Does the Oxygen Functionality Really Improve the Thermodynamics of Reversible Hydrogen Storage with Liquid Organic Hydrogen Carriers?
Abstract
:1. Introduction
- -
- Step I: The typical representatives of the promising LOHC systems are studied using the conventional experimental methods (the high-precision combustion calorimetry for (liq/cr, 298.15 K) values and the transpiration method for (298.15 K) and (298.15 K) in this work)
- -
- Step II: The high-level QC calculations are performed to derive (g, 298.15 K)QC values and (g, 298.15 K)QC values and the possible experimental and empirical methods are involved to validate these theoretical results.
- -
- Step III: The liquid-phase (liq, 298.15 K) and (liq, 298.15 K) values are derived and used to calculate (liq, T). In addition, the equilibrium constants are derived according to Equation (1), and a thermodynamic analysis of the LOHC system is carried out.
2. Theoretical and Experimental Methods
Quantum-Chemical Calculations
3. Results and Discussion
3.1. Step I: Evaluation of Experimental and Empirical Thermochemical Results
3.1.1. Enthalpies of Formation from Combustion Calorimetry
3.1.2. Absolute Vapour Pressures and Vaporisation Enthalpies
3.1.3. Validation of Vaporisation Enthalpies by Structure–Property Correlations
3.2. Step II: Quantum-Chemical Calculations of the Gas-Phase Enthalpies of Formation
3.3. Step III: Thermodynamic Analysis of the LOHC Systems
3.3.1. Comparison of the Energetics of Dehydrogenation Reactions with and without Oxygen Functionality
3.3.2. Comparison of the Reaction Entropies of Dehydrogenation of Lohc Based on Ethers and Hydrocarbons
3.3.3. Comparison of the Gibbs Energies of Dehydrogenation Reactions R1 to R10
3.3.4. Thermodynamic Analysis of the Reversible Hydrogenation/Dehydrogenation Process in the Gas Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-Free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Hodoshima, S.; Takaiwa, S.; Shono, A.; Satoh, K.; Saito, Y. Hydrogen Storage by Decalin/Naphthalene Pair and Hydrogen Supply to Fuel Cells by Use of Superheated Liquid-Film-Type Catalysis. Appl. Catal. A Gen. 2005, 283, 235–242. [Google Scholar] [CrossRef]
- Sebastián, D.; Bordejé, E.G.; Calvillo, L.; Lázaro, M.J.; Moliner, R. Hydrogen Storage by Decalin Dehydrogenation/Naphthalene Hydrogenation Pair over Platinum Catalysts Supported on Activated Carbon. Int. J. Hydrogen Energy 2008, 33, 1329–1334. [Google Scholar] [CrossRef]
- Sung, J.; Choo, K.; Kim, T.; Tarasov, A.; Tkachenko, O.; Kustov, L. A New Hydrogen Storage System Based on Efficient Reversible Catalytic Hydrogenation/Dehydrogenation of Terphenyl. Int. J. Hydrogen Energy 2008, 33, 2721–2728. [Google Scholar] [CrossRef]
- Kustov, L.M.; Kalenchuk, A.N.; Bogdan, V.I. Systems for Accumulation, Storage and Release of Hydrogen. Russ. Chem. Rev. 2020, 89, 897–916. [Google Scholar] [CrossRef]
- Pez, G.P.; Scott, A.R.; Cooper, A.C.; Cheng, H.; Wilchelm, F.C.; Abdourazak, A.H. Hydrogen storage by reversible hydrogenation of pi-conjugated substrates. US patent US7351395B1, 2008. Available online: https://patents.google.com/patent/US7351395B1/en (accessed on 26 June 2024).
