Abstract
Ampakines are a class of orally available positive allosteric modulators of the AMPA-glutamate receptor (AMPAR) and have therapeutic implications for neurological/neuropsychiatric disorders in which AMPAR signaling is compromised. Low-impact ampakines are a distinct subclass of drugs that only modestly offset receptor desensitization and do not alter agonist binding affinity and thus lack the neurotoxicity and epileptogenic effects associated with other AMPAR modulators. In these studies, we describe the pre-clinical pharmacology of ampakine 1-(benzofurazan-5-ylcarbonyl)morpholine (CX717). CX717 modestly offsets desensitization in hippocampal patches and augments synaptic transmission in vivo. CX717 also enhances long-term potentiation in rats, which is crucial for learning and memory. CX717 enhances performance in the eight-arm radial maze and abrogates amphetamine-induced locomotor activity while being devoid of cataleptic activity in rats. CX717 also ameliorates alfentanil-induced respiratory depression in rats and is not toxic to cultured rat neurons. CX717 is active at doses of 0.3–10 mg/kg and lacked serious adverse events in safety studies in mice up to 2000 mg/kg. CX717 was also previously shown to be safe in humans and effective in reversing opiate-induced respiratory depression and hyperactivity and inattentiveness in adults with ADHD. These findings support the continued clinical investigation of CX717 in the treatment of ADHD, dementia, and opiate-induced respiratory depression.
1. Introduction
AMPA-glutamate receptors (AMPARs) govern the majority of fast excitatory neurotransmission in the mammalian CNS [1]. AMPAR signaling is important for a myriad of neuronal processes, and AMPAR dysregulation is thought to underlie a multitude of neurological/neuropsychiatric disorders including ADHD [2,3,4], schizophrenia [5,6,7,8,9], Alzheimer’s Disease [10,11,12,13,14,15,16,17], and Fragile X syndrome [18,19,20]. Ampakines, positive allosteric modulators of AMPAR, are a class of orally bioavailable small molecules that readily penetrate into the CNS and enhance the effects of glutamate. Ampakines have demonstrated positive effects in several pre-clinical studies, including in models of Alzheimer’s Disease [21], Parkinson’s Disease [22,23,24,25], Rett syndrome [26,27,28], ischemic stroke [29,30], Huntington Disease [31,32,33], and Angelman Syndrome [34]. Ampakines are also effective at reversing opiate-induced respiratory depression (OIRD) in animals [35,36,37,38,39] and humans [40] without compromising opiate analgesia.
The widespread therapeutic effects of AMPAR modulation via ampakines is promising, though some first generation ampakines were shown to possess poor therapeutic ratios, exhibiting seizure induction at disease-modifying doses [41,42,43,44,45]. Pro-convulsive properties of experimental ampakines have drastically halted their translation into the clinical setting. However, some experimental ampakines have been shown to be devoid of epileptogenic activity at doses that are far above doses that produce therapeutic effects [39,46]. This subclass of ampakines is termed low-impact ampakines, since they do not markedly offset AMPAR desensitization and do not alter agonist binding affinity for the receptor [47]. One such low-impact ampakine, CX516, was tested in the clinic in schizophrenia and Fragile X syndrome but was ineffective, findings attributed to its low potency and rapid excretion [48,49].
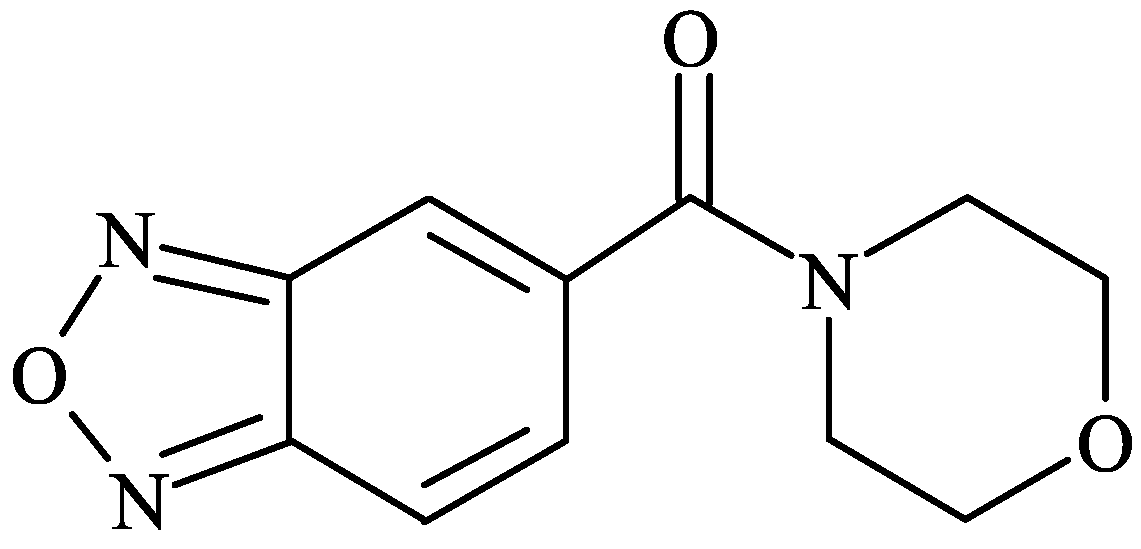
RespireRx Pharmaceuticals is developing low-impact ampakines for the treatment of OIRD and other sequelae of spinal cord injury [50,51,52,53,54,55]. CX717 (Figure 1) is a low-impact ampakine that has shown positive effects in preclinical studies of opiate-induced respiratory depression, reversing sleep deprivation-associated memory impairment and ameliorating respiratory dysfunction after spinal cord injury [35,36,37,50,51,52,55,56,57,58,59,60,61,62,63,64]. In these studies, CX717 was typically effective between 5 and 30 mg/kg. CX717 was further investigated in the clinical setting, where it has shown activity in adult ADHD [65] and OIRD [40]. In concomitant animal studies it was, however, discovered that in rodents treated with markedly higher doses (1500 mg/kg/day for 14 days), astrocytic vacuoles were observed [66], and CX717 was subsequently placed on an FDA clinical hold. Further evaluation revealed that these vacuoles only occurred in mice after formalin fixation and did not occur when tissue was flash frozen, which led us to conclude that the interaction between CX717 and formalin caused vacuolation [66].
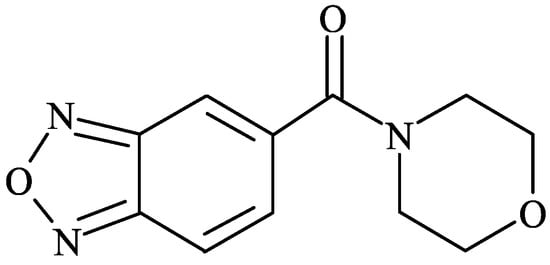
Figure 1.
Chemical structure of 1-(benzofurazan-5-ylcarbonyl)morpholine (CX717).
Given the positive effects of CX717 in pre-clinical and clinical studies, we sought to explore the electrophysiological and pharmacological effects of this molecule. In these studies, we explore the effects of CX717 on patches excised from the CA1 pyramidal neurons. We further explore the effects of this ampakine on synaptic transmission and LTP in vivo, the latter of which having important implications for the use of ampakines to enhance memory, especially in neurological disorders underpinned by memory dysfunction [10,12,15,16,21,57,67,68,69,70]. We tested CX717 in the eight-arm radial maze task to screen for pro-cognitive effects. As ampakines have been examined for their antipsychotic effects [46,71,72], we examined the ability of CX717 to attenuate amphetamine-induced locomotor activity and whether CX717 possessed cataleptic activity. CX717 was also tested for its ability to impede alfentanil-induced respiratory depression. Additionally, we evaluated the propensity of CX717 to produce seizures in mice and discussed future implications for CX717 development.
2. Materials and Methods
All experiments using rodents were performed according to the Guide for the Care and Use of Laboratory Animals issued by the National Institutes of Health and with protocols approved by the University of California at Irvine Institutional Animal Care and Use Committee (Irvine, CA, USA). We made efforts to reduce the suffering of the animals and sought to reduce the number of animals used in our studies. Specific animal strains and species were selected based upon previous characterizations in the studies described below.
2.1. Hippocampal Slice Patch Clamp Experiments
Outside-out patches excised from CA1 pyramidal neurons of organotypic hippocampal slices were used for patch clamp studies as described previously [73,74]. Sprague-Dawley rats, 13–14 days old, were utilized to prepare hippocampal slice cultures which were grown on cellulose membrane inserts for two weeks (Millipore CM) in an incubator. On the day of experimentation, slices were transferred and placed in a medium with the following components (in mM): KCl 2.5, KH2PO4 1.25, MgCl2 1, CaCl2 2, NaHCO3 5, D-glucose 25, HEPES 20, and NaCl 125 (pH 7.3). A patch was excised and transferred to a recording chamber perfused with recording medium which had the following (in mM): KCl 3.5, HEPES 20, and NaCl 130. Moreover, 10 μM MK-801 and 50 μM D-AP5 was included in order to antagonize NMDA receptors in the patches. Patch pipettes have a resistance of 3–8 MOhm and were filled with the following solution (in mM): CsCl 65, CsF 65, MgCl 2, EGTA 10, ATP disodium salt 2, and HEPES 10 (pH 7.3). A piezo system (50 μm translation in 0.4 ms) was utilized to quickly alternate solutions applied to the patch [74,75,76]. Briefly, glutamate and background containing medium flowed through a double-barrel pipette through two lines continuously.
The patch was initially exposed to background stream. Piezo translator actuation exposed the patch to glutamate for a predetermined amount of time. Five responses were typically collected and averaged. For experiments using CX717, flow lines were switched to solutions with indicated concentrations of CX717. After 30 s for equilibration, patches were exposed to glutamate. For data analysis, responses with CX717 were compared with the average control response taken before and after CX717 treatment. The holding potential was −50 mV. A patch amplifier was used to acquire data (AxoPatch-1D) at a filter frequency of 5 kHz and was digitized at 10 kHz with PClamp/Digidata 1200 (Axon Instrument).
2.2. Electrically Evoked Field Excitatory Postsynaptic Potentials (fEPSPs)
Evaluation of CX717 effects on hippocampal EPSPs were described previously [29,39]. This study utilized male Long Evan Rats weighing 250–350 g. All animals were maintained under anesthesia via pentobarbital (50 mg/mL) administered at a rate of 0.1 to 0.15 mL/h with a Hamilton syringe pump. Stimulating electrodes were placed into the perforant path and recording electrodes were placed into the dentate gyrus of the hippocampus. After electrode implantation, a stable baseline of evoked responses was obtained using single monophasic pulses (pulse duration of 100 μs) delivered at three pulses per minute to the stimulating electrode. Field EPSPs were recorded until a stable baseline was established (which typically occurred after about twenty to thirty minutes), after which a solution of CX717 in hydroxypropyl-P-cyclodextrin (HPCD) was injected IP (1–2 mL/kg), and evoked field potentials were recorded. Evoked potentials were recorded for approximately 2 h following CX717 administration. Data were acquired and subsequently analyzed using available software (NAC and NACSHOW, Irvine, CA, USA). Elevation of EPSP amplitudes via CX717 was assessed with ANOVA and Dunnett’s multiple comparison test.
2.3. In Vivo LTP Experiments
LTP experiments using male Long Evan Rats (250–350 g) were conducted as described previously [39,77] using similar methods of anesthesia, electrode implantation, and baseline EPSP measurements as described above. Field EPSPs were monitored until a stable baseline was achieved (as above, about twenty to thirty minutes), after which a suboptimal tetanic protocol was used to produce a decremental form of LTP (or STP). Specifically, 4 trains at 400 Hz and of 20 msec duration were delivered at 0.1 Hz. The pulse width during the tetanus was increased to evoke a hint of a population spike to single-pulse stimulation. Field potential recording was continued at the same rate and intensity of stimulation as during the baseline period, to observe the amount and duration of the induced STP.
