Molecular Oxygen as a Probe Molecule in EPR Spin Labeling Studies of Membrane Structure and Dynamics
Abstract
:1. Introduction
2. Molecular Oxygen
3. T1-Sensitive Method for Monitoring the Oxygen Diffusion-Concentration Product
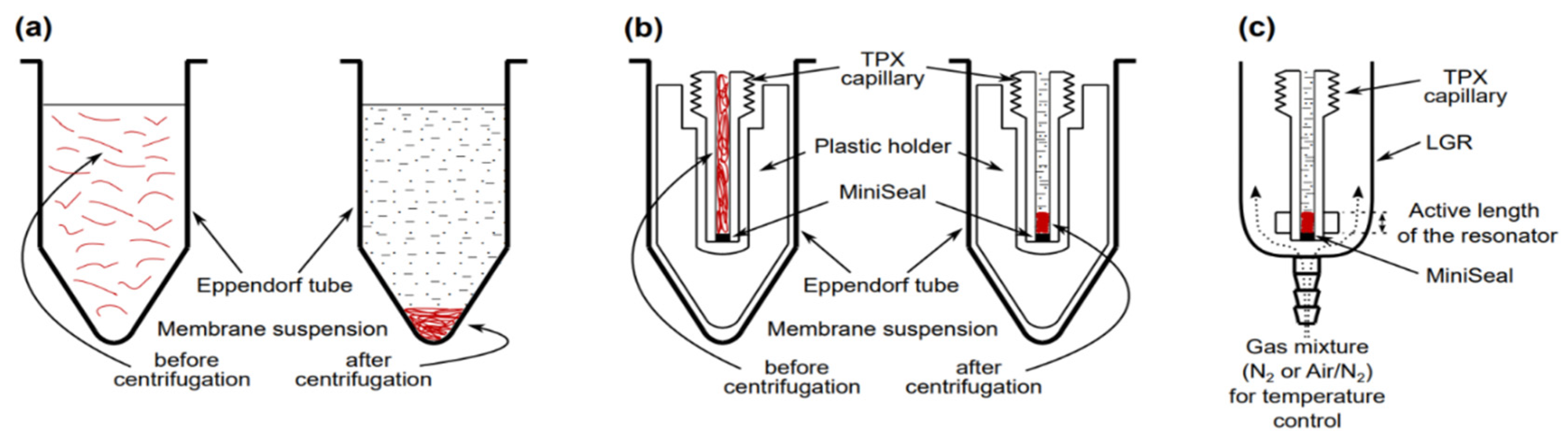
4. Methods of Controlling Oxygen Concentration (Oxygen Partial Pressure) in Investigated Samples
5. The Oxygen Transport Parameter (Outline of Theory)
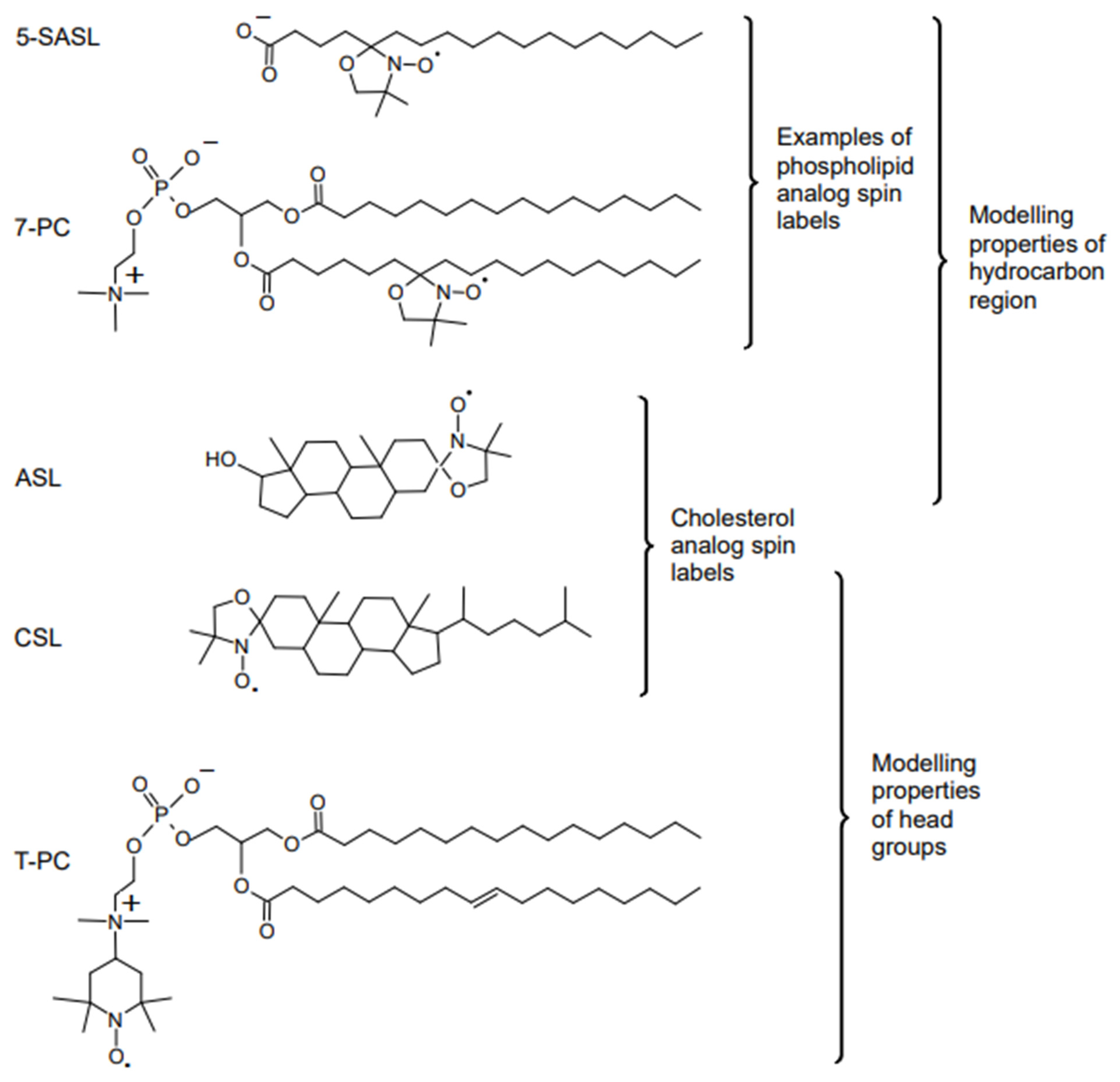
6. Molecular Oxygen Differently Monitors Membrane Fluidity and Dynamics of Acyl Chains
7. Domain Structure of Model and Biological Membranes
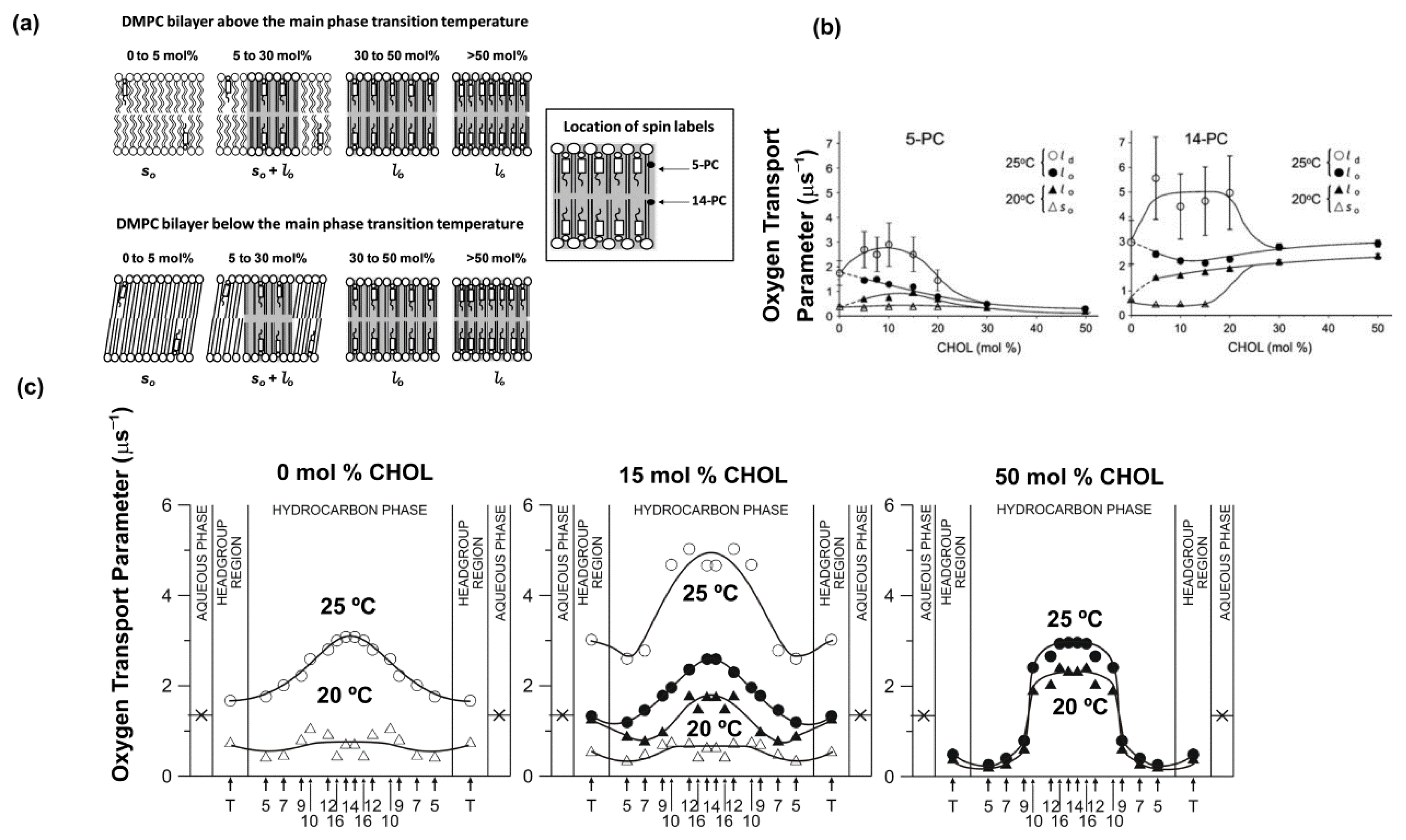
7.1. Cholesterol Induced Phases in Lipid Bilayers
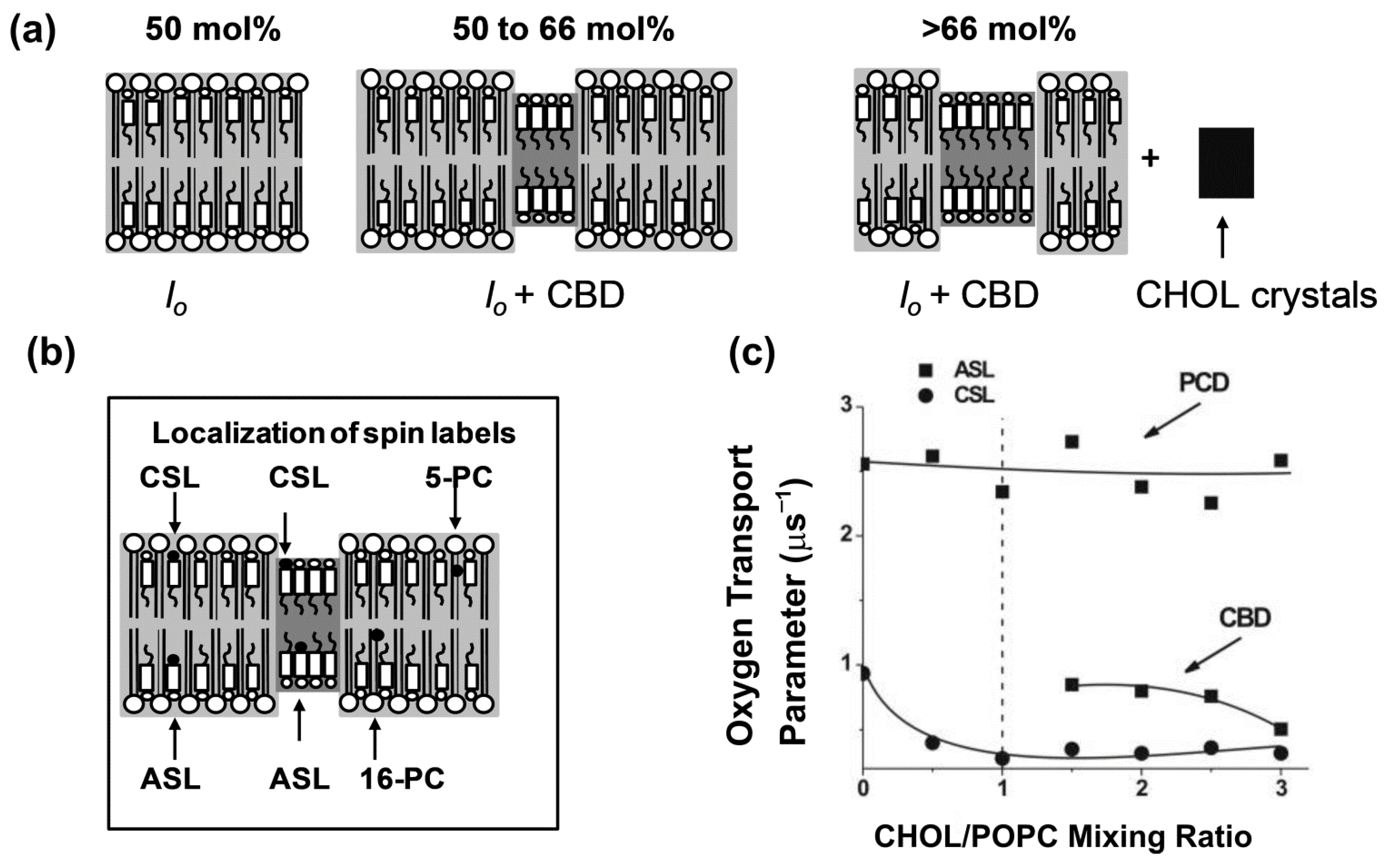
7.2. Cholesterol Bilayer Domain
7.3. Boundary and Trapped Lipid Domains Induced by Membrane Integral Proteins
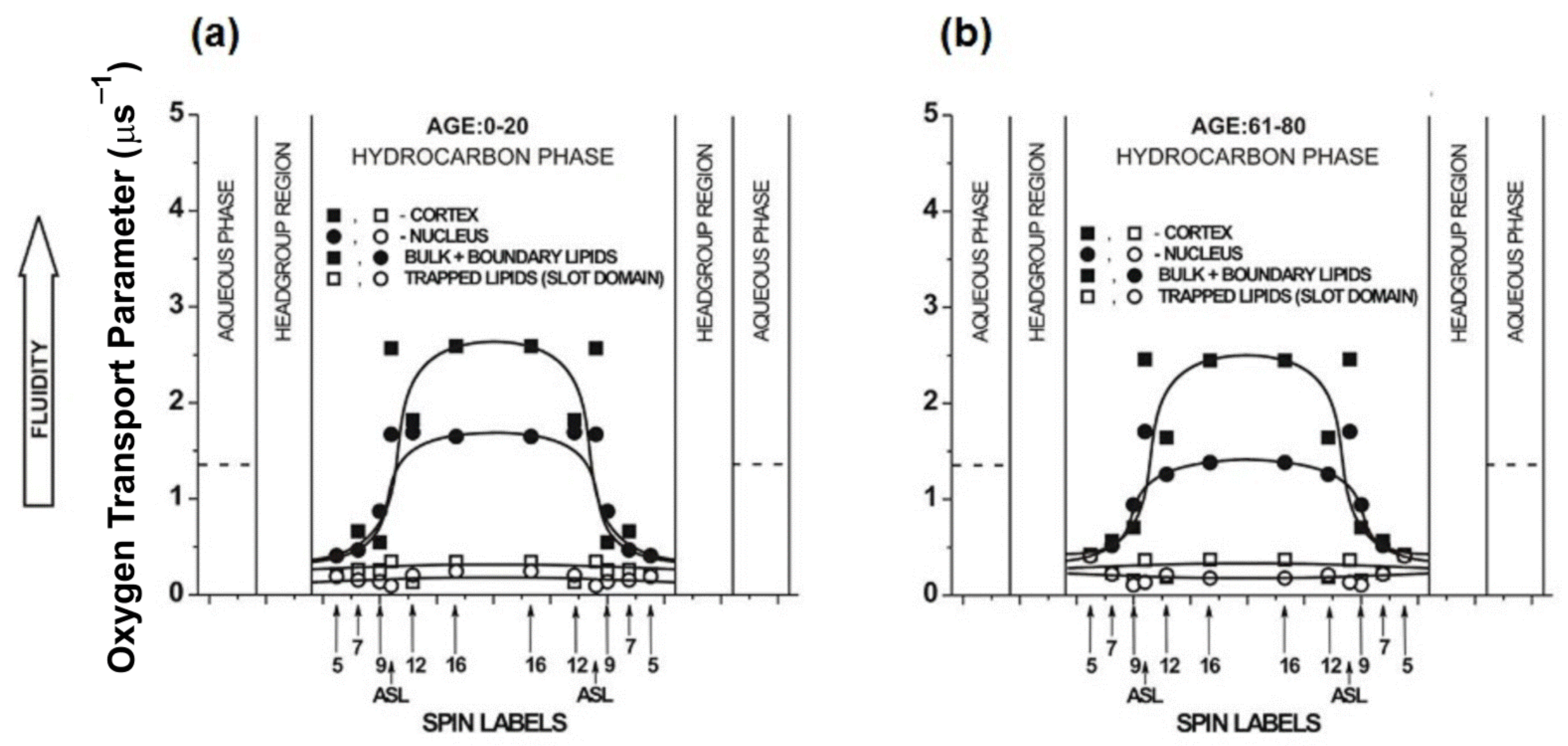
8. Unique Information Obtained from Profiles of OTP across Membrane Domains
9. Other Applications of O2 as a Probe Molecule in EPR Spin Labeling Studies of Membranes
10. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gorter, E.; Grendel, F. On biomolecular layers of lippods on the chromocytes of the blood. J. Exp. Med. 1925, 41, 439–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danielli, J.F.; Davson, H. A contribution to the theory of permeability of thin films. J. Cell. Comp. Physiol. 1935, 5, 495–508. [Google Scholar] [CrossRef]
- Singer, S.J.; Nicolson, G.L. The fluid mosaic model of the structure of cell membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.N. The Lively Membranes; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Kusumi, A.; Suzuki, K.G.; Kasai, R.S.; Ritchie, K.; Fujiwara, T.K. Hierarchical mesoscale domain organization of the plasma membrane. Trends Biochem. Sci. 2011, 36, 604–615. [Google Scholar] [CrossRef]
- Freeman, S.A.; Vega, A.; Riedl, M.; Collins, R.F.; Ostrowski, P.P.; Woods, E.C.; Bertozzi, C.R.; Tammi, M.I.; Lidke, D.S.; Johnson, P.; et al. Transmembrane Pickets Connect Cyto- and Pericellular Skeletons Forming Barriers to Receptor Engagement. Cell 2018, 172, 305–317.e10. [Google Scholar] [CrossRef] [Green Version]
- Bieberich, E. Sphingolipids and lipid rafts: Novel concepts and methods of analysis. Chem. Phys. Lipids 2018, 216, 114–131. [Google Scholar] [CrossRef]
- Pike, L.J. The challenge of lipid rafts. J. Lipid Res. 2009, 50, S323–S328. [Google Scholar] [CrossRef] [Green Version]
- Levental, I.; Levental, K.R.; Heberle, F.A. Lipid Rafts: Controversies Resolved, Mysteries Remain. Trends Cell Biol. 2020, 30, 341–353. [Google Scholar] [CrossRef]
- Pike, L.J. Rafts defined: A report on the Keystone symposium on lipid rafts and cell function. J. Lipid Res. 2006, 47, 1597–1598. [Google Scholar] [CrossRef] [Green Version]
- Pathak, P.; London, E. The Effect of Membrane Lipid Composition on the Formation of Lipid Ultrananodomains. Biophys. J. 2015, 109, 1630–1638. [Google Scholar] [CrossRef] [Green Version]
- Cebecauer, M.; Amaro, M.; Jurkiewicz, P.; Sarmento, M.J.; Šachl, R.; Cwiklik, L.; Hof, M. Membrane Lipid Nanodomains. Chem. Rev. 2018, 118, 11259–11297. [Google Scholar] [CrossRef]
- Sezgin, E.; Schwille, P. Fluorescence Techniques to Study Lipid Dynamics. Cold Spring Harb. Perspect. Biol. 2011, 3, a009803. [Google Scholar] [CrossRef] [Green Version]
- Frederix, P.L.; Bosshart, P.D.; Engel, A. Atomic Force Microscopy of Biological Membranes. Biophys. J. 2009, 96, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Tamm, L.K. NMR as a tool to investigate the structure, dynamics and function of membrane proteins. Nat. Struct. Mol. Biol. 2016, 23, 468–474. [Google Scholar] [CrossRef] [Green Version]
