β-Cyclodextrin-Aided Aqueous Extraction of Antioxidant Polyphenols from Peppermint (Mentha × piperita L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals–Reagents
2.2. Plant Material and Handling
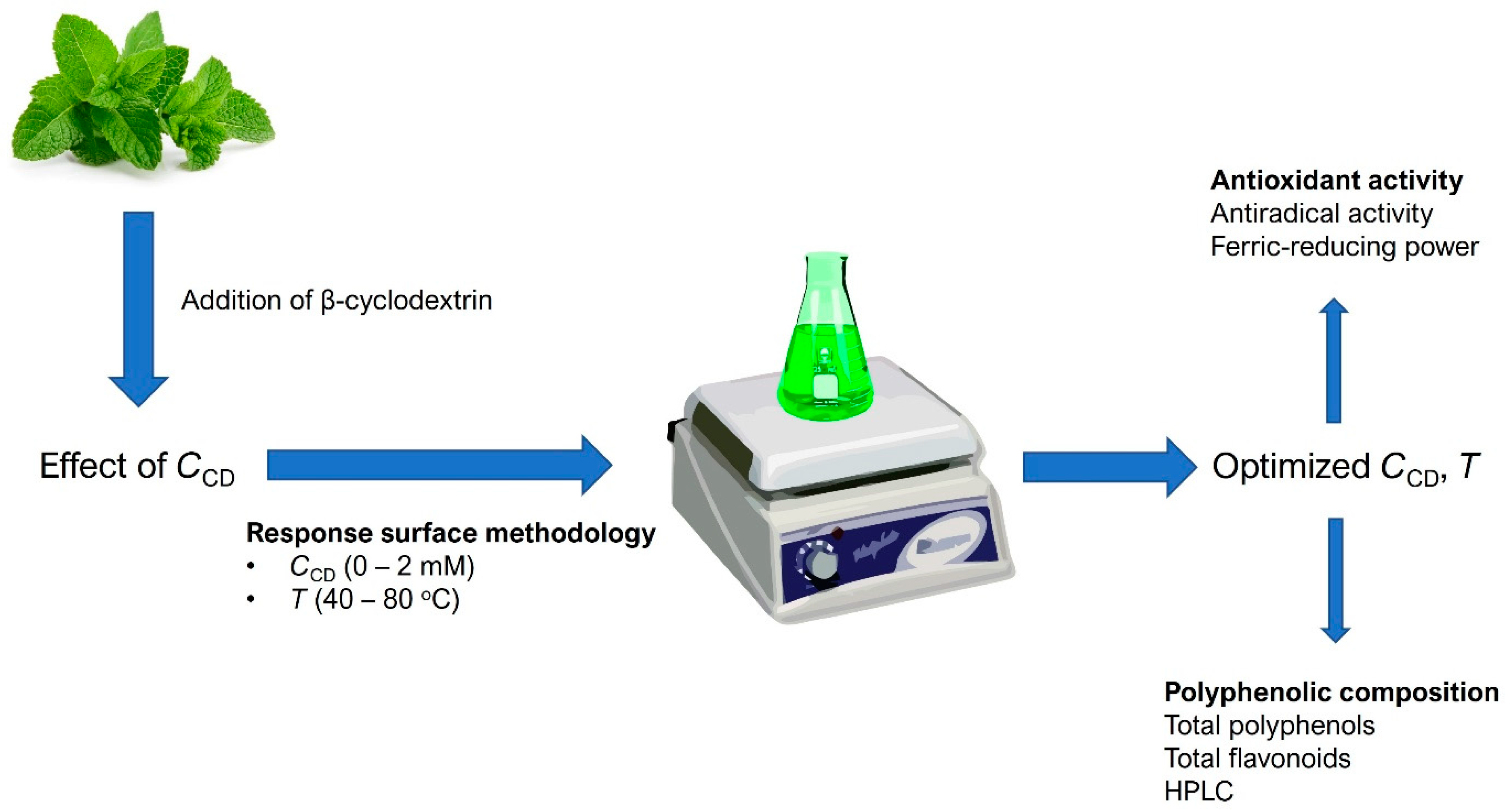
2.3. Extraction Procedures
2.4. Experimental Design and Response Surface Methodology (RSM)
2.5. Spectrophotometric Analyses
2.5.1. Total Polyphenols
2.5.2. Total Flavonoids
2.5.3. Antioxidant Activity
2.6. Quantitative High-Performance Liquid Chromatography (HPLC)
2.7. Statistical Elaboration
3. Results and Discussion
3.1. The Effect of β-CD Concentration
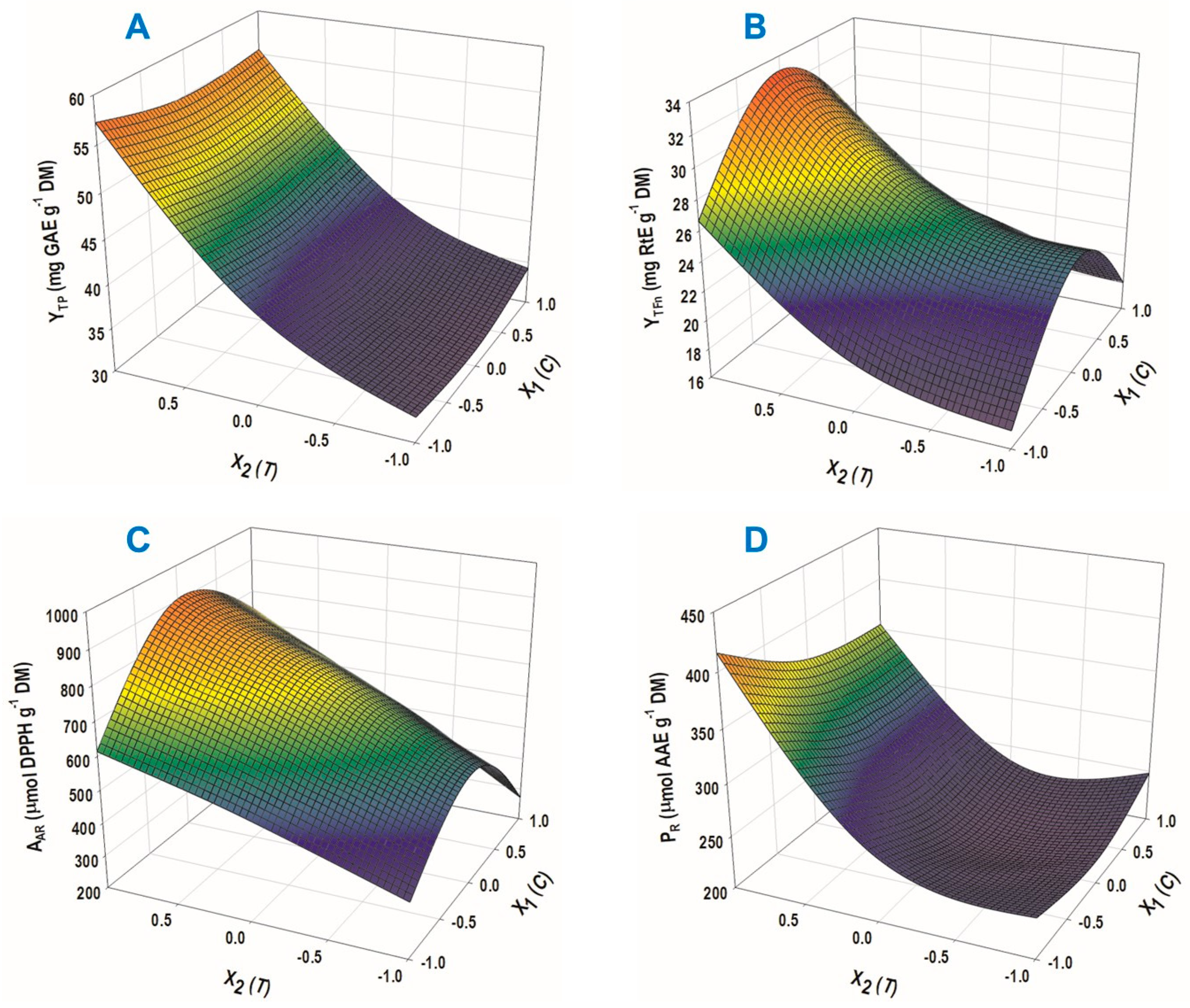
3.2. Extraction Optimization by Response Surface Methodology
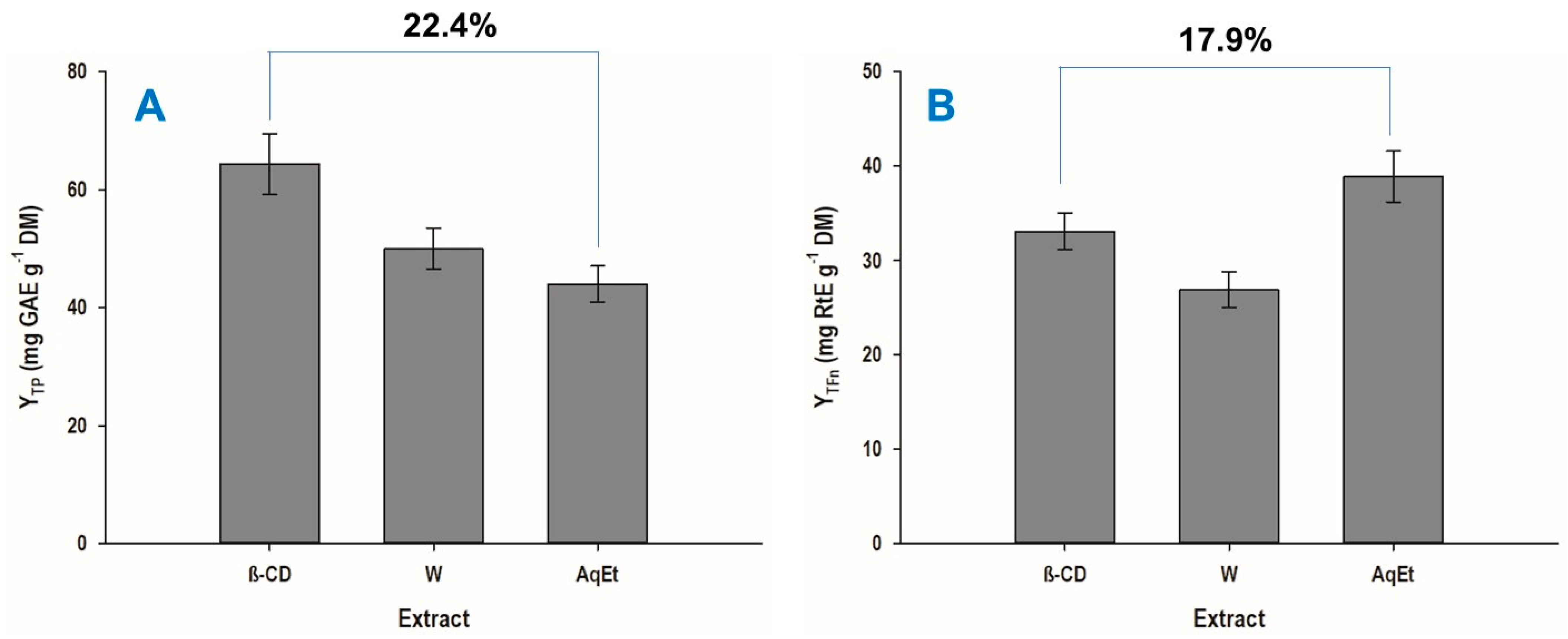
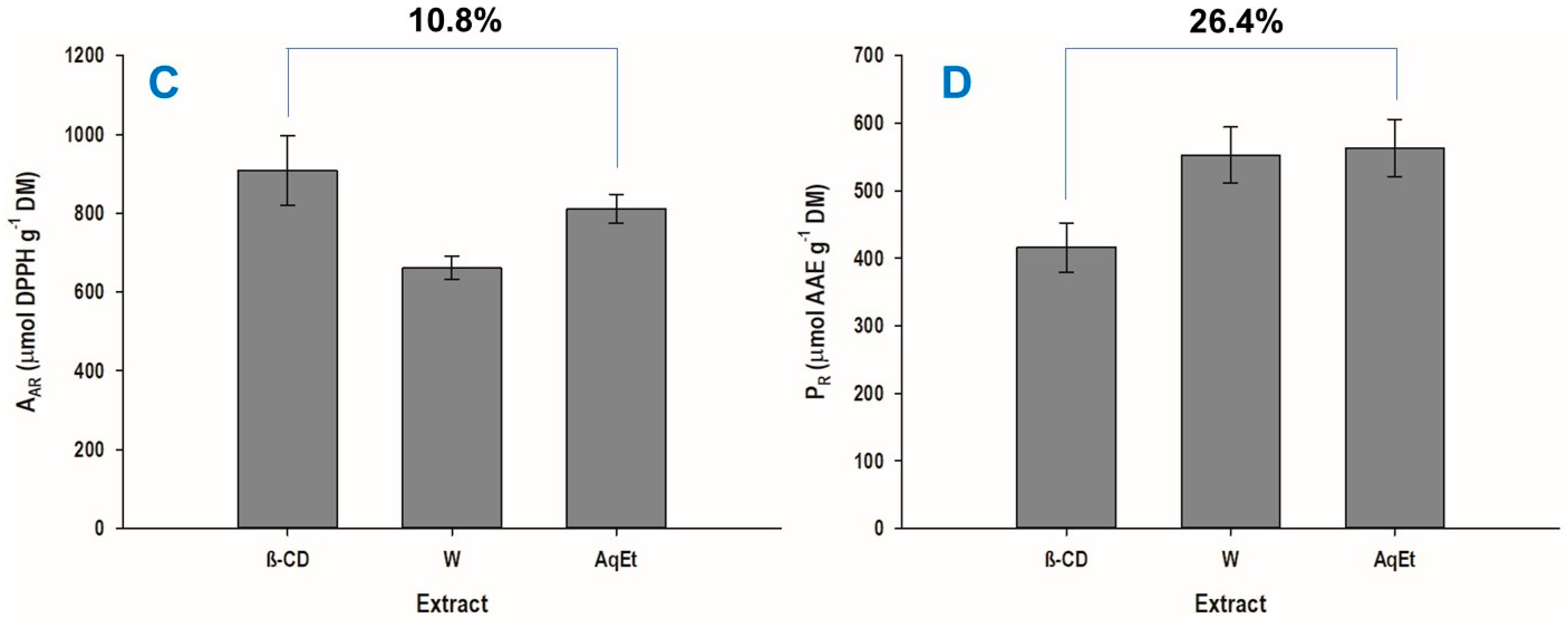
3.3. Extraction Yield and Antioxidant Properties
3.4. Polyphenolic Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anton, R.; Mathioudakis, B.; Pramono, S.; Sezik, E.; Sharma, S. Traditional use of botanicals and botanical preparations. Eur. Food Feed Law Rev. 2019, 14, 132–141. [Google Scholar]
- Colombo, F.; Restani, P.; Biella, S.; Di Lorenzo, C. Botanicals in Functional Foods and Food Supplements: Tradition, Efficacy and Regulatory Aspects. Appl. Sci. 2020, 10, 2387. [Google Scholar] [CrossRef]
- Campa, M.; Baron, E. Anti-aging Effects of Select Botanicals: Scientific Evidence and Current Trends. Cosmetics 2018, 5, 54. [Google Scholar] [CrossRef]
- Gholamipourfard, K.; Salehi, M.; Banchio, E. Mentha piperita phytochemicals in agriculture, food industry and medicine: Features and applications. S. Afr. J. Bot. 2021, 141, 183–195. [Google Scholar] [CrossRef]
