The Effect of Additives on the Hydrothermal Synthesis and Thermochromic Performance of Monoclinic Vanadium Dioxide Powder
Abstract
:1. Introduction
2. Materials and Methods
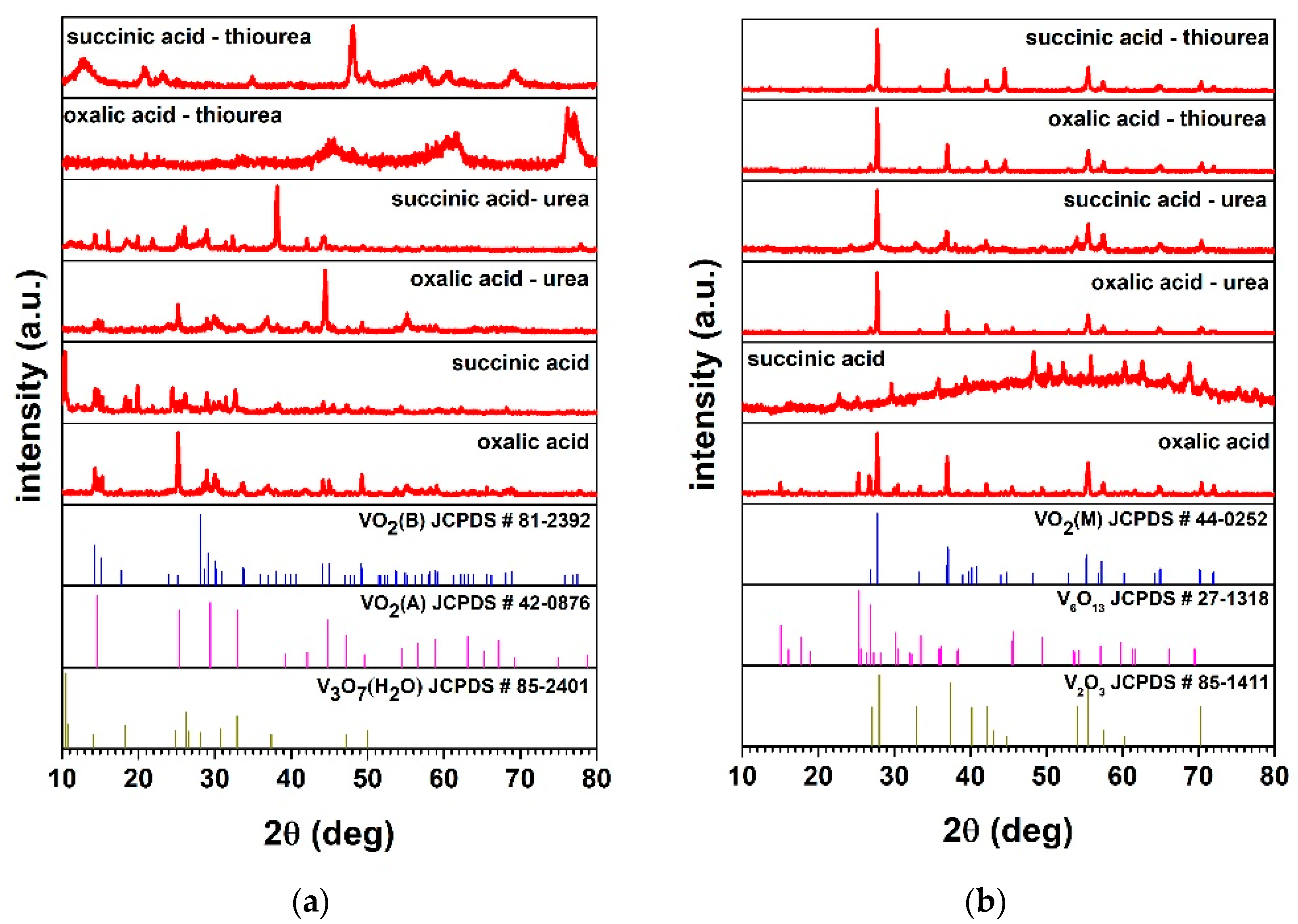
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, H.; Long, L.; Zhang, H.; Gao, Y. The energy saving index and the performance evaluation of thermochromic windows in passive buildings. Renew. Energy 2014, 66, 215–221. [Google Scholar] [CrossRef]
- Long, L.; Ye, H.; Zhang, H.; Gao, Y. Performance demonstration and simulation of thermochromic double glazing in building applications. Sol. Energy 2015, 120, 55–64. [Google Scholar] [CrossRef]
- Iken, O.; Dlimi, M.; Agounoun, R.; Kadiri, I.; Fertahi, S.E.-D.; Zoubir, A.; Sbai, K. Numerical investigation of energy performance and cost analysis of Moroccan’s building smart walls integrating vanadium dioxide. Sol. Energy 2019, 179, 249–263. [Google Scholar] [CrossRef]
- Granqvist, C.G. Recent progress in thermochromics and electrochromics: A brief survey. Thin Solid Films 2016, 614, 90–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Zhang, X.; Deng, Y.; Zhong, Y.; Huang, C.; Liu, X.; Liu, X.; Mo, S. Influence of different additives on the synthesis of VO2 polymorphs. Ceram. Int. 2013, 39, 8363–8376. [Google Scholar] [CrossRef]
- Morin, F.J. Oxides which show a metal-to-insulator transition at the neel temperature. Phys. Rev. Lett. 1959, 3, 34–36. [Google Scholar] [CrossRef]
- Goodenough, J.B. The Two Components of the Crystallographic Transition in VO2. J. Solid State Chem. 1971, 500, 490–500. [Google Scholar] [CrossRef]
- Zylbersztejn, A.; Mott, N.F. Metal-insulator transition in vanadium dioxide. Phys. Rev. B 1975, 11, 4383–4395. [Google Scholar] [CrossRef]
- Babulanam, S.M.; Eriksson, T.S.; Niklasson, G.A.; Granqvist, C.G. Thermochromic VO2 films for energy-efficient windows. Sol. Energy Mater. 1987, 16, 347–363. [Google Scholar] [CrossRef]
- Granqvist, C.; Niklasson, G. Thermochromic Oxide-Based Thin Films and Nanoparticle Composites for Energy-Efficient Glazings. Buildings 2016, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Gagaoudakis, E.; Aperathitis, E.; Michail, G.; Panagopoulou, M.; Katerinopoulou, D.; Binas, V.; Raptis, Y.S.; Kiriakidis, G. Low-temperature rf sputtered VO2 thin films as thermochromic coatings for smart glazing systems. Sol. Energy 2018, 165, 115–121. [Google Scholar] [CrossRef]
- Panagopoulou, M.; Gagaoudakis, E.; Boukos, N.; Aperathitis, E.; Kiriakidis, G.; Tsoukalas, D.; Raptis, Y.S. Thermochromic performance of Mg-doped VO2 thin films on functional substrates for glazing applications. Sol. Energy Mater. Sol. Cells 2016, 157, 1004–1010. [Google Scholar] [CrossRef]
