Photosynthetic Production of Molecular Oxygen by Water Oxidation
Abstract
:1. Introduction
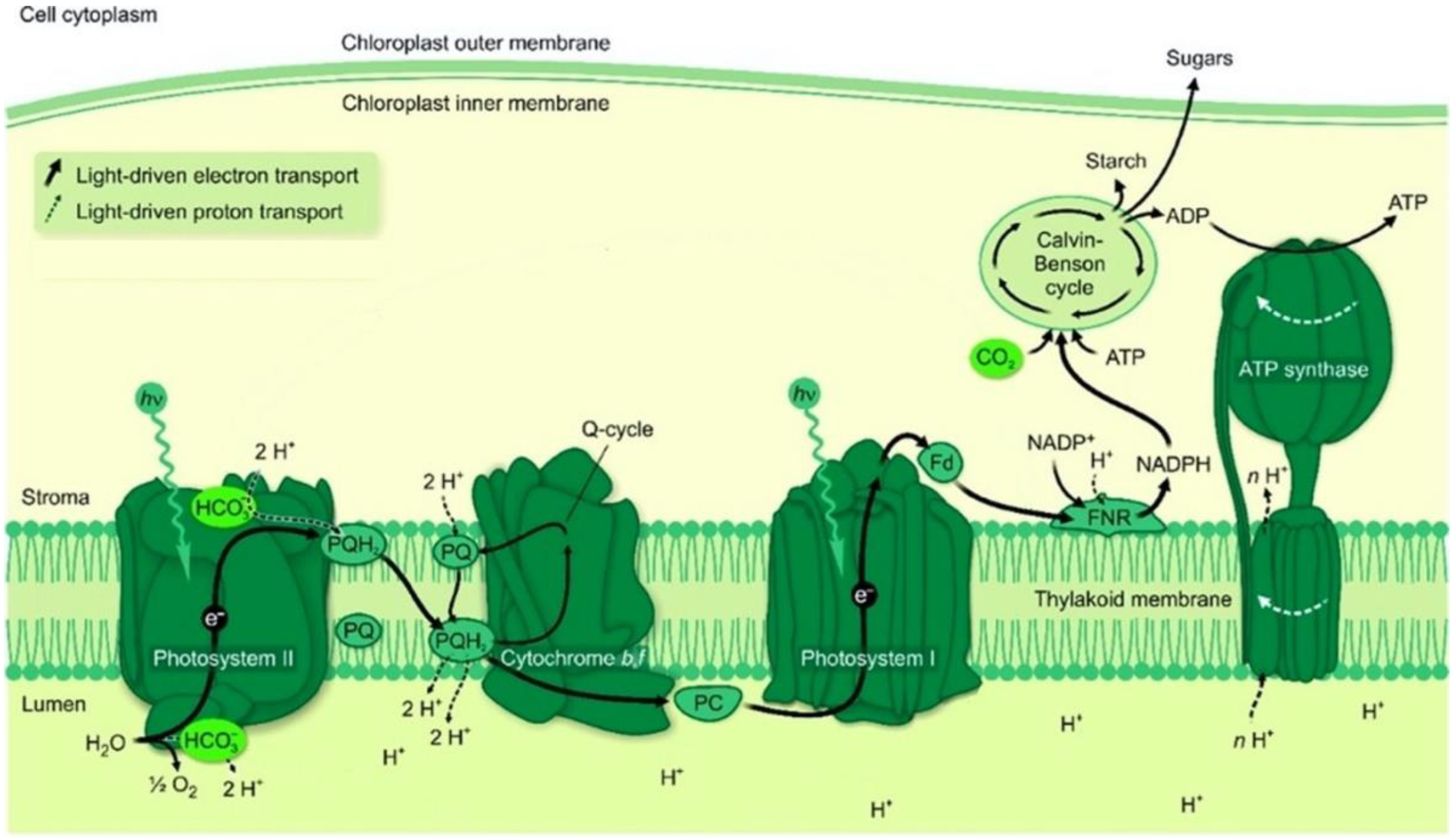
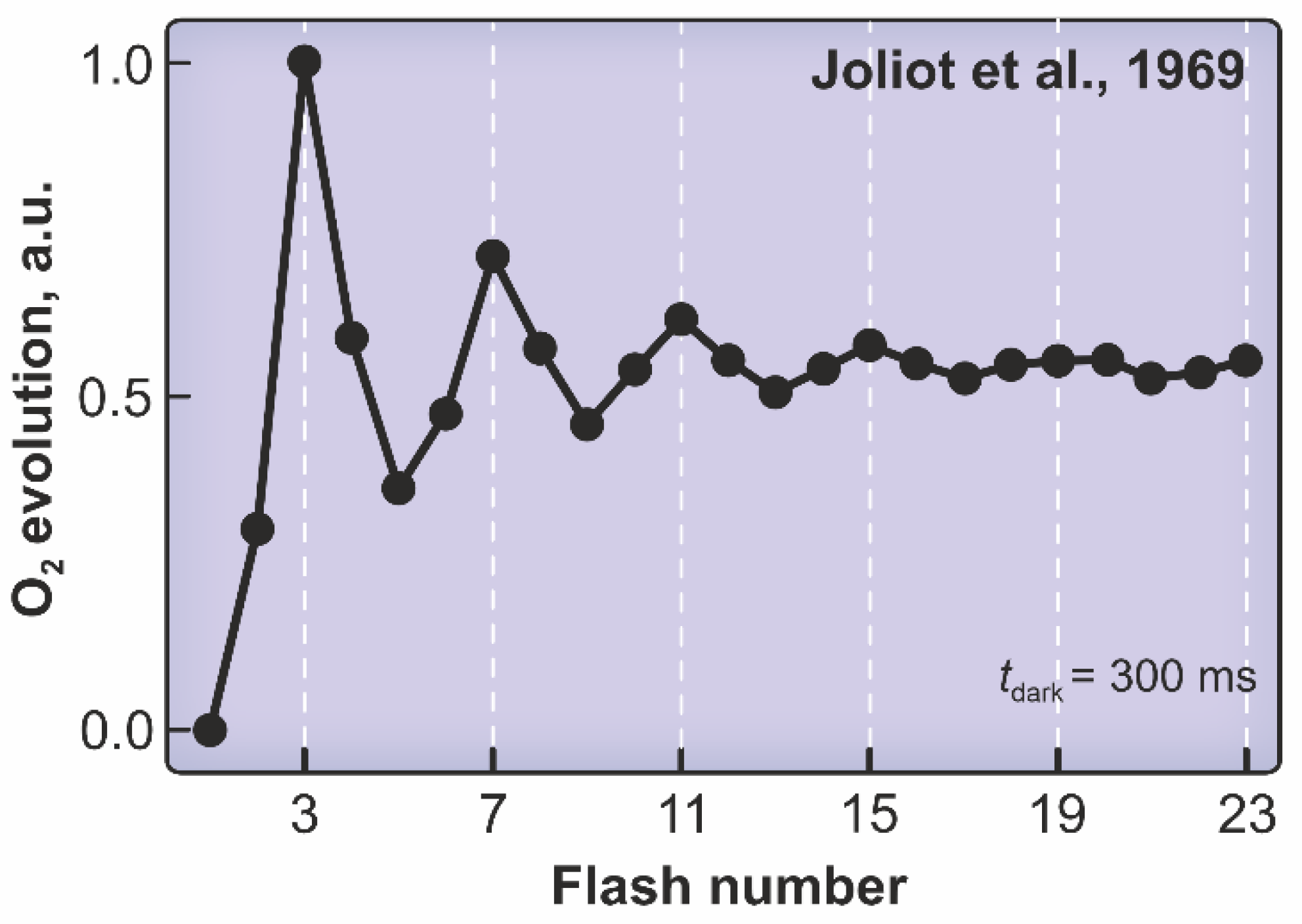
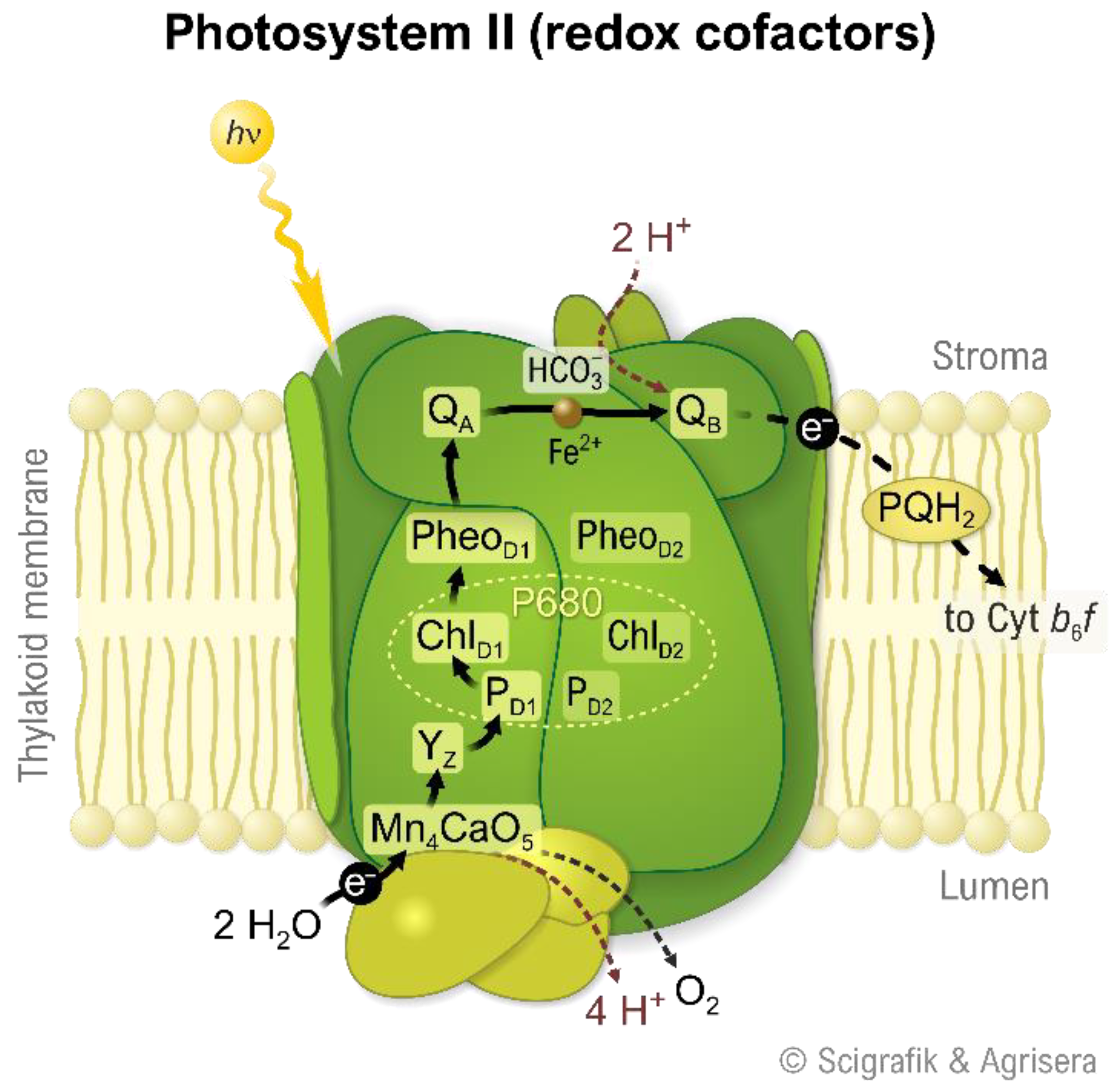
2. Photosystem II
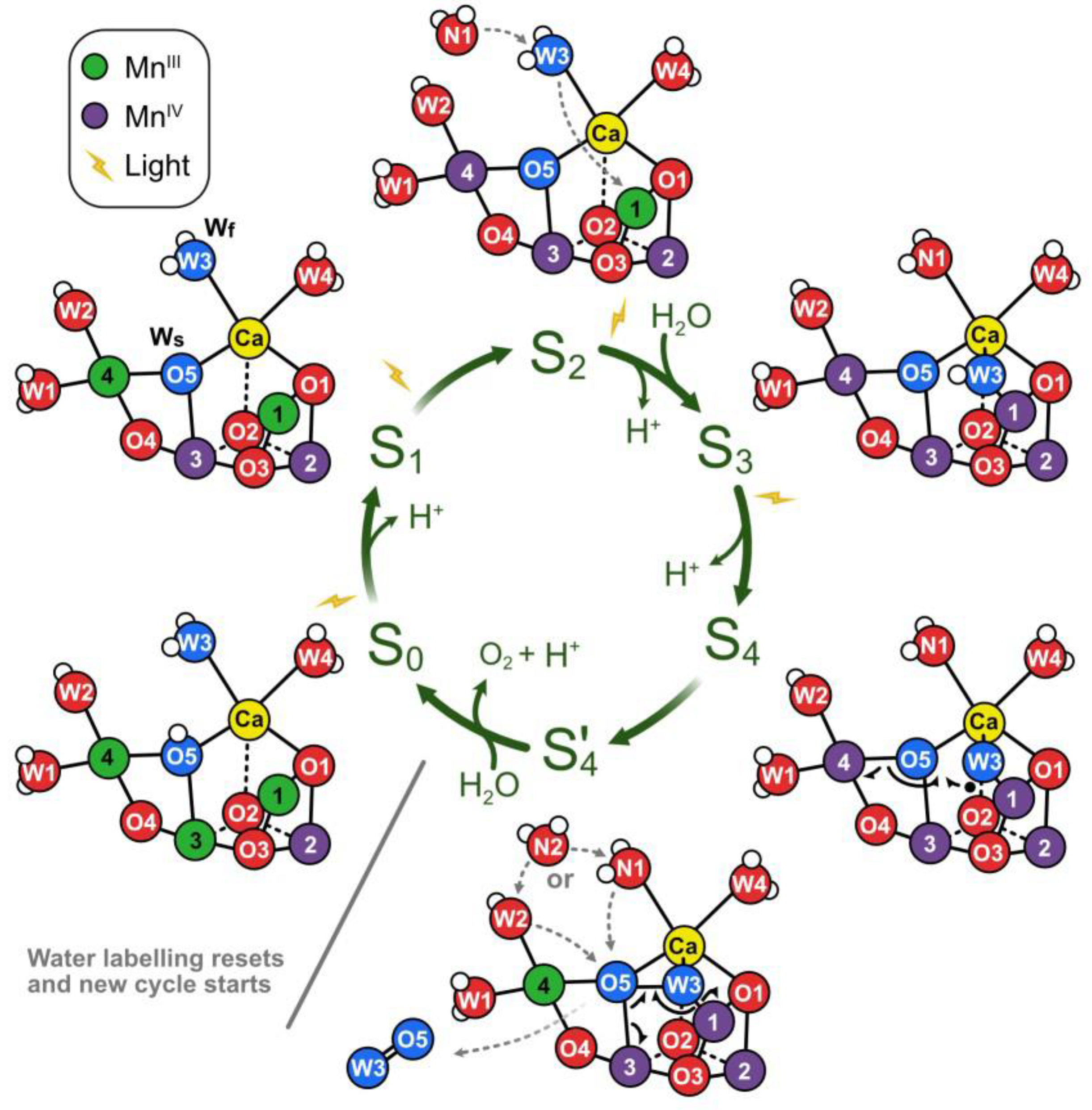
3. The Role of Manganese
4. The Role of Calcium
5. The Role of Bicarbonate in PSII Function
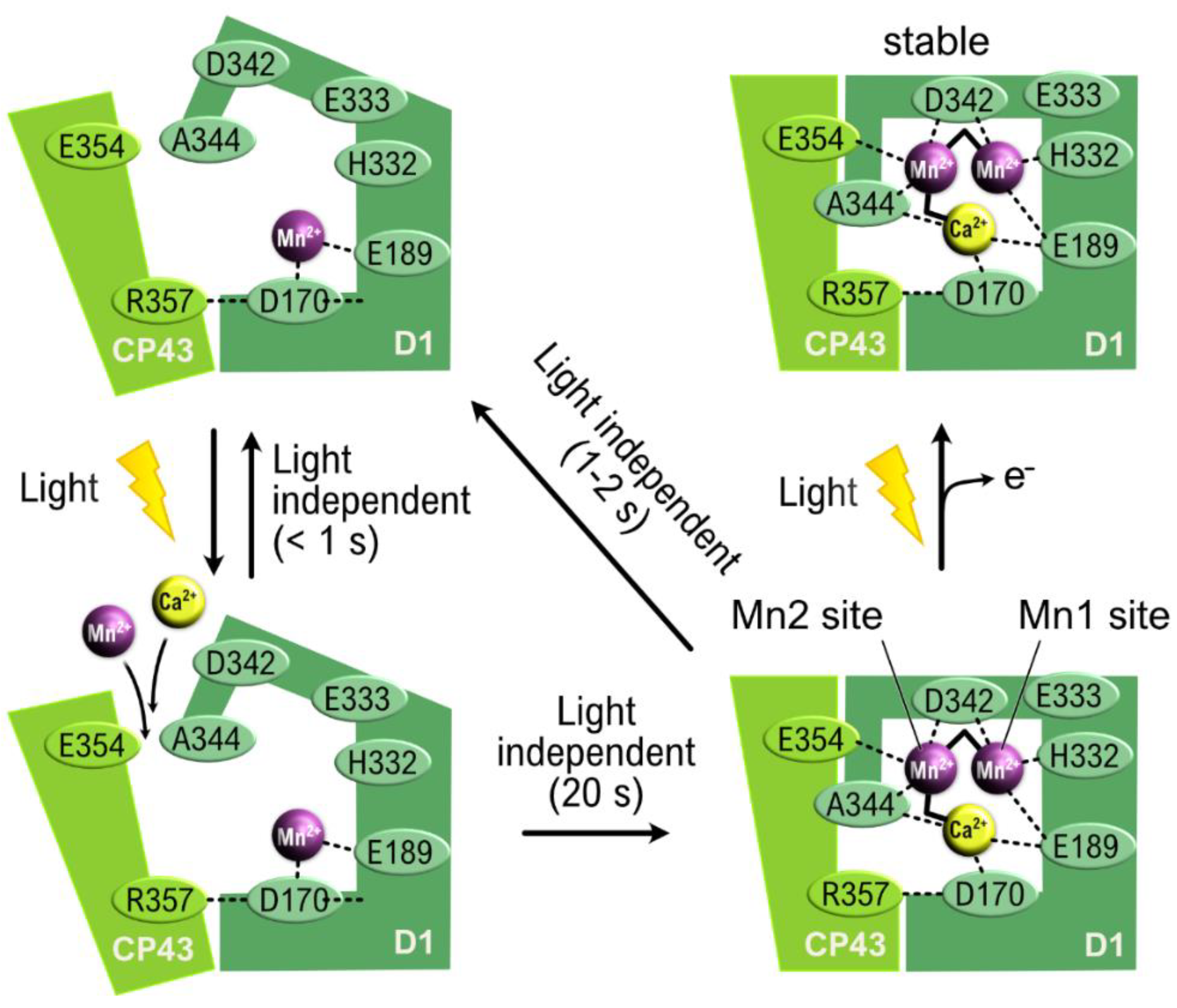
6. Assembly of the Oxygen-Evolving Complex
7. Evolution of Oxygen Evolution
Funding
Acknowledgments
Conflicts of Interest
References
- Dvorák, P.; Casamatta, D.A.; Poulícková, A.; Hasler, P.; Ondrej, V.; Sanges, R. Synechococcus: 3 billion years of global dominance. Molec. Ecol. 2014, 23, 5538–5551. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Planavsky, N.J.; Hofmann, A.; Saupe, E.E.; De Corte, B.P.; Philippot, P.; LaLonde, S.V.; Jemison, N.E.; Zou, H.; Ossa, F.O.; et al. A Mesoarchean shift in uranium isotope systematics. Geochim. Cosmochim. Acta 2018, 238, 438–452. [Google Scholar] [CrossRef]
- Satkoski, A.; Beukes, N.J.; Li, W.; Beard, B.L.; Johnson, C.M. A redox-stratified ocean 3.2 billion years ago. Earth Planet. Sci. Lett. 2015, 430, 43–53. [Google Scholar]
- Shevela, D.; Do, H.-N.; Fantuzzi, A.; Rutherford, A.W.; Messinger, J. Bicarbonate-mediated CO2 formation on both sides of photosystem II. Biochemistry 2020, 59, 2442–2449. [Google Scholar] [PubMed]
- Joliot, P.; Barbieri, G.; Chabaud, R. Un nouveau modèle des centres photochimiques du système II. Photochem. Photobiol. 1969, 10, 309–329. [Google Scholar]
- Kok, B.; Forbush, B.; McGloin, M. Cooperation of charges in photosynthetic O2 evolution—I. A linear four step mechanism. Photochem. Photobiol. 1970, 11, 457–475. [Google Scholar]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9A. Nature 2011, 273, 55–60. [Google Scholar]
- Suga, M.; Akita, F.; Hirata, K.; Ueno, G.; Murakami, H.; Nakajima, Y.; Shimizu, T.; Yamashita, K.; Yamamoto, M.; Ago, H.; et al. Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 2015, 517, 99–103. [Google Scholar]