- Pereira, R.O.; Cardoso, S. Overview on Mentha and Thymus polyphenols. Cur. Anal. Chem. 2013, 9, 382–396. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef]
- Eftekhari, A.; Khusro, A.; Ahmadian, E.; Dizaj, S.M.; Hasanzadeh, A.; Cucchiarini, M. Phytochemical and nutra-pharmaceutical attributes of Mentha spp.: A comprehensive review. Arab. J. Chem. 2021, 14, 103106. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Espino, M.; de los Ángeles Fernández, M.; Gomez, F.J.; Boiteux, J.; Silva, M.F. Green analytical chemistry metrics: Towards a sustainable phenolics extraction from medicinal plants. Microchem. J. 2018, 141, 438–443. [Google Scholar] [CrossRef]
- Li, Z.; Smith, K.H.; Stevens, G.W. The use of environmentally sustainable bio-derived solvents in solvent extraction applications—A review. Chin. J. Chem. Eng. 2016, 24, 215–220. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidović, S.; Redovniković, I.R.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in aqueous solution: A review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gandara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Ezhilarasi, P.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation techniques for food bioactive components: A review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Cai, R.; Yuan, Y.; Cui, L.; Wang, Z.; Yue, T. Cyclodextrin-assisted extraction of phenolic compounds: Current research and future prospects. Trends Food Sci. Technol. 2018, 79, 19–27. [Google Scholar] [CrossRef]
- Bozinou, E.; Lakka, A.; Poulianiti, K.; Lalas, S.; Makris, D.P. Cyclodextrins as high-performance green co-solvents in the aqueous extraction of polyphenols and anthocyanin pigments from solid onion waste. Eur. Food Res. Technol. 2021, 247, 2831–2845. [Google Scholar] [CrossRef]
