Chasing Uterine Cancer with NK Cell-Based Immunotherapies
Abstract
:1. Introduction
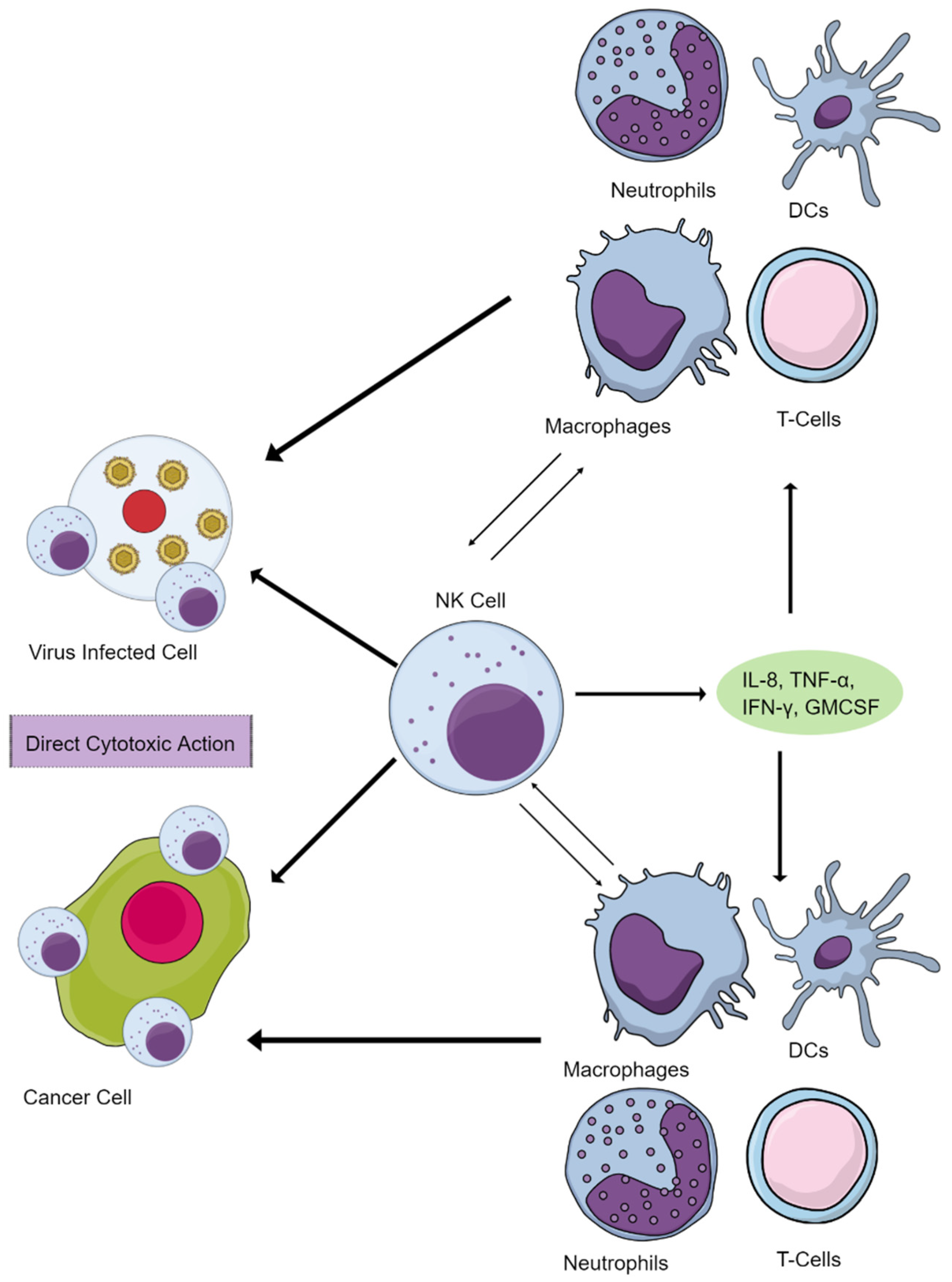
2. NK Cells Maintain Immune Homeostasis and Function
3. Human Uterine NK (uNK) Cells
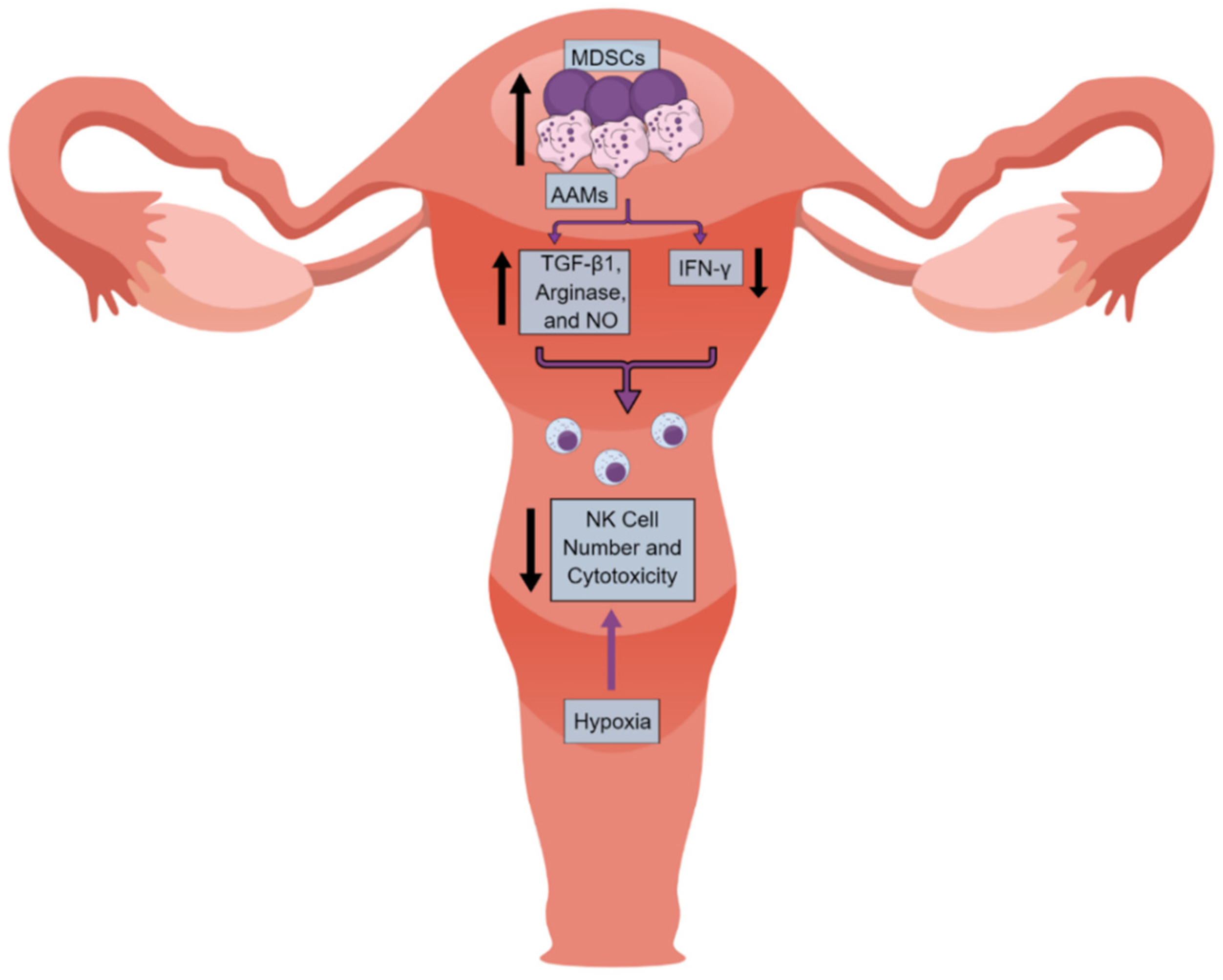
4. NK Cells in the UC Immune Microenvironment (UCIM)
5. NK Cell-Based Therapies in Different Cancers
6. Future Perspective and Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAMs | Alternatively activated macrophages |
| ADCC | Antibody-dependent cellular cytotoxicity |
| ANKETs | Antibody-based natural killer cell engager therapeutics |
| APCs | Antigen presenting cells |
| CAR | Chimeric antigen receptor |
| cDCs | Conventional dendritic cells |
| CIML NK cells | Cytokine-induced memory-like NK cells |
| CIN | Cervical intraepithelial neoplasia |
| CLPs | Common lymphoid progenitors |
| DAMPs | Damage/death-associated molecular patterns |
| DCs | Dendritic cells |
| EC | Endometrial Cancer |
| ECIM | Endometrial cancer immune microenvironment |
| EVs | Extracellular vesicles |
| FRT | Female reproductive tract |
| GM-CSF | granulocyte-monocyte colony stimulating factor |
| GVHD | Graft-versus-host disease |
| haNK | High affinity NK |
| HLA | Human Leukocyte Antigen |
| IDO | Indoleamine 2,3-dioxygenase |
| ILCs | Innate lymphoid cells |
| LILRB1 | Leukocyte immunoglobulin-like receptor B1 |
| MDSCs | Myeloid-derived suppressor cells |
| NCT | Normal ectocervical tissue |
| NKCC | Natural Killer cell cytotoxicity |
| PD-1 | Programmed death-1 |
| PSCs | Pluripotent stem cells |
| PTdNKs | Pregnancy trained decidual or uterine NK cells |
| RAGs | Recombination activating genes |
| TIME | Tumor Immune Microenvironment |
| TME | Tumor microenvironment |
| UC | Uterine Cancer |
| UCIM | Endometrial cancer immune microenvironment |
| uNK | Uterine NK |
| VLPs | Virus-like particles |
References
- Centers for Disease Control and Prevention. Gynecologic Cancer Incidence, United States—2012–2016; Centers for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2019; Volume 2022.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Pruemer, J.M.; Hincapie, A.L.; Almalki, Z.S.; Guo, J.J. Economic burden and treatment patterns of gynecologic cancers in the United States: Evidence from the Medical Expenditure Panel Survey 2007–2014. J. Gynecol. Oncol. 2020, 31, e52. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar]
- Kumar, V. Inflammation research sails through the sea of immunology to reach immunometabolism. Int. Immunopharmacol. 2019, 73, 128–145. [Google Scholar] [CrossRef]
- Swann, J.B.; Smyth, M.J. Immune surveillance of tumors. J. Clin. Investig. 2007, 117, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Fridman, W.H. From Cancer Immune Surveillance to Cancer Immunoediting: Birth of Modern Immuno-Oncology. J. Immunol. 2018, 201, 825–826. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.; Emi, M.; Tanabe, K. Cancer immunoediting from immune surveillance to immune escape. Immunology 2007, 121, 1–14. [Google Scholar] [CrossRef]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef]
- Darcy, P.K.; Neeson, P.; Yong, C.S.M.; Kershaw, M.H. Manipulating immune cells for adoptive immunotherapy of cancer. Curr. Opin. Immunol. 2014, 27, 46–52. [Google Scholar] [CrossRef]
- Sun, H.; Sun, C.; Tian, Z.; Xiao, W. NK cells in immunotolerant organs. Cell. Mol. Immunol. 2013, 10, 202–212. [Google Scholar] [CrossRef] [Green Version]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Carotta, S. Targeting NK Cells for Anticancer Immunotherapy: Clinical and Preclinical Approaches. Front. Immunol. 2016, 7, 152. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Wu, S.Y.; Fu, T.; Jiang, Y.Z.; Shao, Z.M. Natural killer cells in cancer biology and therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef]
- Kim, K.W.; Jeong, J.U.; Lee, K.H.; Uong, T.N.T.; Rhee, J.H.; Ahn, S.J.; Kim, S.K.; Cho, D.; Quang Nguyen, H.P.; Pham, C.T.; et al. Combined NK Cell Therapy and Radiation Therapy Exhibit Long-Term Therapeutic and Antimetastatic Effects in a Human Triple Negative Breast Cancer Model. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 115–125. [Google Scholar] [CrossRef]
- Lamb, M.G.; Rangarajan, H.G.; Tullius, B.P.; Lee, D.A. Natural killer cell therapy for hematologic malignancies: Successes, challenges, and the future. Stem Cell Res. Ther. 2021, 12, 211. [Google Scholar] [CrossRef]
- Veluchamy, J.P.; Spanholtz, J.; Tordoir, M.; Thijssen, V.L.; Heideman, D.A.M.; Verheul, H.M.W.; de Gruijl, T.D.; van der Vliet, H.J. Combination of NK Cells and Cetuximab to Enhance Anti-Tumor Responses in RAS Mutant Metastatic Colorectal Cancer. PLoS ONE 2016, 11, e0157830. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, R.; Petranyi, G.; Kärre, K.; Jondal, M.; Tracey, D.; Wigzell, H. Killer cells: A functional comparison between natural, immune T-cell and antibody-dependent in vitro systems. J. Exp. Med. 1976, 143, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiessling, R.; Klein, E.; Pross, H.; Wigzell, H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur. J. Immunol. 1975, 5, 117–121. [Google Scholar] [CrossRef]
