Insights for Future Pharmacology: Exploring Phytochemicals as Potential Inhibitors Targeting SARS-CoV-2 Papain-like Protease
Abstract
1. Introduction
2. Methodology
2.1. Data Retrieval
2.2. Inclusion and Exclusion Criteria
2.3. Data Presentation
2.4. Supporting Data
3. Results and Discussion
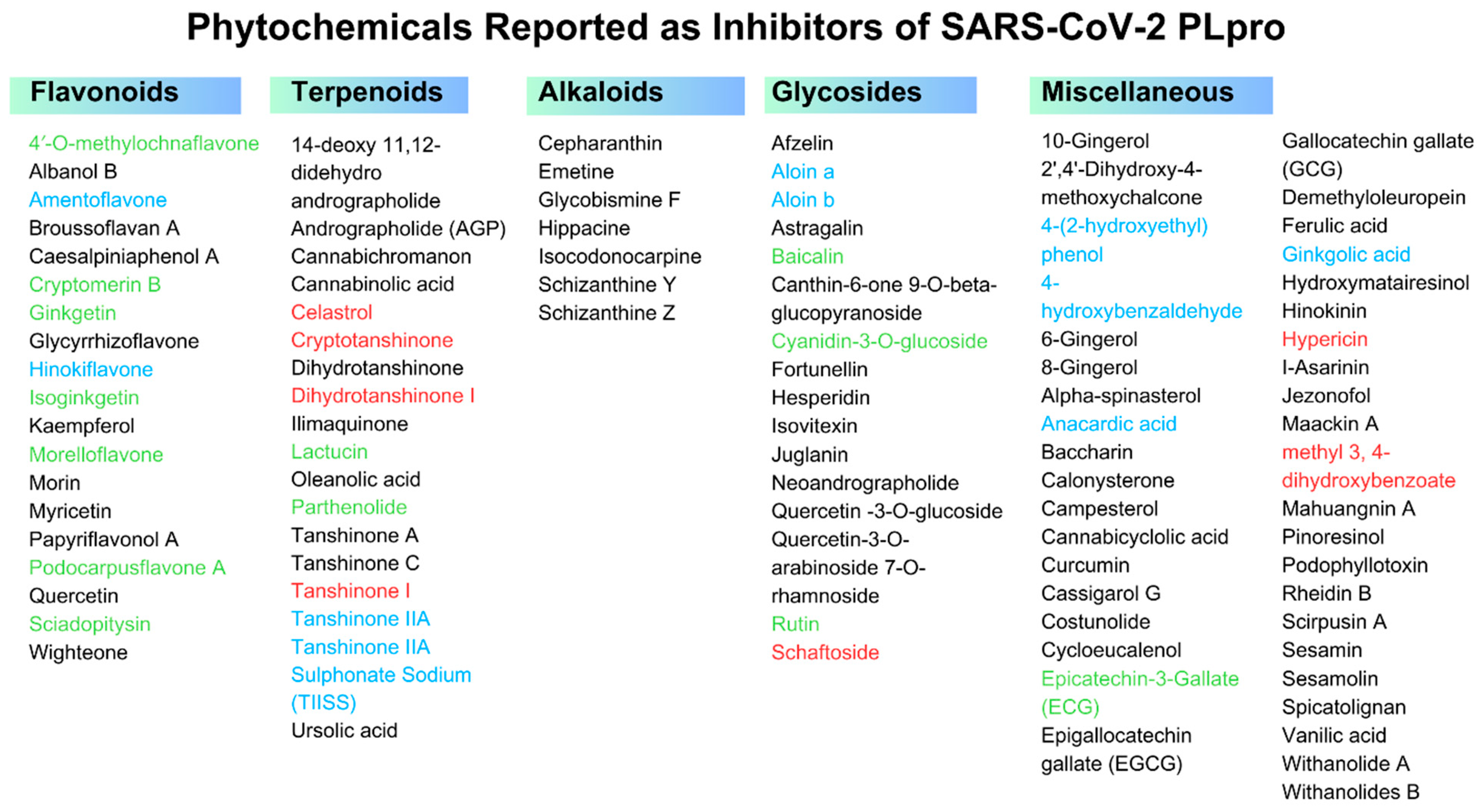
3.1. Potential Phytochemicals against SARS-CoV-2 PLpro
3.2. Flavonoids
| Phytochemicals Reported against SARS-CoV-2 PLpro | PubChem CID | Pharmacognostic Class | Plant Scientific Name | Plant Family | Docking Score (Kcal/mol) | Control | Reference |
|---|---|---|---|---|---|---|---|
| 10-Gingerol | 168115 | Phenolic Compound | Zingiber officinale Roscoe | Zingiberaceae | −42 | Chloroquine −27 kJ/mol | [47] |
| 14-deoxy 11,12-didehydro andrographolide | 5708351 | Diterpenoid | A. paniculata | Acanthaceae | −6.7 | - | [48] |
| 2′,4′-Dihydroxy-4-methoxychalcone | 5711223 | Chalcone | Astragalus laxmannii Jacq. | Fabaceae | −14.1 | Cocrystallized ligand TTT −9.30 kcal/mol | [45] |
| 6-Gingerol | 442793 | Phenolic Compound | Z. officinale | Zingiberaceae | −39 | Chloroquine −27 kJ/mol | [47] |
| 8-Gingerol | 168114 | Phenolic Compound | Z. officinale | Zingiberaceae | −43 | Chloroquine −27 kJ/mol | [47] |
| Afzelin | 5316673 | Flavonoid glycoside | S. androgynus | Phyllanthaceae | −190.23 | Chloroquine −231 kcal/mol | [49] |
| Albanol B | 480819 | Arylbenzofuran Flavonoid | M. alba | Moraceae | −9.3 | - | [50] |
| Alpha-spinasterol | 5281331 | Phytosterol | Nigella Sativa L. | Ranunculaceae | −9.6 | Ivermectin −9.8 kcal/mol | [51] |
| Andrographolide (AGP) | 5318517 | Diterpenoid | A. paniculata | Acanthaceae | −6.5 | - | [48] |
| Astragalin | 5282102 | Flavonoid glycoside | P. amarus | Phyllanthaceae | −9.7 | Remdesivir −9.50 kcal/mol | [52] |
| Baccharin | 117587576 | Resins | Propolis (Apis mellifera L.) | Apidae | −8.2 | Darunavir −3.8 kcal/mol Favipiravir −4.0 kcal/mol | [53] |
| Broussoflavan A | 44257178 | Flavandiol | B. papyrifera | Moraceae | −8.5 | Lopinavir −6.8 kcal/mol GRL0617 −6.5 kcal/mol | [46] |
| Caesalpiniaphenol A | 71454364 | Isoflavonoid | C. sappan | Fabaceae | −9.2 | GRL0617 −6.9 kcal/mol | [54] |
| Calonysterone | 101281312 | Steroid | Senna obtusifolia (L.) H.S.Irwin & Barneby | Fabaceae | −6.9 | GRL0617 −6.5 kcal/mol | [55] |
| Campesterol | 173183 | Phytosterol | N. Sativa | Ranunculaceae | −9.8 | Ivermectin −9.8 kcal/mol | [51] |
| Cannabichromanon | 25105340 | Terpene phenolic compound | C. sativa | Cannabaceae | −28.3 | Y97 Ligand −4.08 kcal/mol | [56] |
| Cannabicyclolic acid | 71437560 | Salicylic acid | C. sativa | Cannabaceae | −19.8 | Y97 Ligand −4.08 kcal/mol | [56] |
| Cannabinolic acid | 3081990 | Meroterpenoids | C. sativa | Cannabaceae | −22.8 | Y97 Ligand −4.08 kcal/mol | [56] |
| Canthin-6-one 9-O-beta-glucopyranoside | 637482 | Alkaloidal glycoside | Eurycoma harmandiana Pierre | Simaroubaceae | −9.4 | Remdesivir −8.3 kcal/mol | [57] |
| Cassigarol G | 10005549 | Piceatannol dimers | Cocos nucifera L. | Arecaceae | −10.5 | Reference Inhibitor −8.2 kcal/mol | [58] |
| Cepharanthin | 10206 | Biscoclaurine alkaloid | E. lathyris | Euphorbiaceae | −8.1 | GRL0617 −6.5 kcal/mol Lopinavir −6.8 kcal/mol | [46] |
| Costunolide | 5281437 | Steroid | Costus speciosus (J.Koenig) Sm. | Zingiberaceae | −8.2 | Disulfiram −3.11 kcal/mol | [59] |
| Curcumin | 969516 | Diarylheptanoid | Curcuma longa L. | Zingiberaceae | −8.0 | - | [60] |
| Cycloeucalenol | 101690 | Phytosterol | N. Sativa | Ranunculaceae | −9.8 | Ivermectin −9.8 kcal/mol | [51] |
| Dihydrotanshinone | 5316743 | Terpenoids | G. pensilis | Cupressaceae | −7.3 | Remdesivir −6.8 kcal/mol | [61] |
| Demethyloleuropein | 6450302 | Olive secoiridoid | Olea europaea L. | Oleaceae | −94.54 | - | [62] |
| Emetine | 10219 | Alkaloid | Carapichea ipecacuanha (Brot.) L.Andersson | Rubiaceae | −9.0 | - | [63] |
| Epigallocatechin gallate (EGCG) | 65064 | Gallic acid esters | C. sinensis | Theaceae | −8.6 | GRL0617 −6.5 kcal/mol | [64] |
| Ferulic acid | 445858 | Phenolic acid | Sesamum indicum L. | Pedaliaceae | −4.8 | VER 250 (Co-Crystallized Ligand) −7.2 kcal/mol | [65] |