- Kern, J.; Chatterjee, R.; Young, I.D.; Fuller, F.D.; Lassalle, L.; Ibrahim, M.; Gul, S.; Fransson, T.; Brewster, A.S.; Alonso-Mori, R.; et al. Structures of the Intermediates of Kok’s Photosynthetic Water Oxidation Clock. Nature 2018, 563, 421–425. [Google Scholar]
- Li, Y.; Yao, R.; Chen, Y.; Xu, B.; Chen, C.; Zhang, C. Mimicking the catalytic center for the water-Splitting reaction in photosystem II. Catalysts 2020, 10, 185. [Google Scholar] [CrossRef]
- Joliot, P.; Kok, B. Oxygen evolution in photosynthesis. In Bioenergetics of Photosynthesis; Govindjee, Ed.; Academic Press: New York, NY, USA, 1975; pp. 387–412. [Google Scholar]
- Pham, L.V.; Olmos, J.D.J.; Chernev, P.; Kargul, J.; Messinger, J. Unequal misses during the flash-induced advancement of photosystem II: Effects of the S state and acceptor side cycles. Photosynth. Res. 2019, 139, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Klauss, A.; Haumann, M.; Dau, H. Alternating electron and proton transfer steps in photosynthetic water oxidation. Proc. Natl. Acad. Sci. USA 2012, 109, 16035–16040. [Google Scholar] [PubMed]
- Amin, M.; Kaur, D.; Yang, K.R.; Wang, J.; Mohamed, Z.; Brudvig, G.W.; Gunner, M.R.; Batista, V. Thermodynamics of the S2-to-S3 state transition of the oxygen-evolving complex of photosystem II. Phys. Chem. Chem. Phys. 2019, 37, 20840–20848. [Google Scholar]
- Ibrahim, M.; Fransson, T.; Chatterjee, R.; Cheah, M.H.; Hussein, R.; Lassalle, L.; Sutherlin, K.D.; Young, I.D.; Fuller, F.D.; Gul, S.; et al. Untangling the sequence of events during the S2 → S3 transition in photosystem II and implications for the water oxidation mechanism. Proc. Natl. Acad. Sci. USA 2020, 117, 12624–12635. [Google Scholar]
- Mandal, M.; Saito, K.; Ishikita, H. Requirement of chloride for the downhill electron transfer pathway from the water-splitting center in natural photosynthesis. J. Phys. Chem. B 2022, 126, 123–131. [Google Scholar] [CrossRef]