- Bourane, A.; Elanany, M.; Pham, T.V.; Katikaneni, S.P. An Overview of Organic Liquid Phase Hydrogen Carriers. Int. J. Hydrogen Energy 2016, 41, 23075–23091. [Google Scholar] [CrossRef]
- Cooper, A.C.; Campbell, K.M.; Pez, G.P. An Integrated Hydrogen Storage and Delivery Approach Using Organic Liquid-Phase Carriers. In Proceedings of the 16th World Hydrogen Energy Conference 2006, Lyon, France, 13–16 June 2006; Volume 3, pp. 2164–2175. [Google Scholar]
- Products, A.; Cooper, A.C.; Manager, P. Design and Development of New Carbon-Based Sorbent Systems for an Effective Containment of Hydrogen Final Report Revised April 2012 Hydrogen Storage by Reversible Hydrogenation of Solid- and Liquid-Phase Hydrogen Carriers; Air Products and Chemicals, Inc.: Allentown, PA, USA, 2012. [Google Scholar]
- Crabtree, R.H. Hydrogen Storage in Liquid Organic Heterocycles. Energy Environ. Sci. 2008, 1, 134. [Google Scholar] [CrossRef]
- Zakgeym, D.; Hofmann, J.D.; Maurer, L.A.; Auer, F.; Müller, K.; Wolf, M.; Wasserscheid, P. Better through Oxygen Functionality? The Benzophenone/Dicyclohexylmethanol LOHC-System. Sustain. Energy Fuels 2023, 7, 1213–1222. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Zaitseva, K.V.; Agapito, F.; Martinho Simões, J.A.; Verevkin, S.P. Benchmark Thermodynamic Properties of Methylanisoles: Experimental and Theoretical Study. J. Chem. Thermodyn. 2015, 85, 155–162. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitsau, D.H.; Emel’yanenko, V.N.; Zhabina, A.A. Thermodynamic Properties of Glycerol: Experimental and Theoretical Study. Fluid Phase Equilib. 2015, 397, 87–94. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N.; Diky, V.; Muzny, C.D.; Chirico, R.D.; Frenkel, M. New Group-Contribution Approach to Thermochemical Properties of Organic Compounds: Hydrocarbons and Oxygen-Containing Compounds. J. Phys. Chem. Ref. Data 2013, 42, 033102. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Verevkin, S.P.; Heintz, A. The Gaseous Enthalpy of Formation of the Ionic Liquid 1-Butyl-3-Methylimidazolium Dicyanamide from Combustion Calorimetry, Vapor Pressure Measurements, and Ab Initio Calculations. J. Am. Chem. Soc. 2007, 129, 3930–3937. [Google Scholar] [CrossRef] [PubMed]
- Verevkin, S.P.; Emel’yanenko, V.N. Transpiration Method: Vapor Pressures and Enthalpies of Vaporization of Some Low-Boiling Esters. Fluid Phase Equilib. 2008, 266, 64–75. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Zaitsau, D.H.; Shoifet, E.; Meurer, F.; Verevkin, S.P.; Schick, C.; Held, C. Benchmark Thermochemistry for Biologically Relevant Adenine and Cytosine. A Combined Experimental and Theoretical Study. J. Phys. Chem. A 2015, 119, 9680–9691. [Google Scholar] [CrossRef] [PubMed]
