Induced Sex Reversal in Adult Males of the Protandric Hermaphrodite Centropomus undecimalis Using 17 β-Estradiol: Enhancing Management Strategies for Captive Broodstock
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warner, R.R. The adaptive significance of sequential hermaphroditism in animals. Am. Nat. 1975, 109, 61–82. [Google Scholar] [CrossRef]
- Munday, P.L.; Buston, P.M.; Warner, R.R. Diversity and flexibility of sex-change strategies in animals. Trends Ecol. Evol. 2006, 21, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Baroiller, J.F.; D’Cotta, H.; Saillant, E. Environmental effects on fish sex determination and differentiation. Sex. Dev. 2009, 3, 118–135. [Google Scholar] [CrossRef]
- Smith, C.L. The evolution of hermaphroditism in fishes. In Intersexuality in the Animal Kingdom; Reinboth, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1975; pp. 295–310. [Google Scholar]
- Chan, S.; Yeung, W. Sex control and sex reversal in fish under natural conditions. In Fish Physiology; Academic Press: Cambridge, MA, USA, 1983; pp. 171–222. [Google Scholar]
- Alonso-Fernández, A.; Alós, J.; Grau, A.; Domínguez-Petit, R.; Saborido-Rey, F. The use of histological techniques to study the reproductive biology of the hermaphroditic Mediterranean fishes Coris julis, Serranus scriba, and Diplodus annularis. Mar. Coast. Fish. 2011, 1, 145–159. [Google Scholar] [CrossRef]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Kikuchi, K.; Koyama, T. Revisiting the role of steroid hormones in gonadal fate determination. In Spectrum of Sex: The Molecular Bases that Induce Various Sexual Phenotypes; Springer Nature: Singapore, 2022; pp. 87–110. [Google Scholar]
- Peters, K.M.; Matheson, R.E.; Taylor, R.G. Reproduction and early life history of common snook, Centropomus undecimalis (Bloch), in Florida. Bull. Mar. Sci. 1998, 62, 509–529. [Google Scholar]
- Taylor, R.G.; Whittington, J.A.; Grier, H.J.; Crabtree, R.E. Age, growth and protandric sex reversal in common snook Centropomus undecimalis, from the east and west coasts of Florida. Fish. Bull 2000, 98, 612–624. [Google Scholar]
- De Assis, D.A.S.D.; Nobre, D.M.; de Freitas, M.C.; Moraes, L.E.; Santos, A.C.D.A. Reproductive biology of the protandric hermaphrodite fat snook Centropomus parallelus Poey 1860 in a tropical estuary, northeastern Brazil. Stud. Neotrop. Fauna Environ. 2019, 54, 225–235. [Google Scholar] [CrossRef]
- Young, J.M.; Yeiser, B.G.; Whittington, J.A.; Dutka-Gianelli, J. Maturation of female common snook Centropomus undecimalis: Implications for managing protandrous fishes. J. Fish Biol. 2020, 97, 1317–1331. [Google Scholar] [CrossRef]