Once the STP returned to baseline (usually within 20–40 min), 2 mg/kg CX717 was injected IP (1 mL/kg), and field potentials were continuously recorded every 20 sec for approximately 15 min. This was followed by administration of a second episode of suboptimal tetanic stimulation, identical in all aspects to the first one. Field potential recording was continued at the same frequency and intensity of stimulation as during the baseline period. Data acquisition and analysis was performed using commercially available software (NAC and NACSHOW).
2.4. Eight-Arm Radial Maze
Each compound dose was tested during a one-week period using a cross-over design on an alternate-day dosing schedule using 10 two-year-old male Long Evan rats (250–350 g). Two-year old rats were selected to better approximate an aged population, for which dementia medications would be most needed. According to this schedule, rats were subjected to alternate-day drug (Tuesday and Thursday) and vehicle (Monday, Wednesday and Friday) treatments. Monday experiments were not used in evaluations. The behavioral test used a standard eight-arm radial maze wherein five arms were open for the first morning session. Each animal was allowed to consume the food rewards present in the open arms and was then returned to its home cage. Six hours later, animals were replaced in the maze, wherein all eight arms were open, but only the three arms that were previously closed contained food rewards. Scoring was based on the number of correct choices before an incorrect choice (re-entering an arm that was visited during the morning acquisition session) was made and on the total number of incorrect choices made before all three of the baited arms were visited. The rats were administered one of five doses of CX717 (0.1, 0.3, 1, 3, and 10 mg/kg, IP, 1–2 mL/kg) on each of the two drug treatment days (Tuesday and Thursday). CX717 was administered 15 min prior to the start of the morning acquisition session and no drug was administered prior to the second trial 6 h later. Data were analyzed with ANOVA followed by Wilcoxon signed-rank test.
2.5. Amphetamine-Induced Locomotor Activity
Male Sprague-Dawley rats with a typical mass of 250–300 g were used in this assay. This study involved utilizing amphetamine or methamphetamine to induce locomotion, captured on a computerized Photobeam Activity System (San Diego Instruments, San Diego, CA, USA) as described previously [46]. Standard test cages (26 × 48 × 20 cm W × L × H) were surrounded by two photobeam arrays. The lower array was used to detect ambulation locomotor behavior with the upper array being used to assess rearing behavior. The activities were continuously assessed via a computer. Cages with photobeam arrays were positioned in a somewhat darkened room. On the day of experimentation, rats were positioned in the test cages and permitted to acclimate for thirty minutes. Rats were then randomly assigned to experimental groups and were injected IP (1–2 mL/kg) with the indicated drugs and returned to their respective cages and monitored for 90 min. Total photobeam breaks were calculated for each group and tabulated. Amphetamine sulfate (1 mg/kg), methamphetamine HCl (2 mg/kg) and clozapine (0.1 mg/kg) were purchased from Research Biochemicals (Natick, MA, USA).
2.6. Catalepsy Studies
For determination of catalepsy studies, Sprague-Dawley rats were used (300–350 g). Catalepsy studies were previously described [46]. Briefly, the potential cataleptic effects of CX717 was assessed using the bar and grid tests [78]. For bar test studies, a metal rod bar placed into tygon tubing (1 cm diameter) was positioned horizontally across the width of the animal cage. Rats were injected IP with vehicle, CX717, haloperidol (used as a positive control, 10 mL/kg), or the combination and were tested for 3 h at 30 min intervals. The rats’ front paws were positioned on the rod and the rear paws on the bedding of the cage. We then measured the time (seconds) the rat kept its front paws on the bar. For any 30 min interval, the maximum time documented was 180 s; 0 s was the time recorded if, after 3 tries, the rat still did not allow the investigator to place its front paws on the bar. Investigators were blind to the drug treatment the rats received in this assay.
The grid test was conducted after the bar test during each 30 min interval. This test involved a 50-degree incline wire mesh, 15 × 22 in with 0.5 cm-size mesh encompassed on three sides with a 10.2 cm strip of black Plexiglas. The rat was placed in the middle of the grid and recorded the time until the rat moved. The maximum score possible was 180 s. The grid test was also performed for three hours at thirty-minute intervals. The catalepsy score, or the seconds immobile was tabulated for each rat.
2.7. In Vivo Plethysmography Studies
This study was performed similarly as previously described [39]. A day prior to the experiment, Charles River rats had two PE30 cannulas inserted into the right jugular vein. The jugular vein cannulas are used for CX717 administration and continuous alfentanil infusion. These cannulas are exteriorized through the skin between the shoulder blades, cut to a length just short of reaching the eyes, filled with 10 U/mL heparin, and plugged with bone wax. On the day of testing, rats are placed into a rodent-sized whole-body plethysmograph (Buxco) chamber to record several different respiratory parameters before and after the testing drugs are administered. After a base line is established (20 min), alfenfanil is administered intravenously via an infusion pump at a rate of 250 μg/kg/20 min. Then, 20 min later, a bolus CX717 in HPCD is injected through the other cannula. Then, 40 min after CX717 injection, alfentanil infusion is stopped and the experiment is ended 30 min later.
2.8. Assessment of CX717 Toxicity in Cultured Neurons
We have described cortical neuronal isolation previously [79]. Briefly, cortices and hippocampi were removed from E15-E17 Wistar rats. The tissue was manually cut into smaller pieces and incubated for 8–10 min with papain in calcium- and magnesium-free HBSS at 37 °C. After this incubation, HBSS was replaced with DMEM + 10% serum. The tissue was further dissociated by passaging it through a pipette after adding DNAse. The resultant suspension of cells was centrifuged through a 5% BSA (bovine serum albumin) solution and the pellet was again re-suspended in DMEM + 10% serum. Neurons were counted and seeded in a 96-well plate coated with poly-D/L-lysine. Following incubation overnight, cultures were treated for 24 h with 500 μM CX717 or increasing concentrations of LY503430 [22,24,68,80,81] or LY451395 [81,82,83,84,85,86,87]. After treatment, XTT assay was performed according to standard protocols. Briefly, phenazine methosulfate electron coupling reagent was mixed with XTT solution. Then, 25 μL of the solution was added to each well, and the plates were incubated for a further 2 h, after which absorbance was measured at 450 nm (reference 650 nm). Data were normalized to control values.
2.9. Single Dose Toxicity in Adult Mice
Single-dose toxicity experiments were performed with adult CD1 mice (n = 2–3 mice per group). CX717 was administered at the indicated doses via oral gavage and mice were placed singly into cages for observation. They were monitored for seizure for 2 h using the scale we previously described [39]. On each of the subsequent days, mice were observed for 10 min. The vehicle was an aqueous solution of 0.5% methylcellulose + 0.1% Tween 20. For 2000 mg/kg dosing, a 100 mg/mL suspension was used. For 2500 and 5000 mg/kg dosing, a 250 mg/mL suspension was used.
2.10. Data Analysis
Data with dose-responses were typically analyzed with ANOVA followed by Dunnett’s or bonferroni’s multiple comparison test using Prism. Comparing two different groups was conducted with Student’s t-test. King’s synergy test, described previously [88], was used to assess the interaction between clozapine and CX717. The alpha value was set at 0.05.
3. Results
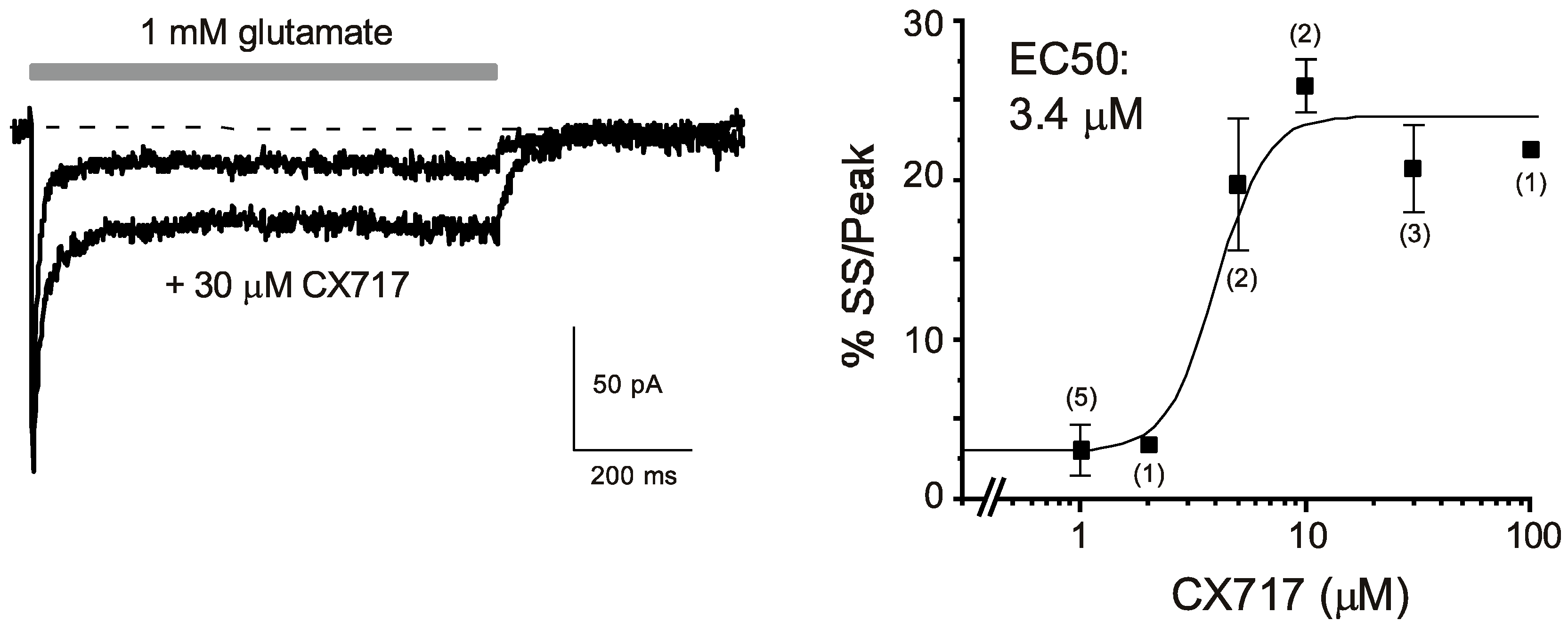
CX717 (Figure 1) was first tested for its effects on glutamate-induced currents on patches taken from the CA1 region of hippocampal slices. A 1 s pulse of 1 mM glutamate treatment resulted in an inward current that reached a peak within 1 ms (Figure 2 Left). The response then decayed to a steady-state level that typically was 3–5% of the peak current. Upon termination of glutamate application, the response returned to the baseline. CX717 enhanced glutamate-induced inward currents at concentrations of 5 μM or higher. The main effect was an increase in the steady-state/peak current which was highly significant (p < 0.001, F (6, 24) = 23.7, one-way ANOVA). A logistic equation fitted to the data points provided an EC50 of 3.4 µM. The increase reached a maximum at 10–100 µM, at which the steady-state/peak ratio was about five-times larger than in control responses (e.g., 20.7 ± 2.7% for 30 µM CX717 vs. 4.3 ± 2.7% without drug, 3 pairs, p = 0.007, paired t-test). Taken together, these experiments indicate that CX717 positively modulates AMPA receptors in hippocampal CA1 pyramidal cells. Its kinetic profile resembles that of CX516 [75], but its potency is about 60-times higher than that of CX516.
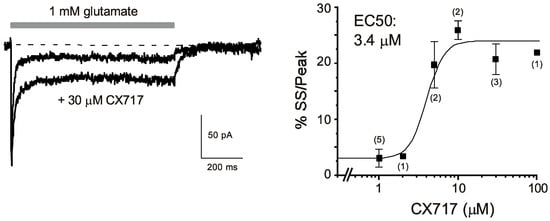
Figure 2.