- Subczynski, W.K.; Raguz, M.; Widomska, J. Studying Lipid Organization in Biological Membranes Using Liposomes and EPR Spin Labeling, in Liposomes: Methods and Protocols. In Biological Membrane Models; Weissig, V., Ed.; Humana Press: Totowa, NJ, USA, 2010; Volume 2, pp. 247–269. [Google Scholar]
- Schmidt, T.; Schütz, G.J.; Baumgartner, W.; Gruber, H.J.; Schindler, H. Imaging of single molecule diffusion. Proc. Natl. Acad. Sci. USA 1996, 93, 2926–2929. [Google Scholar] [CrossRef] [Green Version]
- Sankaran, J.; Wohland, T. Fluorescence strategies for mapping cell membrane dynamics and structures. APL Bioeng. 2020, 4, 020901. [Google Scholar] [CrossRef]
- Busch, S.; Smuda, C.; Pardo, L.C.; Unruh, T. Molecular Mechanism of Long-Range Diffusion in Phospholipid Membranes Studied by Quasielastic Neutron Scattering. J. Am. Chem. Soc. 2010, 132, 3232–3233. [Google Scholar] [CrossRef]
- Tieleman, D.; Marrink, S.; Berendsen, H. A computer perspective of membranes: Molecular dynamics studies of lipid bilayer systems. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1997, 1331, 235–270. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Swartz, H.M. EPR Oximetry in Biological and Model Samples. In Biomedical EPR, Part A: Free Radicals, Metals, Medicine, and Physiology; Eaton, S.R., Eaton, G.R., Berliner, L.J., Eds.; Springer US: Boston, MA, USA, 2005; pp. 229–282. [Google Scholar]
- Subczynski, W.K.; Widomska, J.; Wisniewska, A.; Kusumi, A. Saturation-Recovery Electron Paramagnetic Resonance Discrimination by Oxygen Transport (DOT) Method for Characterizing Membrane Domains. In Lipid Rafts; Humana Press: Totowa, NJ, USA, 2007; Volume 398, pp. 143–157. [Google Scholar] [CrossRef]
- Subczynski, W.; Mainali, L.; Camenisch, T.; Froncisz, W.; Hyde, J. Spin-label oximetry at Q- and W-band. J. Magn. Reson. 2011, 209, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Hyde, J.S.; Subczynski, W.K. Spin-Label Oximetry. In Spin Labeling: Theory and Applications; Berliner, L.J., Reuben, J., Eds.; Springer: Boston, MA, USA, 1989; pp. 399–425. [Google Scholar]
- Eaton, S.S.; Eaton, G.R. EPR Spectra and Electron Spin Relaxation of O2. Appl. Magn. Reson. 2021, 52, 1223–1236. [Google Scholar] [CrossRef]
- Carlsson, D.J. Singlet oxygen–reactions with organic compounds and polymers. J. Polym. Sci. Polym. Lett. Ed. 1978, 16, 485–486. [Google Scholar] [CrossRef]
- Beringer, R.; Castle, J.J.G. Microwave Magnetic Resonance Spectrum of Oxygen. Phys. Rev. 1951, 81, 82–88. [Google Scholar] [CrossRef]
- Ashikawa, I.; Yin, J.-J.; Subczynski, W.K.; Kouyama, T.; Hyde, J.S.; Kusumi, A. Molecular Organization and Dynamics in Bacteriorhodopsin-Rich Reconstituted Membranes: Discrimination of Lipid Environments by the Oxygen Transport Parameter Using a Pulse ESR Spin-Labeling Technique. Biochemistry 1994, 33, 4947–4952. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Wisniewska, A.; Hyde, J.S.; Kusumi, A. Three-Dimensional Dynamic Structure of the Liquid-Ordered Domain in Lipid Membranes as Examined by Pulse-EPR Oxygen Probing. Biophys. J. 2007, 92, 1573–1584. [Google Scholar] [CrossRef] [Green Version]
- Knopp, J.; Longmuir, I. Intracellular measurement of oxygen by quenching of fluorescence of pyrenebutyric acid. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1972, 279, 393–397. [Google Scholar] [CrossRef]
- Fischkoff, S.; Vanderkooi, J.M. Oxygen diffusion in biological and artificial membranes determined by the fluorochrome pyrene. J. Gen. Physiol. 1975, 65, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Povich, M.J. Electron spin resonance oxygen broadening. J. Phys. Chem. 1975, 79, 1106–1109. [Google Scholar] [CrossRef]
- Backer, J.; Budker, V.; Eremenko, S.; Molin, Y. Detection of the kinetics of biochemical reactions with oxygen using exchange broadening in the ESR spectra of nitroxide radicals. Biochim. Biophys. Acta 1977, 460, 152–156. [Google Scholar] [CrossRef]