- Klauss, A.; Sikora, T.; Süss, B.; Dau, H. Fast structural changes (200–900 ns) may prepare the photosynthetic manganese complex for oxidation by the adjacent tyrosine radical. Biochim. Biophys. Acta 2012, 1817, 1196–1207. [Google Scholar] [CrossRef]
- Klauss, A.; Haumann, M.; Dau, H. Seven steps of alternating electron and proton transfer in photosystem II water oxidation traced by time-resolved photothermal beam deflection at improved sensitivity. J. Phys. Chem. B 2015, 119, 2677–2689. [Google Scholar] [CrossRef]
- Siegbahn, P.E.M. Water oxidation mechanism in photosystem II, including oxidations, proton release pathways, O–O bond formation and O2 release. Biochim. Biophys. Acta 2013, 1827, 1003–1019. [Google Scholar]
- Barber, J. A mechanism for water splitting and oxygen production in photosynthesis. Nat. Plants 2017, 3, 17041. [Google Scholar]
- Pushkar, Y.; Davis, K.M.; Palenik, M.C. Model of the oxygen evolving complex which is highly predisposed to O–O bond formation. J. Phys. Chem. Lett. 2018, 9, 3525–3531. [Google Scholar]
- Guo, Y.; Messinger, J.; Kloo, L.; Sun, L. Reversible structural isomerization of Nature’s water oxidation catalyst prior to O–O bond formation. J. Am. Chem. Soc. 2022, 144, 11736–11747. [Google Scholar] [PubMed]
- Allgöwer, F.; Gamiz-Hernandez, A.P.; Rutherford, A.W.; Kaila, V.R.I. Molecular principles of redox-coupled protonation dynamics in photosystem II. J. Am. Chem. Soc. 2022, 144, 7171–7180. [Google Scholar] [PubMed]
- Han, G.; Chernev, P.; Styring, S.; Messinger, J.; Mamedov, F. Molecular basis for turnover inefficiencies (misses) during water oxidation in photosystem II. Chem. Sci. 2022, 13, 8667–8678. [Google Scholar] [PubMed]
- De Lichtenberg, C.; Kim, C.J.; Chernev, P.; Debus, R.J.; Messinger, J. The exchange of the fast substrate water in the S2 state of photosystem II is limited by diffusion of bulk water through channels–implications for the water oxidation mechanism. Chem. Sci. 2021, 12, 12763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, L. Why Nature chose the Mn4CaO5 cluster as water-splitting catalyst in photosystem II: A new hypothesis for the mechanism of O–O bond formation. Dalton Trans. 2018, 47, 14381–14387. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-C.; Li, J.; Siegbahn, P.E.M. A theoretical study of the recently suggested MnVII mechanism for O–O bond formation in photosystem II. J. Phys. Chem. A 2020, 124, 8011–8018. [Google Scholar]