- Pracht, P.; Bohle, F.; Grimme, S. Automated Exploration of the Low-Energy Chemical Space with Fast Quantum Chemical Methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef] [PubMed]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A Complete Basis Set Model Chemistry. I. The Total Energies of Closed-shell Atoms and Hydrides of the First-row Elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Curtiss, L.A.; Raghavachari, K.; Redfern, P.C.; Pople, J.A. Gaussian-3 Theory Using Scaled Energies. J. Chem. Phys. 2000, 112, 1125–1132. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N.; Notario, R.; Roux, M.V.; Chickos, J.S.; Liebman, J.F. Rediscovering the Wheel. Thermochemical Analysis of Energetics of the Aromatic Diazines. J. Phys. Chem. Lett. 2012, 3, 3454–3459. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, G. Assignment of Uncertainties. In Combustion Calorimetry: Experimental Chemical Thermodynamics; Sunner, S., Månsson, M., Eds.; Pergamon: New York, NY, USA, 1979; pp. 137–161. [Google Scholar]
- Cox, J.D.; Wagman, D.D.; Medvedev, V.A. CODATA Key Values for Thermodynamics: Final Report of the CODATA Task Group on Key Values for Thermodynamics; CODATA Series on Thermodynamic; Hemisphere Publishing Corp.: New York, NY, USA, 1989. [Google Scholar]
- Hubbard, W.N.; Scott, D.W.; Waddington, G. Standard States and Corrections for Combustions in a Bomb at Constant Volume. In Experimental Thermochemistry; Rossini, F.D., Ed.; Interscience Publishers: New York, NY, USA, 1956; pp. 75–128. [Google Scholar]
- Stull, D.R. Vapor Pressure of Pure Substances. Organic and Inorganic Compounds. Ind. Eng. Chem. 1947, 39, 517–540. [Google Scholar] [CrossRef]
- Stephenson, R.M.; Malanowski, S. Handbook of the Thermodynamics of Organic Compounds; Springer: Dordrecht, The Netherlands, 1987; ISBN 978-94-010-7923-5. [Google Scholar]
- Barontini, F.; Cozzani, V. Thermogravimetry as a Screening Tool for the Estimation of the Vapor Pressures of Pure Compounds. J. Therm. Anal. Calorim. 2007, 89, 309–314. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Zaitsau, D.H.; Pimerzin, A.A.; Verevkin, S.P. Benchmark Properties of Diphenyl Oxide as a Potential Liquid Organic Hydrogen Carrier: Evaluation of Thermochemical Data with Complementary Experimental and Computational Methods. J. Chem. Thermodyn. 2018, 125, 149–158. [Google Scholar] [CrossRef]
- Osborn, A.G.; Scott, D.W. Vapor Pressures of 17 Miscellaneous Organic Compounds. J. Chem. Thermodyn. 1980, 12, 429–438. [Google Scholar] [CrossRef]
- Bolmatenkov, D.N.; Yagofarov, M.I.; Notfullin, A.A.; Solomonov, B.N. Calculation of the Vaporization Enthalpies of Alkylaromatic Hydrocarbons as a Function of Temperature from Their Molecular Structure. Fluid Phase Equilib. 2022, 554, 113303. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Samarov, A.A.; Vostrikov, S. V Thermodynamics of Reversible Hydrogen Storage: Does Alkoxy-Substitution of Naphthalene Yield Functional Advantages for LOHC Systems? J. Chem. Phys. 2024, 160, 154709. [Google Scholar] [CrossRef] [PubMed]
- Samarov, A.A.; Verevkin, S.P. Hydrogen Storage Technologies: Methyl-Substituted Biphenyls as an Auspicious Alternative to Conventional Liquid Organic Hydrogen Carriers (LOHC). J. Chem. Thermodyn. 2022, 165, 106648. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Samarov, A.A.; Vostrikov, S.V.; Wasserscheid, P.; Müller, K. Comprehensive Thermodynamic Study of Alkyl-Cyclohexanes as Liquid Organic Hydrogen Carriers Motifs. Hydrogen 2023, 4, 42–59. [Google Scholar] [CrossRef]