- Windrem, D.A.; Plachy, W.Z. The diffusion-solubility of oxygen in lipid bilayers. Biochim. Biophys. Acta (BBA)-Biomembr. 1980, 600, 655–665. [Google Scholar] [CrossRef]
- Kimmich, R.; Peters, A. Solvation of oxygen in lecithin bilayers. Chem. Phys. Lipids 1975, 14, 350–362. [Google Scholar] [CrossRef]
- Peters, A.; Kimmich, R. The heterogenious solubility of oxygen in aqeuous lecithin dispersions and its relation to chain mobility. A NMR relaxation and wide-line study. Eur. Biophys. J. 1977, 4, 67–85. [Google Scholar]
- McDonald, G.G.; Vanderkooi, J.M.; Oberholtzer, J.C. Oxygen diffusion in phospholipid artificial membranes studied by fourier transform nuclear magnetic resonance. Arch. Biochem. Biophys. 1979, 196, 281–283. [Google Scholar] [CrossRef]
- Epel, B.; Kotecha, M.; Halpern, H.J. In vivo preclinical cancer and tissue engineering applications of absolute oxygen imaging using pulse EPR. J. Magn. Reson. 2017, 280, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Epel, B.; Halpern, H.J. In Vivo pO2 Imaging of Tumors: Oxymetry with Very Low-Frequency Electron Paramagnetic Resonance. Methods Enzymol. 2015, 564, 501–527. [Google Scholar]
- Epel, B.; Redler, G.; Pelizzari, C.; Tormyshev, V.M.; Halpern, H.J. Approaching Oxygen-Guided Intensity-Modulated Radiation Therapy. Single Mol. Single Cell Seq. 2016, 876, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Popp, C.A.; Hyde, J.S. Effects of oxygen on EPR spectra of nitroxide spin-label probes of model membranes. J. Magn. Reson. (1969) 1981, 43, 249–258. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Felix, C.C.; Klug, C.S.; Hyde, J.S. Concentration by centrifugation for gas exchange EPR oximetry measurements with loop–gap resonators. J. Magn. Reson. 2005, 176, 244–248. [Google Scholar] [CrossRef]
- Froncisz, W.; Hyde, J.S. The loop-gap resonator: A new microwave lumped circuit ESR sample structure. J. Magn. Reson. (1969) 1982, 47, 515–521. [Google Scholar] [CrossRef]
- Froncisz, W.; Oles, T.; Hyde, J.S. Q-band loop-gap resonator. Rev. Scic. Instrum. 1986, 57, 1095–1099. [Google Scholar] [CrossRef]
- Hyde, J.S.; Yin, J.-J.; Subczynski, W.K.; Camenisch, T.G.; Ratke, A.J.J.; Froncisz, W. Spin-Label EPR T1 Values Using Saturation Recovery from 2 to 35 GHz. J. Phys. Chem. B 2004, 108, 9524–9529. [Google Scholar] [CrossRef]
- Eaton, S.S.; Eaton, G.R. Saturation Recovery EPR. In Biomedical EPR, Part B: Methodology, Instrumentation, and Dynamics; Eaton, S.R., Eaton, G.R., Berliner, L.J., Eds.; Springer: Boston, MA, USA, 2005; pp. 3–18. [Google Scholar]
- Mainali, L.; Camenisch, T.G.; Hyde, J.S.; Subczynski, W.K. Saturation Recovery EPR Spin-Labeling Method for Quantification of Lipids in Biological Membrane Domains. Appl. Magn. Reson. 2017, 48, 1355–1373. [Google Scholar] [CrossRef]
- Kusumi, A.; Subczynski, W.K.; Hyde, J.S. Oxygen transport parameter in membranes as deduced by saturation recovery measurements of spin-lattice relaxation times of spin labels. Proc. Natl. Acad. Sci. USA 1982, 79, 1854–1858. [Google Scholar] [CrossRef] [Green Version]
- Ligeza, A.; Tikhonov, A.N.; Hyde, J.S.; Subczynski, W.K. Oxygen permeability of thylakoid membranes: Electron paramagnetic resonance spin labeling study. Biochim. Biophys. Acta 1998, 1365, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Hyde, J.S.; Subczynski, W. Simulation of ESR spectra of the oxygen-sensitive spin-label probe CTPO. J. Magn. Reson. (1969) 1984, 56, 125–130. [Google Scholar] [CrossRef]
- Subczynski, W.; Hyde, J. Diffusion of oxygen in water and hydrocarbons using an electron spin resonance spin-label technique. Biophys. J. 1984, 45, 743–748. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, K.; Yin, J.-J.; Subczynski, W.K.; Hyde, J.S.; Kusumi, A. Pulse EPR Detection of Lipid Exchange between Protein-Rich Raft and Bulk Domains in the Membrane: Methodology Development and Its Application to Studies of Influenza Viral Membrane. Biophys. J. 2001, 80, 738–748. [Google Scholar] [CrossRef] [Green Version]