- Vidal-López, J.M.; Contreras-Sánchez, W.M.; Hernández-Franyutti, A.; Contreras-García, M.J.; Uribe-Aranzábal, M.D.C. Functional feminization of the Mexican snook (Centropomus poeyi) using 17β-estradiol in the diet. Lat. Am. J. Aquat. Res. 2019, 47, 240–250. [Google Scholar] [CrossRef]
- Costa-Silva, G.H.; Freitas, M.O.; Abilhoa, V. Reproductive biology of the fat snook Centropomus parallelus Poey, 1860 (Teleostei, Centropomidae) and implications for its management in the southern Atlantic Ocean. J. Fish Biol. 2021, 99, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Oliveira, C.D.; Souto, D.; Rangely, J.; Fabré, N.N. Growth stanza in fish life history using otoliths shape: The protandric Centropomus case (Carangaria: Centropomidae). Neotrop. Ichthyol. 2021, 19, 1–19. [Google Scholar] [CrossRef]
- Gilmore, R.G.; Christopher, J.; Donohoe, J.C.D. Observation on the distribution and biology of the East-Central Florida population of the common Centropomus undecimalis. Fla. Sci. 1983, 46, 306–313. [Google Scholar]
- Tucker, J.W.; Campbell, C.S. Spawning season of common snook along the east-central Florida Coast. Fla. Sci. 1988, 51, 1–6. [Google Scholar]
- Blewett, D.A.; Hensley, R.A.; Stevens, P.W. Feeding Habits of Common Snook, Centropomus undecimalis, in Charlotte Harbor, Florida. Gulf Caribb. Res. 2006, 18, 1–14. [Google Scholar] [CrossRef]
- Álvarez-Lajonchère, L.S.; Hernández-Molejón, G. Producción de Juveniles de peces Estuarinos para un Centro de Producción en América Latina y el Caribe: Diseño, Operación y Tecnologías. [Production of Juvenile Estuarine Fishes for a Production Center in Latin America and the Caribbean: Design, Operation and Technologies]; The World Aquaculture Society: Baton Rouge, LA, USA, 2001; p. 424. [Google Scholar]
- Álvarez-Lajonchère, L.S.; Tsuzuki, M.Y. A review of methods for Centropomus spp. (snooks) aquaculture and recommendations for the establishment of their culture in Latin America. Aquac. Res. 2008, 39, 684–700. [Google Scholar] [CrossRef]
- Polonía-River, C.; Gaitán, S.; Chaparro-Muñoz, N.; Villamizar, N. Captura, transporte y aclimatación de juveniles y adultos de róbalo Centropomus undecimalis (Bloch, 1792). [Capture, transport and acclimatization of juvenile and adult snook Centropomus undecimalis (Bloch, 1792). Intropica 2017, 12, 61–64. [Google Scholar] [CrossRef]
- Chávez, R.H. Contribución al conocimiento de la biología de los robalos, chucumite y constantino (Centropomus undecimalis) del Estado de Veracruz, México. Ciencia 1963, 22, 141–161. [Google Scholar]
- Perera-García, M.A.; Mendoza-Carranza, M.; Contreras-Sánchez, W.M.; Huerta-Ortiz, M.; Pérez-Sánchez, E. Reproductive biology of common snook Centropomus undecimalis (Perciformes: Centropomidae) in two tropical habitats. Rev. Biol. Trop. 2011, 59, 669–681. [Google Scholar] [CrossRef]