Effects of CX717 on AMPA receptor-mediated currents in excised patches from hippocampal pyramidal cells in field CA1. (Left): Representative traces in the presence and absence of CX717. The horizontal bar indicates the application of 1 mM glutamate. Moreover, 30 μM CX717 was included in both the background and glutamate-containing solution. Holding potential: −50 mV. (Right): Concentration response relations. %SS/Peak denotes the percentage of steady-state current relative to the peak amplitude. Numbers in parentheses indicate the number of experiments. p < 0.001, F (6, 24) = 23.7, one-way ANOVA.
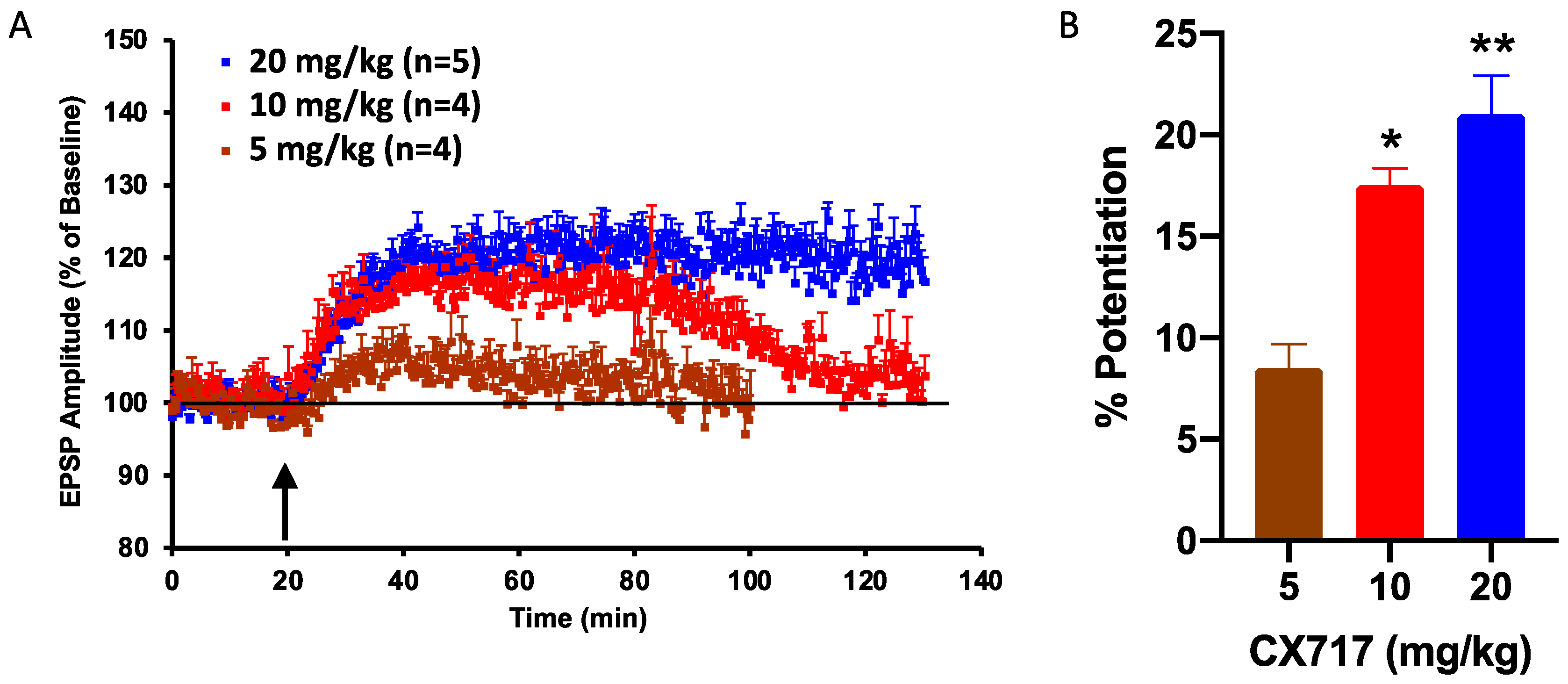
After determining the effects of CX717 in vitro, we tested the ability of CX717 to potentiate synaptic responses in vivo in anesthetized animals following IP administration. CX717 produced a long-lasting increase in the evoked EPSPs in a dose-dependent manner when dosed IP at 5, 10, or 20 mg/kg. CX717 increased the amplitude of the evoked EPSPs by 8.5 ± 1.2% (n = 4), 17.5 ± 0.9% (n = 4), and 21 ± 1.9% (n = 5), respectively, in the dentate gyrus of rats. There was a significant dose-dependent relationship (p < 0.01, F (2, 10) = 17.95, one-way ANOVA). Of note, the amplitude enhancement after 20 mg/kg remained elevated throughout the duration of the experiment (Figure 3A). At 10 mg/kg and 20 mg/kg, CX717 significantly potentiated EPSP amplitudes above baseline (Figure 3B).
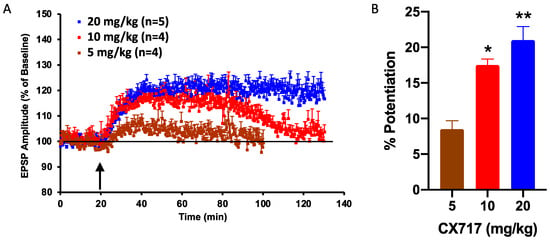
Figure 3.
Effect of CX717 on EPSPs in rat hippocampus in vivo. (A). The time course of the measured increase in the amplitude of the EPSP in the dentate gyrus following 5, 10, or 20 mg/kg CX717. (B). Potentiation of EPSP amplitudes 15 min after CX717 administration. Bars represent the mean ± SEM from 4–5 animals. p = 0.0005, F (2, 10) = 18.0, one-way ANOVA. * p < 0.05, ** p < 0.01, Dunnett’s multiple comparison test, compared to baseline.
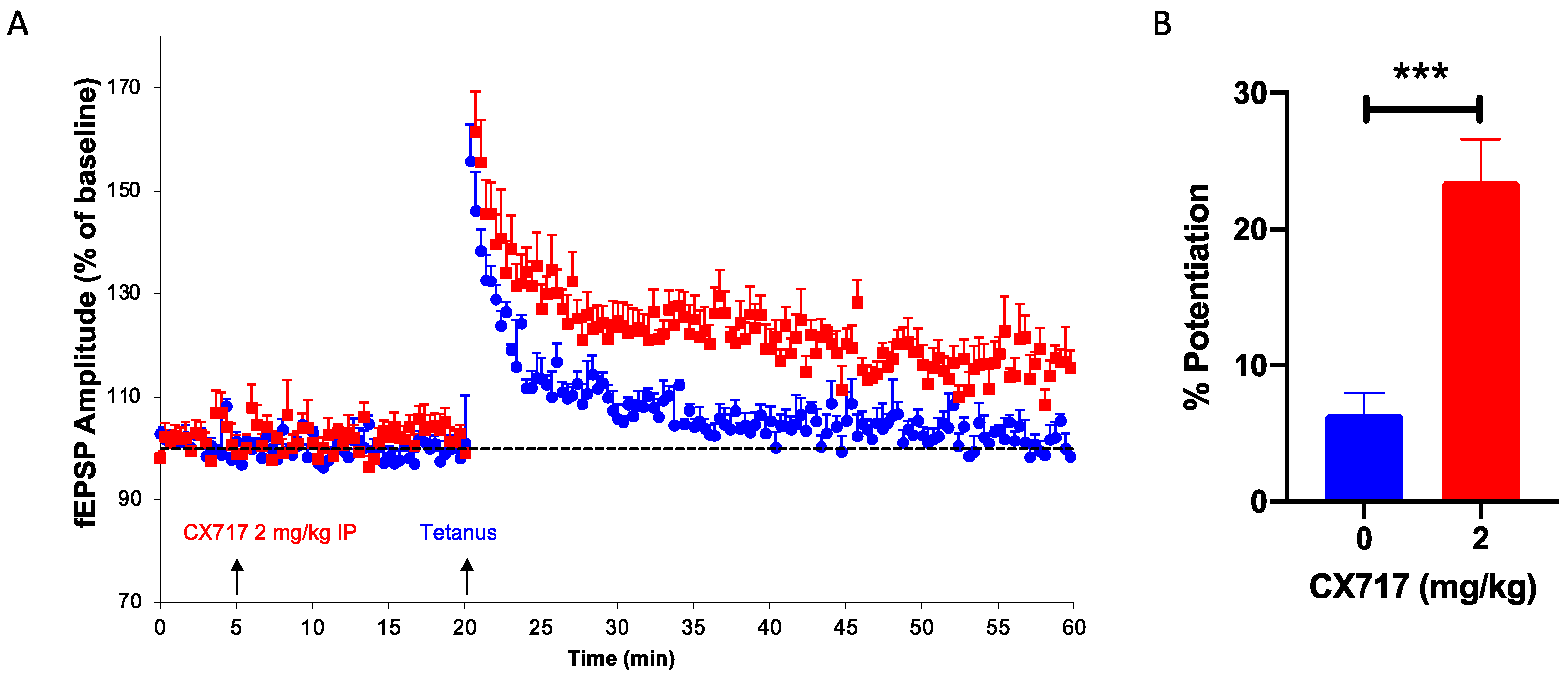
We have previously shown that high-impact [77] and low-impact ampakines [39] augment the induction of LTP, a process critical for learning and memory. In the presence of CX717, suboptimal tetanic stimuli produced robust LTP in all six animals tested (Figure 4A). CX717 did not affect baseline values, but after brief tetanic stimulation, a stable, long-lasting increase in the fEPSP of about 20% was observed (Figure 4B).
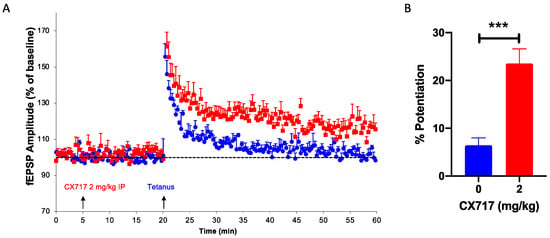
Figure 4.
Effect of CX717 on long-term potentiation (LTP) in vivo. (A). Time-course of dentate gyrus EPSP after stimulating the perforant path. (B). Mean increases in EPSP after treatment with vehicle or 2 mg/kg CX717. Mean increases in EPSP measured over ten minutes starting ten minutes after tetanic stimulation. Bars represent the mean ± SEM from 6 animals per group. *** p < 0.001, Student’s t-test.