- Gennis, R.B. Biomembranes: Molecular Structure and Function. In Springer Advanced Texts in Chemistry; Springer: New York, NY, USA, 1989. [Google Scholar]
- Clarke, J.B.; Hastie, J.W.; Kihlborg, L.H.E.; Metselaar, R.; Thackeray, M.M. Definitions of terms relating to phase transitions of the solid state (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 577–594. [Google Scholar] [CrossRef] [Green Version]
- Marsh, D. Electron Spin Resonance: Spin Labels. In Membrane Spectroscopy; Grell, E., Ed.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 51–142. [Google Scholar]
- Schneider, D.J.; Freed, J.H. Calculating Slow Motional Magnetic Resonance Spectra. In Spin Labeling: Theory and Applications; Berliner, L.J., Reuben, J., Eds.; Springer: Boston, MA, USA, 1989; pp. 1–76. [Google Scholar]
- Meirovitch, E.; Freed, J.H. Analysis of slow-motional electron spin resonance spectra in smectic phases in terms of molecular configuration, intermolecular interactions, and dynamics. J. Phys. Chem. 1984, 88, 4995–5004. [Google Scholar] [CrossRef]
- Earle, K.A.; Budil, D.E. ChemInform Abstract: Calculating Slow-Motion ESR Spectra of Spin-Labeled Polymers. In Advanced ESR Methods in Polymer Research; Schlick, S., Ed.; Wiley: New York, NY, USA, 2006; pp. 53–83. [Google Scholar]
- Mainali, L.; Feix, J.B.; Hyde, J.S.; Subczynski, W.K. Membrane fluidity profiles as deduced by saturation-recovery EPR measurements of spin-lattice relaxation times of spin labels. J. Magn. Reson. 2011, 212, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Mainali, L.; Hyde, J.S.; Subczynski, W.K. Using spin-label W-band EPR to study membrane fluidity profiles in samples of small volume. J. Magn. Reson. 2013, 226, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Marsh, D. Molecular order and T 1 -relaxation, cross-relaxation in nitroxide spin labels. J. Magn. Reson. 2018, 290, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mailer, C.; Nielsen, A.R.D.; Robinson, B.H. Explanation of Spin-Lattice Relaxation Rates of Spin Labels Obtained with Multifrequency Saturation Recovery EPR. J. Phys. Chem. A 2005, 109, 4049–4061. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.; Haas, D.; Mailer, C. Molecular dynamics in liquids: Spin-lattice relaxation of nitroxide spin labels. Science 1994, 263, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Plesnar, E.; Szczelina, R.; Subczynski, W.K.; Pasenkiewicz-Gierula, M. Is the cholesterol bilayer domain a barrier to oxygen transport into the eye lens? Biochim. Biophys. Acta 2018, 1860, 434–441. [Google Scholar] [CrossRef]
- Träuble, H. The movement of molecules across lipid membranes: A molecular theory. J. Membr. Biol. 1971, 4, 193–208. [Google Scholar] [CrossRef]
- Pace, R.J.; Chan, S.I. Molecular motions in lipid bilayers. III. Lateral and transverse diffusion in bilayers. J. Chem. Phys. 1982, 76, 4241–4247. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Hyde, J.S.; Kusumi, A. Effect of alkyl chain unsaturation and cholesterol intercalation on oxygen transport in membranes: A pulse ESR spin labeling study. Biochemistry 1991, 30, 8578–8590. [Google Scholar] [CrossRef]
- Raguz, M.; Mainali, L.; O’Brien, W.J.; Subczynski, W.K. Lipid domains in intact fiber-cell plasma membranes isolated from cortical and nuclear regions of human eye lenses of donors from different age groups. Exp. Eye Res. 2015, 132, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Raguz, M.; Mainali, L.; O’Brien, W.J.; Subczynski, W.K. Lipid–protein interactions in plasma membranes of fiber cells isolated from the human eye lens. Exp. Eye Res. 2014, 120, 138–151. [Google Scholar] [CrossRef] [Green Version]