| Fortunellin | 5317385 | Flavonoid glycoside | Citrus japonica Thunb. | Rutaceae | −28.1 | - | [66] |
| Gallocatechin gallate (GCG) | 199472 | Gallic acid esters | C. sinensis | Theaceae | –8.8 | - | [67] |
| Glycobismine F | 12111778 | Acridone alkaloids | G. pentaphylla | Rutaceae | −9.6 | - | [50] |
| Glycyrrhizoflavone | 5317764 | Flavone | G. glabra | Fabaceae | −51.63 | - | [45] |
| Hesperidin | 10621 | Flavanone glycoside | Citrus aurantium L. | Rutaceae | −10.6 | - | [68] |
| Hinokinin | 442879 | Lignan | P. amarus | Phyllanthaceae | −9.8 | Remdesivir −9.50 kcal/mol | [52] |
| Hippacine | 10015025 | Quinoline alkaloid | Ammocharis coranica Herb. | Amaryllidaceae | −13.22 | Cocrystallized ligand TTT −9.30 kcal/mol | [69] |
| Hydroxymatairesinol | 10948757 | Furanoid lignans | S. indicum | Pedaliaceae | −7.2 | VER250-Ligand −7.2 kcal/mol | [65] |
| I-Asarinin | 11869417 | Furofuranoid lignans | Piper longum L. | Piperaceae | −10.8 | - | [70] |
| Ilimaquinone | 72291 | Diterpenoid | Hippospongia metachromia de Laubenfels | Spongiidae | −8.1 | Remdesivir −9.9 kcal/mol | [71] |
| Isocodonocarpine | - | Spermidine Alkaloid | Capparis decidua Edgew. | Capparaceae | −7.0 | GRL0617 −6.5 kcal/mol | [55] |
| Isovitexin | 162350 | Flavonoid glycoside | V. negundo | Lamiaceae | −9.3 | - | |
| Jezonofol | 46226510 | Piceatannol dimers | C. nucifera | Arecaceae | −10.4 | Reference Inhibitor −8.2 kcal/mol | [58] |
| Juglanin | 5318717 | Flavonoid glycoside | Polygonum aviculare L. | Polygonaceae | −7.8 | Lopinavir −6.8 kcal/mol GRL0617 −6.5 kcal/mol | [46] |
| Kaempferol | 5280863 | Flavonol | P. amarus | Phyllanthaceae | −9.6 | Remdesivir −9.50 kcal/mol | [52] |
| Maackin A | 56666152 | Piceatannol dimers | C. nucifera | Arecaceae | −9.3 | Reference Inhibitor −8.2 kcal/mol | [58] |
| Mahuangnin A | 5319217 | - | Murraya microphylla (Merr. & Chun) Swingle | Rutaceae | −9.3 | - | [50] |
| Morin | 5281670 | Flavonoid | M. pomifera. | Moraceae | −6.8 | - | [72] |
| Myricetin | 5281672 | Flavonoid | Ficus auriculata Lour. | Moraceae | −7.3 | - | [73] |
| Neoandrographolide | 9848024 | Terpene glycoside | A. paniculata | Acanthaceae | −7.3 | Remdesivir −7.5 kcal/mol | [74] |
| Oleanolic acid | 10494 | Terpenoid | V. negundo | Lamiaceae | −10 | - | [75] |
| Papyriflavonol A | 10343070 | Prenylated Flavonol | B. papyrifera | Moraceae | −8.6 | Lopinavir −6.8 kcal/mol GRL0617 −6.5 kcal/mol | [46] |
| Pinoresinol | 73399 | Furanoid lignans | S. indicum | Pedaliaceae | −6.51 | VER250-Ligand −7.2 kcal/mol | [65] |
| Podophyllotoxin | 10607 | Lignan | P. peltatum | Berberidaceae | −8.1 | - | [76] |
| Quercetin | 5280343 | Flavonol | P. amarus | Phyllanthaceae | −4.6 | - | [77] |
| Quercetin-3-O-glucoside | 5280804 | Flavonoid glycoside | P. amarus | Phyllanthaceae | −10.3 | Remdesivir −9.50 kcal/mol | [52] |
| Quercetin-3-O-arabinoside 7-O-rhamnoside | - | Flavonoid glycoside | P. amarus | Phyllanthaceae | −8.2 | Remdesivir −5.8 kcal/mol | [78] |
| Rheidin B | 5320958 | Dianthrone | Rheum palmatum L. | Polygonaceae | −9.3 | - | [50] |
| Schizanthine y | - | Tropane alkaloids | S. porrigens | Asteraceae | −7.1 | Lopinavir −7.0 kcal/mol | [79] |
| Schizanthine z | - | Tropane alkaloids | S. porrigens | Asteraceae | −7.5 | Lopinavir −7.0 kcal/mol | [79] |
| Scirpusin A | 5458896 | Piceatannol dimers | C. nucifera | Arecaceae | −10.5 | Reference Inhibitor −8.2 kcal/mol | [58] |
| Sesamin | 72307 | Furofuranoid lignans | S. indicum | Pedaliaceae | −6.5 | VER250-Ligand −7.2 kcal/mol | [65] |
| Sesamolin | 101746 | Furofuranoid lignans | S. indicum | Pedaliaceae | −6.4 | VER250-Ligand −7.2 kcal/mol | [65] |
| Spicatolignan | 72729358 | Lignan | S. indicum | Pedaliaceae | −6.6 | VER250-Ligand −7.2 kcal/mol | [65] |
| Tanshinone A | 114917 | Terpenoids | G. pensilis | Cupressaceae | −7.2 s | Remdesivir −6.8 kcal/mol | [61] |
| Tanshinone C | 160254 | Terpenoids | G. pensilis | Cupressaceae | −7.1 | Remdesivir −6.8 kcal/mol | [61] |
| Ursolic acid | 64945 | Triterpenoid | V. negundo | Lamiaceae | −9.7 | - | [75] |