- Benson, S.W. Thermochemical Kinetics, 2nd ed.; John Wiley & Sons: New York, NY, USA; London, UK; Sydney, Australia; Toronto, ON, Canada, 1976; pp. 1–320. [Google Scholar]
- Verevkin, S.P.; Emel’yanenko, V.N.; Pimerzin, A.A.; Vishnevskaya, E.E. Thermodynamic Analysis of Strain in the Five-Membered Oxygen and Nitrogen Heterocyclic Compounds. J. Phys. Chem. A 2011, 115, 1992–2004. [Google Scholar] [CrossRef]
- Steele, W.V.; Chirico, R.D.; Knipmeyer, S.E.; Nguyen, A.; Smith, N.K.; Tasker, I.R. Thermodynamic Properties and Ideal-Gas Enthalpies of Formation for Cyclohexene, Phthalan (2,5-Dihydrobenzo-3,4-Furan), Isoxazole, Octylamine, Dioctylamine, Trioctylamine, Phenyl Isocyanate, and 1,4,5,6-Tetrahydropyrimidine. J. Chem. Eng. Data 1996, 41, 1269–1284. [Google Scholar] [CrossRef]
- Chirico, R.; Archer, D.; Hossenlopp, I.; Nguyen, A.; Steele, W.; Gammon, B. The Thermodynamic Properties of Chroman and Isochroman. J. Chem. Thermodyn. 1990, 22, 665–682. [Google Scholar] [CrossRef]
- Wheeler, S.E.; Houk, K.N.; Schleyer, P.v.R.; Allen, W.D. A Hierarchy of Homodesmotic Reactions for Thermochemistry. J. Am. Chem. Soc. 2009, 131, 2547–2560. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Vostrikov, S.V.; Leinweber, A.; Wasserscheid, P.; Müller, K. Thermochemical Properties of Benzyltoluenes and Their Hydro- and Perhydro-Derivatives as Key Components of a Liquid Organic Hydrogen Carrier System. Fuel 2023, 335, 126618. [Google Scholar] [CrossRef]
- Roux, M.V.; Temprado, M.; Chickos, J.S.; Nagano, Y. Critically Evaluated Thermochemical Properties of Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. Ref. Data 2008, 37, 1855–1996. [Google Scholar] [CrossRef]
- Pedley, J.B.; Naylor, R.D.; Kirby, S.P. Thermochemical Data of Organic Compounds; Chapman and Hall: New York, NY, USA, 1986; pp. 1–792. [Google Scholar]
- Oliver, G.D.; Eaton, M.; Huffman, H.M. The Heat Capacity, Heat of Fusion and Entropy of Benzene 1. J. Am. Chem. Soc. 1948, 70, 1502–1505. [Google Scholar] [CrossRef]
- Ambrose, D.; Ellender, J.; Sprake, C.H.; Townsend, R. Thermodynamic Properties of Organic Oxygen Compounds XLIII. Vapour Pressures of Some Ethers. J. Chem. Thermodyn. 1976, 8, 165–178. [Google Scholar] [CrossRef]
- Chirico, R.D.; Steele, W.V. Thermodynamic Properties of Diphenylmethane. J. Chem. Eng. Data 2005, 50, 1052–1059. [Google Scholar] [CrossRef]
- Chirico, R.D.; Knipmeyer, S.E.; Nguyen, A.; Steele, W.V. The Thermodynamic Properties to the Temperature 700 K of Naphthalene and of 2,7-Dimethylnaphthalene. J. Chem. Thermodyn. 1993, 25, 1461–1494. [Google Scholar] [CrossRef]
- Ruehrwein, R.A.; Huffman, H.M. Thermal Data. XVII. The Heat Capacity, Entropy, and Free Energy of Formation of Cyclohexane. A New Method of Heat Transfer in Low Temperature Calorimetry. J. Am. Chem. Soc. 1943, 65, 1620–1625. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Samarov, A.A.; Vostrikov, S.V. Thermodynamics of Reversible Hydrogen Storage: Are Methoxy-Substituted Biphenyls Better through Oxygen Functionality? Hydrogen 2023, 4, 862–880. [Google Scholar] [CrossRef]
