, J.J.; Kvingedal, E. Hatchery tank enrichment affects cortisol levels and shelter-seeking in Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 2013, 70, 585–590. [Google Scholar] [CrossRef] [Green Version]
- Gerber, B.; Stamer, A.; Stadtlander, T. Environmental Enrichment and its Effects on Welfare in Fish; FiBL: Frick, Switzerland, 2015; pp. 1–74. [Google Scholar]
- Kientz, J.L.; Barnes, M.E. Structural Complexity Improves the Rearing Performance of Rainbow Trout in Circular Tanks. N. Am. J. Aquac. 2016, 78, 203–207. [Google Scholar] [CrossRef]
- Hyvärinen, P.; Rodewald, P. Enriched rearing improves survival of hatchery-reared Atlantic salmon smolts during migration in the River Tornionjoki. Can. J. Fish. Aquat. Sci. 2013, 70, 1386–1395. [Google Scholar] [CrossRef]
- Roberts, L.J.; Taylor, J.; Gough, P.J.; Forman, D.W.; de Leaniz, C.G. Silver spoons in the rough: Can environmental enrichment improve survival of hatchery Atlantic salmon Salmo salar in the wild? J. Fish Biol. 2014, 85, 1972–1991. [Google Scholar] [CrossRef]
- Salvanes, A.G.V.; Moberg, O.; Ebbesson, L.O.E.; Nilsen, T.O.; Jensen, K.H.; Braithwaite, V.A. Environmental enrichment promotes neural plasticity and cognitive ability in fish. Proc. R. Soc. B Boil. Sci. 2013, 280, 20131331. [Google Scholar] [CrossRef]
- Bergendahl, I.A.; Salvanes, A.G.V.; Braithwaite, V.A. Determining the effects of duration and recency of exposure to environmental enrichment. Appl. Anim. Behav. Sci. 2016, 176, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Baynes, S.M.; Howell, B.R. Observations on the growth, survival and disease resistance of juvenile common sole, Solea solea (L.), fed Mytilus edulis L. Aquac. Res. 1993, 24, 95–100. [Google Scholar] [CrossRef]
- Tuckey, L.M.; Smith, T.I.J. Effects of Photoperiod and Substrate on Larval Development and Substrate Preference of Juvenile Southern Flounder, Paralichthys lethostigma. J. Appl. Aquac. 2001, 11, 1–20. [Google Scholar] [CrossRef]
- Krebs, E.; Huysman, N.; Voorhees, J.M.; Barnes, M.E. Suspended arrays improve rainbow trout growth during hatchery rearing in circular tanks. Int. J. Aquac. Fish. Sci. 2018, 4, 27–30. [Google Scholar]
- Crank, K.M.; Kientz, J.L.; Barnes, M.E. An evaluation of vertically suspended environmental enrichment structures during rainbow trout rearing. N. Am. J. Aquac. 2019, 81, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Huysman, N.; Krebs, E.; Voorhees, J.M.; Barnes, M.E. Use of large vertically suspended rod array in circular tanks during juvenile rainbow trout rearing. Int. J. Mar. Biol. Res. 2019, 4, 1–5. [Google Scholar]
- Huysman, N.; Voorhees, J.M.; Krebs, E.; Barnes, M.E. Vertically-suspended environmental enrichment improves growth of landlocked fall chinook salmon during initial hatchery rearing. Open J. Appl. Sci. 2020, 10, 725–731. [Google Scholar] [CrossRef]
- Jones, M.D.; Krebs, E.; Huysman, N.; Voorhees, J.M.; Barnes, M.E. Rearing performance of Atlantic salmon grown in circular tanks with vertically-suspended environmental enrichment. Open J. Anim. Sci. 2019, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- White, S.C.; Krebs, E.; Huysman, N.; Voorhees, J.M.; Barnes, M.E. Use of suspended plastic conduit arrays during brown trout and rainbow trout rearing in circular tanks. N. Am. J. Aquac 2019, 81, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Voorhees, J.M.; Huysman, N.; Krebs, E.; Barnes, M.E. Use of exercise and structure during rainbow trout rearing. Open J. Appl. Sci. 2020, 10, 258–269. [Google Scholar] [CrossRef]
- Moine, J.; Barnes, M.E.; Kientz, J.; Simpson, G. Flow patterns in circular rearing tanks containing vertical structure. J. Fish. Livest. Prod. 2016, 4, 204–207. [Google Scholar] [CrossRef]
- Muggli, A.M.; Barnes, J.M.; Barnes, M.E. Vertically-suspended environmental enrichment alters the velocity profiles of circular fish rearing tanks. World J. Eng. Technol. 2019, 7, 208–226. [Google Scholar] [CrossRef] [Green Version]
- Caasi, J.M.A.; Barnes, J.M.; Barnes, M.E. Impact of vertically-suspended environmental enrichment and two densities of fish on circular tank velocity profiles. Engineering 2020, 12, 723–738. [Google Scholar] [CrossRef]
- Morris, B.; Huysman, N.; Voorhees, J.M.; Krebs, E.; Barnes, M.E. Rainbow trout locations in a circular tank containing vertically-suspended environmental enrichment. J. Agric. Res. Aquat. Sci. 2021, 1. [Google Scholar]