- Morsli, F.; Grigorakis, S.; Halahlah, A.; Poulianiti, K.P.; Makris, D.P. Appraisal of the combined effect of time and temperature on the total polyphenol yield in batch stirred-tank extraction of medicinal and aromatic plants: The extraction efficiency factor. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100340. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Development of a Low-Temperature and High-Performance Green Extraction Process for the Recovery of Polyphenolic Phytochemicals from Waste Potato Peels Using Hydroxypropyl β-Cyclodextrin. Appl. Sci. 2020, 10, 3611. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Manousaki, A.; Jancheva, M.; Grigorakis, S.; Makris, D.P. Extraction of Antioxidant Phenolics from Agri-Food Waste Biomass Using a Newly Designed Glycerol-Based Natural Low-Transition Temperature Mixture: A Comparison with Conventional Eco-Friendly Solvents. Recycling 2016, 1, 194–204. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron Processing Wastes as a Bioresource of High-Value Added Compounds: Development of a Green Extraction Process for Polyphenol Recovery Using a Natural Deep Eutectic Solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Hydroxypropyl-β-Cyclodextrin as a Green Co-Solvent in the Aqueous Extraction of Polyphenols from Waste Orange Peels. Beverages 2020, 6, 50. [Google Scholar] [CrossRef]
- Grigorakis, S.; Benchennouf, A.; Halahlah, A.; Makris, D.P. High-Performance Green Extraction of Polyphenolic Antioxidants from Salvia fruticosa Using Cyclodextrins: Optimization, Kinetics, and Composition. Appl. Sci. 2020, 10, 3447. [Google Scholar] [CrossRef]