- Ghanotakis, D.F.; Babcock, G.T.; Yocum, C.F. Calcium reconstitutes high rates of oxygen evolution in polypeptide depleted Photosystem II preparations. FEBS 1984, 167, 127–130. [Google Scholar]
- Gates, C.; Ananyev, G.; Dismukes, G.C. The strontium inorganic mutant of the water oxidizing center (CaMn4O5) of PSII improves WOC efficiency but slows electron flux through the terminal acceptors. Biochim. Biophys. Acta 2016, 1857, 1550–1560. [Google Scholar]
- Lockett, C.J.; Demetriou, C.; Simon, J.; Bowden, S.J.; Nugent, J.H.A. Studies on calcium depletion of PS II by pH 8.3 treatment. Biochim. Biophys. Acta 1990, 1016, 213–218. [Google Scholar]
- Miqyass, M.; Marosvolgyi, M.A.; Nagel, Z.; Yocum, C.F.; van Gorkom, H.J. S-state dependence of the calcium requirement and binding characteristics in the oxygen-evolving complex of photosystem II. Biochemistry 2008, 47, 7915–7924. [Google Scholar]
- Latimer, M.J. The Role of Calcium in the Oxygen Evolving Center of Photosystem II. Ph.D. Thesis, Lawrence Berkeley Laboratory, University of California, Berkeley, CA, USA, May 1995. [Google Scholar]
- Yachandra, V.K.; Yano, J. Calcium in the oxygen-evolving complex: Structural and mechanistic role determined by X-ray spectroscopy. J. Photochem. Photobiol. B Biol. 2011, 104, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Mino, H. Local Structural Modification of Ca2+-Depleted Photosystem II Detected by Proton Matrix ENDOR. Appl. Magn. Reson. 2018, 49, 803–812. [Google Scholar] [CrossRef]