- Ho, H.-C.; Lai, Y.-C.; Chen, K.; Dao, T.D.; Hsueh, C.-H.; Nagao, T. High quality thermochromic VO2 films prepared by magnetron sputtering using V2O5 target with in situ annealing. Appl. Surf. Sci. 2019, 495, 143436. [Google Scholar] [CrossRef]
- Shigesato, Y.; Enomoto, M.; Odaka, H. Thermochromic VO2 Films Deposited by RF Magnetron Sputtering Using V2O3 or V2O5 Targets. Jpn. J. Appl. Phys. 2000, 39, 6016–6024. [Google Scholar] [CrossRef]
- Borek, M.; Qian, F.; Nagabushnam, V.; Singh, R.K. Pulsed laser deposition of oriented VO2 thin films on R-cut sapphire substrates. Appl. Phys. Lett. 1993, 63, 3288–3290. [Google Scholar] [CrossRef]
- Masina, B.N.; Lafane, S.; Wu, L.; Akande, A.A.; Mwakikunga, B.; Abdelli-Messaci, S.; Kerdja, T.; Forbes, A. Phase-selective vanadium dioxide (VO2) nanostructured thin films by pulsed laser deposition. J. Appl. Phys. 2015, 118, 165308. [Google Scholar] [CrossRef]
- Maaza, M.; Bouziane, K.; Maritz, J.; McLachlan, D.; Swanepool, R.; Frigerio, J.; Every, M. Direct production of thermochromic VO2 thin film coatings by pulsed laser ablation. Opt. Mater. 2000, 15, 41–45. [Google Scholar] [CrossRef]
- Vernardou, D.; Pemble, M.E.; Sheel, D.W. The Growth of Thermochromic VO2 Films on Glass by Atmospheric-Pressure CVD: A Comparative Study of Precursors, CVD Methodology, and Substrates. Chem. Vap. Depos. 2006, 12, 263–274. [Google Scholar] [CrossRef]
- Sahana, M.B.; Dharmaprakash, M.S.; Shivashankar, S.A. Microstructure and properties of VO2 thin films deposited by MOCVD from vanadyl acetylacetonate. J. Mater. Chem. 2002, 12, 333–338. [Google Scholar] [CrossRef]
- Louloudakis, D.; Vernardou, D.; Spanakis, E.; Katsarakis, N.; Koudoumas, E. Thermochromic Vanadium Oxide Coatings Grown by APCVD at Low Temperatures. Phys. Procedia. 2013, 46, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Velichko, A.; Pergament, A.; Putrolaynen, V.; Berezina, O.; Stefanovich, G. Effect of memory electrical switching in metal/vanadium oxide/silicon structures with VO2 films obtained by the sol–gel method. Mater. Sci. Semicond. Process. 2015, 29, 315–320. [Google Scholar] [CrossRef]
- Li, D.; Huang, W.; Song, L.; Shi, Q. Thermal stability of VO2 thin films deposited by sol–gel method. J. Sol-Gel Sci. Technol. 2015, 75, 189–197. [Google Scholar] [CrossRef]
- Wu, J.; Huang, W.; Shi, Q.; Cai, J.; Zhao, D.; Zhang, Y.; Yan, J. Effect of annealing temperature on thermochromic properties of vanadium dioxide thin films deposited by organic sol-gel method. Appl. Surf. Sci. 2013, 268, 556–560. [Google Scholar] [CrossRef]