- Raguz, M.; Mainali, L.; Widomska, J.; Subczynski, W.K. The immiscible cholesterol bilayer domain exists as an integral part of phospholipid bilayer membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2011, 1808, 1072–1080. [Google Scholar] [CrossRef] [Green Version]
- Raguz, M.; Mainali, L.; Widomska, J.; Subczynski, W.K. Using spin-label electron paramagnetic resonance (EPR) to discriminate and characterize the cholesterol bilayer domain. Chem. Phys. Lipids 2011, 164, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Mainali, L.; Raguz, M.; Subczynski, W.K. Formation of Cholesterol Bilayer Domains Precedes Formation of Cholesterol Crystals in Cholesterol/Dimyristoylphosphatidylcholine Membranes: EPR and DSC Studies. J. Phys. Chem. B 2013, 117, 8994–9003. [Google Scholar] [CrossRef] [Green Version]
- Subczynski, W.K.; Raguz, M.; Widomska, J.; Mainali, L.; Konovalov, A. Functions of Cholesterol and the Cholesterol Bilayer Domain Specific to the Fiber-Cell Plasma Membrane of the Eye Lens. J. Membr. Biol. 2012, 245, 51–68. [Google Scholar] [CrossRef] [Green Version]
- Widomska, J.; Subczynski, W.K.; Mainali, L.; Raguz, M. Cholesterol Bilayer Domains in the Eye Lens Health: A Review. Cell Biochem. Biophys. 2017, 75, 387–398. [Google Scholar] [CrossRef]
- Mainali, L.; Pasenkiewicz-Gierula, M.; Subczynski, W.K. Formation of cholesterol Bilayer Domains Precedes Formation of Cholesterol Crystals in Membranes Made of the Major Phospholipids of Human Eye Lens Fiber Cell Plasma Membranes. Curr. Eye Res. 2020, 45, 162–172. [Google Scholar] [CrossRef]
- Gaffney, B.J.; Marsh, D. High-frequency, spin-label EPR of nonaxial lipid ordering and motion in cholesterol-containing membranes. Proc. Natl. Acad. Sci. USA 1998, 95, 12940–12943. [Google Scholar] [CrossRef] [Green Version]
- Ge, M.; Field, K.; Aneja, R.; Holowka, D.; Baird, B.; Freed, J.H. Electron Spin Resonance Characterization of Liquid Ordered Phase of Detergent-Resistant Membranes from RBL-2H3 Cells. Biophys. J. 1999, 77, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Veiga, M.P.; Arrondo, J.L.R.; Goñi, F.M.; Alonso, A.; Marsh, D. Interaction of Cholesterol with Sphingomyelin in Mixed Membranes Containing Phosphatidylcholine, Studied by Spin-Label ESR and IR Spectroscopies. A Possible Stabilization of Gel-Phase Sphingolipid Domains by Cholesterol. Biochemistry 2001, 40, 2614–2622. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Kusumi, A. Effects of very small amounts of cholesterol on gel-phase phosphatidylcholine membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 1986, 854, 318–320. [Google Scholar] [CrossRef]
- Simons, K.; Vaz, W.L.C. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Antholine, W.E.; Hyde, J.S.; Kusumi, A. Microimmiscibility and three-dimensional dynamic structures of phosphatidylcholine-cholesterol membranes: Translational diffusion of a copper complex in the membrane. Biochemistry 1990, 29, 7936–7945. [Google Scholar] [CrossRef] [PubMed]
- Loura, L.M.; Fedorov, A.; Prieto, M. Fluid–Fluid Membrane Microheterogeneity: A Fluorescence Resonance Energy Transfer Study. Biophys. J. 2001, 80, 776–788. [Google Scholar] [CrossRef] [Green Version]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Changes in the Properties and Organization of Human Lens Lipid Membranes Occurring with Age. Curr. Eye Res. 2017, 42, 721–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mainali, L.; Raguz, M.; Subczynski, W.K. Phase-Separation and Domain-Formation in Cholesterol-Sphingomyelin Mixture: Pulse-EPR Oxygen Probing. Biophys. J. 2011, 101, 837–846. [Google Scholar] [CrossRef] [Green Version]
- Widomska, J.; Raguz, M.; Dillon, J.; Gaillard, E.R.; Subczynski, W.K. Physical properties of the lipid bilayer membrane made of calf lens lipids: EPR spin labeling studies. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 1454–1465. [Google Scholar] [CrossRef] [Green Version]