- Greenberg, A. The origins of the NK cell, or a Canadian in King Ivan’s court. Clin. Investig. Med. 1994, 17, 626–631. [Google Scholar]
- Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 1989, 47, 187–376. [Google Scholar]
- Biron, C.A. Activation and function of natural killer cell responses during viral infections. Curr. Opin. Immunol. 1997, 9, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Biron, C.A.; Nguyen, K.B.; Pien, G.C.; Cousens, L.P.; Salazar-Mather, T.P. Natural killer cells in antiviral defense: Function and regulation by innate cytokines. Annu. Rev. Immunol. 1999, 17, 189–220. [Google Scholar] [CrossRef] [PubMed]
- Fogler, W.E.; Volker, K.; McCormick, K.L.; Watanabe, M.; Ortaldo, J.R.; Wiltrout, R.H. NK cell infiltration into lung, liver, and subcutaneous B16 melanoma is mediated by VCAM-1/VLA-4 interaction. J. Immunol. 1996, 156, 4707–4714. [Google Scholar]
- Castriconi, R.; Carrega, P.; Dondero, A.; Bellora, F.; Casu, B.; Regis, S.; Ferlazzo, G.; Bottino, C. Molecular Mechanisms Directing Migration and Retention of Natural Killer Cells in Human Tissues. Front. Immunol. 2018, 9, 2324. [Google Scholar] [CrossRef]
- Gismondi, A.; Santoni, A. Migration of NK cells. In Lymphocyte Trafficking in Health and Disease; Badolato, R., Sozzani, S., Eds.; Birkhäuser Basel: Basel, Switzerland, 2006; pp. 95–112. [Google Scholar]
- Ran, G.H.; Lin, Y.Q.; Tian, L.; Zhang, T.; Yan, D.M.; Yu, J.H.; Deng, Y.C. Natural killer cell homing and trafficking in tissues and tumors: From biology to application. Signal Transduct. Target. Ther. 2022, 7, 205. [Google Scholar] [CrossRef]
- Shannon, M.J.; Mace, E.M. Natural Killer Cell Integrins and Their Functions in Tissue Residency. Front. Immunol. 2021, 12, 647358. [Google Scholar] [CrossRef] [PubMed]
- Cursons, J.; Souza-Fonseca-Guimaraes, F.; Foroutan, M.; Anderson, A.; Hollande, F.; Hediyeh-Zadeh, S.; Behren, A.; Huntington, N.D.; Davis, M.J. A Gene Signature Predicting Natural Killer Cell Infiltration and Improved Survival in Melanoma Patients. Cancer Immunol. Res. 2019, 7, 1162–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maghazachi, A.A. Role of chemokines in the biology of natural killer cells. Curr. Top. Microbiol. Immunol. 2010, 341, 37–58. [Google Scholar] [PubMed]
- Robertson, M.J. Role of chemokines in the biology of natural killer cells. J. Leukoc. Biol. 2002, 71, 173–183. [Google Scholar] [CrossRef]
- Lupo, K.; Matosevic, S. Chapter 16—Immunometabolic targeting of NK cells to solid tumors. In Successes and Challenges of NK Immunotherapy; Bonavida, B., Jewett, A., Eds.; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- van den Heuvel, M.J.; Chantakru, S.; Xuemei, X.; Evans, S.S.; Tekpetey, F.; Mote, P.A.; Clarke, C.L.; Croy, B.A. Trafficking of circulating pro-NK cells to the decidualizing uterus: Regulatory mechanisms in the mouse and human. Immunol. Investig. 2005, 34, 273–293. [Google Scholar] [CrossRef] [Green Version]
- Bernardini, G.; Antonangeli, F.; Bonanni, V.; Santoni, A. Dysregulation of Chemokine/Chemokine Receptor Axes and NK Cell Tissue Localization during Diseases. Front. Immunol. 2016, 7, 402. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Matosevic, S. Chemokine networks modulating natural killer cell trafficking to solid tumors. Cytokine Growth Factor Rev. 2021, 59, 36–45. [Google Scholar] [CrossRef]
- Fauriat, C.; Long, E.O.; Ljunggren, H.G.; Bryceson, Y.T. Regulation of human NK cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–2176. [Google Scholar] [CrossRef] [Green Version]
- Langers, I.; Renoux, V.M.; Thiry, M.; Delvenne, P.; Jacobs, N. Natural killer cells: Role in local tumor growth and metastasis. Biologics 2012, 6, 73–82. [Google Scholar]
- Mariani, E.; Pulsatelli, L.; Meneghetti, A.; Dolzani, P.; Mazzetti, I.; Neri, S.; Ravaglia, G.; Forti, P.; Facchini, A. Different IL-8 production by T and NK lymphocytes in elderly subjects. Mech. Ageing Dev. 2001, 122, 1383–1395. [Google Scholar] [CrossRef]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E.; et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef]
- Kumar, V. Innate lymphoid cells: New paradigm in immunology of inflammation. Immunol. Lett. 2014, 157, 23–37. [Google Scholar] [CrossRef]
- Kumar, V. Innate Lymphoid Cells: Immunoregulatory Cells of Mucosal Inflammation. Eur. J. Inflamm. 2014, 12, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V. Innate lymphoid cell and adaptive immune cell cross-talk: A talk meant not to forget. J. Leukoc. Biol. 2020, 108, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Innate Lymphoid Cells and Adaptive Immune Cells Cross-Talk: A Secret Talk Revealed in Immune Homeostasis and Different Inflammatory Conditions. Int. Rev. Immunol. 2021, 40, 217–251. [Google Scholar] [CrossRef]