- McCullough, J.P.; Finke, H.L.; Messerly, J.F.; Todd, S.S.; Kincheloe, T.C.; Waddington, G. The Low-Temperature Thermodynamic Properties of Naphthalene, l-Methylnaphthalene, 2-Methylnaphthalene, 1,2,3,4-Tetrahydronaphthalene,Trans-Decahydronaphthalene and Cis-Decahydronaphthalene. J. Phys. Chem. 1957, 61, 1105–1116. [Google Scholar] [CrossRef]

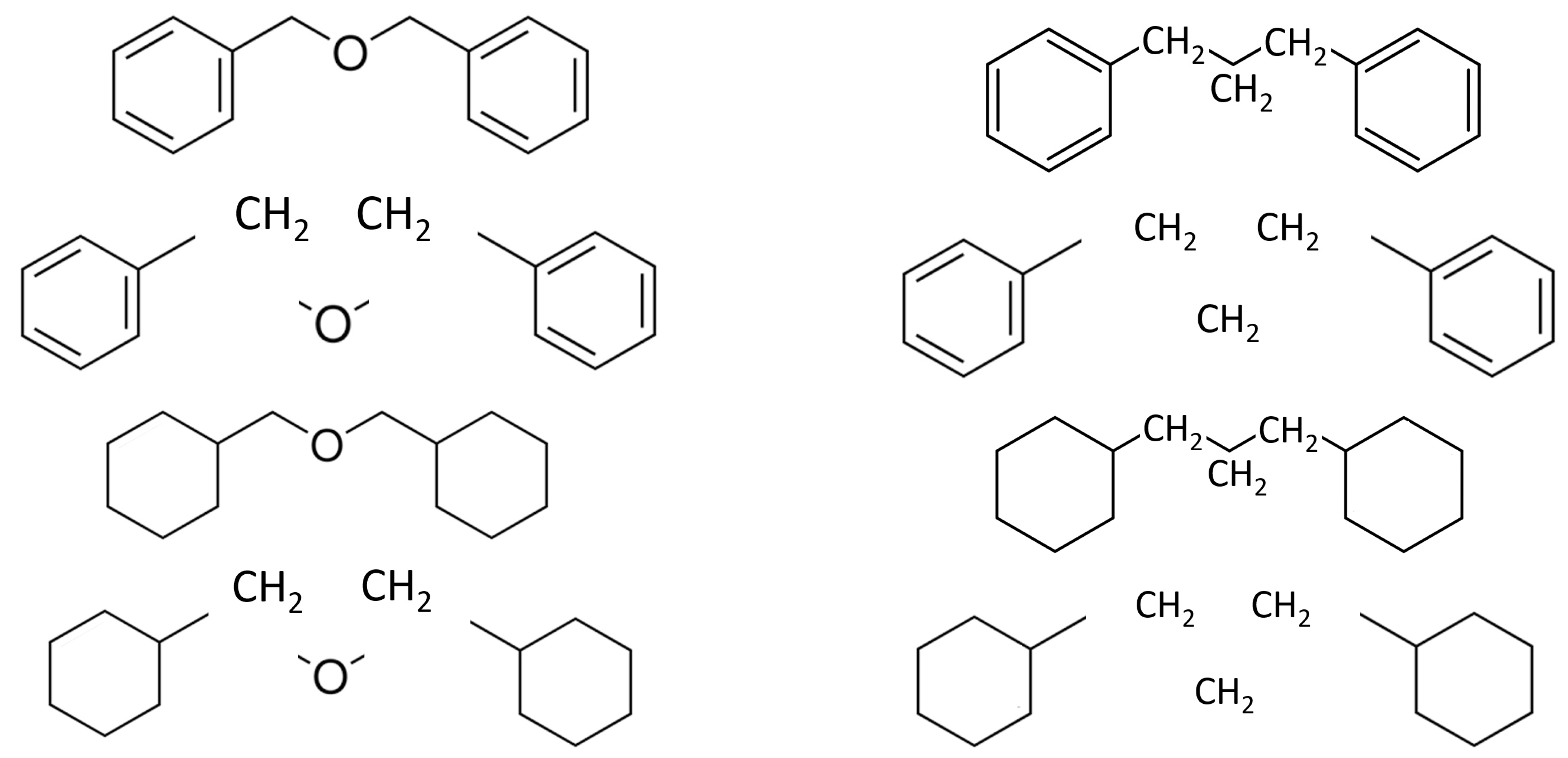
| Compound | (cr/liq) | (cr/liq) | (cr/liq)exp |
|---|---|---|---|
| J·g−1 | kJ·mol−1 | kJ·mol−1 | |
| benzyl phenyl ether (cr) | −36,691.3 ± 5.1 | −6765.9 ± 2.4 | −64.7 ± 3.0 |
| dibenzyl ether (liq) | −37,589.6 ± 3.8 | −7459.9 ± 2.3 | −50.0 ± 2.9 |
| Compounds | Method a | T-Range/ K | Tav | 298.15 K b | |
|---|---|---|---|---|---|
| benzyl phenyl ether | n/a | 368.6–560.2 | 59.2 ± 1.0 | 71.3 ± 2.6 | [26] |
| 946-80-5 | T | 318.3–363.2 | 70.6 ± 0.3 | 73.8 ± 0.4 | Table S3 |
| BP | 358–561 | 62.8 ± 0.6 | 74.7 ± 2.4 | Table S4 | |
| 73.7 ± 0.5 c | average | ||||
| GA | 298.15 | 75.8 ± 1.5 | this work | ||
| dibenzyl ether | n/a | 275–417 | 50.1 ± 1.0 | (54.2 ± 1.3) | [27] |
| 103-50-4 | n/a | 413–561 | 57.4 ± 1.0 | 73.8 ± 3.4 | [27] |
| TGA | 392–425 | 69.4 ± 1.0 | 78.9 ± 2.1 | [28] | |
| T | 309.4 ± 358.1 | 75.6 ± 0.4 | 78.6 ± 0.6 | Table S3 | |
| BP | 365–571 | 67.0 ± 0.4 | 79.5 ± 2.5 | Table S4 | |
| 78.5 ± 0.5 | average | ||||
| GA | 298.15 | 78.2 ± 1.5 | this work | ||
| dicyclohexyl ether | GA | 68.3 ± 1.5 | [29] | ||
| 4645-15-2 | BP | 348–517 | 57.4 ± 1.1 | 68.1 ± 2.4 | Table S4 |
| 68.2 ± 1.3 c | average | ||||
| 1,2-diphenylethane | IP | 333.2–413.2 | 64.0 ± 0.1 | 69.2 ± 1.0 | [30] |
| 103-29-7 | BP | 338–558 | 59.6 ± 0.5 | 68.8 ± 1.9 | Table S4 |
| 69.1 ± 0.9 c | average | ||||
| GA | 298.15 | 71.2 ± 1.5 | this work | ||
| 1,2-dicyclohexylethane | BP | 348–547 | 56.7 ± 0.8 | 68.2 ± 2.4 | Table S4 |
| 3321-50-4 | GA | 298.15 | 67.2 ± 1.5 | this work | |
| Tb | 298.15 | 67.9 ± 2.0 | Equation (6) | ||
| 67.6 ± 1.1 c | average | ||||
| cyclohexylmethoxy–cyclohexane | GA | 298.15 | 69.7 ± 1.5 | this work | |
| 118161-77-6 | |||||
| 1,3-diphenylpropane | n/a | 342–577 | 60.0 ± 1.0 | 71.4 ± 2.5 | [27] |
| 1081-75-0 | SC | 298.15 | 74.2 ± 2.0 | [31] | |
| BP | 342–576 | 63.4 ± 0.7 | 73.8 ± 2.2 | Table S4 | |
| 73.3 ± 1.3 | average | ||||
| GA | 298.15 | 75.7 ± 1.5 | this work | ||
| 1,3-dicyclohexylpropane | BP | 382–565 | 58.1 ± 0.5 | 72.9 ± 3.0 | Table S4 |
| 3178-24-3 | GA | 298.15 | 71.8 ± 1.5 | this work | |
| Tb | 298.15 | 71.3 ± 2.0 | Equation (6) | ||
| 71.8 ± 1.1 c | average | ||||
| dicyclohexylmethyl ether | BP | 356–574 | 59.2 ± 2.1 | 73.9 ± 3.6 | Table S4 |
| 14315-63-0 | GA | 298.15 | 72.8 ± 1.5 | this work | |
| 73.0 ± 1.4 c | average | ||||
| 2-methoxy-naphthalene | BP | 365–549 | 59.8 ± 1.1 | 68.9 ± 2.1 | [32] |
| 93-04-9 | T | 348.2–378.2 | 65.4 ± 0.8 | 69.1 ± 0.9 | [32] |
| T | 348.7–372.4 | 66.1 ± 0.5 | 69.6 ± 0.6 | Table S3 | |