- Widomska, J.; Raguz, M.; Subczynski, W.K. Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 2635–2645. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-K.; So, L.; Spector, A. Age-dependent changes in the distribution and concentration of human lens cholesterol and phospholipids. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1987, 917, 112–120. [Google Scholar] [CrossRef]
- Yappert, M.; Rujoi, M.; Borchman, D.; Vorobyov, I.; Estrada, R. Glycero- versus sphingo-phospholipids: Correlations with human and non-human mammalian lens growth. Exp. Eye Res. 2003, 76, 725–734. [Google Scholar] [CrossRef]
- Rujoi, M.; Jin, J.; Borchman, U.; Tang, D.; Yappert, M.C. Isolation and lipid characterization of cholesterol-enriched fractions in cortical and nuclear human lens fibers. Investig. Opthalmol. Vis. Sci. 2003, 44, 1634–1642. [Google Scholar] [CrossRef] [Green Version]
- Borchman, D.; Yappert, M.C. Lipids and the ocular lens. J. Lipid Res. 2010, 51, 2473–2488. [Google Scholar] [CrossRef] [Green Version]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Properties of membranes derived from the total lipids extracted from the human lens cortex and nucleus. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 1432–1440. [Google Scholar] [CrossRef] [Green Version]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Properties of membranes derived from the total lipids extracted from clear and cataractous lenses of 61–70-year-old human donors. Eur. Biophys. J. 2014, 44, 91–102. [Google Scholar] [CrossRef] [Green Version]
- East, J.M.; Melville, D.; Lee, A.G. Exchange rates and numbers of annular lipids for the calcium and magnesium ion dependent adenosine triphosphatase. Biochemistry 1985, 24, 2615–2623. [Google Scholar] [CrossRef]
- Ryba, N.J.P.; Horvath, L.I.; Watts, A.; Marsh, D. Molecular exchange at the lipid-rhodopsin interface: Spin-label electron spin resonance studies of rhodopsin-dimyristoylphosphatidylcholine recombinants. Biochemistry 1987, 26, 3234–3240. [Google Scholar] [CrossRef]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Properties of fiber cell plasma membranes isolated from the cortex and nucleus of the porcine eye lens. Exp. Eye Res. 2012, 97, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Aloni, B.; Eitan, A.; Livne, A. The erythrocyte membrane site for the effect of temperature on osmotic fragility. Biochim. Biophys. Acta (BBA)-Biomembr. 1977, 465, 46–53. [Google Scholar] [CrossRef]
- Bieri, V.G.; Wallach, D.F.H. Variations of lipid-protein interactions in erythrocyte ghosts as a function of temperature and pH in physiological and non-physiological ranges: A study using paramagnetic quenching of protein fluorescence by nitroxide lipid analogues. Biochim. Biophys. Acta (BBA)-Biomembr. 1975, 406, 415–423. [Google Scholar] [CrossRef]
- Dergunov, A.D.; Taveirne, J.; Vanloo, B.; Caster, H.; Rosseneu, M. Structural organization of lipid phase and protein-lipid interface in apolipoprotein-phospholipid recombinants: Influence of cholesterol. Biochim. Biophys. Acta 1997, 134, 131–146. [Google Scholar] [CrossRef]
- Massey, J.B.; Gotto, A.M., Jr.; Pownall, H.J. Thermodynamics of lipid-protein association. Enthalphy of association of apolipoprotein A-II with dimyristoylphosphatidylcholine-cholesterol mixtures. Biochim. Biophys. Acta. 1984, 794, 137–141. [Google Scholar] [CrossRef]
- Tall, A.R.; Lange, Y. Interaction of cholesterol, phospholipid and apoprotein in high density lipoprotein recombinants. Biochim. Biophys. Acta (BBA)-Biomembr. 1978, 513, 185–197. [Google Scholar] [CrossRef]
- Warren, G.B.; Houslay, M.D.; Metcalfe, J.C.; Birdsall, N.J.M. Cholesterol is excluded from the phospholipid annulus surrounding an active calcium transport protein. Nature 1975, 255, 684–687. [Google Scholar] [CrossRef]