- O’Sullivan, T.E. Dazed and Confused: NK Cells. Front. Immunol. 2019, 10, 2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V. Natural killer cells in sepsis: Underprivileged innate immune cells. Eur. J. Cell Biol. 2019, 98, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Geiger, T.L.; Sun, J.C. Development and maturation of natural killer cells. Curr. Opin. Immunol. 2016, 39, 82–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V. Chapter 8—Innate lymphoid cells in autoimmune diseases. In Translational Autoimmunity; Rezaei, N., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 1, pp. 143–175. [Google Scholar]
- Perera Molligoda Arachchige, A.S. Human NK cells: From development to effector functions. Innate Immun. 2021, 27, 212–229. [Google Scholar] [CrossRef]
- Sun, J.C.; Lanier, L.L. NK cell development, homeostasis and function: Parallels with CD8⁺ T cells. Nat. Rev. Immunol. 2011, 11, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, B. The Development and Diversity of ILCs, NK Cells and Their Relevance in Health and Diseases. In Regulation of Inflammatory Signaling in Health and Disease; Xu, D., Ed.; Springer: Singapore, 2017; pp. 225–244. [Google Scholar]
- Lam, V.C.; Lanier, L.L. NK cells in host responses to viral infections. Curr. Opin. Immunol. 2017, 44, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Sun, C.; Xiao, W.; Sun, R. Tissue-resident lymphocytes: From adaptive to innate immunity. Cell. Mol. Immunol. 2019, 16, 205–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK cell–cancer cycle: Advances and new challenges in NK cell–based immunotherapies. Nat. Immunol. 2020, 21, 835–847. [Google Scholar] [CrossRef]
- Chiossone, L.; Dumas, P.-Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- López-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef]
- Moon, W.Y.; Powis, S.J. Does Natural Killer Cell Deficiency (NKD) Increase the Risk of Cancer? NKD May Increase the Risk of Some Virus Induced Cancer. Front. Immunol. 2019, 10, 1703. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.; Chen, Y.; Xiao, W.; Sun, R.; Tian, Z. NK cell-based immunotherapy for malignant diseases. Cell. Mol. Immunol. 2013, 10, 230–252. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.; Gao, F.; Yan, M.; Zhao, S.; Yan, Z.; Shi, B.; Liu, Y. Natural killer cells: A promising immunotherapy for cancer. J. Transl. Med. 2022, 20, 240. [Google Scholar] [CrossRef]
- Pegram, H.J.; Andrews, D.M.; Smyth, M.J.; Darcy, P.K.; Kershaw, M.H. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2011, 89, 216–224. [Google Scholar] [CrossRef]
- Farag, S.S.; Fehniger, T.A.; Ruggeri, L.; Velardi, A.; Caligiuri, M.A. Natural killer cell receptors: New biology and insights into the graft-versus-leukemia effect. Blood 2002, 100, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Thielens, A.; Vivier, E.; Romagné, F. NK cell MHC class I specific receptors (KIR): From biology to clinical intervention. Curr. Opin. Immunol. 2012, 24, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Mohammed, R.; Delforce, S.J.; Skerrett-Byrne, D.A.; Coribisier de Meaulsart, C.; Almzi, J.G.; Stephens, A.N.; Verills, N.M.; Dimitriadis, E.; Wang, Y.; et al. Role of the prorenin receptor in endometrial cancer cell growth. Oncotarget 2022, 13, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Rajalingam, R. Overview of the killer cell immunoglobulin-like receptor system. Methods Mol. Biol. 2012, 882, 391–414. [Google Scholar]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Vilches, C.; Parham, P. KIR: Diverse, Rapidly Evolving Receptors of Innate and Adaptive Immunity. Annu. Rev. Immunol. 2002, 20, 217–251. [Google Scholar] [CrossRef]
- Chou, Y.C.; Chen, C.H.; Chen, M.J.; Chang, C.W.; Chen, P.H.; Yu, M.H.; Chen, Y.J.; Tsai, E.M.; Yang, P.S.; Lin, S.Y.; et al. Killer cell immunoglobulin-like receptors (KIR) and human leukocyte antigen-C (HLA-C) allorecognition patterns in women with endometriosis. Sci. Rep. 2020, 10, 4897. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.C.; Lin, C.Y.; Chang, W.C.; Shen, B.J.; Chang, W.P.; Chuang, C.M. Increased association between endometriosis and endometrial cancer: A nationwide population-based retrospective cohort study. Int. J. Gynecol. Cancer 2015, 25, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Hermens, M.; van Altena, A.M.; Velthuis, I.; van de Laar DC, M.; Bulten, J.; van Vliet, H.; Siebers, A.G.; Bekkers, R.L.M. Endometrial Cancer Incidence in Endometriosis and Adenomyosis. Cancers 2021, 13, 4592. [Google Scholar] [CrossRef]
- Ye, J.; Peng, H.; Huang, X.; Qi, X. The association between endometriosis and risk of endometrial cancer and breast cancer: A meta-analysis. BMC Womens Health 2022, 22, 455. [Google Scholar] [CrossRef] [PubMed]