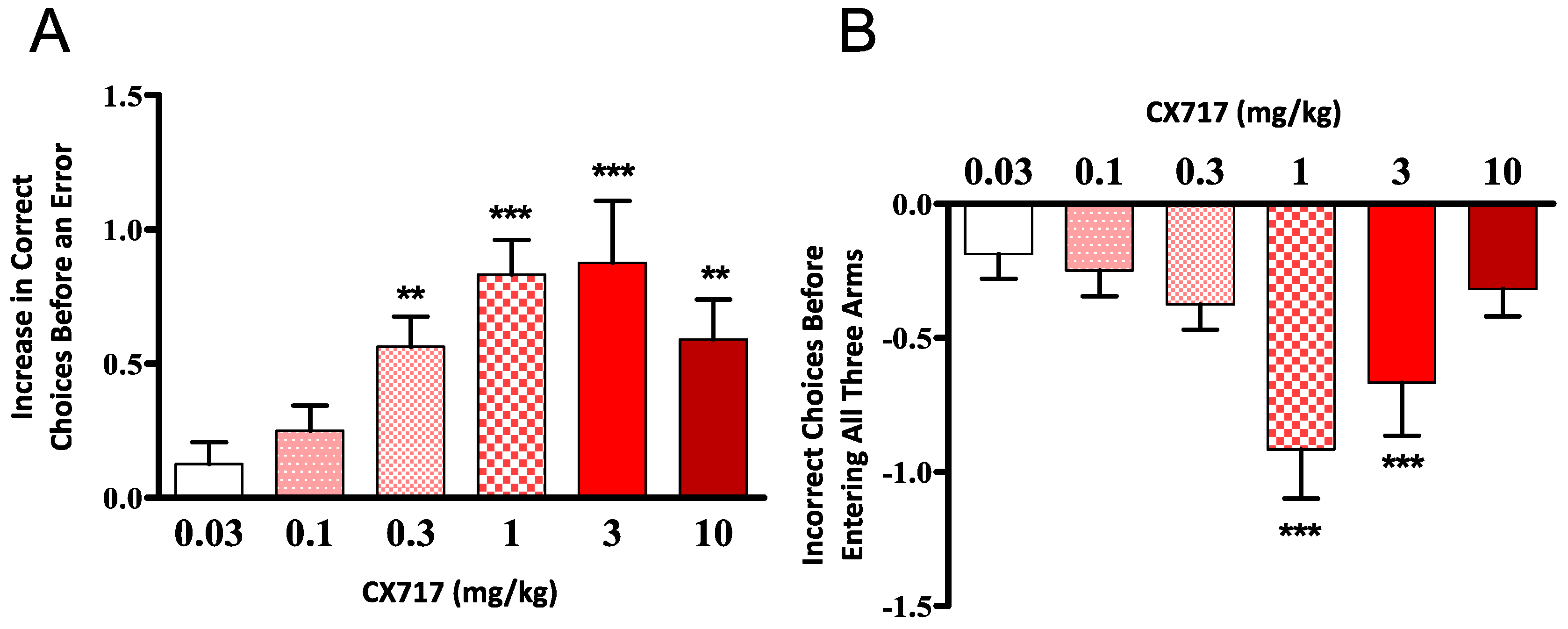
CX717 was tested for its ability to improve the performance of aged rats in the eight-arm radial maze. CX717 produced a dose-dependent increase in the number of correct choices (before making an error) by the subjects in the second maze session and a decrease in the total number of errors (Figure 5A). The difference in performance between drug and vehicle days was significant (p < 0.01, F (5, 54) = 4.48, one-way ANOVA) for doses of 0.3 mg/kg and above (Wilcoxon signed-rank test). There was a dose-dependent decrease in re-entry errors (p < 0.01, F (5, 54) = 4.641, one-way ANOVA) for doses of 1 and 3 mg/kg (Wilcoxon signed-rank test) (Figure 5B). These results demonstrate that CX717 potently enhances acquisition and retention of spatial memory in rats over the dose range of 0.3 to 10 mg/kg.
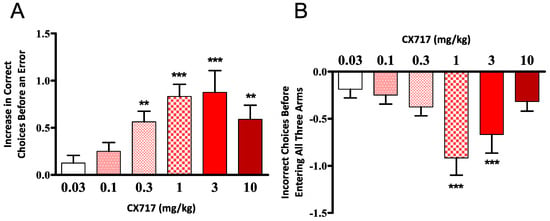
Figure 5.
Effect of CX717 on performance in the eight–arm radial maze. Data represent mean ± SEM from 10 rats showing the number of correct choices before an error (A) and the total number of incorrect choices made before all three baited arms were visited (B). (A) One-way ANOVA p < 0.01 F (5, 54) = 4.48. (B) One-Way ANOVA p < 0.01, F (5, 54) = 4.64. ** p < 0.01, *** p < 0.001, two-tail, Wilcoxon signed-rank test.
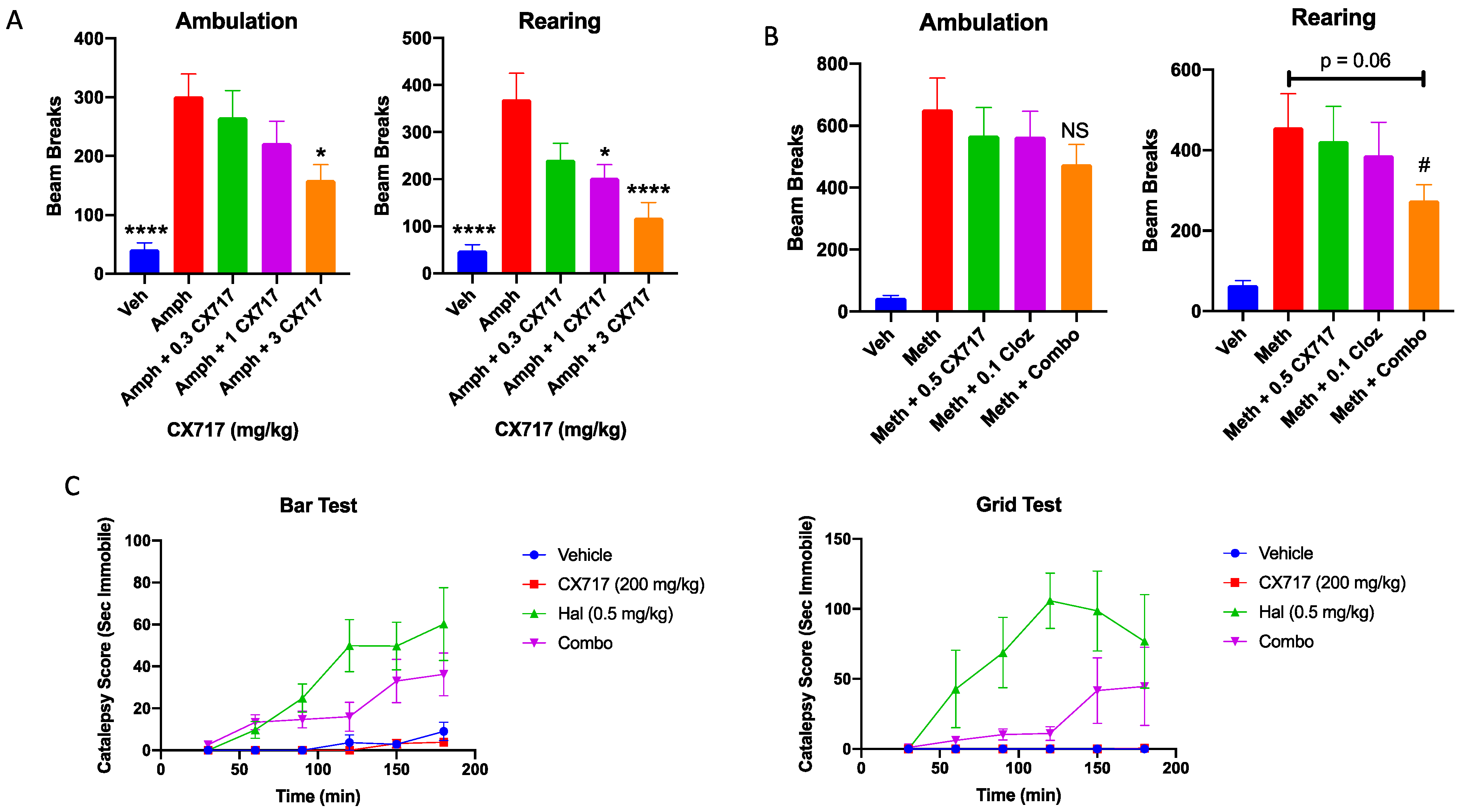
Low-impact ampakines have been evaluated for their potential antipsychotic effects in pre-clinical [46] and clinical studies [48,89,90]. Low-impact ampakines such as CX516 have also shown synergistic interactions with approved antipsychotics [46]. CX717 was assessed for its ability to antagonize amphetamine-induced hyperactivity and for a potential interaction with clozapine. Figure 6A clearly demonstrates that 1 mg/kg amphetamine was sufficient to significantly increase both rearing and ambulation in rats during the study period. CX717 dose-dependently antagonized amphetamine-induced locomotor activity. The AD50 (dose required to antagonize amphetamine effect by 50%) for rearing activity and ambulation was 2.6 and 0.9 mg/kg, respectively (Figure 6A). CX717 (1 mg/kg) significantly reduced rearing while 3 mg/kg significantly reduced both rearing and ambulation. We also examined a potential interaction with clozapine in ameliorating methamphetamine-induced hyperactivity. Methamphetamine alone produced a significant increase in rearing and ambulation activity (Figure 6B). Low-dose CX717 (0.5 mg/kg) and clozapine (0.1 mg/kg) reduced methamphetamine-induced ambulation alone by 14% and together by 29%, an additive interaction (king’s synergy test, p > 0.05). However, CX717 and clozapine reduced rearing by 9% and 18%, respectively. Interestingly, the combination reduced rearing by 46% (Figure 6B), a significantly synergistic interaction (king’s synergy test, p < 0.05). These preliminary results suggest that, in line with prior work [46], CX717 may interact favorably with approved antipsychotics.
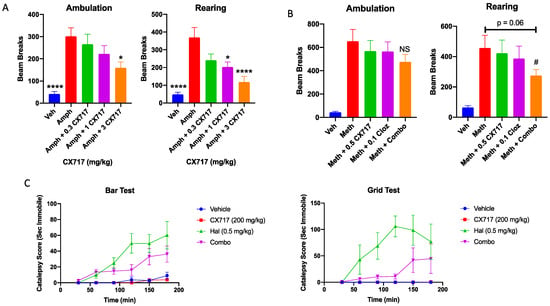
Figure 6.
Locomotor and catalepsy studies. (A) CX717 dose-dependently reduces amphetamine-induced locomotor activity (LMA) in rats. Each data point represents the mean ± SEM from 8 to 12 rats. Statistical differences were determined using a one-way ANOVA followed by Bonferroni’s multiple comparison test versus amphetamine alone; * p < 0.05. **** p < 0.0001. (B) Effects of CX717 and clozapine on methamphetamine-induced locomotor activity. Each data point represents the mean ± SEM from 10 rats. # p < 0.05, King’s synergy test. (C) Effects of CX717 on haloperidol-induced catalepsy in bar and grid tests. Graphs depict ± SEM from 6 rats per group.
Catalepsy is an undesirable side effect of approved antipsychotics. As the potential for ampakines to treat psychotic disorders is further investigated, it is important to determine whether drug candidates like CX717 possess cataleptic activity or whether they may augment the cataleptic activity associated with approved antipsychotics. In the bar test, two-way ANOVA revealed a significant time x treatment interaction (p < 0.0001, F (15, 100) = 4.5). Post hoc Bonferroni mean effect comparison illustrates that haloperidol induced significant catalepsy in this test (p < 0.0001), whereas 200 mg/kg CX717 did not (p > 0.99) (Figure 6C). Haloperidol + 200 mg/kg CX717 produced slightly less catalepsy than Haloperidol alone, though this difference was not statistically significant (p = 0.27). In the grid test, there was also a significant time x treatment interaction (p = 0.0002, F (15, 100) = 3.26, two-way ANOVA). Similar post hoc Bonferroni tests were used to compare mean effects of each treatment over time. In the grid test, haloperidol produced significant cataleptic effects (p < 0.0001), an effect not produced with 200 mg/kg CX717 (p > 0.99). Interestingly, 200 mg/kg CX717 antagonized catalepsy induced via haloperidol (p = 0.0041), demonstrating that CX717 may possess antipsychotic activity and reduce side effects of approved antipsychotics. Furthermore, these data illustrate that high doses of CX717 are devoid of catalepsy (Figure 6C).