- Byrappa, K.; Keerthiraj, N.; Byrappa, S.M. Hydrothermal Growth of Crystals—Design and Processing. In Handbook of Crystal Growth; Elsevier: Amsterdam, The Netherlands, 2015; pp. 535–575. [Google Scholar] [CrossRef]
- Byrappa, K.; Yoshimura, M. Hydrothermal Technology—Principles and Applications. In Handbook of Hydrothermal Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1–52. [Google Scholar] [CrossRef]
- Malarde, D.; Johnson, I.D.; Godfrey, I.J.; Powell, M.J.; Cibin, G.; Quesada-Cabrera, R.; Darr, J.A.; Carmalt, C.J.; Sankar, G.; Parkin, I.P.; et al. Direct and continuous hydrothermal flow synthesis of thermochromic phase pure monoclinic VO2 nanoparticles. J. Mater. Chem. C 2018, 6, 11731–11739. [Google Scholar] [CrossRef]
- Guo, H.J.D.; Ling, C.; Wang, C.; Wang, D.; Li, J.; Zhao, Z.; Wang, Z.; Zhao, Y.; Zhang, J. Hydrothermal One-Step Synthesis of Highly Dispersed M-Phase VO2 Nanocrystals and Application to Flexible Thermochromic Film. Appl. Mater. Interfaces 2018, 10, 28627–28634. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Wang, Q.; Zhang, J.; Shi, J.; Lu, Y.; Li, G. Effect of Mie Scattering on Thermochromic Performance of Branched VO2 Prepared by One-Step Hydrothermal Method. Eur. J. Inorg. Chem. 2020, 2020, 1783–1789. [Google Scholar] [CrossRef]
- Numan, N.; Madiba, I.G.; Khanyile, B.S.; Khumalo, Z.M.; Maaza, M. Hydrothermal synthesis and characterization of undoped and W-doped vanadium dioxide nanorods for thermochromic application. J. Cryst. Growth 2022, 590, 126702. [Google Scholar] [CrossRef]
- Barra, H.M.; Chen, S.K.; Tamchek, N.; Talib, Z.A.; Lee, O.J.; Tan, K.B. Phase, Microstructure, Thermochromic, and Thermophysical Analyses of Hydrothermally Synthesized W-Doped VO2 Nanopowder. Adv. Mater. Sci. Eng. 2021, 2021, 8582274. [Google Scholar] [CrossRef]
- Wu, X.; Weng, X.; Yuan, L.; Zhang, J.; Qi, L.; Wei, B. Phase- and shape-controlled synthesis of VO2 by a hydrothermal-calcination method. Vacuum 2020, 176, 109352. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, J.; Guo, Y.; Xia, F.; Cui, Y.; Liu, B.; Gao, Y. VO2(A) nanorods: One-pot synthesis, formation mechanism and thermal transformation to VO2(M). Ceram. Int. 2018, 44, 19301–19306. [Google Scholar] [CrossRef]
- Bragaggia, G.; Cacciatore, A.; Poffe, E.; Capone, C.; Zorzi, F.; Causin, V.; Gross, S. Systematic exploration of the synthetic parameters for the production of dynamic VO2 (M1). Molecules 2021, 26, 4513. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Q.; Li, X.; Mudasar, M.; Zhao, X.; Cheng, X.; Liu, J. Synthesis, characterization and electromagnetic absorbing performance of multi-step petaloid morphology VO2(M). Ceram. Int. 2020, 46, 25493–25502. [Google Scholar] [CrossRef]