- Pende, D.; Falco, M.; Vitale, M.; Cantoni, C.; Vitale, C.; Munari, E.; Bertaina, A.; Moretta, F.; Del Zotto, G.; Pietra, G.; et al. Killer Ig-Like Receptors (KIRs): Their Role in NK Cell Modulation and Developments Leading to Their Clinical Exploitation. Front. Immunol. 2019, 10, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, K.K.; Pal, S.; Moulik, S.; Chatterjee, A. Integrins and metastasis. Cell Adhes. Migr. 2013, 7, 251–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamai, L.; Ponti, C.; Mirandola, P.; Gobbi, G.; Papa, S.; Galeotti, L.; Cocco, L.; Vitale, M. NK Cells and Cancer. J. Immunol. 2007, 178, 4011–4016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weizman, O.E.; Adams, N.M.; Schuster, I.S.; Krishna, C.; Pritykin, Y.; Lau, C.; Degli-Esposti, M.A.; Leslie, C.S.; Sun, J.C.; O’Sullivan, T.E. ILC1 Confer Early Host Protection at Initial Sites of Viral Infection. Cell 2017, 171, 795–808.e712. [Google Scholar] [CrossRef]
- Prager, I.; Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef]
- Albini, A.; Noonan, D.M. Decidual-Like NK Cell Polarization: From Cancer Killing to Cancer Nurturing. Cancer Discov. 2021, 11, 28–33. [Google Scholar] [CrossRef]
- Sojka, D.K. Uterine Natural Killer Cell Heterogeneity: Lessons From Mouse Models. Front. Immunol. 2020, 11, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Medhi, B. Emerging role of uterine natural killer cells in establishing pregnancy. Iran J. Immunol. 2008, 5, 71–81. [Google Scholar]
- Moffett-King, A. Natural killer cells and pregnancy. Nat. Rev. Immunol. 2002, 2, 656–663. [Google Scholar] [CrossRef]
- Gamliel, M.; Goldman-Wohl, D.; Isaacson, B.; Gur, C.; Stein, N.; Yamin, R.; Berger, M.; Grunewald, M.; Keshet, E.; Rais, Y.; et al. Trained Memory of Human Uterine NK Cells Enhances Their Function in Subsequent Pregnancies. Immunity 2018, 48, 951–962.e955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husby, A.; Wohlfahrt, J.; Melbye, M. Pregnancy duration and endometrial cancer risk: Nationwide cohort study. BMJ 2019, 366, l4693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Male, V.; Sharkey, A.; Masters, L.; Kennedy, P.R.; Farrell, L.E.; Moffett, A. The effect of pregnancy on the uterine NK cell KIR repertoire. Eur. J. Immunol. 2011, 41, 3017–3027. [Google Scholar] [CrossRef] [Green Version]
- Male, V.; Hughes, T.; McClory, S.; Colucci, F.; Caligiuri, M.A.; Moffett, A. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J. Immunol. 2010, 185, 3913–3918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, N.; Zenclussen, A.C. Immune Cells in the Uterine Remodeling: Are They the Target of Endocrine Disrupting Chemicals? Front. Immunol. 2020, 11, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manaster, I.; Mizrahi, S.; Goldman-Wohl, D.; Sela, H.Y.; Stern-Ginossar, N.; Lankry, D.; Gruda, R.; Hurwitz, A.; Bdolah, Y.; Haimov-Kochman, R.; et al. Endometrial NK cells are special immature cells that await pregnancy. J. Immunol. 2008, 181, 1869–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlino, C.; Stabile, H.; Morrone, S.; Bulla, R.; Soriani, A.; Agostinis, C.; Bossi, F.; Mocci, C.; Sarazani, F.; Tedesco, F.; et al. Recruitment of circulating NK cells through decidual tissues: A possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood 2008, 111, 3108–3115. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine Immunity and Microbiota: A Shifting Paradigm. Front. Immunol. 2019, 10, 2387. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; O’Donnell, E.; Leon, G.; Kisovar, A.; Melo, P.; Zondervan, K.; Granne, I.; Southcombe, J. The role of endometrial B cells in normal endometrium and benign female reproductive pathologies: A systematic review. Hum. Reprod. Open 2022, 2022, hoab043. [Google Scholar] [CrossRef]
- Wang, Y.; He, M.; Zhang, G.; Cao, K.; Yang, M.; Zhang, H.; Liu, H. The immune landscape during the tumorigenesis of cervical cancer. Cancer Med. 2021, 10, 2380–2395. [Google Scholar] [CrossRef]
- De Leo, B.; Esnal-Zufiaurre, A.; Collins, F.; Critchley, H.O.D.; Saunders, P.T.K. Immunoprofiling of human uterine mast cells identifies three phenotypes and expression of ERβ and glucocorticoid receptor. F1000Research 2017, 6, 667. [Google Scholar] [CrossRef] [PubMed]
- Drudy, L.; Sheppard, B.; Bonnar, J. Mast cells in the normal uterus and in dysfunctional uterine bleeding. Eur. J. Obstet. Gynecol. Reprod. Biol. 1991, 39, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Schulke, L.; Manconi, F.; Markham, R.; Fraser, I.S. Endometrial dendritic cell populations during the normal menstrual cycle. Hum. Reprod. 2008, 23, 1574–1580. [Google Scholar] [CrossRef] [Green Version]
- Friedenreich, C.M.; Langley, A.R.; Speidel, T.P.; Lau, D.C.; Courneya, K.S.; Csizmadi, I.; Magliocco, A.M.; Yasui, Y.; Cook, L.S. Case-control study of inflammatory markers and the risk of endometrial cancer. Eur. J. Cancer Prev. 2013, 22, 374–379. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Qian, H.; Di, G.; Zhou, R.; Dong, Y.; Chen, W.; Ren, Q. C-Reactive Protein as a Prognostic Biomarker for Gynecologic Cancers: A Meta-Analysis. Comput. Intell. Neurosci. 2022, 2022, 6833078. [Google Scholar] [CrossRef] [PubMed]