- Lorán-Núñez, R.M.; Martínez-Isunza, F.R.; Valdez-Guzmán, A.J.; Garduño-Dionate, M.; Martínez-Lorán, R.E. Reproducción y madurez sexual de robalo prieto (Centropomus poeyi) y robalo blanco (C. undecimalis) en el Sistema Lagunar de Alvarado, Veracruz. [Reproduction and sexual maturity of Mexican snook (Centropomus poeyi) and common snook (C. undecimalis) in the Alvarado Lagoon System, Veracruz]. Ciencia Pesq. 2012, 20, 49–64. [Google Scholar]
- McMichael, R.H., Jr.; Peters, K.M.; Parsons, G.R. Early life history of the snook Centropomus undecimalis, in Tampa Bay, Florida. Gulf Mex. Sci. 1986, 10, 113–125. [Google Scholar] [CrossRef]
- Taylor, R.G.; Grier, H.J.; Whittington, J.A. Spawning rhythms of common snook in Florida. J. Fish Biol. 1998, 53, 502–520. [Google Scholar] [CrossRef]
- Neidig, C.L.; Skapura, D.P.; Grier, H.J.; Sprinkel, J.M. A preliminary study—A comparison of doses of Human Chorionic Gonadotropin (HGC) on ovulation, eggs quality and larval survival in common snook, Centropomus undecimalis (Bloch). In Proceedings of the Sixth International Symposium on Reproductive Physiology of Fish, Bergen, Norway, 4–9 July 1999; p. 429. [Google Scholar]
- Álvarez-Lajonchère, L.S.; Cerqueira, R.V.; Reis, M. Desarrollo embrionario y primeros estudios larvales del robalo chucumite, Centropomus parallelus Poey (Pisces, Centropomidae) con interés para su cultivo. [Embryonic development and first larval studies of the chucumite snook, Centropomus parallelus Poey (Pisces, Centropomidae) with interest for its culture]. Hidrobiológica 2002, 132, 89–99. [Google Scholar]
- Cerqueira, V.R.; Tsuzuki, M.Y. A review of spawning induction, larviculture, and juvenile rearing of the fat snook, Centropomus parallelus. Fish Physiol. Biochem. 2009, 35, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Main, K.; Yanes-Roca, C.; Rhody, N. Status and challenges in larval rearing and fingerling aquaculture of common snook (Centropomus undecimalis) in Florida. In Segundo Simposio Internacional sobre Biología y Cultivo de robalos. Villahermosa, Tabasco, México. AquaFish Collaborative Research Support Program; Universidad Juárez Autónoma de Tabasco: Tabasco, Mexico, 2009; p. 26. [Google Scholar]
- Blewett, D.A.; Stevens, P.W.; Champeau, T.R.; Taylor, R.G. Use of rivers by common snook Centropomus undecimalis in southwest Florida: A first step in addressing the overwintering paradigm. Fla. Sci. 2009, 72, 310–324. [Google Scholar]
- Yanes, C.; Main, K. Improving larval culture and rearing techniques on common snook (Centropomus undecimalis). Aquaculture 2012, 1, 187–216. [Google Scholar]
- Passini, G.; Carvalho, C.V.A.; Sterzelecki, F.C.; Cerqueira, V.R. Induction of sex inversion in common snook (Centropomus undecimalis) males, using 17-β oestradiol implants. Aquac. Res. 2014, 47, 1–10. [Google Scholar] [CrossRef]