- Alibante, A.; Lakka, A.; Bozinou, E.; Chatzilazarou, A.; Lalas, S.; Makris, D.P. Integrated Green Process for the Extraction of Red Grape Pomace Antioxidant Polyphenols Using Ultrasound-Assisted Pretreatment and β-Cyclodextrin. Beverages 2021, 7, 59. [Google Scholar] [CrossRef]
- Kowalska, G.; Baj, T.; Kowalski, R.; Szymańska, J. Optimization of Glycerol–Water Extraction of Selected Bioactive Compounds from Peppermint and Common Nettle. Antioxidants 2021, 10, 817. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Pałecz, B.; Rachwał-Rosiak, D.; Hodurek, P.; Miśkiewicz, K.; Oracz, J.; Żyżelewicz, D. Inclusion complexes of β-cyclodextrin with chlorogenic acids (CHAs) from crude and purified aqueous extracts of green Robusta coffee beans (Coffea canephora L.). Food Res. Int. 2014, 61, 202–213. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Lalas, S.; Makris, D.P. Effect of Methyl β-cyclodextrin on Radical Scavenging Kinetics of Olive Leaf Extracts and Interactions with Ascorbic Acid. ChemEngineering 2017, 1, 6. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, J.; Fang, Z.; Sun, P. Complexing of chlorogenic acid with β-cyclodextrins: Inclusion effects, antioxidative properties and potential application in grape juice. Food Hydrocoll. 2014, 41, 132–139. [Google Scholar] [CrossRef]
- Celik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Antioxidant capacity of quercetin and its glycosides in the presence of β-cyclodextrins: Influence of glycosylation on inclusion complexation. J. Incl. Phenom. Macrocycl. Chem. 2015, 83, 309–319. [Google Scholar] [CrossRef]