- Njoku, K.; Ramchander, N.C.; Wan, Y.L.; Barr, C.E.; Crosbie, E.J. Pre-treatment inflammatory parameters predict survival from endometrial cancer: A prospective database analysis. Gynecol. Oncol. 2022, 164, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Gupta, S.K.; Perretti, M.; Godson, C.; Brennan, E.; Li, Y.; Soehnlein, O.; Shimizu, T.; Werz, O.; Chiurchiù, V.; et al. The Atlas of Inflammation Resolution (AIR). Mol. Asp. Med. 2020, 74, 100894. [Google Scholar] [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Wang, F.; Qualls, A.E.; Marques-Fernandez, L.; Colucci, F. Biology and pathology of the uterine microenvironment and its natural killer cells. Cell. Mol. Immunol. 2021, 18, 2101–2113. [Google Scholar] [CrossRef]
- Kumar, V.; Kiran, S.; Kumar, S.; Singh, U.P. Extracellular vesicles in obesity and its associated inflammation. Int. Rev. Immunol. 2022, 41, 30–44. [Google Scholar] [CrossRef]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Source of Chronic Inflammation in Aging. Front. Cardiovasc. Med. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.V.; Shen, Z.; Wira, C.R. Do endometrial immune changes with age prior to menopause compromise fertility in women? Explor. Immunol. 2022, 2, 677–692. [Google Scholar] [CrossRef]
- van der Woude, H.; Hally, K.E.; Currie, M.J.; Gasser, O.; Henry, C.E. Importance of the endometrial immune environment in endometrial cancer and associated therapies. Front. Oncol. 2022, 12, 975201. [Google Scholar] [CrossRef] [PubMed]
- Vanderstraeten, A.; Luyten, C.; Verbist, G.; Tuyaerts, S.; Amant, F. Mapping the immunosuppressive environment in uterine tumors: Implications for immunotherapy. Cancer Immunol. Immunother. 2014, 63, 545–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krneta, T.; Gillgrass, A.; Poznanski, S.; Chew, M.; Lee, A.J.; Kolb, M.; Ashkar, A.A. M2-polarized and tumor-associated macrophages alter NK cell phenotype and function in a contact-dependent manner. J. Leukoc. Biol. 2017, 101, 285–295. [Google Scholar] [CrossRef]
- Chen, X.-J.; Wu, S.; Yan, R.-M.; Fan, L.-S.; Yu, L.; Zhang, Y.-M.; Wei, W.-F.; Zhou, C.-F.; Wu, X.-G.; Zhong, M.; et al. The role of the hypoxia-Nrp-1 axis in the activation of M2-like tumor-associated macrophages in the tumor microenvironment of cervical cancer. Mol. Carcinog. 2019, 58, 388–397. [Google Scholar] [CrossRef]
- Mise, Y.; Hamanishi, J.; Daikoku, T.; Takamatsu, S.; Miyamoto, T.; Taki, M.; Yamanoi, K.; Yamaguchi, K.; Ukita, M.; Horikawa, N.; et al. Immunosuppressive tumor microenvironment in uterine serous carcinoma via CCL7 signal with myeloid-derived suppressor cells. Carcinogenesis 2022, 43, 647–658. [Google Scholar] [CrossRef]
- Terrén, I.; Orrantia, A.; Vitallé, J.; Zenarruzabeitia, O.; Borrego, F. NK Cell Metabolism and Tumor Microenvironment. Front. Immunol. 2019, 10, 2278. [Google Scholar] [CrossRef]
- Ni, Y.; Soliman, A.; Joehlin-Price, A.; Abdul-Karim, F.; Rose, P.G.; Mahdi, H. Immune cells and signatures characterize tumor microenvironment and predict outcome in ovarian and endometrial cancers. Immunotherapy 2021, 13, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Zaiatz-Bittencourt, V.; Finlay, D.K.; Gardiner, C.M. Canonical TGF-β Signaling Pathway Represses Human NK Cell Metabolism. J. Immunol. 2018, 200, 3934–3941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelmini, S.; Mangoni, M.; Castiglione, F.; Beltrami, C.; Pieralli, A.; Andersson, K.L.; Fambrini, M.; Taddei, G.L.; Serio, M.; Orlando, C. The CXCR4/CXCL12 axis in endometrial cancer. Clin. Exp. Metastasis 2009, 26, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Medina-Gutiérrez, E.; Céspedes, M.V.; Gallardo, A.; Rioja-Blanco, E.; Pavón, M.; Asensio-Puig, L.; Farré, L.; Alba-Castellón, L.; Unzueta, U.; Villaverde, A.; et al. Novel Endometrial Cancer Models Using Sensitive Metastasis Tracing for CXCR4-Targeted Therapy in Advanced Disease. Biomedicines 2022, 10, 1680. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Omatsu, Y.; Sugiyama, T.; Oishi, S.; Fujii, N.; Nagasawa, T. CXCL12-CXCR4 chemokine signaling is essential for NK-cell development in adult mice. Blood 2011, 117, 451–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degos, C.; Heinemann, M.; Barrou, J.; Boucherit, N.; Lambaudie, E.; Savina, A.; Gorvel, L.; Olive, D. Endometrial Tumor Microenvironment Alters Human NK Cell Recruitment, and Resident NK Cell Phenotype and Function. Front. Immunol. 2019, 10, 877. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.Q.; Tsuda, Y.; Han, M.; Xu, D.H.; Kanagawa, N.; Hatanaka, Y.; Tani, Y.; Mizuguchi, H.; Tsutsumi, Y.; Mayumi, T.; et al. NK cells are migrated and indispensable in the anti-tumor activity induced by CCL27 gene therapy. Cancer Immunol. Immunother. 2009, 58, 291–299. [Google Scholar] [CrossRef]
- Parodi, M.; Raggi, F.; Cangelosi, D.; Manzini, C.; Balsamo, M.; Blengio, F.; Eva, A.; Varesio, L.; Pietra, G.; Moretta, L.; et al. Hypoxia Modifies the Transcriptome of Human NK Cells, Modulates Their Immunoregulatory Profile, and Influences NK Cell Subset Migration. Front. Immunol. 2018, 9, 2358. [Google Scholar] [CrossRef] [Green Version]
- Jedlička, M.; Feglarová, T.; Janstová, L.; Hortová-Kohoutková, M.; Frič, J. Lactate from the tumor microenvironment—A key obstacle in NK cell-based immunotherapies. Front. Immunol. 2022, 13, 932055. [Google Scholar] [CrossRef]