- Bassnett, S.; Shi, Y.; Vrensen, G.F.J.M. Biological glass: Structural determinants of eye lens transparency. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1250–1264. [Google Scholar] [CrossRef] [Green Version]
- Buzhynskyy, N.; Hite, R.K.; Walz, T.; Scheuring, S. The supramolecular architecture of junctional microdomains in native lens membranes. EMBO Rep. 2007, 8, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Buzhynskyy, N.; Sens, P.; Behar-Cohen, F.; Scheuring, S. Eye lens membrane junctional microdomains: A comparison between healthy and pathological cases. New J. Phys. 2011, 13, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Costello, M.J.; McIntosh, T.J.; Robertson, J.D. Distribution of gap junctions and square array junctions in the mammalian lens. Investig. Ophthalmol. Vis. Sci. 1989, 30, 975–989. [Google Scholar]
- Dunia, I.; Cibert, C.; Gong, X.; Xia, C.-H.; Recouvreur, M.; Levy, E.; Kumar, N.; Bloemendal, H.; Benedetti, E.L. Structural and immunocytochemical alterations in eye lens fiber cells from Cx46 and Cx50 knockout mice. Eur. J. Cell Biol. 2006, 85, 729–752. [Google Scholar] [CrossRef] [Green Version]
- Zampighi, G.A.; Eskandarib, S.; Hall, J.E.; Zampighia, L.; Kremana, M. Micro-domains of AQP0 in Lens Equatorial Fibers. Exp. Eye Res. 2002, 75, 505–519. [Google Scholar] [CrossRef]
- Mainali, L.; O’Brien, W.J.; Subczynski, W.K. Detection of cholesterol bilayer domains in intact biological membranes: Methodology development and its application to studies of eye lens fiber cell plasma membranes. Exp. Eye Res. 2018, 178, 72–81. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Wisniewska, A.; Yin, J.-J.; Hyde, J.S.; Kusumi, A. Hydrophobic Barriers of Lipid Bilayer Membranes Formed by Reduction of Water Penetration by Alkyl Chain Unsaturation and Cholesterol. Biochemistry 1994, 33, 7670–7681. [Google Scholar] [CrossRef]
- Plesnar, E.; Subczynski, W.K.; Pasenkiewicz-Gierula, M. Saturation with cholesterol increases vertical order and smoothes the surface of the phosphatidylcholine bilayer: A molecular simulation study. Biochim. Biophys. Acta (BBA)-Biomembr. 2012, 1818, 520–529. [Google Scholar] [CrossRef] [Green Version]
- Subczynski, W.K.; Widomska, J.; Feix, J.B. Physical properties of lipid bilayers from EPR spin labeling and their influence on chemical reactions in a membrane environment. Free Radic. Biol. Med. 2009, 46, 707–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Miller, M.J.S.; Joshi, M.S.; Thomas, D.D.; Lancaster, J.R. Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc. Natl. Acad. Sci. USA 1998, 95, 2175–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möller, M.N.; Li, Q.; Vitturi, D.A.; Robinson, J.M.; Lancaster, J.R.; Denicola, A. Membrane “Lens” Effect: Focusing the Formation of Reactive Nitrogen Oxides from the •NO/O2 Reaction. Chem. Res. Toxicol. 2007, 20, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.K.; Lomnicka, M.; Hyde, J.S. Permeability of Nitric Oxide through Lipid Bilayer Membranes. Free Radic. Res. 1996, 24, 343–349. [Google Scholar] [CrossRef]
- Zhang, H.; Joseph, J.; Feix, J.; Hogg, N.; Kalyanaraman, B. Nitration and Oxidation of a Hydrophobic Tyrosine Probe by Peroxynitrite in Membranes: Comparison with Nitration and Oxidation of Tyrosine by Peroxynitrite in Aqueous Solution. Biochemistry 2001, 40, 7675–7686. [Google Scholar] [CrossRef]
- Zhang, H.; Bhargava, K.; Keszler, A.; Feix, J.; Hogg, N.; Joseph, J.; Kalyanaraman, B. Transmembrane Nitration of Hydrophobic Tyrosyl Peptides. J. Biol. Chem. 2003, 278, 8969–8978. [Google Scholar] [CrossRef] [Green Version]
- Altenbach, C.; Greenhalgh, D.A.; Khorana, H.G.; Hubbell, W.L. A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: Application to spin-labeled mutants of bacteriorhodopsin. Proc. Natl. Acad. Sci. USA 1994, 91, 1667–1671. [Google Scholar] [CrossRef] [Green Version]