- Barra, H.M.; Chen, S.K.; Tamchek, N.; Talib, Z.A.; Lee, O.J.; Tan, K.B. Nanostructured VO2 (A) and VO2 (m) derived from VO2 (b): Facile preparations and analyses of structural, thermal, optical and thermophysical properties. Medziagotyra 2021, 27, 269–275. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Tian, S.; Liu, B.; Zhao, X. Facile synthesis of VO2 (D) and its transformation to VO2 (M) with enhanced thermochromic properties for smart windows. Ceram. Int. 2020, 46, 14739–14746. [Google Scholar] [CrossRef]
- Alie, D.; Gedvilas, L.; Wang, Z.; Tenent, R.; Engtrakul, C.; Yan, Y.; Shaheen, S.E.; Dillon, A.C.; Ban, C. Direct synthesis of thermochromic VO2 through hydrothermal reaction. J. Solid State Chem. 2014, 212, 237–241. [Google Scholar] [CrossRef]
- Mjejri, I.; Etteyeb, N.; Sediri, F. Mesoporous vanadium oxide nanostructures: Hydrothermal synthesis, optical and electrochemical properties. Ceram. Int. 2014, 40, 1387–1397. [Google Scholar] [CrossRef]
- Ji, S.; Zhang, F.; Jin, P. Selective formation of VO2(A) or VO2(R) polymorph by controlling the hydrothermal pressure. J. Solid State Chem. 2011, 184, 2285–2292. [Google Scholar] [CrossRef]
- Popuri, S.R.; Miclau, M.; Artemenko, A.; Labrugere, C.; Villesuzanne, A.; Pollet, M. Rapid Hydrothermal Synthesis of VO2 (B) and Its Conversion to Thermochromic VO2 (M1). Inorg. Chem. 2013, 2, 4780–4785. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Huang, Y.F.; Zhang, J.C.; Wu, W.B.; Niu, F.; Zhong, Y.L.; Liu, X.H.; Liu, X.H.; Huang, C. Facile synthesis, phase transition, optical switching and oxidation resistance properties of belt-like VO2(A) and VO2(M) with a rectangular cross section. Mater. Res. Bull. 2012, 47, 1978–1986. [Google Scholar] [CrossRef]
- Banerjee, S.; Sambandamurthy, G.; Patridge, C.J.; Wu, T.-L.; Whittaker, L. Distinctive finite size effects on the phase diagram and metal–insulator transitions of tungsten-doped vanadium(iv) oxide. J. Mater. Chem. 2011, 21, 5580. [Google Scholar] [CrossRef]
- Yan, X.P.; Chen, J.; Liu, J.M.; Zhou, P.P.; Liu, X.; Su, Z.X. Fabrication of hollow VO2 microspheres by a facile template-free process. Mater. Lett. 2010, 64, 278–280. [Google Scholar] [CrossRef]
- Lv, W.; Huang, D.; Chen, Y.; Qiu, Q.; Luo, Z. Synthesis and characterization of Mo-W co-doped VO2(R) nano-powders by the microwave-assisted hydrothermal method. Ceram. Int. 2014, 40, 12661–12668. [Google Scholar] [CrossRef]