- Nagashima, H.; Nakajima, Y.; Shen, J.-R.; Mino, H. Proton matrix ENDOR studies on Ca2+-depleted and Sr2+-substituted manganese cluster in photosystem II. J. Biol. Chem. 2015, 290, 28166–28174. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Li, Y.; Chen, Y.; Xu, B.; Chen, C.; Zhang, C. Rare-earth elements can structurally and energetically replace the calcium in a synthetic Mn4CaO4-cluster mimicking the oxygen-evolving center in photosynthesis. J. Am. Chem. Soc. 2021, 143, 17360–17365. [Google Scholar] [CrossRef]
- Tsui, E.Y.; Agapie, T. Reduction potentials of heterometallic manganese–oxido cubane complexes modulated by redox-inactive metals. Proc. Natl. Acad. Sci. USA 2013, 110, 10084–10088. [Google Scholar] [CrossRef]
- Bang, S.; Lee, Y.-M.; Hong, S.; Cho, K.-B.; Nishida, Y.; Seo, M.S.; Sarangi, R.; Fukuzumi, S.; Nam, W. Redox-inactive metal ions modulate the reactivity and oxygen release of mononuclear non-haem iron(III)–peroxo complexes. Nat. Chem. 2014, 6, 394–939. [Google Scholar] [CrossRef] [Green Version]
- Begouin, J.-M.; Niggemann, M. Calcium-based lewis acid catalysts. Chem. Eur. J. 2013, 19, 8030–8041. [Google Scholar] [CrossRef]
- Hill, M.S.; Liptrot, D.J.; Weetman, C. Alkaline earths as main group reagents in molecular catalysis. Chem. Soc. Rev. 2016, 45, 972–988. [Google Scholar] [CrossRef]
- Boyle, F.P. Some Factors involved in oxygen evolution from triturated spinach leaves. Science 1948, 108, 359–360. [Google Scholar] [CrossRef]
- Warburg, O.; Krippahl, G. Notwendigkeit der Kohlensäure für die Chinon- und Ferricyanid-Reaktionen in grünen Grana. Zschr. Naturforsch. 1960, 15, 367–369. [Google Scholar] [CrossRef]
- Govindjee, W.T. A new site of bicarbonate effect in photosystem II of photosynthesis: Evidence from chlorophyll fluorescence transients in spinach chloroplasts. Biochim. Biophys. Acta 1975, 387, 403–408. [Google Scholar]
- Brinkert, K.; De Causmaecker, S.; Krieger-Liszkay, A.; Fantuzzia, A.; Rutherford, A.W. Bicarbonate-induced redox tuning in Photosystem II for regulation and protection. Proc. Natl. Acad. Sci. USA 2016, 113, 12144–12149. [Google Scholar] [CrossRef] [PubMed]
- Vermaas, W.F.J.; Rutherford, A.W. EPR measurements on the effects of bicarbonate and triazine resistance on the acceptor side of Photosystem II. FEBS 1984, 175, 243–248. [Google Scholar] [CrossRef]