| PhT | 69.2 ± 0.8 | Table S5 | |||
| 69.4 ± 0.4 c | average | ||||
| 2-methoxy-decalin (liq) | GA | 58.4 ± 1.0 | [32] | ||
| 55473-38-6 | Tb | 58.8 ± 1.0 | [32] | ||
| BP | 349–509 | 49.6 ± 1.7 | 58.7 ± 2.5 | Table S4 | |
| 58.6 ± 0.7 c | average |
| CAS | Compound | (g, AT)G3MP2 a | (g)exp b | (g)QC c | Δ d |
|---|---|---|---|---|---|
| 496-16-2 | dihydrobenzofuran | −57.5 | −47.0 ± 1.4 [36] | −46.3 | −0.7 |
| 496-14-0 | dihydroisobenzofuran | −36.9 | −30.1 ± 1.0 [37] | −25.3 | −4.8 |
| 493-05-0 | isochromane | −78.2 | −63.1 ± 1.1 [38] | −67.4 | 4.3 |
| 2216-69-5 | 1-methoxynaphthalene | −19.0 | −6.7 ± 1.8 [32] | −7.1 | 0.4 |
| 93-04-9 | 2-methoxynaphthalene | −20.3 | −6.5 ± 1.4 [32] | −8.4 | 1.9 |
| 493-08-3 | dihydrobenzopyran | −96.2 | −82.4 ± 1.2 [38] | −85.8 | 3.4 |
| 21720-89-8 | 1-methoxydecalin | −333.6 | −328.7 ± 3.5 [32] | −327.8 | −0.9 |
| 55473-38-6 | 2-methoxydecalin | −334.6 | −329.5 ± 3.5 [32] | −328.8 | −0.7 |
| 101-84-8 | diphenyl ether | 38.6 | 50.9 ± 1.4 [29] | 51.6 | −0.7 |
| 4645-15-2 | dicyclohexyl ether | −361.4 | - | −356.1 | |
| 946-80-5 | phenyl benzyl ether | 20.7 | 30.6 ± 3.1 [Table 4] | 33.4 | −2.8 |
| 118161-77-6 | cyclohexylmethoxy–cyclohexane | −377.0 | - | −372.0 | |
| 103-50-4 | dibenzyl ether | 15.0 | 28.5 ± 3.0 [Table 4] | 27.6 | 0.9 |
| 14315-63-0 | dicyclohexylmethyl ether | −391.5 | - | −386.8 |
| Compound | (cr/liq)exp b | c | d | (g)exp e | (g)QC f |
|---|---|---|---|---|---|
| benzyl phenyl ether (cr) | −64.7 ± 3.0 | 73.7 ± 0.5 | 21.6 ± 0.4 | 30.6 ± 3.1 | 33.4 ± 4.1 |
| dibenzyl ether (liq) | −50.0 ± 2.9 | 78.5 ± 0.5 | 28.5 ± 3.0 | 27.6 ± 4.1 | |
| 2-methoxy-naphthalene (cr) | −96.6 ± 1.3 [32] | 90.1 ± 0.5 [32] | −6.5 ± 1.4 | −8.4 ± 4.1 |
| Compound; CAS | (g) b | c | (liq) d |
|---|---|---|---|
| diphenyl–methane; 101-81-5 | 164.7 ± 0.9 [40] | 68.1 ± 0.3 [40] | 96.6 ± 0.8 [40] |
| dicyclohexyl–methane; 3178-23-2 | −242.4 ± 2.0 [34] | 64.7 ± 1.0 [34] | −307.1 ± 2.2 [34] |
| diphenyl ether, 101-84-8 | 50.9 ± 1.4 [29] | 66.7 ± 0.2 [29] | −15.8 ± 1.4 [29] |
| dicyclohexyl ether; 4645-15-2 | −356.1 ± 4.1 [Table 3] | 68.2 ± 1.3 | −424.3 ± 4.3 |
| 1,2-diphenyl-ethane; 103-29-7 | 133.7 ± 4.1 [Table S10] | 69.1 ± 0.9 | 64.6 ± 4.2 |
| 1,2-dicyclohexyl-ethane; 3321-50-4 | −261.0 ± 4.1 [Table S11] | 67.6 ± 1.1 | −328.6 ± 4.2 |
| benzyl phenyl ether; 946-80-5 | 30.6 ± 3.1 [Table 4] | 73.7 ± 0.5 | −43.1 ± 3.1 |
| cyclohexylmethoxy–cyclohexane; 118161-77-6 | −372.0 ± 4.1 [Table 3] | 69.7 ± 1.5 | −441.7 ± 4.4 |
| 1,3-diphenyl-propane; 1081-75-0 | 114.7 ± 4.1 [Table S10] | 73.3 ± 1.3 | 41.4 ± 4.3 |
| 1,3-dicyclohexyl-propane; 3178-24-3 | −281.6 ± 4.1 [Table S11] | 71.8 ± 1.1 | −353.4 ± 4.2 |
| dibenzyl ether; 103-50-4 | −50.0 ± 2.9 [Table 4] | 78.5 ± 0.5 | −128.5 ± 2.9 |
| dicyclohexylmethyl ether; 14315-63-0 | −386.8 ± 4.1 [Table 3] | 73.0 ± 1.4 | −459.8 ± 4.3 |
| naphthalene; 91-20-3 | 150.6 ± 1.5 [41] | 55.4 ± 1.4 [41] | 94.9 ± 1.6 [41] |
| trans-decalin; 493-02-7 | −182.0 ± 1.0 [42] | 48.6 ± 0.2 [42] | −230.6 ± 1.0 [42] |
| 2-methoxy-naphthalene; 93-04-9 | −6.5 ± 1.4 [32] | 69.4 ± 0.4 | −75.9 ± 1.5 |