| Vanilic acid | 8468 | Phenolic acid | S indicum | Pedaliaceae | −4.8 | VER250-Ligand −7.2 kcal/mol | [65] |
| Wighteone | 5281814 | Isoflavonoid | E. suberosa | Fabaceae | −16.52 | Cocrystallized ligand TTT −9.30 kcal/mol | [69] |
| Withanolide A | 11294368 | Steroid | Datura innoxia Mill. Withania somnifera (L.) Dunal | Solanaceae | −7.4 | GRL0617 −6.5 kcal/mol | [55] |
| Withanolides B | 14236711 | Steroid | W. somnifera | Solanaceae | −10.3 | Procainamide −5.03 kcal/mol | [80] |
3.3. Terpenoids
| Phytochemicals Reported against SARS-CoV-2 PLpro | PubChem CID | Pharmacognostic Class | Plant Scientific Name | Plant Family | Docking Score (Kcal/mol) | In Vitro/Ex Vivo/In Vivo Data on PLpro Inhibition | Control | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Assay | Result | ||||||||
| 4′-O-methylochnaflavone | 5384799 | Biflavone | L. japonica | Caprifoliaceae | −105.2 | FBA | IC50 = 22.8 μM | Psoralidin IC50 = 27.8 μM Plpro + pro-ISG15 95% proteolysis | [41] |
| PICA | ~100% at 20 μM | ||||||||
| Baicalin | 64982 | Flavonoid glycoside | Scutellaria baicalensis Georgi | Lamiaceae | −10.8 | IEIA | IC50 = 178 µM | HY-17542 IC50 = 1.73 µM | [68,101] |
| Cryptomerin B | 5316145 | Biflavone | Platycladus orientalis (L.) Franco | Cupressaceae | −87.9 | FBA | IC50 = 26.3 μM | Psoralidin IC50 = 27.8 μM Plpro + pro-ISG15 95% proteolysis | [41] |
| PICA | 74.8% at 20 μM | ||||||||
| Cyanidin-3-O-glucoside | 12303220 | Anthocyanin glycoside | M. alba | Moraceae | −7.3 | DUA | 42% at 100 µM | GRL0617 DUA 90% at 100 µM | [102] |
| Epicatechin-3-Gallate (ECG) | 65056 | Gallic acid esters | C. sinensis | Theaceae | −8.5 | IEIA | IC50 = 11.62 μg mL−1 (37.73 μM) | ECG fraction IC50 0.13 ± 0.001 µg mL−1 (0.42 μM) | [64,103] |
| Ginkgetin | 5271805 | Biflavone | Ginkgo. biloba L. | Ginkgoaceae | −117.2 | FBA | IC50 = 29.8 μM | Psoralidin IC50 = 27.8 μM Plpro + pro-ISG15 95% proteolysis | [41] |
| PICA | ~100% at 20 μM | ||||||||
| Isoginkgetin | 5318569 | Biflavone | G. biloba | Ginkgoaceae | −123.5 | FBA | IC50 = 31.2 μM | Psoralidin IC50 = 27.8 μM Plpro + pro-ISG15 95% proteolysis | [41] |
| PICA | ~100% at 20 μM | ||||||||
| Lactucin | 442266 | Sesquiterpenoids | Cichorium intybus L. | Asteraceae | −8.5 | IEIA | IC50 = 174 µM | GRL0617 DS = −7.5 kcal/mol | [100] |
| Morelloflavone | 5464454 | Biflavone | Garcinia lateriflora Blume | Clusiaceae | −81.6 | FBA | IC50 = 36.4 μM | Psoralidin IC50 = 27.8 μM Plpro + pro-ISG15 95% proteolysis | [41] |
| PICA | 34.1% at 20 μM | ||||||||
| Parthenolide | 7251185 | Sesquiterpene lactones | Tanacetum parthenium (L.) Sch.Bip. | Asteraceae | −7.5 | ICA | IC50 = 132.5 μM | - | [99] |
| Podocarpusflavone A | 5320644 | Biflavone | Podocarpus nakaii Hayata | Podocarpaceae | −66.2 | FBA | IC50 = 43.2 μM | Psoralidin IC50 = 27.8 μM Plpro + pro-ISG15 95% proteolysis | [41] |
| PICA | 64.9% at 20 μM | ||||||||
| Rutin | 5280805 | Flavonoid glycoside | Azadirachta indica A.Juss. | Meliaceae | −8.8 | DUA | 50% at 100 µM | GRL0617 DUA 90% at 100 µM | [102] |
| Sciadopitysin | 5281696 | Biflavone | G. biloba | Ginkgoaceae | −113.4 | FBA | IC50 = 34.8 μM | Psoralidin IC50 = 27.8 μM Plpro + pro-ISG15 95% proteolysis | [41] |
| PICA | 32.3% at 20 μM | ||||||||
3.4. Alkaloids
| Phytochemicals | PubChem CID | Pharmacognostic Class | Plant Scientific Name | Plant Family | Docking Score (Kcal/mol) | In Vitro/Ex Vivo/In Vivo Data on PLpro Inhibition | Control | Cellular Toxicity/Selectivity Index | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| Assay | Outcome | |||||||||
| 4-(2-hydroxyethyl)phenol (YRL) | 10393 | Phenolic Compound | Lawsonia alba Lam. | Lythraceae | −7.17 | ICA | 70% Inhibition IC50 = 6.68 μM | GRL0617 IC50 = 0.82 μM | No cellular toxicity at 100 µM SI = not studied | [111] |
| qRT-PCR | IC50 = 1 μM | |||||||||
| 4-hydroxybenzaldehyde (HBA) | 126 | Phenolic Compound | Acalypha torta hort. ex-Pax & K.Hoffm. | Euphorbiaceae | −6.97 | ICA | 73% Inhibition IC50 = 3.99 μM | GRL0617 IC50 = 0.82 μM | ~80% cell viability at 100 µM SI = not studied | [111] |
| CCAA | - | |||||||||