- Shevela, D.; Nöring, B.; Koroidov, S.; Shutova, T.; Samuelsson, G.; Messinger, J.J. Efficiency of photosynthetic water oxidation at ambient and depleted levels of inorganic carbon. Photosynth. Res. 2013, 117, 401–412. [Google Scholar] [CrossRef]
- Aoyama, C.; Suzuki, H.; Sugiura, M.; Noguchi, T. Flash-induced FTIR difference spectroscopy shows no evidence for the structural coupling of bicarbonate to the oxygen-evolving Mn cluster in photosystem II. Biochemistry 2008, 47, 2760–2765. [Google Scholar] [CrossRef]
- Shevela, D.; Su, J.H.; Klimov, V.; Messinger, J. Hydrogencarbonate is not a tightly bound constituent of the water-oxidizing complex in photosystem II. Biochim. Biophys. Acta 2008, 1777, 532–539. [Google Scholar] [CrossRef]
- Ulas, G.; Olack, G.; Brudvig, G.W. Evidence against bicarbonate bound in the O2-evolving complex of photosystem II. Biochemistry 2008, 47, 3073–3075. [Google Scholar] [CrossRef]
- Koroidov, S.; Shevela, D.; Shutova, T.; Samuelsson, G.; Messinger, J. Mobile hydrogen carbonate acts as proton acceptor in photosynthetic water oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 6299–6304. [Google Scholar] [CrossRef]
- Tikhonov, K.; Shevela, D.; Klimov, V.V.; Messinger, J. Quantification of bound bicarbonate in photosystem II. Photosynthetica 2018, 56, 210–216. [Google Scholar] [CrossRef]
- Khorobrykh, A.; Dasgupta, J.; Kolling, D.R.J.; Terentyev, V.; Klimov, V.V.; Dismukes, C.C. Evolutionary origins of the photosynthetic water oxidation cluster: Bicarbonate permits Mn2+ photooxidation by anoxygenic bacterial reaction centers. ChemBioChem 2013, 14, 1725–1731. [Google Scholar] [CrossRef]
- Mizrahi, A.; Meyerstein, D. Plausible roles of carbonate in catalytic water oxidation. Adv. Inorg. Chem. 2013, 74, 343–360. [Google Scholar] [CrossRef]
- Kálmán, L.; Williams, J.C.; Allen, J.P. Energetics for oxidation of a bound manganese cofactor in modified bacterial reaction centers. Biochemistry 2011, 50, 3310–3320. [Google Scholar] [CrossRef]