- Medronho, B.; JM Valente, A.; Costa, P.; Romano, A. Inclusion complexes of rosmarinic acid and cyclodextrins: Stoichiometry, association constants, and antioxidant potential. Colloid Polym. Sci. 2014, 292, 885–894. [Google Scholar] [CrossRef]
- Dorman, H.D.; Koşar, M.; Başer, K.H.C.; Hiltunen, R. Phenolic profile and antioxidant evaluation of Mentha x piperita L.(peppermint) extracts. Nat. Prod. Commun. 2009, 4, 1934578X0900400419. [Google Scholar] [CrossRef]
- Kapp, K.; Hakala, E.; Orav, A.; Pohjala, L.; Vuorela, P.; Püssa, T.; Vuorela, H.; Raal, A. Commercial peppermint (Mentha × piperita L.) teas: Antichlamydial effect and polyphenolic composition. Food Res. Int. 2013, 53, 758–766. [Google Scholar] [CrossRef]
- Lv, J.; Huang, H.; Yu, L.; Whent, M.; Niu, Y.; Shi, H.; Wang, T.T.; Luthria, D.; Charles, D.; Yu, L.L. Phenolic composition and nutraceutical properties of organic and conventional cinnamon and peppermint. Food Chem. 2012, 132, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choe, E. Interaction effect of tocopherol homologs with peppermint extract on the iron-catalyzed oxidation of soybean oil-in-water emulsion. Food Sci. Biotechnol. 2019, 28, 1679–1685. [Google Scholar] [CrossRef]


| Process Variables | Codes | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| CCD (mM) | X1 | 0 | 1 | 2 |
| T (°C) | X2 | 40 | 60 | 80 |
| Design Point | Process Variable | Responses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 (C) | X2 (T) | YTP (mg GAE g−1 DM) | YTFn (mg RtE g−1 DM) | AAR (μmol DPPH g−1 DM) | PR (μmol AAE g−1 DM) | |||||
| Measured | Predicted | Measured | Predicted | Measured | Predicted | Measured | Predicted | |||