| Aloin a | Anthraquinone Glycoside | Aloe barbadensis Mill. | Asphodelaceae | - | IEIA | IC50 = 13.16μM | - | No significant cytotoxicity after 48 h at 50 μM and 100 μM SI = not studied | [112] | |
| 12305761 | DUA | IC50 = 15.68 μM | ||||||||
| Aloin b | 14989 | Anthraquinone Glycoside | A. barbadensis Mill. | Asphodelaceae | - | IEIA | IC50 = 16.08μM | - | No significant cytotoxicity after 48 h at 50 μM and 100 μM SI = not studied | [112] |
| DUA | IC50 = 17.51μM | |||||||||
| Amentoflavone | 5281600 | Biflavone | G. biloba | Ginkgoaceae | −129.6 | FBA | IC50 = 13.0 μM | Psoralidin IC50 = 27.8 μM PLpro + pro-ISG15 95% proteolysis | CC50 > 200 μM EC50 46.79 μM SI > 4.27 | [41,113] |
| ICA | 48.1% at 20 μM | |||||||||
| Anacardic acid | 167551 | Phenolic acid | Anacardium occidentale L. | Anacardiaceae | - | IEIA | IC50 = 17.08 ± 1.30 μM | - | SI = 2.83 | [114] |
| CA | CC50 = 25.48 ± 0.69 µΜ | |||||||||
| PRA | 13% at 7.5 µΜ EC50 = 9.0 ± 2.5 μM | |||||||||
| Celastrol | 122724 | Triterpenoid | T. wilfordii | Celastraceae | −7.4 | FBA | IC50 = 8.9 ± 0.8 μM | - | CC50 > 1000 nM SI > 4.52 | [97] |
| CCAA | EC50 = 221 nM | |||||||||
| Ginkgolic acid | 5281858 | Phenolic acid | A. occidentale | Anacardiaceae | −4.9 | IEIA | IC50 = 16.30 ± 0.64 µM | - | CC50 = 27.88 ± 0.77 µM SI = 3.35 | [114] |
| PRA | 42% at 7.5 µM EC50 = 8.3 ± 0.03 μM | |||||||||
| Hinokiflavone | 5281627 | Biflavone | P. orientalis | Cupressaceae | −119.1 | FBA | IC50 = 9.5 μM | Psoralidin IC50 = 27.8 μM PLpro + pro-ISG15 95% proteolysis | - | [41] |
| PICA | ~100% at 20 μM | |||||||||
| Tanshinone IIA | 164676 | Terpenoids | S. miltiorrhiza | Lamiaceae | - | FBA | IC50 = 1.57 µM | GRL-0617 IC50 = 1.789 µM EC50 = 32.6 µM | CC50 > 300 µM SI = 1.5 | [87] |
| CCAA | EC50 > 200 µM | |||||||||
| Tanshinone IIA Sulphonate Sodium (TIISS) | 40580588 | Terpenoids | S. miltiorrhiza | Lamiaceae | −8.6 | PAA | IC50 = 1.65 ± 0.13 µM | - | - | [11] |
3.5. Glycosides
3.6. Miscellaneous
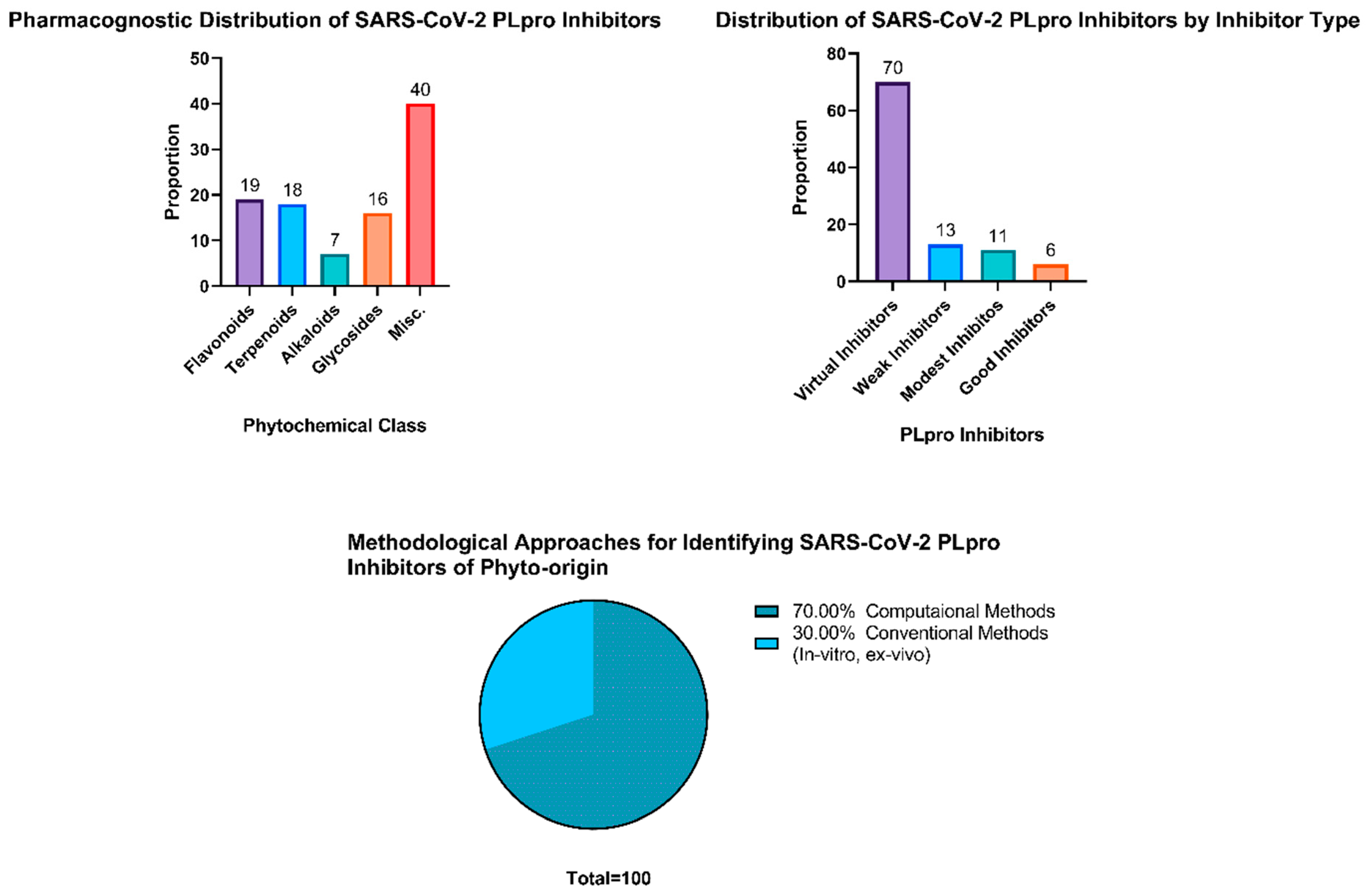
3.7. Flavonoids and Terpenoids Possess a Rich Reservoir of SARS-CoV-2 PLpro Inhibitors among Other Phytochemical Classes
3.8. Promising Natural Candidates as Inhibitors of SARS-CoV-2 PLpro
| Phytochemicals | PubChem CID | Pharmacognostic Class | Plant Scientific Name | Plant Family | Docking Score (Kcal/mol) | Assay | Outcome | Control | Cellular Toxicity/Selectivity Index | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Schaftoside | 442658 | Flavonoid glycoside | G. uralensis | Fabaceae | −8.5 | IEIA | 60% at 8 μmol/L IC50 = 3.91 μmol/L | GRL0617 84.75% at 8 μmol/L IC50 = 0.34 μmol/L | CC50 > 200 μmol/L SI > 16.90 | [120] |
| CCAA | EC50 = 11.83 μmol/L | |||||||||
| Dihydrotanshinone I | 11425923 | Terpenoid | S. miltiorrhiza | Lamiaceae | - | FBA | IC50 = 0.59 µM | GRL0617 IC50 = 1.789 µM EC50 = 32.6 µM | CC50 > 300 μmol/L SI > 37.5 | [87] |
| CCAA | EC50 = 8 µM | |||||||||
| Tanshinone I | 114917 | Terpenoid | S. miltiorrhiza | Lamiaceae | - | FBA | IC50 = 2.21 μmol/L | GRL0617 IC50 = 1.39 μmol/L EC50 = 3.18 | CC50 > 300 μmol/L SI > 132 | [89] |
| PRA | EC50 = 2.26 μmol/L | |||||||||
| Cryptotanshinone | 160254 | Diterpenoid | S. miltiorrhiza | Lamiaceae | - | FBA | IC50 = 5.63 μmol/L | GRL0617 IC50 = 1.39 μmol/L EC50 = 3.18 | CC50 > 300 μmol/L SI > 428.5 | [89] |
| PRA | EC50 = 0.70 μM | |||||||||
| Hypericin | 3663 | Anthraquinone derivative | H. perforatum | Hypericaceae | −6.5 | DUA | 90% at 100 μM | GRL0617 90% inhibition of deubiquitinase activity at 100 μM | CC50 > 100 μg/mL SI = >178,858.88 | [102,128] |
| ICA | IC50 = 559.1 pg/mL | |||||||||
| methyl 3, 4-dihydroxybenzoate (HE9) | 287064 | Phenolic Compound | Tagetes patula L. | Asteraceae | −6.15 | ICA | 55% Inhibition IC50 = 3.76 μM | GRL0617 IC50 = 0.82 μM | CC50 > 100 µM SI > 9.64 | [111] |
| qRT-PCR | IC50 = 0.13 μM | |||||||||
| CPE | IC50 = 10.37 μM |
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J. Gen. Intern. Med. 2020, 35, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Director General Opening Remarks at the Media Briefing on COVID-19. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 7 March 2022).