| trans-2-methoxy-decalin; 55473-38-6 | −329.5 ± 3.6 [32] | 58.6 ± 0.7 | −388.1 ± 3.6 |
| Compound | (liq)HL a | (liq)HR b | (liq) c | (liq)/H2 d |
|---|---|---|---|---|
| benzene | 49.0 ± 0.9 [Table S12] | −156.4 ± 0.8 [Table S12] | 205.4 | 68.5 |
| methoxy–benzene | −116.9 ± 0.7 [Table S12] | −313.7 ± 3.5 [Table S12] | 196.8 | 65.6 |
| diphenyl–methane | 96.6 ± 0.8 | −307.1 ± 2.2 | 403.7 | 67.3 |
| diphenyl ether | −15.8 ± 1.4 | −424.3 ± 4.3 | 408.5 | 68.1 |
| 1,2-diphenyl-ethane | 64.6 ± 4.2 | −328.6 ± 4.2 | 393.2 | 65.5 |
| benzyl phenyl ether | −43.1 ± 3.1 | −441.7 ± 4.4 | 398.6 | 66.4 |
| 1,3-diphenyl-propane | 41.4 ± 4.3 | −353.4 ± 4.2 | 394.8 | 65.8 |
| dibenzyl ether | −50.0 ± 2.9 | −459.8 ± 4.3 | 409.8 | 68.3 |
| naphthalene | 94.9 ± 1.6 | −230.6 ± 1.0 | 325.5 | 65.1 |
| 2-methoxy-naphthalene | −75.9 ± 1.5 | −388.1 ± 3.6 | 312.2 | 62.4 |
| Compound | a | (g) b | (liq) c |
|---|---|---|---|
| benzene | 173.3 [43] | ||
| methoxy–benzene | 111.8 ± 0.1 [44] | 346.6 | 234.8 |
| diphenyl–methane | 140.5± 0.5 [45] | 440.3 | 299.8 |
| diphenyl ether | 134.5± 4.3 [29] | 439.8 | 305.3 |
| 1,2-diphenyl-ethane | 134.9 ± 1.8 | 466.4 | 331.5 |
| benzyl-phenyl ether | 142.2 ± 1.1 | 456.9 | 314.7 |
| 1,3-diphenyl-propane | 140.9 ± 2.5 | 498.9 | 358.0 |
| dibenzyl ether | 151.8 ± 1.3 | 502.4 | 350.6 |
| naphthalene | 217.6 [46] | ||
| 2-methoxy-naphthalene | 137.9 ± 1.6 | 401.5 | 263.6 |
| cyclohexane | 204.4 [47] | ||
| methoxy–cyclohexane | 106.7 ± 3.2 [48] | 376.8 | 270.1 |
| dicyclohexyl-methane | 136.3 ± 2.8 [34] | 470.5 | 334.2 |
| 1,2-dicyclohexyl-ethane | 137.1 ± 2.6 | 510.5 | 373.4 |
| 1,3-dicayclohexyl-propane | 144.1 ± 1.7 | 544.4 | 400.3 |
| dicyclohexyl ether | 142.9 ± 3.9 | 475.1 | 332.2 |
| cyclohexylmethoxy–cyclohexane | 145.0 ± 5.0 d | 511.1 | 366.1 |
| dicyclohexylmethyl ether | 147.9 ± 7.1 | 539.2 | 391.3 |
| trans-decalin | 264.9 [49] | ||
| 2-methoxy-decalin | 123.8 ± 5.7 | 446.3 | 322.5 |
| Reactants | a (298 K) | b (298 K) | (298 K) | c (298 K) | d (400 K) | e (500 K) |
|---|---|---|---|---|---|---|
| kJ·mol−1 | J·mol−1·K−1 | kJ·mol−1 | kJ·mol−1 | kJ·mol−1 | kJ·mol−1 | |
| benzene | 205.4 | 360.9 | 107.6 | 97.8 | 60.1 | 21.1 |
| cyclohexane | 68.5 | 120.3 | 35.9 | 32.6 | 20.0 | 7.0 |
| methoxy–benzene | 197.7 | 356.7 | 106.4 | 91.3 | 23.9 | 14.6 |
| methoxy–cyclohexane | 65.9 | 118.9 | 35.5 | 30.4 | 18.0 | 4.9 |
| diphenyl–methane | 403.7 | 749.7 | 223.5 | 180.2 | 102.2 | 22.1 |
| dicyclohexyl–methane | 67.3 | 125.0 | 37.3 | 30.0 | 17.0 | 3.7 |
| diphenyl–ether | 408.5 | 757.2 | 225.8 | 182.7 | 103.8 | 22.5 |
| dicyclohexyl ether | 68.1 | 126.2 | 37.6 | 30.5 | 17.3 | 3.8 |
| 1,2-diphenyl-ethane | 393.2 | 742.2 | 221.3 | 171.9 | 94.5 | 14.5 |
| 1,2-dicyclohexyl-ethane | 65.5 | 123.7 | 36.9 | 28.7 | 15.7 | 2.4 |
| benzyl phenyl ether | 398.6 | 732.7 | 218.5 | 180.2 | 103.6 | 24.3 |
| Cyclohexylmethoxy–cyclohexane | 66.4 | 122.1 | 36.4 | 30.0 | 17.3 | 4.1 |
| 1,3-diphenyl-propane | 394.8 | 741.8 | 221.2 | 173.6 | 96.2 | 16.3 |
| 1,3-dicyclohexyl-propane | 65.8 | 123.6 | 36.9 | 28.9 | 16.0 | 2.7 |