- Inoue, T.; Adachi, K.; Kawana, K.; Taguchi, A.; Nagamatsu, T.; Fujimoto, A.; Tomio, K.; Yamashita, A.; Eguchi, S.; Nishida, H.; et al. Cancer-associated fibroblast suppresses killing activity of natural killer cells through downregulation of poliovirus receptor (PVR/CD155), a ligand of activating NK receptor. Int. J. Oncol. 2016, 49, 1297–1304. [Google Scholar] [CrossRef] [Green Version]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef] [PubMed]
- Dersh, D.; Hollý, J.; Yewdell, J.W. A few good peptides: MHC class I-based cancer immunosurveillance and immunoevasion. Nat. Rev. Immunol. 2021, 21, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, M.L.; Cook, R.S.; Johnson, D.B.; Balko, J.M. Biological Consequences of MHC-II Expression by Tumor Cells in Cancer. Clin. Cancer Res. 2019, 25, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, B.K.; Barahmand-Pour, F.; Paulsene, W.; Medley, S.; Geraghty, D.E.; Strong, R.K. Interactions between NKG2x immunoreceptors and HLA-E ligands display overlapping affinities and thermodynamics. J. Immunol. 2005, 174, 2878–2884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, B.K.; Pizarro, J.C.; Kerns, J.; Strong, R.K. Structural basis for NKG2A/CD94 recognition of HLA-E. Proc. Natl. Acad. Sci. USA 2008, 105, 6696–6701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.; Llano, M.; Carretero, M.; Ishitani, A.; Navarro, F.; López-Botet, M.; Geraghty, D.E. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl. Acad. Sci. USA 1998, 95, 5199–5204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwaszko, M.; Bogunia-Kubik, K. Clinical Significance of the HLA-E and CD94/NKG2 Interaction. Arch. Immunol. Ther. Exp. 2011, 59, 353. [Google Scholar] [CrossRef]
- Wada, H.; Matsumoto, N.; Maenaka, K.; Suzuki, K.; Yamamoto, K. The inhibitory NK cell receptor CD94/NKG2A and the activating receptor CD94/NKG2C bind the top of HLA-E through mostly shared but partly distinct sets of HLA-E residues. Eur. J. Immunol. 2004, 34, 81–90. [Google Scholar] [CrossRef]
- Lauterbach, N.; Wieten, L.; Popeijus, H.E.; Voorter, C.E.M.; Tilanus, M.G.J. HLA-E regulates NKG2C+ natural killer cell function through presentation of a restricted peptide repertoire. Hum. Immunol. 2015, 76, 578–586. [Google Scholar] [CrossRef]
- Versluis, M.A.C.; Marchal, S.; Plat, A.; de Bock, G.H.; van Hall, T.; de Bruyn, M.; Hollema, H.; Nijman, H.W. The prognostic benefit of tumour-infiltrating Natural Killer cells in endometrial cancer is dependent on concurrent overexpression of Human Leucocyte Antigen-E in the tumour microenvironment. Eur. J. Cancer 2017, 86, 285–295. [Google Scholar] [CrossRef]
- Ben Yahia, H.; Boujelbene, N.; Babay, W.; Ben Safta, I.; Dhouioui, S.; Zemni, I.; Ali Ayadi, M.; Charfi, L.; Ouzari, H.I.; Rebmann, V.; et al. Expression analysis of immune-regulatory molecules HLA-G, HLA-E and IDO in endometrial cancer. Hum. Immunol. 2020, 81, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Gornalusse, G.G.; Hirata, R.K.; Funk, S.E.; Riolobos, L.; Lopes, V.S.; Manske, G.; Prunkard, D.; Colunga, A.G.; Hanafi, L.A.; Clegg, D.O.; et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 2017, 35, 765–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, Y.; Pan, M.; Song, S.; Hua, J.; Liu, R.; Li, L. CD3+CD56+ natural killer T cell infiltration is increased in cervical cancer and negatively correlated with tumour progression. Biotechnol. Biotechnol. Equip. 2019, 33, 1380–1391. [Google Scholar] [CrossRef] [Green Version]
- Piulats, J.M.; Matias-Guiu, X. Immunotherapy in Endometrial Cancer: In the Nick of Time. Clin. Cancer Res. 2016, 22, 5623–5625. [Google Scholar] [CrossRef] [Green Version]
- Santin, A.D.; Bellone, S.; Buza, N.; Choi, J.; Schwartz, P.E.; Schlessinger, J.; Lifton, R.P. Regression of Chemotherapy-Resistant Polymerase epsilon (POLE) Ultra-Mutated and MSH6 Hyper-Mutated Endometrial Tumors with Nivolumab. Clin. Cancer Res. 2016, 22, 5682–5687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Tucci, C.; Capone, C.; Galati, G.; Iacobelli, V.; Schiavi, M.C.; Di Donato, V.; Muzii, L.; Panici, P.B. Immunotherapy in endometrial cancer: New scenarios on the horizon. J. Gynecol. Oncol. 2019, 30, e46. [Google Scholar] [CrossRef] [Green Version]
- Langers, I.; Renoux, V.; Reschner, A.; Touze, A.; Coursaget, P.; Boniver, J.; Koch, J.; Delvenne, P.; Jacobs, N. Natural killer and dendritic cells collaborate in the immune response induced by the vaccine against uterine cervical cancer. Eur. J. Immunol. 2014, 44, 3585–3595. [Google Scholar] [CrossRef]
- Laskowski, T.J.; Biederstädt, A.; Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 2022, 22, 557–575. [Google Scholar] [CrossRef]
- Maskalenko, N.A.; Zhigarev, D.; Campbell, K.S. Harnessing natural killer cells for cancer immunotherapy: Dispatching the first responders. Nat. Rev. Drug Discov. 2022, 21, 559–577. [Google Scholar] [CrossRef]