- Wipf, M.M.; Barnes, M.E. Competitor density and size effects on aggression and feeding in cutthroat trout: Implications for aquaculture. Open Fish. Sci. J. 2011, 4, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Walker, L.M.; Parker, T.M.; Barnes, M.E. Full and partial overhead tank cover improves Rainbow Trout rearing performance. N. Am. J. Aquac. 2016, 78, 20–24. [Google Scholar] [CrossRef]
- Odling-Smee, L.; Braithwaite, V.A. The role of learning in fish orientation. Fish Fish. 2003, 4, 235–246. [Google Scholar] [CrossRef]
- Salas, C.; Broglio, C.; Durán, E.; Gómez, A.; Ocana, F.M.; Jimenez-Moya, F.; Rodriguez, F. Neuropsychology of learning and memory in teleost fish. Zebrafish 2006, 3, 157–171. [Google Scholar] [CrossRef]
- Fausch, K.D. Profitable stream positions for salmonids: Relating specific growth rate to net energy gain. Can. J. Zool. 1984, 62, 441–445. [Google Scholar] [CrossRef]
- Hartman, G.E. Observations on the behavior of juvenile brown trout in a stream or aquarium during winter and spring. J. Fish. Res. Board Can. 1963, 20, 769–787. [Google Scholar] [CrossRef]
- Bachman, R.A. Foraging behavior of free-ranging wild and hatchery brown trout in a stream. Trans. Am. Fish. Soc. 1984, 113, 1–32. [Google Scholar] [CrossRef]
- O’Hara, K. Fish Behavior and the Management of Freshwater Fisheries. In The Behavior of Teleost Fishes; Pitcher, T.J., Ed.; John Hopkins University Press: Baltimore, MD, USA, 1986; pp. 496–523. [Google Scholar]
- Millidine, K.J.; Armstrong, J.D.; Metcalf, N.B. Presence of shelter reduces maintenance metabolism of juvenile salmon. Funct. Ecol. 2006, 20, 839–845. [Google Scholar] [CrossRef]
- Fausch, K.D. Experimental analysis of microhabitat selection by juvenile steelhead (Oncorhynchus mykiss) and Coho salmon (O. kisutch) in a British Columbia stream. Can. J. Fish. Aquat. Sci. 1993, 50, 1198–1207. [Google Scholar] [CrossRef]
- Webb, P.W. Entrainment by river chub Nocomis micropogon and smallmouth bass Micropterus dolomieu on cylinders. J. Exp. Biol 1998, 201, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.W. Control of posture, depth, and swimming trajectories of fishes. Integr. Comp. Biol. 2002, 42, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Hinch, S.G.; Bratty, J. Effects of swim speed and activity pattern on success of adult sockeye salmon migration through an area of difficult passage. Trans. Am. Fish. Soc. 2000, 129, 598–606. [Google Scholar] [CrossRef]
- Heggenes, J. Flexible summer habitat selection by wild, allopatric brown trout in lotic environments. Trans. Am. Fish. Soc. 2002, 131, 287–298. [Google Scholar] [CrossRef]
- Liao, J.C.; Beal, D.N.; Lauder, G.V.; Triantafyllou, M.S. Fish exploiting vortices decrease muscle activity. Science 2003, 302, 1566–1569. [Google Scholar] [CrossRef]
- Gilmore, K.M.; Di Battista, J.D.; Thomas, J.B. Physiological causes and consequences of social status in salmonid fish. Integr. Comp. Biol. 2005, 45, 263–273. [Google Scholar] [CrossRef]
- McIntyre, D.C.; Healy, L.M.; Saari, M. Intraspecies aggression and monoamine levels in rainbow trout (Salmo gairdneri) fingerlings. Behav. Neural Biol. 1979, 25, 90–98. [Google Scholar] [CrossRef]
- Schaerf, T.M.; Dillingham, P.W.; Ward, A.J. The effects of external cues on individual and collective behavior of shoaling fish. Sci. Adv. 2017, 3, e1603201. [Google Scholar] [CrossRef] [Green Version]
- Rowland, W.J. Studying visual cues in fish behavior: A review of ethological techniques. Environ. Biol. Fishes 1999, 56, 285–305. [Google Scholar] [CrossRef]


| Enrichment | A | B | C | D | χ2 | p | |
|---|---|---|---|---|---|---|---|
| Naïve | Yes | 9 | 45 | 11 | 15 | 2.057 | 0.561 |
| No | 10 | 42 | 17 | 11 | |||
| Experienced | Yes | 25 | 36 | 8 | 11 | 19.169 | 0.001 |
| No | 10 | 61 | 7 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huysman, N.; Robidoux, M.; Morris, B.; Voorhees, J.M.; Krebs, E.; Barnes, M.E. Behavioral Response of Multiple Rainbow Trout to Vertically Suspended Environmental Enrichment. Aquac. J. 2022, 2, 180-185. https://doi.org/10.3390/aquacj2020009
Huysman N, Robidoux M, Morris B, Voorhees JM, Krebs E, Barnes ME. Behavioral Response of Multiple Rainbow Trout to Vertically Suspended Environmental Enrichment. Aquaculture Journal. 2022; 2(2):180-185. https://doi.org/10.3390/aquacj2020009
Chicago/Turabian StyleHuysman, Nathan, Michael Robidoux, Benj Morris, Jill M. Voorhees, Eric Krebs, and Michael E. Barnes. 2022. "Behavioral Response of Multiple Rainbow Trout to Vertically Suspended Environmental Enrichment" Aquaculture Journal 2, no. 2: 180-185. https://doi.org/10.3390/aquacj2020009