- Kozlov, Y.N.; Zharmukhamedov, S.K.; Tikhonov, K.G.; Dasgupta, J.; Kazakova, A.A.; Dismukes, G.C.; Klimov, V.V. Oxidation potentials and electron donation to photosystem II of manganese complexes containing bicarbonate and carboxylate ligands. Phys. Chem. Chem. Phys. 2004, 6, 4905–4911. [Google Scholar] [CrossRef]
- Nickelsen, J.; Rengstl, B. Photosystem II assembly: From cyanobacteria to plants. Annu. Rev. Plant Biol. 2013, 64, 609–635. [Google Scholar] [CrossRef] [PubMed]
- Ono, T. Metallo-radical hypothesis for photoassembly of (Mn)4-cluster of photosynthetic oxygen evolving complex. Biochim. Biophys. Acta 2001, 1503, 40–51. [Google Scholar] [CrossRef]
- Burnap, R.L. D1 protein processing and Mn cluster assembly in light of the emerging photosystem II structure. Phys. Chem. Chem. Phys. 2004, 6, 4803–4809. [Google Scholar] [CrossRef]
- Huang, G.; Xiao, Y.; Pi, X.; Zhao, L.; Zhu, Q.; Wang, W.; Kuang, T.; Han, G.; Sui, S.-F.; Shen, J.-R. Structural insights into a dimeric Psb27-photosystem II complex from a cyanobacterium Thermosynechococcus vulcanus. Proc. Natl. Acad. Sci. USA 2021, 118, e2018053118. [Google Scholar] [CrossRef]
- Baranov, S.V.; Tyryshkin, A.M.; Katz, D.; Dismukes, G.C.; Ananyev, G.M.; Klimov, V.V. Bicarbonate is a native cofactor for assembly of the manganese cluster of the photosynthetic water oxidizing complex. Kinetics of reconstitution of O2 evolution by photoactivation. Biochemistry 2004, 43, 2070–2079. [Google Scholar] [CrossRef]
- Hwang, H.J.; Burnap, R.L. Multiflash experiments reveal a new kinetic phase of photosystem II manganese cluster assembly in Synechocystis sp. PCC6803 in vivo. Biochemistry 2005, 44, 9766–9774. [Google Scholar] [CrossRef]
- Avramov, A.P.; Hwanga, H.J.; Burnap, R.L. The role of Ca2+ and protein scaffolding in the formation of nature’s water oxidizing complex. Proc. Natl. Acad. Sci. USA 2020, 117, 28036–28045. [Google Scholar] [CrossRef]
- Russell, B.P.; Vinyard, D.J. Chloride facilitates Mn(III) formation during photoassembly of the photosystem II oxygen-evolving complex. Photosynth. Res. 2021, 1–6. [Google Scholar] [CrossRef]