- Li, M.; Wang, H.; Tian, L.; Pang, Z.; Yang, Q.; Huang, T.; Fan, J.; Song, L.; Tong, Y.; Fan, H. COVID-19 Vaccine Development: Milestones, Lessons and Prospects. Signal Transduct. Target. Ther. 2022, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO COVID-19 Dashboard. Available online: https://covid19.who.int (accessed on 28 December 2022).
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 Pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A.; Ibrahim, N.S. Anti-SARS and Anti-HCV Drugs Repurposing against the Papain-like Protease of the Newly Emerged Coronavirus (2019-NCoV). Res. Sq. 2020. [Google Scholar] [CrossRef]
- Li, G.; De Clercq, E. Therapeutic Options for the 2019 Novel Coronavirus (2019-NCoV). Nat. Rev. Drug Discov. 2020, 19, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lucas, J.; Castañeda, D.; Hormigo, D. New Trends for a Classical Enzyme: Papain, a Biotechnological Success Story in the Food Industry. Trends Food Sci. Technol. 2017, 68, 91–101. [Google Scholar] [CrossRef]
- Rut, W.; Lv, Z.; Zmudzinski, M.; Patchett, S.; Nayak, D.; Snipas, S.J.; El Oualid, F.; Huang, T.T.; Bekes, M.; Drag, M.; et al. Activity Profiling and Crystal Structures of Inhibitor-Bound SARS-CoV-2 Papain-like Protease: A Framework for Anti–COVID-19 Drug Design. Sci. Adv. 2020, 6, 4596–4612. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Sacco, M.D.; Xia, Z.; Lambrinidis, G.; Townsend, J.A.; Hu, Y.; Meng, X.; Szeto, T.; Ba, M.; Zhang, X.; et al. Discovery of SARS-CoV-2 Papain-like Protease Inhibitors through a Combination of High-Throughput Screening and a FlipGFP-Based Reporter Assay. ACS Cent. Sci. 2021, 7, 1245–1260. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, K.; Pan, J.; Lei, Y.; Zhang, D.; Fang, L.; Tang, J.; Chen, X.; Ma, Y.; Zheng, Y.; et al. Repurposing Clinically Approved Drugs for COVID-19 Treatment Targeting SARS-CoV-2 Papain-like Protease. Int. J. Biol. Macromol. 2021, 188, 137–146. [Google Scholar] [CrossRef]
- Freitas, B.T.; Durie, I.A.; Murray, J.; Longo, J.E.; Miller, H.C.; Crich, D.; Hogan, R.J.; Tripp, R.A.; Pegan, S.D. Characterization and Noncovalent Inhibition of the Deubiquitinase and DeISGylase Activity of SARS-CoV-2 Papain-like Protease. ACS Infect. Dis. 2020, 6, 2099–2109. [Google Scholar] [CrossRef]
- Elseginy, S.A.; Anwar, M.M. In Silico Analysis of SARS-CoV-2 Papain-like Protease Potential Inhibitors. RSC Adv. 2021, 11, 38616–38631. [Google Scholar] [CrossRef] [PubMed]
- Ratia, K.; Kilianski, A.; Baez-Santos, Y.M.; Baker, S.C.; Mesecar, A. Structural Basis for the Ubiquitin-Linkage Specificity and DeISGylating Activity of SARS-CoV Papain-like Protease. PLoS Pathog. 2014, 10, e1004113. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Coronavirus (COVID-19) Drugs. Available online: https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs (accessed on 23 December 2022).
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Höbartner, C.; Cramer, P. Mechanism of SARS-CoV-2 Polymerase Stalling by Remdesivir. Nat. Commun. 2021, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Rosas, I.O.; Bräu, N.; Waters, M.; Go, R.C.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Aziz, M.S.; Cooper, N.; Douglas, I.S.; et al. Tocilizumab in Hospitalized Patients with Severe COVID-19 Pneumonia. N. Engl. J. Med. 2021, 384, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Finn, A.; Lal, A.; Khan, M.S.; Dapaah-Afriyie, K.; Carino, G.P. Baricitinib in Hospitalised Patients with COVID-19: A Meta-Analysis of Randomised Controlled Trials. EClinicalMedicine 2022, 49, 101489. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Emergency Use Authorizations for Drugs and Non-Vaccine Biological Products. Available online: https://www.fda.gov/drugs/emergency-preparedness-drugs/emergency-use-authorizations-drugs-and-non-vaccine-biological-products (accessed on 23 December 2022).