- Kang, X.J.; Zhang, J.M.; Sun, X.W.; Zhang, F.R.; Zhang, Y.X. One-pot synthesis of vanadium dioxide nanoflowers on graphene oxide. Ceram. Int. 2016, 42, 7883–7887. [Google Scholar] [CrossRef]
- Zou, J.; Xiao, L.; Zhu, L.; Chen, X. One-step rapid hydrothermal synthesis of monoclinic VO2 nanoparticles with high precursors concentration. J. Sol-Gel Sci. Technol. 2019, 91, 302–309. [Google Scholar] [CrossRef]
- Li, W.; Ji, S.; Li, Y.; Huang, A.; Luo, H.; Jin, P. Synthesis of VO2 nanoparticles by a hydrothermal-assisted homogeneous precipitation approach for thermochromic applications. RSC Adv. 2014, 4, 13026. [Google Scholar] [CrossRef]
- Son, J.; Wei, J.; Cobden, D.; Cao, G.; Xia, Y.; Engineering, B.; Louis, S. Hydrothermal Synthesis of Monoclinic VO2 Micro- and Nanocrystals in One Step and Their Use in Fabricating Inverse Opals. Chem. Mater. 2010, 22, 3043–3050. [Google Scholar] [CrossRef]
- Karahan, O.; Tufani, A.; Unal, S.; Misirlioglu, I.B.; Menceloglu, Y.Z.; Sendur, K. Synthesis and morphological control of VO2 nanostructures via a one-step hydrothermal method. Nanomaterials 2021, 11, 752. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.I. Perovskite Oxides Prepared by Hydrothermal and Solvothermal Synthesis: A Review of Crystallisation, Chemistry, and Compositions. Chem. Eur. J. 2020, 26, 9041–9069. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Bragg, W.H.; Bragg, W.L. The Reflection of X-rays by Crystals. Proc. R. Soc. A Math. Phys. Eng. Sci. 1913, 88, 428–438. [Google Scholar] [CrossRef]
- Tracey, A.S.; Willsky, G.R.; Takeuchi, E.S. Vanadium. In Chemistry, Biochemistry, Pharmacology and Practical Applications; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Mhaske, S.T.; Mestry, S.U.; Borse, P.Y. Reducing Agents in Colloidal Nanoparticle Synthesis; Chapter 7, Acids; The Royal Society of Chemistry: Washington, DC, USA, 2021; pp. 157–183. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Dai, J.; Yang, J.; Wu, Z.; Wei, S.; Xie, Y. Direct hydrothermal synthesis of monoclinic VO2(M) single-domain nanorods on large scale displaying magnetocaloric effect. J. Mater. Chem. 2011, 21, 4509. [Google Scholar] [CrossRef]
- Zhang, S.; Shang, B.; Yang, J.; Yan, W.; Wei, S.; Xie, Y. From VO2 (B) to VO2 (A) nanobelts: First hydrothermal transformation, spectroscopic study and first principles calculation. Phys. Chem. Chem. Phys. 2011, 13, 15873–15881. [Google Scholar] [CrossRef]
- Kumbour, P.; Sikong, L. Effect of Oxalic Acid and Temperature on Hydrothermal VO2 (B) Transformation to VO2 (M). Adv. Mater. Res. 2013, 785, 335–338. [Google Scholar] [CrossRef]
- Kam, K.C.; Cheetham, A.K. Thermochromic VO2 nanorods and other vanadium oxides nanostructures. Mater. Res. Bull. 2006, 41, 1015–1021. [Google Scholar] [CrossRef]