| 1 | −1 | −1 | 32.66 | 32.85 | 17.95 | 17.23 | 402.93 | 368.26 | 241.17 | 238.46 |
| 2 | −1 | 1 | 57.66 | 57.27 | 27.12 | 26.63 | 615.67 | 613.93 | 407.22 | 415.96 |
| 3 | 1 | −1 | 33.02 | 34.25 | 18.05 | 17.97 | 259.87 | 272.60 | 260.55 | 247.96 |
| 4 | 1 | 1 | 54.83 | 55.49 | 26.94 | 27.09 | 700.70 | 746.26 | 355.52 | 354.46 |
| 5 | −1 | 0 | 40.09 | 40.29 | 18.07 | 19.29 | 465.04 | 503.81 | 266.82 | 260.58 |
| 6 | 1 | 0 | 42.00 | 40.11 | 19.96 | 19.89 | 571.85 | 522.14 | 221.85 | 234.59 |
| 7 | 0 | −1 | 33.46 | 32.04 | 23.00 | 23.80 | 522.50 | 544.14 | 207.76 | 222.58 |
| 8 | 0 | 1 | 55.14 | 54.87 | 32.72 | 33.06 | 946.79 | 903.81 | 373.28 | 364.54 |
| 9 | 0 | 0 | 38.37 | 38.69 | 26.53 | 25.79 | 729.40 | 736.68 | 245.48 | 226.95 |
| 10 | 0 | 0 | 37.02 | 38.69 | 25.05 | 25.79 | 733.12 | 736.68 | 234.56 | 226.95 |
| 11 | 0 | 0 | 39.04 | 38.69 | 27.02 | 25.79 | 739.16 | 736.68 | 223.24 | 226.95 |
| Response | Equation (Model) | R2 | p |
|---|---|---|---|
| YTP (mg GAE g−1 DM) | 38.69 + 11.42 X2 + 4.77 X22 | 0.99 | <0.0001 |
| YTFn (mg RtE g−1 DM) | 25.79 + 4.63 X2 − 6.21 X12 + 2.64 X22 | 0.98 | 0.0005 |
| AAR (μmol DPPH g−1 DM) | 736.68 + 179.83 X2 − 223.71 X12 | 0.97 | 0.0009 |
| PR (μmol AAE g−1 DM) | 226.95 + 71.00 X2 + 66.63 X22 | 0.97 | 0.0006 |
| Response | Maximum Predicted Value | Optimal Conditions | |
|---|---|---|---|
| C (mM) | T (°C) | ||
| YTP (mg GAE g−1 DM) | 57.27 ± 3.38 | 0 | 80 |
| YTFn (mg RtE g−1 DM) | 33.07 ± 1.96 | 1.02 | 80 |
| AAR (μmol DPPH g−1 DM) | 908.70 ± 87.46 | 1.15 | 80 |
| PR (μmol AAE g−1 DM) | 415.96 ± 36.55 | 0 | 80 |
| # | Compound | Yield (mg g−1 dm) | ||
|---|---|---|---|---|
| Water | AqEt | β-CD | ||