- Contreras-Sánchez, W.M.; Contreras-García, M.J.; Mcdonal-Vera, A.; Hernández-Vidal, U.; Cruz-Rosado, L.; Martínez-García, R. Manual para la producción de robalo blanco (Centropomus undecimalis) en cautiverio. [Manual for the production of common snook (Centropomus undecimalis) in captivity]. In Colección José N. Rovirosa: Biodiversidad, Desarrollo Sustentable y Trópico Húmedo; UJAT: Tabasco, Mexico, 2015; 31p. [Google Scholar]
- De Carvalho, C.V.A.; Passini, G.; De Melo, C.W.; Vieira, B.N.; Cerqueira, V.R. Effect of estradiol-17β on the sex ratio, growth and survival of juvenile common snook (Centropomus undecimalis). Acta Sci. Anim. Sci. 2014, 36, 239–245. [Google Scholar] [CrossRef][Green Version]
- De Carvalho, C.V.A.; Passini, G.; De Melo, C.W.; Cerqueira, V.R. Feminization and growth of juvenile fat snook Centropomus parallelus fed diets with different concentrations of estradiol-17β. Aquac. Int. 2014, 22, 1391–1401. [Google Scholar] [CrossRef]
- Akhavan, S.R.; Falahatkar, B.; Gilani, M.H.T.; Lokman, P.M. Effects of estradiol-17β implantation on ovarian growth, sex steroid levels, and vitellogenin proxies in previtellogenic sturgeon Huso Anim. Reprod. Sci. 2015, 157, 1–10. [Google Scholar] [CrossRef]
- Todd, E.V.; Liu, H.; Muncaster, S.; Gemmell, N.J. Bending Genders: The Biology of natural sex change in fish. Sex. Dev. 2016, 10, 223–241. [Google Scholar] [CrossRef]
- Hansen, T.; Stefansson, S.; Taramger, G.L. Growth and sexual maturation in Atlantic salmon, Salmon salar L., reared in sea cages at two different light regimes. Aquac. Res. 1992, 23, 275–280. [Google Scholar] [CrossRef]
- Turker, A. Effects of photoperiod on growth and feed utilization of juvenile Black Sea turbot (Psetta maeotica). Isr. J. Aquac. 2005, 57, 156–163. [Google Scholar] [CrossRef]
- Akhoundian, M.; Salamat, N.; Savari, A.; Movahedinia, A.; Salari, M.A. Influence of photoperiod and temperature manipulation on gonadal development and spawning in Caspian roach (Rutilus caspicus): Implications for artificial propagation. Aquac. Res. 2020, 51, 1623–1642. [Google Scholar] [CrossRef]
- Carrillo, M.; Bromage, N.; Zanuy, S.; Serrano, R.; Prat, F. The effect of modifications in photoperiod on spawning time, ovarian development and egg quality in the sea bass (Dicentrarchus labrax L.). Aquaculture 1989, 81, 351–365. [Google Scholar] [CrossRef]
- Abdollahpour, H.; Falahatkar, B.; Lawrence, C. The effect of photoperiod on zebrafish growth and spawning performance, Danio rerio. Aquac. Rep. 2020, 17, 1–5. [Google Scholar] [CrossRef]