- Reddy, C.V.S.; Jin, A.P.; Han, X.; Zhu, Q.Y.; Mai, L.Q.; Chen, W. Preparation and characterization of (PVP + V2O5) cathode for battery applications. Electrochem. Commun. 2006, 8, 279–283. [Google Scholar] [CrossRef]
- Soltane, L.; Sediri, F. Hydrothermal synthesis, characterization and electrical investigation of poly (para-phenylenediamine)/vanadium oxide nanocomposite nanosheets. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2013, 178, 502–510. [Google Scholar] [CrossRef]
- Vonach, R.; Lendl, B.; Kellner, R. High-performance liquid chromatography with real-time Fourier-transform infrared detection for the determination of carbohydrates, alcohols and organic acids in wines. J. Chromatogr. A 1998, 824, 159–167. [Google Scholar] [CrossRef]
- Taheri, P.; Wielant, J.; Hauffman, T.; Flores, J.R.; Hannour, F.; de Wit, J.H.W.; Mol, J.M.C.; Terryn, H. A comparison of the interfacial bonding properties of carboxylic acid functional groups on zinc and iron substrates. Electrochim. Acta 2011, 56, 1904–1911. [Google Scholar] [CrossRef]
- Ji, Y.; Li, S.; Niklasson, G.A.; Granqvist, C.G. Durability of thermochromic VO2 thin films under heating and humidity : Effect of Al oxide top coatings. Thin Solid Films 2014, 562, 568–573. [Google Scholar] [CrossRef]
- Pan, G.T.; Yang, Y.L.; Chong, S.; Arjun, N.; Yang, T.C.K.; Lai, Y.C. The durability study of thermochromic vanadium dioxide films with the addition of barrier coatings. Vacuum 2017, 145, 158–168. [Google Scholar] [CrossRef]
- Koch, D.; Chaker, M. The Origin of the Thermochromic Property Changes in Doped Vanadium Dioxide. ACS Appl. Mater. Interfaces 2022, 14, 23928–23943. [Google Scholar] [CrossRef]
- Ji, S.; Zhao, Y.; Zhang, F.; Jin, P. Direct formation of single crystal VO2 (R) nanorods by one-step hydrothermal treatment. J. Cryst. Growth 2010, 312, 282–286. [Google Scholar] [CrossRef]
- Li, R.; Liu, C. VO2(B) nanospheres: Hydrothermal synthesis and electrochemical properties. Mater. Res. Bull. 2010, 45, 688–692. [Google Scholar] [CrossRef]
- Tavakoli, M.R.; Dornian, S.; Dreisinger, D.B. The leaching of vanadium pentoxide using sulfuric acid and sulfite as a reducing agent. Hydrometallurgy 2014, 141, 59–66. [Google Scholar] [CrossRef]