| 1 | Caffeic acid | 0.20 ± 0.01 | 0.18 ± 0.03 | 0.31 ± 0.00 a |
| 2 | Eriocitrin | 25.65 ± 2.14 | 36.48 ± 2.97 a | 29.22 ± 1.97 |
| 3 | Luteolin 7-O-rutinoside | 5.80 ± 0.80 | 9.16 ± 0.87 a | 7.07 ± 0.37 |
| 4 | Luteolin 7-O-glucuronide | 2.89 ± 0.19 | 3.43 ± 0.01 | 3.29 ± 0.25 |
| 5 | Narirutin | 0.18 ± 0.00 | 0.30 ± 0.04 a | 0.25 ± 0.04 |
| 6 | Hesperidin | 2.23 ± 0.21 | 3.24 ± 0.44 a | 2.51 ± 0.05 |
| 7 | Rosmarinic acid | 3.30 ± 0.21 | 4.60 ± 0.29 a | 3.44 ± 0.14 |
| 8 | Luteolin derivative | 2.53 ± 0.15 | 4.11 ± 0.08 a | 2.61 ± 0.20 |
| Sum | 44.18 | 63.00 a | 50.12 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasiadis, V.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I.; Makris, D.P. β-Cyclodextrin-Aided Aqueous Extraction of Antioxidant Polyphenols from Peppermint (Mentha × piperita L.). Oxygen 2022, 2, 424-436. https://doi.org/10.3390/oxygen2040029
Athanasiadis V, Palaiogiannis D, Bozinou E, Lalas SI, Makris DP. β-Cyclodextrin-Aided Aqueous Extraction of Antioxidant Polyphenols from Peppermint (Mentha × piperita L.). Oxygen. 2022; 2(4):424-436. https://doi.org/10.3390/oxygen2040029
Chicago/Turabian StyleAthanasiadis, Vassilis, Dimitrios Palaiogiannis, Eleni Bozinou, Stavros I. Lalas, and Dimitris P. Makris. 2022. "β-Cyclodextrin-Aided Aqueous Extraction of Antioxidant Polyphenols from Peppermint (Mentha × piperita L.)" Oxygen 2, no. 4: 424-436. https://doi.org/10.3390/oxygen2040029
APA StyleAthanasiadis, V., Palaiogiannis, D., Bozinou, E., Lalas, S. I., & Makris, D. P. (2022). β-Cyclodextrin-Aided Aqueous Extraction of Antioxidant Polyphenols from Peppermint (Mentha × piperita L.). Oxygen, 2(4), 424-436. https://doi.org/10.3390/oxygen2040029