- Polat, H.; Ozturk, R.C.; Terzi, Y.; Aydin, I.; Kucuk, E. Effect of photoperiod manipulation on spawning time and performance of turbot (Scophthalmus maximus). Aquac. Stud. 2021, 21, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ligo, M.; Fujimoto, Y.; Gunji-Suzuki, M.; Yokosuka, M.; Hara, M.; Ohtani-Kaneko, R.; Tabata, M.; Aida, K.; Hirata, K. Circadian rhythm of melatonin release from the photoreceptive pineal organ of a teleost, ayu (Plecoglossus altivelis) in flow-through culture. J. Neuroendoc. 2004, 16, 45–51. [Google Scholar]
- Falcón, J.; Besseau, L.; Sauzet, S.; Boeuf, G. Melatonin effects on the hypothalamo–pituitary axis in fish. Trends Endocrinol. Metab. 2007, 18, 81–88. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Prentice Hall: Hoboken, NJ, USA, 2010; p. 248. [Google Scholar]
- Grier, H.J.; Taylor, R.G. Testicular maturation and regression in the common snook. J. Fish Biol. 1998, 53, 521–542. [Google Scholar] [CrossRef]
- Nakamura, M.; Kobayashi, T.; Chang, X.T.; Nagahama, Y. Gonadal sex differentiation in teleost fish. J. Exp. Zool. 1998, 281, 362–372. [Google Scholar] [CrossRef]
- Meijide, F.J.; Lo Nostro, F.L.; Guerrero, G.A. Gonadal development and sex differentiation in the cichlid fish Cichlasoma dimerus (Teleostei, Perciformes): A light- and electron-microscopic study. J. Morph. 2005, 264, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, P.; Mårtensson, L.; Mathiasson, L. Improved spectrophotometric vitellogenin determination via alkali-labile phosphate in fish plasma—A cost effective approach for assessment of endocrine disruption. Int. J. Environ. Anal. Chem. 2009, 89, 1023–1042. [Google Scholar] [CrossRef]
- Vidal-López, J.M.; Álvarez-González, C.A.; Contreras-Sánchez, W.M.; Patiño, R.; Hernández-Franyutti, A.A.; Hernández-Vidal, U. Feminización de juveniles del robalo blanco Centropomus undecimalis (Bloch 1792) usando 17β-Estradiol. [Feminization of juvenile common snook Centropomus undecimalis (Bloch 1792) using 17β-Estradiol]. Rev. Cien. Mar. Cost. 2012, 3, 83–93. (In Spanish) [Google Scholar] [CrossRef]
- Moore, R. Natural sex inversion in the giant perch (Lates calcarifer). Aust. J. Mar. Fresh. Res. 1979, 30, 803–813. [Google Scholar] [CrossRef]
- Banh, Q.Q.T.; Domingos, J.A.; Pinto, R.C.C.; Nguyen, K.T.; Jerry, D.R. Dietary 17 β-oestradiol and 17 α-ethinyloestradiol alter gonadal morphology and gene expression of the two sex-related genes, dmrt1 and Cyp19a1a, in juvenile barramundi (Lates calcarifer Bloch). Aquac. Res. 2020, 52, 1414–1430. [Google Scholar] [CrossRef]
- Lai, K.; Scrimshaw, M.; Lester, J. The effects of natural and synthetic steroid estrogens in relation to their environmental occurrence. Crit. Rev. Tox. 2002, 32, 113–132. [Google Scholar] [CrossRef]
- Czarny, K.; Szczukocki, D.; Krawczyk, B.; Zieliński, M.; Miękoś, E.; Gadzała-Kopciuch, R. The impact of estrogens on aquatic organisms and methods for their determination. Crit. Rev. Environ. Sci. Technol. 2017, 47, 909–963. [Google Scholar] [CrossRef]