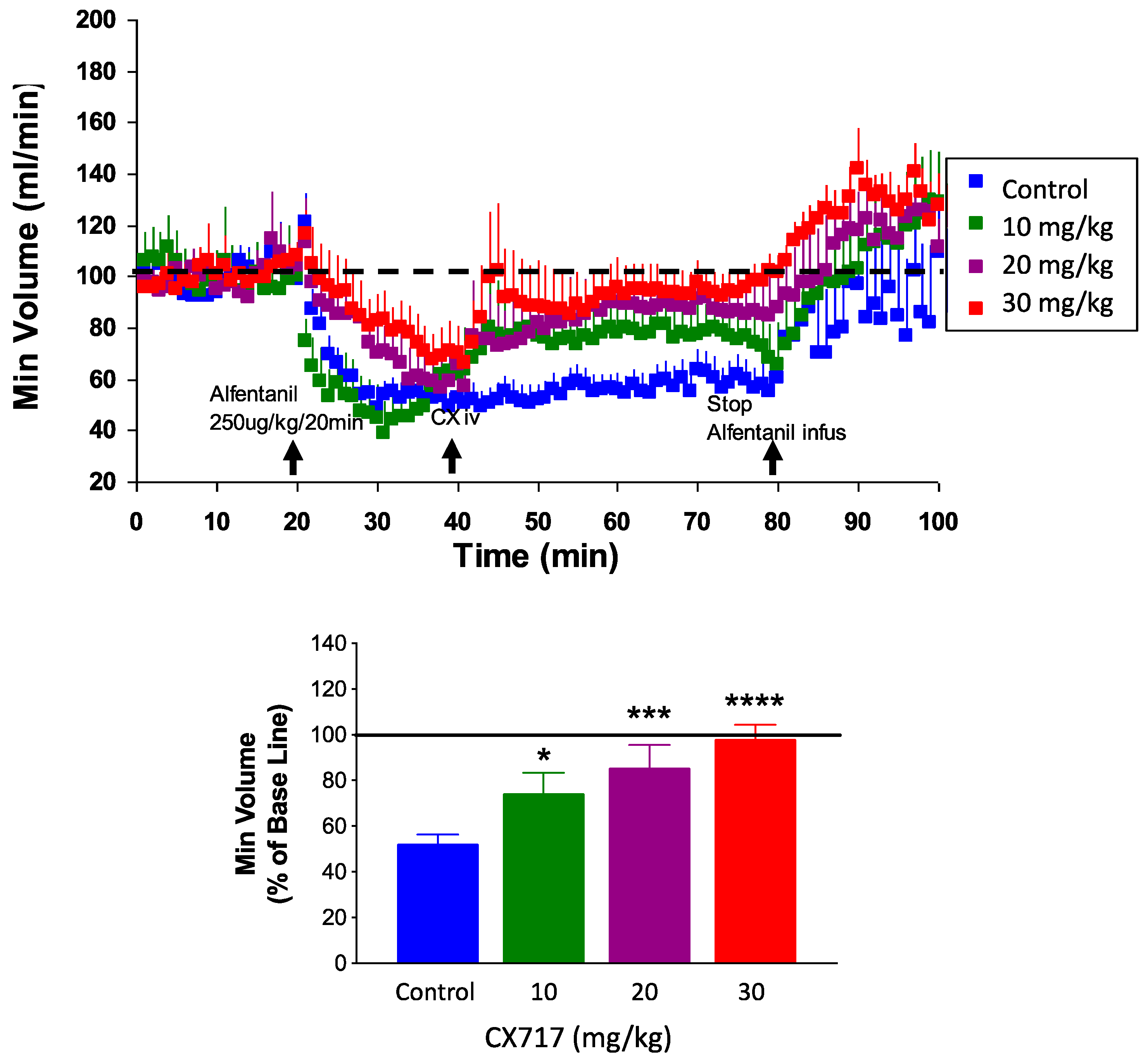
To confirm and expand upon prior work, we tested CX717 for its ability to counteract alfentanil-induced respiratory depression in rats. Potency and onset of CX717 was examined as described previously [35,36,58,59]. Alfentanil depressed breathing by about 50% (Figure 7). IV administration of CX717 dose-dependently reversed alfentanil-induced respiratory depression (p < 0.0001, F (3, 36) = 11.63, one-way ANOVA). Moreover, 10 mg/kg CX717 reduced respiratory depression to 74 ± 9.2% of control. Additionally, 20 mg/kg CX717 reduced respiratory depression to 85 ± 10% of control, and at 30 mg/kg, CX717 reversed respiratory depression to control levels (Figure 7).
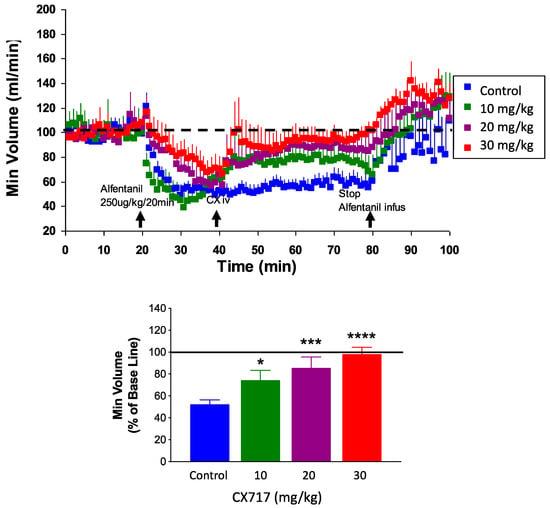
Figure 7.
Effects of CX717 on alfentanil-induced respiratory depression in vivo. Continuous infusion of alfentanil produces approximately 50% respiratory depression in rats. CX717 (10–30 mg/kg) dose-dependently rescues depressed breathing. Bar graph indicates normalized minute volume after alfentanil and increasing doses of CX717. Data are normalized to minute volume prior to alfentanil infusion. Data represent mean ± SEM from 7 to 14 rats per group. One-way ANOVA p < 0.0001, F (3, 36) = 11.63. * p < 0.05, *** p < 0.001, **** p < 0.0001, Bonferroni’s multiple comparison test to control.
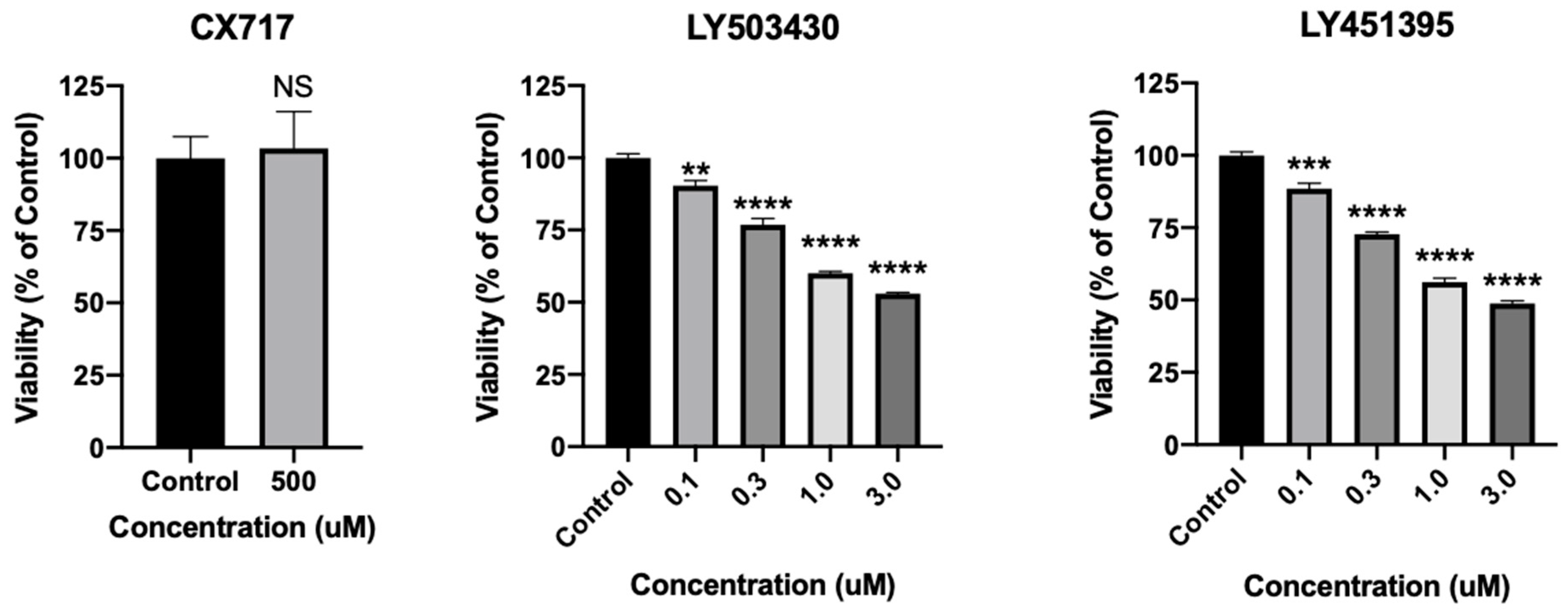
Experimental AMPAR positive allosteric modulators have shown promise previously, yet neurotoxicity has hampered their clinical development [41,42,43,44,45,91]. In order to directly assess the effects of CX717 on neuronal viability, cultured rat cortical neurons were treated with CX717 or other AMPAR PAMs LY503430 and LY451395 (Figure 8). Moreover, 500 μM CX717, a concentration much higher than what is expected to produce therapeutic effects, does not significantly affect neuronal viability (p = 0.81, t-test). However, LY503430 and LY451395 dose-dependently reduce neuronal viability at lower concentrations. LY503430 reduces neuronal viability with an IC50 of ~3.5 μM (p < 0.0001, F (4, 10) = 184.9 one-way ANOVA). LY451395 reduces neuronal viability with an IC50 of ~2.9 μM (p < 0.0001, F (4, 10) = 271.6 one-way ANOVA). Of note, LY451395 and LY503430 completely offset desensitization at 3 μM. Though less is known about the in vitro potency of CX717, 150 μM CX717 significantly reverses reductions in breathing rhythms produced via ethanol and barbiturates [58]. These findings demonstrate that, unlike other more potent AMPAR modulators that markedly offset desensitization, CX717 is not toxic to neurons at high concentrations.
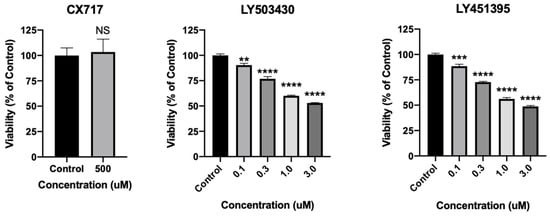
Figure 8.
Effects of CX717 on neuronal viability. Neuronal cultures were treated with CX717, LY503430, or LY451395 for 24 h, after which viability was assessed. Data represent mean ± SEM from 3 to 4 independent experiments. NS, p = 0.81, t-test. One-way ANOVA p < 0.0001 for LY503430 and LY451395. ** p < 0.01, *** p < 0.001, **** p < 0.0001, Dunnett’s multiple comparison test to control.
We additionally performed a single-dose toxicity study with a small group of mice. Mice were treated with increasing doses of CX717 (2000, 2500, and 5000 mg/kg) (Table 1). The minimum lethal dose of CX717 was 2500 mg/kg, in which both mice that were treated died by day 14. Moreover, 5000 mg/kg CX717 induced a seizure and death in one mouse, but the other mouse was alive at day 14. For the three mice treated with 2000 mg/kg CX717, there was no seizure induction nor death of any mouse. These findings highlight that CX717 has a notable therapeutic ratio and does not produce seizures or lethality at therapeutic concentrations.

Table 1.
Single-dose toxicology of CX717.
4. Discussion
A low-impact ampakine with sufficient potency would have tremendous implications for the treatment of several neurological and neuropsychiatric disorders. Low-impact ampakines have been shown to enhance cognition [39,56,57,92,93], restore respiration and bladder function following spinal cord injury [51,52,54,55], reverse OIRD [35,36,37,39], and exhibit a notable lack of epileptic effects at therapeutic doses [39,46]. Our work supports the multitude of therapeutic prospects of CX717 in the treatment of neurological/neuropsychiatric conditions in which AMPAR signaling may be compromised.