- Klug, C.S.; Feix, J.B. Methods and Applications of Site-Directed Spin Labeling EPR Spectroscopy. Methods Cell Biol. 2008, 84, 617–658. [Google Scholar] [CrossRef]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef] [Green Version]
- Graham, K.; Unger, E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int. J. Nanomed. 2018, 13, 6049–6058. [Google Scholar] [CrossRef] [Green Version]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O.C.A. The Concentration of Oxygen Dissolved in Tissues at the Time of Irradiation as a Factor in Radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar] [CrossRef]
- Dang, J.; He, H.; Chen, D.; Yin, L. Manipulating tumor hypoxia toward enhanced photodynamic therapy (PDT). Biomater. Sci. 2017, 5, 1500–1511. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Hyde, J.S.; Kusumi, A. Oxygen permeability of phosphatidylcholine--cholesterol membranes. Proc. Natl. Acad. Sci. USA 1989, 86, 4474–4478. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front. Oncol. 2019, 9, 1563. [Google Scholar] [CrossRef] [Green Version]
- Vozenin, M.-C.; Hendry, J.; Limoli, C. Biological Benefits of Ultra-high Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken. Clin. Oncol. 2019, 31, 407–415. [Google Scholar] [CrossRef]
- Vozenin, M.-C.; Baumann, M.; Coppes, R.P.; Bourhis, J. FLASH radiotherapy International Workshop. Radiother. Oncol. 2019, 139, 1–3. [Google Scholar] [CrossRef]
- Adrian, G.; Konradsson, E.; Lempart, M.; Bäck, S.; Ceberg, C.; Petersson, K. The FLASH effect depends on oxygen concentration. Br. J. Radiol. 2020, 93, 20190702. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Widomska, J.; Stein, N.; Swartz, H.M. Factors Determining Barrier Properties to Oxygen Transport Across Model and Cell Plasma Membranes Based on EPR Spin-Label Oximetry. Appl. Magn. Reson. 2021, 52, 1237–1260. [Google Scholar] [CrossRef]
- Froncisz, W.; Camenisch, T.G.; Ratke, J.J.; Anderson, J.R.; Subczynski, W.K.; Strangeway, R.A.; Sidabras, J.W.; Hyde, J.S. Saturation recovery EPR and ELDOR at W-band for spin labels. J. Magn. Reson. 2008, 193, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Mainali, L.; Raguz, M.; Camenisch, T.G.; Hyde, J.S.; Subczynski, W.K. Spin-label saturation-recovery EPR at W-band: Applications to eye lens lipid membranes. J. Magn. Reson. 2011, 212, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Mainali, L.; Sidabras, J.W.; Camenisch, T.G.; Ratke, J.J.; Raguz, M.; Hyde, J.S.; Subczynski, W.K. Spin-label W-band EPR with Seven-Loop–Six-Gap Resonator: Application to Lens Membranes Derived from Eyes of a Single Donor. Appl. Magn. Reson. 2014, 45, 1343–1358. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subczynski, W.K.; Widomska, J.; Raguz, M.; Pasenkiewicz-Gierula, M. Molecular Oxygen as a Probe Molecule in EPR Spin Labeling Studies of Membrane Structure and Dynamics. Oxygen 2022, 2, 295-316. https://doi.org/10.3390/oxygen2030021
Subczynski WK, Widomska J, Raguz M, Pasenkiewicz-Gierula M. Molecular Oxygen as a Probe Molecule in EPR Spin Labeling Studies of Membrane Structure and Dynamics. Oxygen. 2022; 2(3):295-316. https://doi.org/10.3390/oxygen2030021
Chicago/Turabian StyleSubczynski, Witold K., Justyna Widomska, Marija Raguz, and Marta Pasenkiewicz-Gierula. 2022. "Molecular Oxygen as a Probe Molecule in EPR Spin Labeling Studies of Membrane Structure and Dynamics" Oxygen 2, no. 3: 295-316. https://doi.org/10.3390/oxygen2030021
APA StyleSubczynski, W. K., Widomska, J., Raguz, M., & Pasenkiewicz-Gierula, M. (2022). Molecular Oxygen as a Probe Molecule in EPR Spin Labeling Studies of Membrane Structure and Dynamics. Oxygen, 2(3), 295-316. https://doi.org/10.3390/oxygen2030021