- Guiguen, Y.; Fostier, A.; Piferrer, F.; Chang, C.F. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. End. 2010, 165, 352–366. [Google Scholar] [CrossRef]
- Nagahama, Y. Endocrine regulation of gametogenesis in fish. Int. J. Dev. Biol. 1994, 38, 217–229. [Google Scholar]
- Planas, V.J.; Jaime, A.; Frederick, W.; Goetz, P.S. Regulation of ovarian steroidogenesis in vitro by follicle-stimulating hormone and luteinizing hormone during sexual maturation in salmonid fish. Biol. Rep. 2000, 62, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Guiguen, Y.; Cauty, C.; Fostier, A.; Fuchs, J.; Jalabert, B. Reproductive cycle and sex inversion of the seabass, Lates calcarifer, reared in sea cages in French Polynesia: Histological and morphometric description. Environ. Biol. Fish. 1994, 39, 231–247. [Google Scholar] [CrossRef]
- Liu, H.; Todd, E.V.; Lokman, P.M.; Lamm, M.S.; Godwin, J.R.; Gemmell, N.J. Sexual plasticity: A fishy tale. Mol. Rep. Dev. 2016, 84, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Craik, J.C.A.; Harvey, S.M. A biochemical method for distinguishing between the sexes of fishes by the presence of yolk protein in the blood. J. Phys. Biol. 1984, 25, 293–303. [Google Scholar] [CrossRef]
- Hernández-Vidal, U.; Contreras-Sánchez, W.M.; Chiappa-Carrara, X.; Hernández-Franyutti, A.A.; Uribe, M.C. Common snook reproductive physiology in freshwater and marine environments of Mexico. Mar. Freshw. Behav. Physiol. 2021, 54, 203–225. [Google Scholar] [CrossRef]
- Emmersen, B.K.; Petersen, I.M. Natural occurrence, and experimental induction by estradiol-17-β, of a lip phosphoprotein (vitellogenin) in flounder (Platichtys flesus L.). Comp. Biochem. Physiol. 1976, 54, 443–446. [Google Scholar]
- Whitehead, C.; Bromage, N.R.; Forster, J.R.M. Seasonal changes in reproductive function of the rainbow trout (Salmo gairdneri). J. Fish Biol. 1978, 12, 601–608. [Google Scholar] [CrossRef]
- Nagler, J.J.; Ruby, S.M.; Idler, D.R.; So, Y.P. Serum phosphoprotein phosphorus and calcium levels as reproductive indicators of vitellogenin in highly vitellogenic mature female and estradiol-injected immature rainbow trout (Salmo gairdneri). Can. J. Zool. 1987, 65, 2421–2425. [Google Scholar] [CrossRef]
- Waagboe, R.; Sandnes, K. Determination of vitellogenin in serum of rainbow trout (Salmo gairdneri) by high-performance gel permeation chromatography. J. Chromat. B Biomed. Sci. App. 1988, 427, 138–143. [Google Scholar] [CrossRef]
- Frantzen, M.; Arnesen, A.M.; Damsgard, B.; Tveiten, H.; Johnsen, H.K. Effects of photoperiod on sex steroids and gonad maturation in Arctic charr. Aquaculture 2004, 240, 561–574. [Google Scholar] [CrossRef]