The absence of pro-convulsive effects of CX717 may be at least partially explained by its effects on glutamate-induced currents in patches taken from hippocampal slices. CX717 maximally increases steady-state current to 25% of peak current (Figure 2) while ampakines like cyclothiazide completely offset desensitization and possesses noteworthy seizurogenic activity [41,42,44,91,94,95]. Further, CX717 is devoid of direct neuronal toxicity at high concentrations in contrast to other AMPAR positive modulators such as LY503430 and LY451395 (Figure 8). In vivo, we found that CX717 augmented synaptic transmission in a dose-dependent fashion (Figure 3) and enhanced LTP induction at a low dose of 2 mg/kg (Figure 4). It is thought that AMPAR dysfunction underpins memory dysfunction of neurological disorders, including Alzheimer’s Disease [10,11,12,13,14,15,16,17]. We previously reported that acute treatment of the ampakine CX1846 restores LTP in aged rats [77]. It would be of interest to determine whether acute or chronic treatment of low-impact ampakines such as CX717 or CX1739 are able to restore age-associated defects in LTP. Neurotrophin induction via chronic low-impact ampakine treatment [96] could have profound implications in treating CNS disorders. Our prior work with CX516 and CX691 was conducted with young, three-month-old rats. Whether newer generation low-impact ampakines such as CX717 and CX1739 can augment neurotrophin expression in aged rodents serves as an avenue for future research.
It is interesting to note that in the eight-arm radial maze task, CX717 produced statistically significant improvement at 0.3 mg/kg, a dose lower than that which is needed to augment synaptic transmission and one that is presumably insufficient to enhance LTP in vivo. We observed similar potency in our studies with CX1739 [39]. It is possible that AMPAR modulation via ampakines may have therapeutic effects even at doses that do not acutely alter electrophysiological parameters in vivo. As discussed previously [39], the AMPAR subunits for which low-impact ampakines have the strongest affinity is currently unknown. However, it may be possible to infer their affinities by determining the brain regions in which low-impact ampakines most durably augment neurotrophin induction [96] or glucose metabolism [97] and determining the AMPAR subunit composition in those brain regions.
CX717 has already been shown to ameliorate some of the symptoms of ADHD in adults in a phase 2a study [65]. Therefore, we reasoned that CX717 may be administered as a monotherapy or in combination with approved stimulants. Figure 6 shows that CX717 reduced amphetamine-induced hyperactivity between 1 and 3 mg/kg. CX717 is considerably more potent in this assay than CX516. In one study, CX516 only produced a modest effect at 170 mg/kg [98]. In another report, CX516 produced modest partial effects between 10 and 30 mg/kg [46]. These findings, in addition to the hippocampal slice experiments, demonstrate that CX717 is substantially more potent than CX516. In future work, we might also further explore interactions between CX717 and approved antipsychotics. Our results reported here demonstrate that there may be a favorable interaction between CX717 and clozapine (Figure 6b). Figure 6c demonstrates that CX717 does not possess cataleptic activity at high doses, in contradistinction to this side effect of traditional antipsychotic medications. These findings raise the possibility that at doses in which we observed a reduction in certain ADHD symptoms [65], CX717 may possess antipsychotic effects in humans. CX717 may also antagonize cataleptic effects of approved antipsychotics (Figure 6c).
The OIRD presented here (Figure 7) supports conclusions made previously about CX717′s ability to offset OIRD in pre-clinical models [35,36,37,58] and in humans [40] without compromising the pain alleviating effects of opiates. The doses that produced positive effects in this study, 0.3–10 mg/kg, are well below the dose of 2500 mg/kg that produced lethality in mice (Table 1). These data provide further support to the idea that the therapeutic effects of ampakines can indeed be separated from their epileptogenic and neurotoxic effects. Taken together, these findings highlight that CX717 should be further explored to treat psychiatric conditions like ADHD and the sequelae of spinal cord injury including depressed breathing and bladder dysfunction and can be utilized to treat OIRD while preserving the analgesic effects of opiates.
Author Contributions
Conceptualization, D.P.R., S.Z., R.C., J.M.W. and A.L. Methodology, S.Z. Software, D.P.R. and S.Z. Validation, S.Z. Formal analysis, D.P.R. and S.Z. Investigation, S.Z. Resources, S.Z. and A.L. Data curation, D.P.R., S.Z. and A.L. Writing—original draft preparation, D.P.R., S.Z., R.C., J.L.S., J.M.W. and A.L. Writing—review and editing, D.P.R., S.Z., R.C., J.L.S., J.M.W. and A.L. Visualization, D.P.R., S.Z., J.M.W. and A.L. Supervision, R.C., J.M.W., and A.L. Project administration, J.M.W. and A.L. Funding acquisition, A.L. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by RespireRx Pharmaceuticals Inc.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by UC Irvine IACUC (protocol #61-05-05B, first approved May 2005).
Data Availability Statement
Data are available upon request made to the authors.
Conflicts of Interest
With respect to the manuscript, Daniel Radin, Arnold Lippa, Jeffrey Witkin, and Rok Cerne all are associated with RespireRx, where A.L. is acting CEO, and D.P.R., R.C. and J.M.W. are non-paid researchers who occasionally conduct studies on these compounds. The company had no role in this study’s design, data gathering, interpretation of the results, and writing of the manuscript. All authors of this study consented to publication. Sheng Zhong used to work at RespireRx when this study was being conducted. He now works at Psychogenics.
References
- Bredt, D.S.; Nicoll, R.A. AMPA receptor trafficking at excitatory synapses. Neuron 2003, 40, 361–379. [Google Scholar] [CrossRef]
- Naaijen, J.; Bralten, J.; Poelmans, G.; Consortium, I.; Glennon, J.C.; Franke, B.; Buitelaar, J.K. Glutamatergic and GABAergic gene sets in attention-deficit/hyperactivity disorder: Association to overlapping traits in ADHD and autism. Transl. Psychiatry 2017, 7, e999. [Google Scholar] [CrossRef] [PubMed]
- Medin, T.; Jensen, V.; Skare, O.; Storm-Mathisen, J.; Hvalby, O.; Bergersen, L.H. Altered alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor function and expression in hippocampus in a rat model of attention-deficit/hyperactivity disorder (ADHD). Behav. Brain Res. 2019, 360, 209–215. [Google Scholar] [CrossRef]
- Bai, W.J.; Luo, X.G.; Jin, B.H.; Zhu, K.S.; Guo, W.Y.; Zhu, X.Q.; Qin, X.; Yang, Z.X.; Zhao, J.J.; Chen, S.R.; et al. Deficiency of transmembrane AMPA receptor regulatory protein gamma-8 leads to attention-deficit hyperactivity disorder-like behavior in mice. Zool. Res. 2022, 43, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Drummond, J.B.; Tucholski, J.; Haroutunian, V.; Meador-Woodruff, J.H. Transmembrane AMPA receptor regulatory protein (TARP) dysregulation in anterior cingulate cortex in schizophrenia. Schizophr. Res. 2013, 147, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Tucholski, J.; Simmons, M.S.; Pinner, A.L.; Haroutunian, V.; McCullumsmith, R.E.; Meador-Woodruff, J.H. Abnormal N-linked glycosylation of cortical AMPA receptor subunits in schizophrenia. Schizophr. Res. 2013, 146, 177–183. [Google Scholar] [CrossRef]
- Zeppillo, T.; Schulmann, A.; Macciardi, F.; Hjelm, B.E.; Focking, M.; Sequeira, P.A.; Guella, I.; Cotter, D.; Bunney, W.E.; Limon, A.; et al. Functional impairment of cortical AMPA receptors in schizophrenia. Schizophr. Res. 2022, 249, 25–37. [Google Scholar] [CrossRef]
- Benesh, J.L.; Mueller, T.M.; Meador-Woodruff, J.H. AMPA receptor subunit localization in schizophrenia anterior cingulate cortex. Schizophr. Res. 2022, 249, 16–24. [Google Scholar] [CrossRef]
- Singh, T.; Poterba, T.; Curtis, D.; Akil, H.; Al Eissa, M.; Barchas, J.D.; Bass, N.; Bigdeli, T.B.; Breen, G.; Bromet, E.J.; et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature 2022, 604, 509–516. [Google Scholar] [CrossRef]
- Ikonomovic, M.D.; Mizukami, K.; Davies, P.; Hamilton, R.; Sheffield, R.; Armstrong, D.M. The loss of GluR2(3) immunoreactivity precedes neurofibrillary tangle formation in the entorhinal cortex and hippocampus of Alzheimer brains. J. Neuropathol. Exp. Neurol. 1997, 56, 1018–1027. [Google Scholar] [CrossRef]
- Ikonomovic, M.D.; Nocera, R.; Mizukami, K.; Armstrong, D.M. Age-related loss of the AMPA receptor subunits GluR2/3 in the human nucleus basalis of Meynert. Exp. Neurol. 2000, 166, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Han, H.; Li, H.; Bai, Y.; Wang, W.; Tu, M.; Peng, Y.; Zhou, L.; He, W.; Wu, X.; et al. Long-term potentiation decay and memory loss are mediated by AMPAR endocytosis. J. Clin. Investig. 2015, 125, 234–247. [Google Scholar] [CrossRef]
- Suzuki, K.; Elegheert, J.; Song, I.; Sasakura, H.; Senkov, O.; Matsuda, K.; Kakegawa, W.; Clayton, A.J.; Chang, V.T.; Ferrer-Ferrer, M.; et al. A synthetic synaptic organizer protein restores glutamatergic neuronal circuits. Science 2020, 369, eabb4853. [Google Scholar] [CrossRef]
- Alfaro-Ruiz, R.; Aguado, C.; Martin-Belmonte, A.; Moreno-Martinez, A.E.; Merchan-Rubira, J.; Hernandez, F.; Avila, J.; Fukazawa, Y.; Lujan, R. Alteration in the Synaptic and Extrasynaptic Organization of AMPA Receptors in the Hippocampus of P301S Tau Transgenic Mice. Int. J. Mol. Sci. 2022, 23, 13527. [Google Scholar] [CrossRef]
- Wright, A.L.; Konen, L.M.; Mockett, B.G.; Morris, G.P.; Singh, A.; Burbano, L.E.; Milham, L.; Hoang, M.; Zinn, R.; Chesworth, R.; et al. The Q/R editing site of AMPA receptor GluA2 subunit acts as an epigenetic switch regulating dendritic spines, neurodegeneration and cognitive deficits in Alzheimer's disease. Mol. Neurodegener. 2023, 18, 65. [Google Scholar] [CrossRef]