- Sato, A.; Nakano, Y.; Nakamura, S.; Noguchi, T. Rapid-scan time-resolved ATR-FTIR study on the photoassembly of the water-oxidizing Mn4CaO5 cluster in photosystem II. J. Phys. Chem. B 2021, 125, 4031–4045. [Google Scholar] [CrossRef] [PubMed]
- Cheniae, G.M.; Martin, I.F. Photoactivation of the manganese catalyst of O2 evolution. I. Biochemical and kinetic aspects. Biochim. Biophys. Acta 1971, 253, 167–181. [Google Scholar] [CrossRef]
- Sauer, K.; Yachandra, V.K. A possible evolutionary origin for the Mn4 cluster of the photosynthetic water oxidation complex from natural MnO2 precipitates in the early ocean. Proc. Natl. Acad. Sci. USA 2002, 99, 8631–8636. [Google Scholar] [CrossRef] [PubMed]
- Dismukes, G.C.; Klimov, V.V.; Baranov, S.V.; Kozlov, Y.N.; DasGupta, J.; Tyryshkin, A.; Dismukes, G.C. The origin of atmospheric oxygen on Earth: The innovation of oxygenic photosynthesis. Proc. Natl Acad. Sci. USA 2001, 98, 2170–2175. [Google Scholar] [CrossRef] [PubMed]
- Chernev, P.; Fischer, S.; Hoffmann, J.; Oliver, N.; Assunção, R.; Yu, B.; Burnap, R.L.; Zaharieva, I.; Nürnberg, D.J.; Haumann, M.; et al. Light-driven formation of manganese oxide by today’s photosystem II supports evolutionarily ancient manganese-oxidizing photosynthesis. Nat. Commun. 2020, 11, 6110. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Björn, L.O. Photosynthetic Production of Molecular Oxygen by Water Oxidation. Oxygen 2022, 2, 337-347. https://doi.org/10.3390/oxygen2030024
Björn LO. Photosynthetic Production of Molecular Oxygen by Water Oxidation. Oxygen. 2022; 2(3):337-347. https://doi.org/10.3390/oxygen2030024
Chicago/Turabian StyleBjörn, Lars Olof. 2022. "Photosynthetic Production of Molecular Oxygen by Water Oxidation" Oxygen 2, no. 3: 337-347. https://doi.org/10.3390/oxygen2030024
APA StyleBjörn, L. O. (2022). Photosynthetic Production of Molecular Oxygen by Water Oxidation. Oxygen, 2(3), 337-347. https://doi.org/10.3390/oxygen2030024