- Prayogo, N.A.; Wijayanti, G.E.; Murwantoko, K.M.; Astuti, P. Effect of photoperiods on melatonin levels, the expression cGnRH-II and sGnRH genes and estradiol level in hard-lipped barb (Osteochilus hasselti C.V.). Glob. Vet. 2012, 8, 591–597. [Google Scholar]
- Aragón-Flores, E.A.; Martínez-Cárdenas, L.; Valdez-Hernández, E.F. Photoperiod effect on commercial fishes cultured in different types of experimental systems. Rev. Bio. Cienc. 2014, 3, 17–27. [Google Scholar]
- Maitra, S.K.; Seth, M.; Chattoraj, A. Photoperiod, pineal photoreceptors and melatonin as the signal of photoperiod in the regulation of reproduction in fish. J. Endocrinol. Reprod. 2006, 2, 73–87. [Google Scholar]
- Pandian, T.J.; Sheela, S.G. Hormonal induction of sex reversal in fish. Aquaculture 1995, 138, 1–22. [Google Scholar] [CrossRef]
- George, T.; Sheela, S.G. Dietary administration of androgens induces sterility in the female-heterogametic black molly, Poecilia sphenops (Cuvier and Valenciennes, 1986). Aquac. Res. 1998, 29, 167–175. [Google Scholar]
- García-Hernández, M.P.; Cabas, I.; Rodenas, M.C.; Arizcun, M.; Chaves-Pozo, E.; Power, D.M.; García, A.A. 17α-ethynylestradiol prevents the natural male-to-female sex change in gilthead seabream (Sparus aurata L.). Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Thuong, N.P.; Sung, Y.Y.; Ambak, M.A.; Abol-Munafi, A.B. The hormone 17β-estradiol promotes feminization of juvenile protandrous hermaphrodite false clownfish (Amphiprion ocellaris). Mar. Freshw. Behav. Physiol. 2017, 50, 195–204. [Google Scholar] [CrossRef]
- Contreras-Sánchez, W.M.; Contreras-García, M.J.; Mcdonal-Vera, A.; Hernández-Vidal, U.; Cruz-Rosado, L. Advances in induced spawning and embryo development in captivity of Centropomus poeyi. Kushulkab 2014, 20, 5–9. [Google Scholar]
- Contreras-García, M.J.; Contreras-Sánchez, W.M.; Mcdonal-Vera, A.; Hernández-Vidal, U.; Cruz-Rosado, L. Reproducción inducida del robalo chucumite (Centropomus parallelus) en Tabasco, México. [Induced reproduction of fat snook (Centropomus parallelus) in Tabasco, Mexico]. AquaTic 2020, 56, 15–25. [Google Scholar]
- Contreras-García, M.J.; Contreras-Sánchez, W.M.; Hernández-Vidal, U.; Mcdonal-Vera, A. Induced spawning of the common snook (Centropomus undecimalis) in captivity using GnRH-a implants. Ecosist. Rec. Agrop. 2015, 2, 357–362. [Google Scholar]
- Piferrer, F. Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 2000, 197, 229–281. [Google Scholar] [CrossRef]
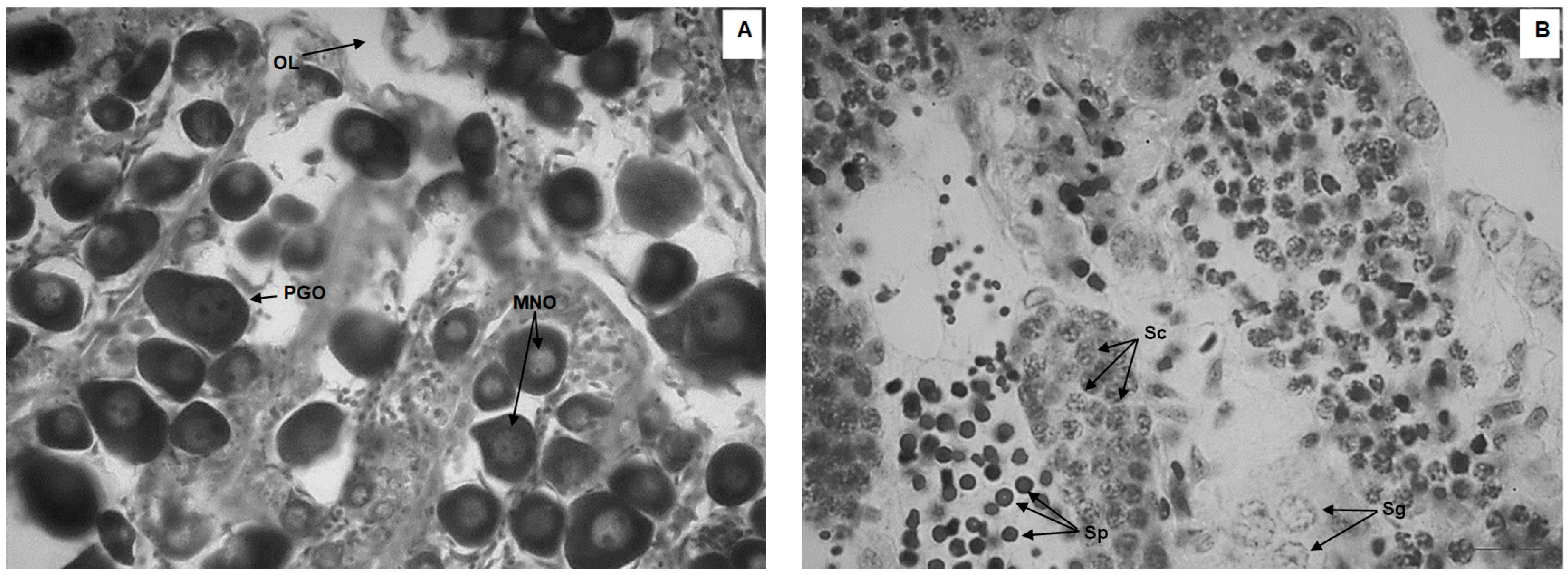
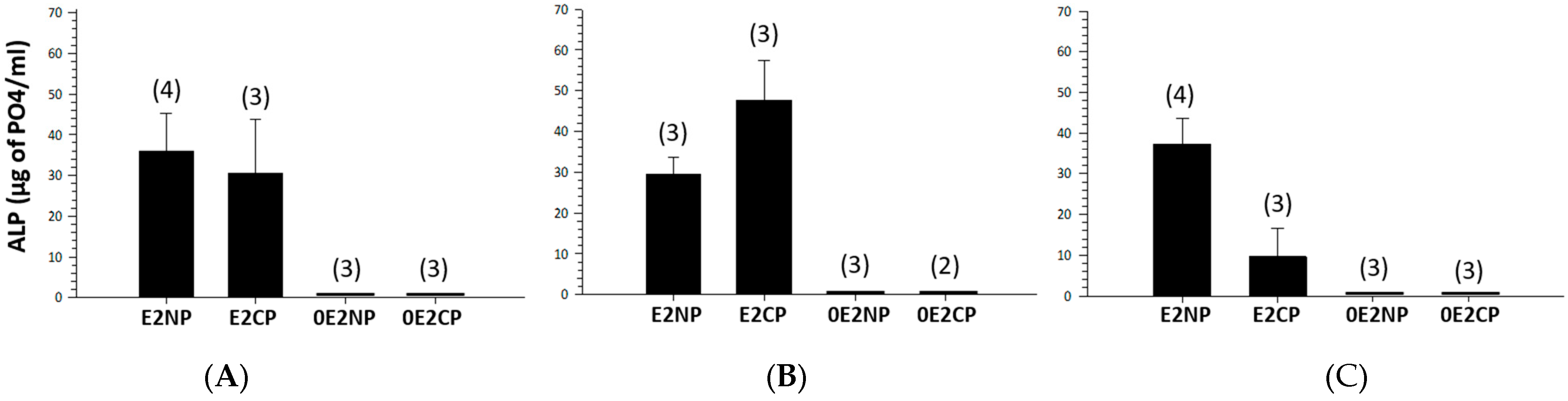
| Treatment | Initial Weight (g) | Final Weight (g) | Weight Gain (g) | Initial TL (cm) | Final TL (cm) |
|---|---|---|---|---|---|
| E2NP | 768.50 ± 58.45 a | 813.20 ± 62.14 a | 44.70 a | 48.91 ± 2.45 a | 49.60 ± 2.55 a |
| 0E2NP | 765.39 ± 58.98 a | 815.85 ± 63.96 a | 50.45 a | 49.31 ± 2.49 a | 50.94 ± 2.24 a |