- Gasmi, A.; Mujawdiya, P.K.; Lysiuk, R.; Shanaida, M.; Peana, M.; Gasmi Benahmed, A.; Beley, N.; Kovalska, N.; Bjørklund, G. Quercetin in the Prevention and Treatment of Coronavirus Infections: A Focus on SARS-CoV-2. Pharmaceuticals 2022, 15, 1049. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J. Cancer Chemoprevention with Dietary Phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Cholevas, C.; Polyzoidis, K.; Politis, A. Long-COVID Syndrome-associated Brain Fog and Chemofog: Luteolin to the Rescue. BioFactors 2021, 47, 232–241. [Google Scholar] [CrossRef]
- Shohan, M.; Nashibi, R.; Mahmoudian-Sani, M.-R.; Abolnezhadian, F.; Ghafourian, M.; Alavi, S.M.; Sharhani, A.; Khodadadi, A. The Therapeutic Efficacy of Quercetin in Combination with Antiviral Drugs in Hospitalized COVID-19 Patients: A Randomized Controlled Trial. Eur. J. Pharmacol. 2022, 914, 174615. [Google Scholar] [CrossRef]
- Di Pierro, F.; Iqtadar, S.; Khan, A.; Ullah Mumtaz, S.; Masud Chaudhry, M.; Bertuccioli, A.; Derosa, G.; Maffioli, P.; Togni, S.; Riva, A.; et al. Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial. Int. J. Gen. Med. 2021, 14, 2807–2816. [Google Scholar] [CrossRef]
- Di Pierro, F.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A.; et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int. J. Gen. Med. 2021, 14, 2359–2366. [Google Scholar] [CrossRef]
- Versace, V.; Ortelli, P.; Dezi, S.; Ferrazzoli, D.; Alibardi, A.; Bonini, I.; Engl, M.; Maestri, R.; Assogna, M.; Ajello, V.; et al. Co-Ultramicronized Palmitoylethanolamide/Luteolin Normalizes GABAB-Ergic Activity and Cortical Plasticity in Long COVID-19 Syndrome. Clin. Neurophysiol. 2023, 145, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.; Laurin, P.; Tardif, J.-C.; Hausermann, L.; Rosa, C.; Guertin, M.-C.; Thibaudeau, K.; Gagnon, L.; Cesari, F.; Robitaille, M.; et al. Fourteen-Day Evolution of COVID-19 Symptoms during the Third Wave in Nonvaccinated Subjects and Effects of Hesperidin Therapy: A Randomized, Double-Blinded, Placebo-Controlled Study. Evid. Based Complement. Altern. Med. 2022, 2022, 3125662. [Google Scholar] [CrossRef]
- Valizadeh, H.; Abdolmohammadi-vahid, S.; Danshina, S.; Ziya Gencer, M.; Ammari, A.; Sadeghi, A.; Roshangar, L.; Aslani, S.; Esmaeilzadeh, A.; Ghaebi, M.; et al. Nano-Curcumin Therapy, a Promising Method in Modulating Inflammatory Cytokines in COVID-19 Patients. Int. Immunopharmacol. 2020, 89, 107088. [Google Scholar] [CrossRef]
- Askari, G.; Sahebkar, A.; Soleimani, D.; Mahdavi, A.; Rafiee, S.; Majeed, M.; Khorvash, F.; Iraj, B.; Elyasi, M.; Rouhani, M.H.; et al. The Efficacy of Curcumin-Piperine Co-Supplementation on Clinical Symptoms, Duration, Severity, and Inflammatory Factors in COVID-19 Outpatients: A Randomized Double-Blind, Placebo-Controlled Trial. Trials 2022, 23, 472. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.-T.; Wu, C.-C.; Wu, S.-F.V.; Lee, M.-C.; Hu, W.-C.; Tsai, K.-W.; Yang, C.-H.; Lu, C.-L.; Chiu, S.-K.; Lu, K.-C. Resveratrol as an Adjunctive Therapy for Excessive Oxidative Stress in Aging COVID-19 Patients. Antioxidants 2021, 10, 1440. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Safety and Efficacy of COVIDEXTM Therapy in Management of Adult COVID-19 Patients in Uganda. Available online: https://clinicaltrials.gov/study/NCT05228626 (accessed on 28 July 2023).
- U.S. National Library of Medicine. The Effect of Berberine on Intestinal Function and Inflammatory Mediators in Severe Patients with COVID-19 (BOIFIM). Available online: https://clinicaltrials.gov/study/NCT04479202 (accessed on 28 July 2023).
- Hernández-Rodríguez, J.; Durán-Sanclemente, J.; Prieto-González, S.; Araújo, O.; Hospital-Vidal, T.; Casanovas, G.; Sapena, V.; Blanco, J.L.; López-Soto, A.; Afonso, F.J.; et al. FRAGILE-COLCOVID19: A Clinical Trial Based on Early Administration of an Oral Combination of Colchicine and Prednisone in Elderly Patients with COVID-19 in Geriatric Facilities. Clin. Drug Investig. 2022, 42, 949–964. [Google Scholar] [CrossRef]
- Das, A.; Khan, S.; Roy, S.; Das, S. Phytochemicals for Mitigating the COVID-19 Crisis: Evidence from Pre-Clinical and Clinical Studies. Explor. Drug Sci. 2023, 1, 336–376. [Google Scholar] [CrossRef]
- Sorokina, M.; Merseburger, P.; Rajan, K.; Yirik, M.A.; Steinbeck, C. COCONUT Online: Collection of Open Natural Products Database. J. Cheminform. 2021, 13, 2. [Google Scholar] [CrossRef]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of Flavonoids against Coronavirus Infection. Chem. Biol. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent Advances in Understanding the Antibacterial Properties of Flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Verma, S.; Twilley, D.; Esmear, T.; Oosthuizen, C.B.; Reid, A.M.; Nel, M.; Lall, N. Anti-SARS-CoV Natural Products with the Potential to Inhibit SARS-CoV-2 (COVID-19). Front. Pharmacol. 2020, 11, 561334. [Google Scholar] [CrossRef]
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.d.S.; Barriga, J.R.d.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological Activities and Therapeutic Potential. Nat. Prod. Res. 2018, 34, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Sandhar, H.; Kumar, B.; Prasher, S.; Tiwari, P.; Salhan, M.; Sharma, P. A Review of Phytochemical and Pharmacology of Flavonoids. Int. Pharm. Sci. 2011, 1, 25–41. [Google Scholar]
- Li, L.; Ma, L.; Hu, Y.; Li, X.; Yu, M.; Shang, H.; Zou, Z. Natural Biflavones Are Potent Inhibitors against SARS-CoV-2 Papain-like Protease. Phytochemistry 2022, 193, 112984. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Eng. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Hu, K.; Guan, W.J.; Bi, Y.; Zhang, W.; Li, L.; Zhang, B.; Liu, Q.; Song, Y.; Li, X.; Duan, Z.; et al. Efficacy and Safety of Lianhuaqingwen Capsules, a Repurposed Chinese Herb, in Patients with Coronavirus Disease 2019: A Multicenter, Prospective, Randomized Controlled Trial. Phytomedicine 2021, 85, 153242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, B.; Hashimoto, K. Current Status of Potential Therapeutic Candidates for the COVID-19 Crisis. Brain Behav. Immun. 2020, 87, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Elkaeed, E.B.; Metwaly, A.M.; Alesawy, M.S.; Saleh, A.M.; Alsfouk, A.A.; Eissa, I.H. Discovery of Potential SARS-CoV-2 Papain-like Protease Natural Inhibitors Employing a Multi-Phase In Silico Approach. Life 2022, 12, 1407. [Google Scholar] [CrossRef]
- Hossain, R.; Sarkar, C.; Hassan, S.M.H.; Khan, R.A.; Arman, M.; Ray, P.; Islam, M.T.; Daştan, S.D.; Sharifi-Rad, J.; Almarhoon, Z.M.; et al. In Silico Screening of Natural Products as Potential Inhibitors of SARS-CoV-2 Using Molecular Docking Simulation. Chin. J. Integr. Med. 2022, 28, 249–256. [Google Scholar] [CrossRef]