- Ames, E.; Canter, R.J.; Grossenbacher, S.K.; Mac, S.; Chen, M.; Smith, R.C.; Hagino, T.; Perez-Cunningham, J.; Sckisel, G.D.; Urayama, S.; et al. NK Cells Preferentially Target Tumor Cells with a Cancer Stem Cell Phenotype. J. Immunol. 2015, 195, 4010–4019. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Dubois, S.; Sato, N.; Sabzevari, H.; Sakai, Y.; Waldmann, T.A.; Tagaya, Y. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood 2005, 105, 721–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamali, A.; Hadjati, J.; Madjd, Z.; Mirzaei, H.R.; Thalheimer, F.B.; Agarwal, S.; Bonig, H.; Ullrich, E.; Hartmann, J. Highly Efficient Generation of Transgenically Augmented CAR NK Cells Overexpressing CXCR4. Front. Immunol. 2020, 11, 2028. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Reger, R.; Segerberg, F.; Lambert, M.; Leijonhufvud, C.; Baumer, Y.; Carlsten, M.; Childs, R. Enhanced Bone Marrow Homing of Natural Killer Cells Following mRNA Transfection With Gain-of-Function Variant CXCR4R334X. Front. Immunol. 2019, 10, 1262. [Google Scholar] [CrossRef] [Green Version]
- Valeri, A.; García-Ortiz, A.; Castellano, E.; Córdoba, L.; Maroto-Martín, E.; Encinas, J.; Leivas, A.; Río, P.; Martínez-López, J. Overcoming tumor resistance mechanisms in CAR-NK cell therapy. Front. Immunol. 2022, 13, 953849. [Google Scholar] [CrossRef]
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine 2020, 59, 102975. [Google Scholar] [CrossRef] [PubMed]
- Biederstädt, A.; Rezvani, K. Engineering the next generation of CAR-NK immunotherapies. Int. J. Hematol. 2021, 114, 554–571. [Google Scholar] [CrossRef] [PubMed]
- Marofi, F.; Abdul-Rasheed, O.F.; Rahman, H.S.; Budi, H.S.; Jalil, A.T.; Yumashev, A.V.; Hassanzadeh, A.; Yazdanifar, M.; Motavalli, R.; Chartrand, M.S.; et al. CAR-NK cell in cancer immunotherapy; A promising frontier. Cancer Sci. 2021, 112, 3427–3436. [Google Scholar] [CrossRef]
- Tonn, T.; Schwabe, D.; Klingemann, H.G.; Becker, S.; Esser, R.; Koehl, U.; Suttorp, M.; Seifried, E.; Ottmann, O.G.; Bug, G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013, 15, 1563–1570. [Google Scholar] [CrossRef]
- Podshivalova, E.S.; Semkina, A.S.; Kravchenko, D.S.; Frolova, E.I.; Chumakov, S.P. Efficient delivery of oncolytic enterovirus by carrier cell line NK-92. Mol. Ther. Oncolytics 2021, 21, 110–118. [Google Scholar] [CrossRef]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Martínez, A.; Fernández, L.; Valentín, J.; Martínez-Romera, I.; Corral, M.D.; Ramírez, M.; Abad, L.; Santamaría, S.; González-Vicent, M.; Sirvent, S.; et al. A phase I/II trial of interleukin-15–stimulated natural killer cell infusion after haplo-identical stem cell transplantation for pediatric refractory solid tumors. Cytotherapy 2015, 17, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, B.C.; Le Luduec, J.B.; Forlenza, C.; Jakubowski, A.A.; Perales, M.A.; Young, J.W.; Hsu, K.C. Phase II Study of Haploidentical Natural Killer Cell Infusion for Treatment of Relapsed or Persistent Myeloid Malignancies Following Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 705–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.A.; Denman, C.J.; Rondon, G.; Woodworth, G.; Chen, J.; Fisher, T.; Kaur, I.; Fernandez-Vina, M.; Cao, K.; Ciurea, S.; et al. Haploidentical Natural Killer Cells Infused before Allogeneic Stem Cell Transplantation for Myeloid Malignancies: A Phase I Trial. Biol. Blood Marrow Transplant. 2016, 22, 1290–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, R.; Wu, H.; Pounds, S.; Inaba, H.; Ribeiro, R.C.; Cullins, D.; Rooney, B.; Bell, T.; Lacayo, N.J.; Heym, K.; et al. A phase II clinical trial of adoptive transfer of haploidentical natural killer cells for consolidation therapy of pediatric acute myeloid leukemia. J. Immunother. Cancer 2019, 7, 81. [Google Scholar] [CrossRef]
- Arai, S.; Meagher, R.; Swearingen, M.; Myint, H.; Rich, E.; Martinson, J.; Klingemann, H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: A phase I trial. Cytotherapy 2008, 10, 625–632. [Google Scholar] [CrossRef]
- Bednarski, J.J.; Zimmerman, C.; Berrien-Elliott, M.M.; Foltz, J.A.; Becker-Hapak, M.; Neal, C.C.; Foster, M.; Schappe, T.; McClain, E.; Pence, P.P.; et al. Donor memory-like NK cells persist and induce remissions in pediatric patients with relapsed AML after transplant. Blood 2022, 139, 1670–1683. [Google Scholar] [CrossRef]
- Parihar, R. Memory NK cells to forget relapsed AML. Blood 2022, 139, 1607–1608. [Google Scholar] [CrossRef]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Ham, J.D.; Hu, G.; Xie, G.; Vergara, J.; Liang, Y.; Ali, A.; Tarannum, M.; Donner, H.; Baginska, J.; et al. Memory-like NK cells armed with a neoepitope-specific CAR exhibit potent activity against NPM1 mutated acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2022, 119, e2122379119. [Google Scholar] [CrossRef]
- Hosseinalizadeh, H.; Habibi Roudkenar, M.; Mohammadi Roushandeh, A.; Kuwahara, Y.; Tomita, K.; Sato, T. Natural killer cell immunotherapy in glioblastoma. Discov. Oncol. 2022, 13, 113. [Google Scholar] [CrossRef]