| 0E2CP | 807.41 ± 62.69 b | 862.90 ± 87.67 a | 55.50 a | 50.22 ± 1.25 a | 51.57 ± 1.47 a |
| E2CP | 799.70 ± 63.82 b | 857.70 ± 94.08 a | 58.00 a | 49.93 ± 1.65 a | 51.11 ± 1.71 a |
| Treatment | Initial Ø (µm) * | Final Ø (µm) ‡ | Spawning | Egg Ø (µm) | Oil Drop Ø (µm) |
|---|---|---|---|---|---|
| E2CP | 96.22 ± 100.30 | n/a | Yes | 681.13 ± 19.91 | 177.78 ± 11.12 |
| E2CP | 73.97 ± 75.73 | n/a | Yes | 687.11 ± 19.91 | 179.47 ± 12.13 |
| E2CP | 69.10 ± 59.65 | 44.30 ± 13.97 | No | n/a | n/a |
| E2NP | 89.72 ± 83.20 | n/a | Yes | 687.74 ± 49.47 | 172.69 ± 12.41 |
| E2NP | 62.34 ± 15.92 | n/a | Yes | 685.48 ± 22.86 | 175.78 ± 13.97 |
| E2NP | 63.81 ± 14.47 | 49.61 ± 12.17 | No | n/a | n/a |
| Treatment | DO (mg/L) | Temperature (°C) | pH (UI) | Ammonia (mg/L) | Nitrates (mg/L) | Nitrites (mg/L) |
|---|---|---|---|---|---|---|
| E2NP | 4.38 ± 1.15 | 26.26 ± 1.87 | 8.56 ± 0.63 | 0.21 ± 0.22 | 1.86 ± 1.38 | 0.11 ± 0.13 |
| 0E2NP | 4.39 ± 1.20 | 26.29 ± 1.91 | 8.52 ± 0.66 | 0.53 ± 0.39 | 1.84 ± 1.39 | 0.08 ± 0.11 |
| 0E2CP | 4.20 ± 1.18 | 27.75 ± 1.65 | 8.79 ± 2.31 | 0.60 ± 0.44 | 2.22 ± 1.92 | 0.21 ± 0.20 |
| E2CP | 4.25 ± 1.16 | 27.70 ± 1.66 | 8.48 ± 0.73 | 0.52 ± 0.40 | 2.30 ± 2.14 | 0.27 ± 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-García, M.d.J.; Contreras-Sánchez, W.M.; Mendoza-Carranza, M.; Mcdonal-Vera, A.; Cruz-Rosado, L. Induced Sex Reversal in Adult Males of the Protandric Hermaphrodite Centropomus undecimalis Using 17 β-Estradiol: Enhancing Management Strategies for Captive Broodstock. Aquac. J. 2023, 3, 196-208. https://doi.org/10.3390/aquacj3030016
Contreras-García MdJ, Contreras-Sánchez WM, Mendoza-Carranza M, Mcdonal-Vera A, Cruz-Rosado L. Induced Sex Reversal in Adult Males of the Protandric Hermaphrodite Centropomus undecimalis Using 17 β-Estradiol: Enhancing Management Strategies for Captive Broodstock. Aquaculture Journal. 2023; 3(3):196-208. https://doi.org/10.3390/aquacj3030016
Chicago/Turabian StyleContreras-García, María de Jesús, Wilfrido Miguel Contreras-Sánchez, Manuel Mendoza-Carranza, Alejandro Mcdonal-Vera, and Leonardo Cruz-Rosado. 2023. "Induced Sex Reversal in Adult Males of the Protandric Hermaphrodite Centropomus undecimalis Using 17 β-Estradiol: Enhancing Management Strategies for Captive Broodstock" Aquaculture Journal 3, no. 3: 196-208. https://doi.org/10.3390/aquacj3030016
APA StyleContreras-García, M. d. J., Contreras-Sánchez, W. M., Mendoza-Carranza, M., Mcdonal-Vera, A., & Cruz-Rosado, L. (2023). Induced Sex Reversal in Adult Males of the Protandric Hermaphrodite Centropomus undecimalis Using 17 β-Estradiol: Enhancing Management Strategies for Captive Broodstock. Aquaculture Journal, 3(3), 196-208. https://doi.org/10.3390/aquacj3030016