| dibenzyl ether | 409.8 | 743.4 | 221.6 | 188.2 | 110.5 | 30.2 |
| dicyclohexylmethyl ether | 68.3 | 123.9 | 36.9 | 31.4 | 18.4 | 5.0 |
| naphthalene | 325.5 | 606.1 | 180.7 | 144.8 | 81.4 | 15.7 |
| trans-decalin | 65.1 | 121.2 | 36.1 | 29.0 | 16.3 | 3.1 |
| 2-methoxy-naphthalene | 312.2 | 594.5 | 177.3 | 134.9 | 72.8 | 8.2 |
| trans-2-methoxy-decalin | 62.4 | 118.9 | 35.5 | 27.0 | 14.6 | 1.6 |
| LOHC System | (298 K) | (400 K) | (500 K) | Ka (298 K) | Ka (400 K) | Ka (500 K) |
|---|---|---|---|---|---|---|
| cyclohexane | 97.8 | 60.1 | 21.1 | 7.2 × 10−18 | 1.4 × 10−8 | 6.2 × 10−3 |
| methoxy–cyclohexane | 91.3 | 23.9 | 14.6 | 9.9 × 10−17 | 7.6 × 10−4 | 3.0 × 10−2 |
| dicyclohexyl–methane | 180.2 | 102.2 | 22.1 | 2.6 × 10−32 | 4.5 × 10−14 | 4.9 × 10−3 |
| dicyclohexyl ether | 182.7 | 103.8 | 22.5 | 9.4 × 10−33 | 2.8 × 10−14 | 4.5 × 10−3 |
| 1,2-dicyclohexyl-ethane | 171.9 | 94.5 | 14.5 | 7.4 × 10−31 | 4.6 × 10−13 | 3.1 × 10−2 |
| Cyclohexylmethoxy–cyclohexane | 180.2 | 103.6 | 24.3 | 2.6 × 10−32 | 3.0 × 10−14 | 2.9 × 10−3 |
| 1,3-dicyclohexyl-propane | 173.6 | 96.2 | 16.3 | 3.7 × 10−31 | 2.7 × 10−13 | 2.0 × 10−2 |
| dicyclohexylmethyl ether | 188.2 | 110.5 | 30.2 | 1.0 × 10−33 | 3.7 × 10−15 | 7.0 × 10−4 |
| decalin | 144.8 | 81.4 | 15.7 | 4.1 × 10−26 | 2.3 × 10−11 | 2.3 × 10−2 |
| 2-methoxy-decalin | 134.9 | 72.8 | 8.2 | 2.3 × 10−24 | 3.1 × 10−10 | 1.4 × 10−1 |
| Reactants | a | b | c |
|---|---|---|---|
| kJ·mol−1 | J·mol−1·K−1 | K | |
| cyclohexane = benzene + 3 × H2 | 205.4 | 360.9 | 569 |
| methoxy–cyclohexane = methoxy–benzene + 3 × H2 | 197.7 | 356.7 | 554 |
| Dicyclohexyl–methane = diphenyl–methane + 6 × H2 | 403.7 | 749.7 | 538 |
| dicyclohexyl ether = diphenyl ether + 6 × H2 | 408.5 | 757.2 | 539 |
| 1,2-dicyclohexyl-ethane = 1,2-diphenyl-ethane + 6 × H2 | 393.2 | 742.2 | 530 |
| cyclohexylmethoxy–cyclohexane = benzyl phenyl ether + 6 × H2 | 398.6 | 732.7 | 544 |
| 1,3-dicyclohexyl-propane = 1,3-diphenyl-propane + 6 × H2 | 394.8 | 741.8 | 532 |
| dibenzyl ether + 6 × H2 = dicyclohexylmethyl ether | 409.8 | 743.4 | 551 |
| trans-decalin = naphthalene + 5 × H2 | 325.5 | 606.1 | 537 |
| trans-2-methoxy-decalin = 2-methoxy-naphthalene + 5 × H2 | 312.2 | 594.5 | 525 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verevkin, S.P.; Samarov, A.A.; Vostrikov, S.V. Does the Oxygen Functionality Really Improve the Thermodynamics of Reversible Hydrogen Storage with Liquid Organic Hydrogen Carriers? Oxygen 2024, 4, 266-284. https://doi.org/10.3390/oxygen4030015
Verevkin SP, Samarov AA, Vostrikov SV. Does the Oxygen Functionality Really Improve the Thermodynamics of Reversible Hydrogen Storage with Liquid Organic Hydrogen Carriers? Oxygen. 2024; 4(3):266-284. https://doi.org/10.3390/oxygen4030015
Chicago/Turabian StyleVerevkin, Sergey P., Artemiy A. Samarov, and Sergey V. Vostrikov. 2024. "Does the Oxygen Functionality Really Improve the Thermodynamics of Reversible Hydrogen Storage with Liquid Organic Hydrogen Carriers?" Oxygen 4, no. 3: 266-284. https://doi.org/10.3390/oxygen4030015