- Qiao, L.; Swihart, M.T. Solution-phase synthesis of transition metal oxide nanocrystals: Morphologies, formulae, and mechanisms. Adv. Colloid Interface Sci. 2017, 244, 199–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.D.; Yoshida, M.; George, B. Theoretical study on the thermal decomposition of thiourea. Comput. Theor. Chem. 2013, 1017, 91–98. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Q.; Wang, J. Thermodynamic analysis of decomposition of thiourea and thiourea oxides. J. Phys. Chem. B 2005, 109, 17281–17289. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.H.R.; Walker, D.G. The Decomposition of Thiourea in Water Solutions. J. Am. Chem. Soc. 1956, 78, 5769–5772. [Google Scholar] [CrossRef]



| Reducing Agent | Additives | “As Obtained” Product Phases by XRD | Hydrothermal Synthesis Yield (%) (Dried Product Mass) |
|---|---|---|---|
| Oxalic acid | No | VO2 (B) and VO2 (A) | 75% (277 mg) |
| Urea | unidentified peaks | 75% (270 mg) | |
| Thiourea | unidentified peaks | 90% (320 mg) | |
| Succinic acid | No | VO2 (B) and VO2 (A) | 3% (11 mg) |
| Urea | unidentified peaks | 50% (191 mg) | |
| Thiourea | unidentified peaks | 70% (245 mg) |
| Reducing Agent | Additives | ‘Final’ Materials Phases | (hkl) | 2θ ** (deg) | Crystallite Size Dhkl (nm) | Interplanar Distance dhkl (nm) |
|---|---|---|---|---|---|---|
| Oxalic acid | No | VO2 (M) (with V6O13 impurities) | (011) | 27.76 | 46.7 | 0.32 |
| Urea | VO2 (M) | (011) | 27.77 | 47.7 | 0.32 | |
| Thiourea | VO2 (M) | (011) | 27.77 | 44.3 | 0.32 | |
| Succinic acid | No | amorphous | (011) | - * | - * | - * |
| Urea | VO2 (M) | (011) | 27.74 | 36.5 | 0.32 | |
| Thiourea | VO2 (M) | (011) | 27.77 | 47.5 | 0.32 |
| Reducing Agent | Additives | TC (°C) | ΔTC (°C) |
|---|---|---|---|
| Oxalic acid | No | 67.8 | 10.1 |
| Urea | 67.6 | 10.4 | |
| Thiourea | 67.9 | 11.4 | |
| Succinic acid | No | - * | - * |
| Urea | 67.9 | 11.3 | |
| Thiourea | 67.7 | 12.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouridi, L.; Gagaoudakis, E.; Mantsiou, E.; Dragani, T.; Maragaki, X.; Aperathitis, E.; Kiriakidis, G.; Binas, V. The Effect of Additives on the Hydrothermal Synthesis and Thermochromic Performance of Monoclinic Vanadium Dioxide Powder. Oxygen 2022, 2, 410-423. https://doi.org/10.3390/oxygen2040028
Zouridi L, Gagaoudakis E, Mantsiou E, Dragani T, Maragaki X, Aperathitis E, Kiriakidis G, Binas V. The Effect of Additives on the Hydrothermal Synthesis and Thermochromic Performance of Monoclinic Vanadium Dioxide Powder. Oxygen. 2022; 2(4):410-423. https://doi.org/10.3390/oxygen2040028
Chicago/Turabian StyleZouridi, Leila, Emmanouil Gagaoudakis, Eleni Mantsiou, Theodora Dragani, Xristina Maragaki, Elias Aperathitis, George Kiriakidis, and Vassilios Binas. 2022. "The Effect of Additives on the Hydrothermal Synthesis and Thermochromic Performance of Monoclinic Vanadium Dioxide Powder" Oxygen 2, no. 4: 410-423. https://doi.org/10.3390/oxygen2040028