- Prinkey, K.; Thompson, E.; Saikia, J.; Cid, T.; Dore, K. Fluorescence lifetime imaging of AMPA receptor endocytosis in living neurons: Effects of Abeta and PP1. Front. Mol. Neurosci. 2024, 17, 1409401. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Da Ros, L.U.; Machado, L.S.; Rocha, A.; Lazzarotto, G.; Carello-Collar, G.; De Bastiani, M.A.; Ferrari-Souza, J.P.; Lussier, F.Z.; Souza, D.O.; et al. The glutamatergic system in Alzheimer's disease: A systematic review with meta-analysis. Mol. Psychiatry 2024. [Google Scholar] [CrossRef]
- Restivo, L.; Ferrari, F.; Passino, E.; Sgobio, C.; Bock, J.; Oostra, B.A.; Bagni, C.; Ammassari-Teule, M. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 11557–11562. [Google Scholar] [CrossRef]
- Chojnacka, M.; Beroun, A.; Magnowska, M.; Stawikowska, A.; Cysewski, D.; Milek, J.; Dziembowska, M.; Kuzniewska, B. Impaired synaptic incorporation of AMPA receptors in a mouse model of fragile X syndrome. Front. Mol. Neurosci. 2023, 16, 1258615. [Google Scholar] [CrossRef]
- Suresh, A.; Dunaevsky, A. Impaired AMPARs Translocation into Dendritic Spines with Motor Skill Learning in the Fragile X Mouse Model. eNeuro 2023, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, N.; Shamsizadeh, A.; Fatemi, I.; Allahtavakoli, M.; Moghadam-Ahmadi, A.; Kaviani, E.; Kaeidi, A. CX691, as an AMPA receptor positive modulator, improves the learning and memory in a rat model of Alzheimer's disease. Iran. J. Basic. Med. Sci. 2018, 21, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.K.; Whalley, K.; Robinson, C.S.; Ward, M.A.; Hicks, C.A.; Lodge, D.; Vandergriff, J.L.; Baumbarger, P.; Siuda, E.; Gates, M.; et al. LY503430, a novel alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor potentiator with functional, neuroprotective and neurotrophic effects in rodent models of Parkinson's disease. J. Pharmacol. Exp. Ther. 2003, 306, 752–762. [Google Scholar] [CrossRef]
- O'Neill, M.J.; Murray, T.K.; Whalley, K.; Ward, M.A.; Hicks, C.A.; Woodhouse, S.; Osborne, D.J.; Skolnick, P. Neurotrophic actions of the novel AMPA receptor potentiator, LY404187, in rodent models of Parkinson's disease. Eur. J. Pharmacol. 2004, 486, 163–174. [Google Scholar] [CrossRef]
- O'Neill, M.J.; Murray, T.K.; Clay, M.P.; Lindstrom, T.; Yang, C.R.; Nisenbaum, E.S. LY503430: Pharmacology, pharmacokinetics, and effects in rodent models of Parkinson's disease. CNS Drug Rev. 2005, 11, 77–96. [Google Scholar] [CrossRef]
- Jourdi, H.; Hamo, L.; Oka, T.; Seegan, A.; Baudry, M. BDNF mediates the neuroprotective effects of positive AMPA receptor modulators against MPP+-induced toxicity in cultured hippocampal and mesencephalic slices. Neuropharmacology 2009, 56, 876–885. [Google Scholar] [CrossRef]
- Ogier, M.; Wang, H.; Hong, E.; Wang, Q.; Greenberg, M.E.; Katz, D.M. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 10912–10917. [Google Scholar] [CrossRef] [PubMed]
- Degano, A.L.; Park, M.J.; Penati, J.; Li, Q.; Ronnett, G.V. MeCP2 is required for activity-dependent refinement of olfactory circuits. Mol. Cell. Neurosci. 2014, 59, 63–75. [Google Scholar] [CrossRef]
- Scaramuzza, L.; De Rocco, G.; Desiato, G.; Cobolli Gigli, C.; Chiacchiaretta, M.; Mirabella, F.; Pozzi, D.; De Simone, M.; Conforti, P.; Pagani, M.; et al. The enhancement of activity rescues the establishment of Mecp2 null neuronal phenotypes. EMBO Mol. Med. 2021, 13, e12433. [Google Scholar] [CrossRef]
- Clarkson, A.N.; Overman, J.J.; Zhong, S.; Mueller, R.; Lynch, G.; Carmichael, S.T. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 3766–3775. [Google Scholar] [CrossRef]
- Clarkson, A.N.; Parker, K.; Nilsson, M.; Walker, F.R.; Gowing, E.K. Combined ampakine and BDNF treatments enhance poststroke functional recovery in aged mice via AKT-CREB signaling. J. Cereb. Blood Flow. Metab. 2015, 35, 1272–1279. [Google Scholar] [CrossRef]
- Simmons, D.A.; Rex, C.S.; Palmer, L.; Pandyarajan, V.; Fedulov, V.; Gall, C.M.; Lynch, G. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington's disease knockin mice. Proc. Natl. Acad. Sci. USA 2009, 106, 4906–4911. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, C.; Cummings, D.M.; Hickey, M.A.; Kleiman-Weiner, M.; Chen, J.Y.; Watson, J.B.; Levine, M.S. Rescuing the Corticostriatal Synaptic Disconnection in the R6/2 Mouse Model of Huntington's Disease: Exercise, Adenosine Receptors and Ampakines. PLoS Curr. 2010, 2. [Google Scholar] [CrossRef]
- Simmons, D.A.; Mehta, R.A.; Lauterborn, J.C.; Gall, C.M.; Lynch, G. Brief ampakine treatments slow the progression of Huntington's disease phenotypes in R6/2 mice. Neurobiol. Dis. 2011, 41, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Baudry, M.; Kramar, E.; Xu, X.; Zadran, H.; Moreno, S.; Lynch, G.; Gall, C.; Bi, X. Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiol. Dis. 2012, 47, 210–215. [Google Scholar] [CrossRef]
- Ren, J.; Ding, X.; Funk, G.D.; Greer, J.J. Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology 2009, 110, 1364–1370. [Google Scholar] [CrossRef]
- Greer, J.J.; Ren, J. Ampakine therapy to counter fentanyl-induced respiratory depression. Respir. Physiol. Neurobiol. 2009, 168, 153–157. [Google Scholar] [CrossRef]
- Lorier, A.R.; Funk, G.D.; Greer, J.J. Opiate-induced suppression of rat hypoglossal motoneuron activity and its reversal by ampakine therapy. PLoS ONE 2010, 5, e8766. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Meng, F.H.; Dai, W.; Yong, Z.; Liu, J.Q.; Zhou, X.B.; Li, S. Design, Synthesis and Biological Evaluation of Brain-Targeted Thiamine Disulfide Prodrugs of Ampakine Compound LCX001. Molecules 2016, 21, 488. [Google Scholar] [CrossRef]
- Radin, D.P.; Zhong, S.; Cerne, R.; Shoaib, M.; Witkin, J.M.; Lippa, A. Low-Impact Ampakine CX1739 Exerts Pro-Cognitive Effects and Reverses Opiate-Induced Respiratory Depression in Rodents. Future Pharmacol. 2024, 4, 173–187. [Google Scholar] [CrossRef]
- Oertel, B.G.; Felden, L.; Tran, P.V.; Bradshaw, M.H.; Angst, M.S.; Schmidt, H.; Johnson, S.; Greer, J.J.; Geisslinger, G.; Varney, M.A.; et al. Selective antagonism of opioid-induced ventilatory depression by an ampakine molecule in humans without loss of opioid analgesia. Clin. Pharmacol. Ther. 2010, 87, 204–211. [Google Scholar] [CrossRef]
- Lasztoczi, B.; Kardos, J. Cyclothiazide prolongs low [Mg2+]-induced seizure-like events. J. Neurophysiol. 2006, 96, 3538–3544. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Qian, B.; Liu, J.; Fan, M.; Chen, G.; Wang, Y. Cyclothiazide induces seizure behavior in freely moving rats. Brain Res. 2010, 1355, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, C.L.; Hurst, R.S.; Scialis, R.J.; Osgood, S.M.; Bryce, D.K.; Hoffmann, W.E.; Lazzaro, J.T.; Hanks, A.N.; Lotarski, S.; Weber, M.L.; et al. Positive allosteric modulation of AMPA receptors from efficacy to toxicity: The interspecies exposure-response continuum of the novel potentiator PF-4778574. J. Pharmacol. Exp. Ther. 2013, 347, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Cheng, Z.; Liu, J.; Wang, Y. Downregulated GABA and BDNF-TrkB pathway in chronic cyclothiazide seizure model. Neural Plast. 2014, 2014, 310146. [Google Scholar] [CrossRef] [PubMed]
- Kunugi, A.; Tanaka, M.; Suzuki, A.; Tajima, Y.; Suzuki, N.; Suzuki, M.; Nakamura, S.; Kuno, H.; Yokota, A.; Sogabe, S.; et al. TAK-137, an AMPA-R potentiator with little agonistic effect, has a wide therapeutic window. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Luu, N.T.; Herbst, T.A.; Knapp, R.; Lutz, D.; Arai, A.; Rogers, G.A.; Lynch, G. Synergistic interactions between ampakines and antipsychotic drugs. J. Pharmacol. Exp. Ther. 1999, 289, 392–397. [Google Scholar] [PubMed]
- Arai, A.C.; Xia, Y.F.; Rogers, G.; Lynch, G.; Kessler, M. Benzamide-type AMPA receptor modulators form two subfamilies with distinct modes of action. J. Pharmacol. Exp. Ther. 2002, 303, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Marenco, S.; Egan, M.F.; Goldberg, T.E.; Knable, M.B.; McClure, R.K.; Winterer, G.; Weinberger, D.R. Preliminary experience with an ampakine (CX516) as a single agent for the treatment of schizophrenia: A case series. Schizophr. Res. 2002, 57, 221–226. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Krause, S.E.; Block, S.S.; Guter, S.; Wuu, J.; Leurgans, S.; Decle, P.; Potanos, K.; Cook, E.; Salt, J.; et al. Effect of CX516, an AMPA-modulating compound, on cognition and behavior in fragile X syndrome: A controlled trial. J. Child. Adolesc. Psychopharmacol. 2006, 16, 525–540. [Google Scholar] [CrossRef]
- Turner, S.M.; ElMallah, M.K.; Hoyt, A.K.; Greer, J.J.; Fuller, D.D. Ampakine CX717 potentiates intermittent hypoxia-induced hypoglossal long-term facilitation. J. Neurophysiol. 2016, 116, 1232–1238. [Google Scholar] [CrossRef]
- Wollman, L.B.; Streeter, K.A.; Fusco, A.F.; Gonzalez-Rothi, E.J.; Sandhu, M.S.; Greer, J.J.; Fuller, D.D. Ampakines stimulate phrenic motor output after cervical spinal cord injury. Exp. Neurol. 2020, 334, 113465. [Google Scholar] [CrossRef]
- Rana, S.; Sunshine, M.D.; Greer, J.J.; Fuller, D.D. Ampakines Stimulate Diaphragm Activity after Spinal Cord Injury. J. Neurotrauma 2021, 38, 3467–3482. [Google Scholar] [CrossRef]
- Witkin, J.M.; Radin, D.P.; Rana, S.; Fuller, D.D.; Fusco, A.F.; Demers, J.C.; Pradeep Thakre, P.; Smith, J.L.; Lippa, A.; Cerne, R. AMPA receptors play an important role in the biological consequences of spinal cord injury: Implications for AMPA receptor modulators for therapeutic benefit. Biochem. Pharmacol. 2024, 116302. [Google Scholar] [CrossRef]
- Rana, S.; Alom, F.; Martinez, R.C.; Fuller, D.D.; Mickle, A.D. Acute ampakines increase voiding function and coordination in a rat model of SCI. eLife 2024, 12, 1–19. [Google Scholar] [CrossRef]
- Rana, S.; Thakre, P.P.; Fuller, D.D. Ampakines increase diaphragm activation following mid-cervical contusion injury in rats. Exp. Neurol. 2024, 376, 114769. [Google Scholar] [CrossRef]
- Porrino, L.J.; Daunais, J.B.; Rogers, G.A.; Hampson, R.E.; Deadwyler, S.A. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005, 3, e299. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Balabhadrapatruni, S.; Masumura, C.; Darlington, C.L.; Smith, P.F. Effects of the putative cognitive-enhancing ampakine, CX717, on attention and object recognition memory. Curr. Alzheimer Res. 2011, 8, 876–882. [Google Scholar] [CrossRef]
- Ren, J.; Ding, X.; Greer, J.J. Respiratory depression in rats induced by alcohol and barbiturate and rescue by ampakine CX717. J. Appl. Physiol. 2012, 113, 1004–1011. [Google Scholar] [CrossRef]