- Rafei, H.; Daher, M.; Rezvani, K. Chimeric antigen receptor (CAR) natural killer (NK)-cell therapy: Leveraging the power of innate immunity. Br. J. Haematol. 2021, 193, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Tarannum, M.; Romee, R. Cytokine-induced memory-like natural killer cells for cancer immunotherapy. Stem. Cell Res. Ther. 2021, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Pahl, J.H.W.; Cerwenka, A.; Ni, J. Memory-like NK Cells: Remembering a Previous Activation by Cytokines and NK Cell Receptors. Front. Immunol. 2018, 9, 2796. [Google Scholar] [CrossRef] [PubMed]
- Berrien-Elliott, M.M.; Wagner, J.A.; Fehniger, T.A. Human Cytokine-Induced Memory-like Natural Killer Cells. J. Innate Immun. 2015, 7, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Ljunggren, H.G.; Malmberg, K.J. Prospects for the use of NK cells in immunotherapy of human cancer. Nat. Rev. Immunol. 2007, 7, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Uppendahl, L.D.; Dahl, C.M.; Miller, J.S.; Felices, M.; Geller, M.A. Natural Killer Cell-Based Immunotherapy in Gynecologic Malignancy: A Review. Front. Immunol. 2017, 8, 1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veluchamy, J.P.; Heeren, A.M.; Spanholtz, J.; van Eendenburg, J.D.; Heideman, D.A.; Kenter, G.G.; Verheul, H.M.; van der Vliet, H.J.; Jordanova, E.S.; de Gruijl, T.D. High-efficiency lysis of cervical cancer by allogeneic NK cells derived from umbilical cord progenitors is independent of HLA status. Cancer Immunol. Immunother. 2017, 66, 51–61. [Google Scholar] [CrossRef]
- Jochems, C.; Hodge, J.W.; Fantini, M.; Fujii, R.; Morillon, Y.M., 2nd; Greiner, J.W.; Padget, M.R.; Tritsch, S.R.; Tsang, K.Y.; Campbell, K.S.; et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget 2016, 7, 86359–86373. [Google Scholar] [CrossRef] [Green Version]
- Farcas, M.; Inngjerdingen, M. Natural killer cell–derived extracellular vesicles in cancer therapy. Scand. J. Immunol. 2020, 92, e12938. [Google Scholar] [CrossRef]
- Dosil, S.G.; Lopez-Cobo, S.; Rodriguez-Galan, A.; Fernandez-Delgado, I.; Ramirez-Huesca, M.; Milan-Rois, P.; Castellanos, M.; Somoza, A.; Gómez, M.J.; Reyburn, H.T.; et al. Natural killer (NK) cell-derived extracellular-vesicle shuttled microRNAs control T cell responses. eLife 2022, 11, e76319. [Google Scholar] [CrossRef]
- Demaria, O.; Gauthier, L.; Vetizou, M.; Blanchard Alvarez, A.; Vagne, C.; Habif, G.; Batista, L.; Baron, W.; Belaïd, N.; Girard-Madoux, M.; et al. Antitumor immunity induced by antibody-based natural killer cell engager therapeutics armed with not-alpha IL-2 variant. Cell Rep. Med. 2022, 3, 100783. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019, 177, 1701–1713.e1716. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoogstad-van Evert, J.S.; Bekkers, R.; Ottevanger, N.; Jansen, J.H.; Massuger, L.; Dolstra, H. Harnessing natural killer cells for the treatment of ovarian cancer. Gynecol. Oncol. 2020, 157, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Rückert, T.; Lareau, C.A.; Mashreghi, M.-F.; Ludwig, L.S.; Romagnani, C. Clonal expansion and epigenetic inheritance of long-lasting NK cell memory. Nat. Immunol. 2022, 23, 1551–1563. [Google Scholar] [CrossRef]

| Receptors | Ligands | |
|---|---|---|
| Activating Receptors | α5β1 integrin ↑ | Vascular Cell Adhesion Molecule 1 (VCAM-1) |
| CD226 (DNAM-1) ↑/↓ | CD112 (Nectin-2), CD155 (Nec15) | |
| CD16 | Immunoglobin B ↑ | |
| NKp46 ↓ | Viral hemagglutinins | |
| KIR2DS2 ↓ | HLA-C1 allotypes and HLA-A*11:01 | |
| CD94-NKG2C | HLA-E ↑ | |
| CD94-NKG2E | HLA-E ↑ | |
| NKG2D ↓ | ULBP (RAET), MICA, MICB | |
| CD96 (Tactile) ↑ | CD155 (Necl5) ↑/↓ | |
| NKp30 ↓ | pp65, BAT-3 | |
| Inhibitory Receptors | KIR-L ↑ | HLA-A, HLA-B, HLA-C |
| CD94-NKG2A | HLA-E ↑ | |
| KLRG1 ↑ | Cadherins | |
| LILRB1 or ILT2 (CD85) ↑ | HLA class 1 ↓ |
| Therapeutic Agent | Goal |
|---|---|
| Peripheral blood or umbilical cord derived NK cells + Cetuximab | Increase cytotoxicity against cancer cells |
| Irradiated haNK cells | Induce CD16 expression to increase cancer cell lysis. |
| NK cell derived extracellular vesicles | Regulate APC response |
| Antibody based NK cell engager therapeutics | Induce NK cell proliferation, cytotoxicity, and cytokine release. |
| Block NKG2A with Monalizumab to enhance NK and T cells | Stimulate T cells and NK cells; promote NK cell antitumor activity. |
| TRACKs, expressing human CXCR4 | Increased NK cell migration to tumor site |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.; Bauer, C.; Stewart, J.H., IV. Chasing Uterine Cancer with NK Cell-Based Immunotherapies. Future Pharmacol. 2022, 2, 642-659. https://doi.org/10.3390/futurepharmacol2040039
Kumar V, Bauer C, Stewart JH IV. Chasing Uterine Cancer with NK Cell-Based Immunotherapies. Future Pharmacology. 2022; 2(4):642-659. https://doi.org/10.3390/futurepharmacol2040039
Chicago/Turabian StyleKumar, Vijay, Caitlin Bauer, and John H. Stewart, IV. 2022. "Chasing Uterine Cancer with NK Cell-Based Immunotherapies" Future Pharmacology 2, no. 4: 642-659. https://doi.org/10.3390/futurepharmacol2040039