- Goswami, D.; Kumar, M.; Ghosh, S.K.; Das, A. Natural Product Compounds in Alpinia Officinarum and Ginger Are Potent SARS-CoV-2 Papain-like Protease Inhibitors. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Khanal, P.; Dey, Y.N.; Patil, R.; Chikhale, R.; Wanjari, M.M.; Gurav, S.S.; Patil, B.M.; Srivastava, B.; Gaidhani, S.N. Combination of System Biology to Probe the Anti-Viral Activity of Andrographolide and Its Derivative against COVID-19. RSC Adv. 2021, 11, 5065–5079. [Google Scholar] [CrossRef]
- Makati, A.C.; Ananda, A.N.; Putri, J.A.; Amellia, S.F.; Setiawan, B. Molecular Docking of Ethanol Extracts of Katuk Leaf (Sauropus androgynus) on Functional Proteins of Severe Acute Respiratory Syndrome Coronavirus 2. S. Afr. J. Bot. 2022, 149, 1. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.; Pan, C.; Liang, A.; Zhang, W.; Sheng, Y. Target-Based in Silico Screening for Phytoactive Compounds Targeting SARS-CoV-2. Interdiscip. Sci. 2022, 14, 64–79. [Google Scholar] [CrossRef]
- Siddiqui, S.; Upadhyay, S.; Ahmad, R.; Gupta, A.; Srivastava, A.; Trivedi, A.; Husain, I.; Ahmad, B.; Ahamed, M.; Khan, M.A. Virtual Screening of Phytoconstituents from Miracle Herb Nigella Sativa Targeting Nucleocapsid Protein and Papain-like Protease of SARS-CoV-2 for COVID-19 Treatment. J. Biomol. Struct. Dyn. 2022, 40, 3928–3948. [Google Scholar] [CrossRef]
- Hiremath, S.; Kumar, H.D.V.; Nandan, M.; Mantesh, M.; Shankarappa, K.S.; Venkataravanappa, V.; Basha, C.R.J.; Reddy, C.N.L. In Silico Docking Analysis Revealed the Potential of Phytochemicals Present in Phyllanthus amarus and Andrographis paniculata, Used in Ayurveda Medicine in Inhibiting SARS-CoV-2. 3 Biotech 2021, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Yosri, N.; El-Wahed, A.A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.M.; et al. Anti-Viral and Immunomodulatory Properties of Propolis: Chemical Diversity, Pharmacological Properties, Preclinical and Clinical Applications, and in Silico Potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef]
- Parmar, P.; Rao, P.; Sharma, A.; Shukla, A.; Rawal, R.M.; Saraf, M.; Patel, B.V.; Goswami, D. Meticulous Assessment of Natural Compounds from NPASS Database for Identifying Analogue of GRL0617, the Only Known Inhibitor for SARS-CoV2 Papain-like Protease (PLpro) Using Rigorous Computational Workflow. Mol. Divers. 2022, 26, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Alamri, M.A.; Altharawi, A.; Alabbas, A.B.; Alossaimi, M.A.; Alqahtani, S.M. Structure-Based Virtual Screening and Molecular Dynamics of Phytochemicals Derived from Saudi Medicinal Plants to Identify Potential COVID-19 Therapeutics. Arab. J. Chem. 2020, 13, 7224–7234. [Google Scholar] [CrossRef] [PubMed]
- Altyar, A.E.; Youssef, F.S.; Kurdi, M.M.; Bifari, R.J.; Ashour, M.L. The Role of Cannabis sativa L. as a Source of Cannabinoids against Coronavirus 2 (SARS-CoV-2): An in-Silico Study to Evaluate Their Activities and ADMET Properties. Molecules 2022, 27, 2797. [Google Scholar] [CrossRef]
- Verma, D.; Mitra, D.; Paul, M.; Chaudhary, P.; Kamboj, A.; Thatoi, H.; Janmeda, P.; Jain, D.; Panneerselvam, P.; Shrivastav, R.; et al. Potential Inhibitors of SARS-CoV-2 (COVID 19) Proteases PL pro and M pro/ 3CL pro: Molecular Docking and Simulation Studies of Three Pertinent Medicinal Plant Natural Components. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100038. [Google Scholar] [CrossRef] [PubMed]
- Elsbaey, M.; Ibrahim, M.A.A.; Bar, F.A.; Elgazar, A.A. Chemical Constituents from Coconut Waste and Their in Silico Evaluation as Potential Antiviral Agents against SARS-CoV-2. S. Afr. J. Bot. 2021, 141, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Chauhan, S.S.; Pandit, S.; Sinha, M.; Gupta, S.; Gupta, A.; Parthasarathi, R. The Dual Role of Phytochemicals on SARS-CoV-2 Inhibition by Targeting Host and Viral Proteins. J. Tradit. Complement. Med. 2022, 12, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Delre, P.; Caporuscio, F.; Saviano, M.; Mangiatordi, G.F. Repurposing Known Drugs as Covalent and Non-Covalent Inhibitors of the SARS-CoV-2 Papain-like Protease. Front. Chem. 2020, 8, 594009. [Google Scholar] [CrossRef] [PubMed]
- Phong, N.V.; Trang, N.M.; Quyen, C.T.; Anh, H.L.T.; Vinh, L.B. SARS-CoV-2 Main Protease and Papain-like Protease Inhibition by Abietane-Type Diterpenes Isolated from the Branches of Glyptostrobus pensilis Using Molecular Docking Studies. Nat. Prod. Res. 2022, 36, 6336–6343. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, N.; Albratty, M. Benchmarked Molecular Docking Integrated Molecular Dynamics Stability Analysis for Prediction of SARS-CoV-2 Papain-like Protease Inhibition by Olive Secoiridoids. J. King Saud Univ. Sci. 2023, 35, 102402. [Google Scholar] [CrossRef] [PubMed]
- Snoussi, M.; Redissi, A.; Mosbah, A.; De Feo, V.; Adnan, M.; Aouadi, K.; Alreshidi, M.; Patel, M.; Kadri, A.; Noumi, E. Emetine, a Potent Alkaloid for the Treatment of SARS-CoV-2 Targeting Papain-like Protease and Non-Structural Proteins: Pharmacokinetics, Molecular Docking and Dynamic Studies. J. Biomol. Struct. Dyn. 2022, 40, 10122–10135. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, M.; Koppula, P.R.; Battu, A.; Ouseph, M.M.; Singh, A.K. EGCG, a Green Tea Catechin, as a Potential Therapeutic Agent for Symptomatic and Asymptomatic SARS-CoV-2 Infection. Molecules 2021, 26, 1200. [Google Scholar] [CrossRef] [PubMed]
- Allam, A.E.; Amen, Y.; Ashour, A.; Assaf, H.K.; Hassan, H.A.; Abdel-Rahman, I.M.; Sayed, A.M.; Shimizu, K. In Silico Study of Natural Compounds from Sesame against COVID-19 by Targeting M pro, PL pro and RdRp. RSC Adv. 2021, 11, 22398–22408. [Google Scholar] [CrossRef]
- Agrawal, S.; Pathak, E.; Mishra, R.; Mishra, V.; Parveen, A.; Mishra, S.K.; Byadgi, P.S.; Dubey, S.K.; Chaudhary, A.K.; Singh, V.; et al. Computational Exploration of the Dual Role of the Phytochemical Fortunellin: Antiviral Activities against SARS-CoV-2 and Immunomodulatory Abilities against the Host. Comput. Biol. Med. 2022, 149, 106049. [Google Scholar] [CrossRef] [PubMed]
- Swargiary, A.; Mahmud, S.; Saleh, M.A. Screening of Phytochemicals as Potent Inhibitor of 3-Chymotrypsin and Papain-like Proteases of SARS-CoV2: An in Silico Approach to Combat COVID-19. J. Biomol. Struct. Dyn. 2022, 40, 2067–2081. [Google Scholar] [CrossRef] [PubMed]