- Ren, J.; Lenal, F.; Yang, M.; Ding, X.; Greer, J.J. Coadministration of the AMPAKINE CX717 with propofol reduces respiratory depression and fatal apneas. Anesthesiology 2013, 118, 1437–1445. [Google Scholar] [CrossRef]
- Gordillo-Salas, M.; Pascual-Anton, R.; Ren, J.; Greer, J.; Adell, A. Antidepressant-Like Effects of CX717, a Positive Allosteric Modulator of AMPA Receptors. Mol. Neurobiol. 2020, 57, 3498–3507. [Google Scholar] [CrossRef] [PubMed]
- Wollman, L.B.; Streeter, K.A.; Fuller, D.D. Ampakine pretreatment enables a single brief hypoxic episode to evoke phrenic motor facilitation. J. Neurophysiol. 2020, 123, 993–1003. [Google Scholar] [CrossRef]
- Thakre, P.P.; Sunshine, M.D.; Fuller, D.D. Ampakine pretreatment enables a single hypoxic episode to produce phrenic motor facilitation with no added benefit of additional episodes. J. Neurophysiol. 2021, 126, 1420–1429. [Google Scholar] [CrossRef]
- Thakre, P.P.; Sunshine, M.D.; Fuller, D.D. Spinally delivered ampakine CX717 increases phrenic motor output in adult rats. Respir. Physiol. Neurobiol. 2022, 296, 103814. [Google Scholar] [CrossRef]
- Thakre, P.P.; Fuller, D.D. Pattern sensitivity of ampakine-hypoxia interactions for evoking phrenic motor facilitation in anesthetized rat. J. Neurophysiol. 2023, 131, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.A.; Stein, M.; Mansbach, H. Treatment of adult ADHD with the novel Ampakine CX717. In Proceedings of the 53rd Annual Meeting of the American Academy of Child and Adolescent Psychiatry, San Diego, CA, USA, 24–29 October 2006; p. A42. [Google Scholar]
- Purcell, R.; Lynch, G.; Gall, C.; Johnson, S.; Sheng, Z.; Stephen, M.R.; Cook, J.; Garman, R.H.; Jortner, B.; Bolon, B.; et al. Brain Vacuolation Resulting From Administration of the Type II Ampakine CX717 Is An Artifact Related to Molecular Structure and Chemical Reaction With Tissue Fixative Agents. Toxicol. Sci. 2018, 162, 383–395. [Google Scholar] [CrossRef]
- Yamada, K.A. Therapeutic potential of positive AMPA receptor modulators in the treatment of neurological disease. Expert. Opin. Investig. Drugs 2000, 9, 765–778. [Google Scholar] [CrossRef]
- O'Neill, M.J.; Bleakman, D.; Zimmerman, D.M.; Nisenbaum, E.S. AMPA receptor potentiators for the treatment of CNS disorders. Curr. Drug Targets. CNS Neurol. Disord. 2004, 3, 181–194. [Google Scholar] [CrossRef]
- O'Neill, M.J.; Dix, S. AMPA receptor potentiators as cognitive enhancers. IDrugs Investig. Drugs J. 2007, 10, 185–192. [Google Scholar]
- Woolley, M.L.; Waters, K.A.; Gartlon, J.E.; Lacroix, L.P.; Jennings, C.; Shaughnessy, F.; Ong, A.; Pemberton, D.J.; Harries, M.H.; Southam, E.; et al. Evaluation of the pro-cognitive effects of the AMPA receptor positive modulator, 5-(1-piperidinylcarbonyl)-2,1,3-benzoxadiazole (CX691), in the rat. Psychopharmacology 2009, 202, 343–354. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Mohn, A.R.; Bohn, L.M.; Caron, M.G. Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proc. Natl. Acad. Sci. USA 2001, 98, 11047–11054. [Google Scholar] [CrossRef]
- Hess, U.S.; Whalen, S.P.; Sandoval, L.M.; Lynch, G.; Gall, C.M. Ampakines reduce methamphetamine-driven rotation and activate neocortex in a regionally selective fashion. Neuroscience 2003, 121, 509–521. [Google Scholar] [CrossRef]
- Arai, A.; Kessler, M.; Xiao, P.; Ambros-Ingerson, J.; Rogers, G.; Lynch, G. A centrally active drug that modulates AMPA receptor gated currents. Brain Res. 1994, 638, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Arai, A.; Kessler, M.; Ambros-Ingerson, J.; Quan, A.; Yigiter, E.; Rogers, G.; Lynch, G. Effects of a centrally active benzoylpyrrolidine drug on AMPA receptor kinetics. Neuroscience 1996, 75, 573–585. [Google Scholar] [CrossRef]
- Arai, A.; Kessler, M.; Rogers, G.; Lynch, G. Effects of a memory-enhancing drug on DL-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor currents and synaptic transmission in hippocampus. J. Pharmacol. Exp. Ther. 1996, 278, 627–638. [Google Scholar]
- Arai, A.; Lynch, G. Response to repetitive stimulation of AMPA receptors in patches excised from fields CA1 and CA3 of the hippocampus. Brain Res. 1996, 716, 202–206. [Google Scholar] [CrossRef]
- Radin, D.P.; Zhong, S.; Purcell, R.; Lippa, A. Acute ampakine treatment ameliorates age-related deficits in long-term potentiation. Biomed. Pharmacother. 2016, 84, 806–809. [Google Scholar] [CrossRef]
- Hoffman, D.C.; Donovan, H. Catalepsy as a rodent model for detecting antipsychotic drugs with extrapyramidal side effect liability. Psychopharmacology 1995, 120, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Radin, D.P.; Zhong, S.; Cerne, R.; Witkin, J.; Lippa, A. High impact AMPAkines induce a Gq-protein coupled endoplasmic calcium release in cortical neurons: A possible mechanism for explaining the toxicity of high impact AMPAkines. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ryder, J.W.; Falcone, J.F.; Manro, J.R.; Svensson, K.A.; Merchant, K.M. Pharmacological characterization of cGMP regulation by the biarylpropylsulfonamide class of positive, allosteric modulators of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. J. Pharmacol. Exp. Ther. 2006, 319, 293–298. [Google Scholar] [CrossRef]
- O'Neill, M.J.; Witkin, J.M. AMPA receptor potentiators: Application for depression and Parkinson's disease. Curr. Drug Targets 2007, 8, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Jhee, S.S.; Chappell, A.S.; Zarotsky, V.; Moran, S.V.; Rosenthal, M.; Kim, E.; Chalon, S.; Toublanc, N.; Brandt, J.; Coutant, D.E.; et al. Multiple-dose plasma pharmacokinetic and safety study of LY450108 and LY451395 (AMPA receptor potentiators) and their concentration in cerebrospinal fluid in healthy human subjects. J. Clin. Pharmacol. 2006, 46, 424–432. [Google Scholar] [CrossRef]
- Jones, N.; Messenger, M.J.; O'Neill, M.J.; Oldershaw, A.; Gilmour, G.; Simmons, R.M.; Iyengar, S.; Libri, V.; Tricklebank, M.; Williams, S.C. AMPA receptor potentiation can prevent ethanol-induced intoxication. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008, 33, 1713–1723. [Google Scholar] [CrossRef]
- Trzepacz, P.T.; Cummings, J.; Konechnik, T.; Forrester, T.D.; Chang, C.; Dennehy, E.B.; Willis, B.A.; Shuler, C.; Tabas, L.B.; Lyketsos, C. Mibampator (LY451395) randomized clinical trial for agitation/aggression in Alzheimer's disease. Int. Psychogeriatr. 2013, 25, 707–719. [Google Scholar] [CrossRef]
- Kunugi, A.; Tajima, Y.; Kuno, H.; Sogabe, S.; Kimura, H. HBT1, a Novel AMPA Receptor Potentiator with Lower Agonistic Effect, Avoided Bell-Shaped Response in In Vitro BDNF Production. J. Pharmacol. Exp. Ther. 2018, 364, 377–389. [Google Scholar] [CrossRef]
- Miraucourt, L.S.; Accardi, M.V.; Asin, K.E.; Pugsley, M.K.; Curtis, M.J.; Authier, S. The application of electrophysiological methods to characterize AMPA receptors in dissociated adult rat and non-human primate cerebellar neurons for use in neuronal safety pharmacology assessments of the central nervous system. J. Pharmacol. Toxicol. Methods 2020, 105, 106883. [Google Scholar] [CrossRef]
- Ishii, T.; Stolz, J.R.; Swanson, G.T. Auxiliary Proteins are the Predominant Determinants of Differential Efficacy of Clinical Candidates Acting as AMPA Receptor Positive Allosteric Modulators. Mol. Pharmacol. 2020, 97, 336–350. [Google Scholar] [CrossRef]
- Radin, D.P.; Smith, G.; Moushiaveshi, V.; Wolf, A.; Bases, R.; Tsirka, S.E. Lucanthone Targets Lysosomes to Perturb Glioma Proliferation, Chemoresistance and Stemness, and Slows Tumor Growth In Vivo. Front. Oncol. 2022, 12, 852940. [Google Scholar] [CrossRef]
- Goff, D.C.; Leahy, L.; Berman, I.; Posever, T.; Herz, L.; Leon, A.C.; Johnson, S.A.; Lynch, G. A placebo-controlled pilot study of the ampakine CX516 added to clozapine in schizophrenia. J. Clin. Psychopharmacol. 2001, 21, 484–487. [Google Scholar] [CrossRef]
- Goff, D.C.; Lamberti, J.S.; Leon, A.C.; Green, M.F.; Miller, A.L.; Patel, J.; Manschreck, T.; Freudenreich, O.; Johnson, S.A. A placebo-controlled add-on trial of the Ampakine, CX516, for cognitive deficits in schizophrenia. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008, 33, 465–472. [Google Scholar] [CrossRef]
- Wan, L.; Liu, X.; Wu, Z.; Ren, W.; Kong, S.; Dargham, R.A.; Cheng, L.; Wang, Y. Activation of extrasynaptic GABA(A) receptors inhibits cyclothiazide-induced epileptiform activity in hippocampal CA1 neurons. Neurosci. Bull. 2014, 30, 866–876. [Google Scholar] [CrossRef]
- Hampson, R.E.; Rogers, G.; Lynch, G.; Deadwyler, S.A. Facilitative effects of the ampakine CX516 on short-term memory in rats: Correlations with hippocampal neuronal activity. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 2748–2763. [Google Scholar] [CrossRef]
- Hampson, R.E.; Rogers, G.; Lynch, G.; Deadwyler, S.A. Facilitative effects of the ampakine CX516 on short-term memory in rats: Enhancement of delayed-nonmatch-to-sample performance. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 2740–2747. [Google Scholar] [CrossRef]
- Arai, A.; Lynch, G. The waveform of synaptic transmission at hippocampal synapses is not determined by AMPA receptor desensitization. Brain Res. 1998, 799, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Sun, Y.; Wu, Z.; Wan, L.; Chen, L.; Kong, S.; Zhang, B.; Zhang, F.; Wang, Z.Y.; Wang, Y. Epileptiform response of CA1 neurones to convulsant stimulation by cyclothiazide, kainic acid and pentylenetetrazol in anaesthetized rats. Seizure 2011, 20, 312–319. [Google Scholar] [CrossRef]
- Radin, D.P.; Johnson, S.; Purcell, R.; Lippa, A.S. Effects of chronic systemic low-impact ampakine treatment on neurotrophin expression in rat brain. Biomed. Pharmacother. 2018, 105, 540–544. [Google Scholar] [CrossRef]
- Jordan, G.R.; McCulloch, J.; Shahid, M.; Hill, D.R.; Henry, B.; Horsburgh, K. Regionally selective and dose-dependent effects of the ampakines Org 26576 and Org 24448 on local cerebral glucose utilisation in the mouse as assessed by 14C-2-deoxyglucose autoradiography. Neuropharmacology 2005, 49, 254–264. [Google Scholar] [CrossRef]
- Vanover, K.E. Effects of AMPA receptor positive modulators on amphetamine- and dizocilpine-induced locomotion. Eur. J. Pharmacol. 1997, 332, 115–119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).