- Ur Rehman, M.F.; Akhter, S.; Batool, A.I.; Selamoglu, Z.; Sevindik, M.; Eman, R.; Mustaqeem, M.; Akram, M.S.; Kanwal, F.; Lu, C.; et al. Effectiveness of Natural Antioxidants against SARS-CoV-2? Insights from the in-Silico World. Antibiotics 2021, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Elkaeed, E.B.; Khalifa, M.M.; Alsfouk, B.A.; Alsfouk, A.A.; El-Attar, A.-A.M.M.; Eissa, I.H.; Metwaly, A.M. The Discovery of Potential SARS-CoV-2 Natural Inhibitors among 4924 African Metabolites Targeting the Papain-like Protease: A Multi-Phase in Silico Approach. Metabolites 2022, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Lakhera, S.; Devlal, K.; Ghosh, A.; Rana, M. In Silico Investigation of Phytoconstituents of Medicinal Herb ‘Piper Longum’ against SARS-CoV-2 by Molecular Docking and Molecular Dynamics Analysis. Results Chem. 2021, 3, 100199. [Google Scholar] [CrossRef] [PubMed]
- Surti, M.; Patel, M.; Adnan, M.; Moin, A.; Ashraf, S.A.; Siddiqui, A.J.; Snoussi, M.; Deshpande, S.; Reddy, M.N. Ilimaquinone (Marine Sponge Metabolite) as a Novel Inhibitor of SARS-CoV-2 Key Target Proteins in Comparison with Suggested COVID-19 Drugs: Designing, Docking and Molecular Dynamics Simulation Study. RSC Adv. 2020, 10, 37707–37720. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Issahaku, A.R.; Agoni, C.; Bendale, A.R.; Nagar, A.; Soliman, M.E.S.S.; Lokwani, D. In Silico Screening of Phytopolyphenolics for the Identification of Bioactive Compounds as Novel Protease Inhibitors Effective against SARS-CoV-2. J. Biomol. Struct. Dyn. 2022, 40, 10437–10453. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.J.O.; Calado, G.P.; Fróes, Y.N.; de Araújo, S.A.; França, L.M.; Paes, A.M.d.A.; de Morais, S.V.; da Rocha, C.Q.; Vasconcelos, C.C. Plant Metabolites as SARS-CoV-2 Inhibitors Candidates: In Silico and In Vitro Studies. Pharmaceuticals 2022, 15, 1045. [Google Scholar] [CrossRef] [PubMed]
- Murugan, N.A.; Pandian, C.J.; Jeyakanthan, J. Computational Investigation on Andrographis Paniculata Phytochemicals to Evaluate Their Potency against SARS-CoV-2 in Comparison to Known Antiviral Compounds in Drug Trials. J. Biomol. Struct. Dyn. 2021, 39, 4415–4426. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Verma, D.; Mahakur, B.; Kamboj, A.; Srivastava, R.; Gupta, S.; Pandey, A.; Arora, B.; Pant, K.; Panneerselvam, P.; et al. Molecular Docking and Simulation Studies of Natural Compounds of Vitex Negundo L. against Papain-like Protease (PL pro) of SARS CoV-2 (Coronavirus) to Conquer the Pandemic Situation in the World. J. Biomol. Struct. Dyn. 2022, 40, 5665–5686. [Google Scholar] [CrossRef]
- Rehman, M.U.; Ali, A.; Ansar, R.; Arafah, A.; Imtiyaz, Z.; Wani, T.A.; Zargar, S.; Ganie, S.A. In Silico Molecular Docking and Dynamic Analysis of Natural Compounds against Major Non-Structural Proteins of SARS-CoV-2. J. Biomol. Struct. Dyn. 2023, 41, 9072–9088. [Google Scholar] [CrossRef]
- Di Pierro, F.; Khan, A.; Bertuccioli, A.; Maffioli, P.; Derosa, G.; Khan, S.; Khan, B.A.; Nigar, R.; Ujjan, I.; Devrajani, B.R. Quercetin Phytosome® as a Potential Candidate for Managing COVID-19. Minerva Gastroenterol. 2021, 67, 19–195. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Bordoloi, R.; Chanu, N.R.; Kalita, R.; Sahariah, B.J.; Bhattacharjee, A. In Silico Discovery of 3 Novel Quercetin Derivatives against Papain-like Protease, Spike Protein, and 3C-like Protease of SARS-CoV-2. J. Genet. Eng. Biotechnol. 2022, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, M.; Alfaro, I.; Angel, C. Identification of Potential Inhibitors of SARS-CoV-2 Papain-like Protease from Tropane Alkaloids from Schizanthus Porrigens: A Molecular Docking Study. Chem. Phys. Lett. 2020, 761, 138068. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Siddiqui, S.; Ahmad, R.; Mehrotra, S.; Ahmad, B.; Srivastava, A.N. Exploring Nature’s Bounty: Identification of Withania somnifera as a Promising Source of Therapeutic Agents against COVID-19 by Virtual Screening and in Silico Evaluation. J. Biomol. Struct. Dyn. 2020, 40, 1858–1908. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.B.; Park, S.J.; Kim, Y.M.; Lee, J.Y.; Seo, W.D.; Chang, J.S.; Park, K.H.; Rho, M.C.; Lee, W.S. SARS-CoV 3CLpro Inhibitory Effects of Quinone-Methide Triterpenes from Tripterygium regelii. Bioorg. Med. Chem. Lett. 2010, 20, 1873. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.C.; Kuo, Y.H.; Jan, J.T.; Liang, P.H.; Wang, S.Y.; Liu, H.G.; Lee, C.K.; Chang, S.T.; Kuo, C.J.; Lee, S.S.; et al. Specific Plant Terpenoids and Lignoids Possess Potent Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.B.; Lee, J.J.; Pickering, J.G.; Borradaile, N.M. Chapter 30—Natural Products in Regeneration. In Regenerative Nephrology, 2nd ed.; Goligorsky, M.S., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 419–437. ISBN 978-0-12-823318-4. [Google Scholar]
- Zhang, Y.; Jiang, P.; Ye, M.; Kim, S.H.; Jiang, C.; Lü, J. Tanshinones: Sources, Pharmacokinetics and Anti-Cancer Activities. Int. J. Mol. Sci. 2012, 13, 13621–13666. [Google Scholar] [CrossRef]
- Islam, M.T.; Sarkar, C.; El-Kersh, D.M.; Jamaddar, S.; Uddin, S.J.; Shilpi, J.A.; Mubarak, M.S. Natural Products and Their Derivatives against Coronavirus: A Review of the Non-Clinical and Pre-Clinical Data. Phytother. Res. 2020, 34, 2471–2492. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Seo, K.H.; Curtis-Long, M.J.; Oh, K.Y.; Oh, J.W.; Cho, J.K.; Lee, K.H.; Park, K.H. Phenolic Phytochemical Displaying SARS-CoV Papain-like Protease Inhibition from the Seeds of Psoralea corylifolia. J. Enzym. Inhib. Med. Chem. 2014, 29, 59–63. [Google Scholar] [CrossRef]
- Lim, C.T.; Tan, K.W.; Wu, M.; Ulferts, R.; Armstrong, L.A.; Ozono, E.; Drury, L.S.; Milligan, J.C.; Zeisner, T.U.; Zeng, J.; et al. Identifying SARS-CoV-2 Antiviral Compounds by Screening for Small Molecule Inhibitors of Nsp3 Papain-like Protease. Biochem. J. 2021, 478, 2517–2531. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.H.; Kim, Y.M.; Jeong, H.J.; Kim, D.W.; Park, K.H.; Kwon, H.J.; Park, S.J.; Lee, W.S.; Ryu, Y.B. Tanshinones as Selective and Slow-Binding Inhibitors for SARS-CoV Cysteine Proteases. Bioorg Med. Chem. 2012, 20, 5928. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, X.; Duan, Y.; Pan, X.; Sun, Y.; You, T.; Han, L.; Jin, Z.; Shang, W.; Yu, J.; et al. High-Throughput Screening Identifies Established Drugs as SARS-CoV-2 PLpro Inhibitors. Protein Cell 2021, 12, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, J. Validation and Invalidation of SARS-CoV-2 Papain-like Protease Inhibitors. ACS Pharmacol. Transl. Sci. 2022, 5, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, S.; Sahoo, S.K. In Silico ADMET and Molecular Docking Study on Searching Potential Inhibitors from Limonoids and Triterpenoids for COVID-19. Comput. Biol. Med. 2020, 124, 103936. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.R.; Yen, C.T.; Ei-Shazly, M.; Lin, W.H.; Yen, M.H.; Lin, K.H.; Wu, Y.C. Anti-Human Coronavirus (Anti-HCoV) Triterpenoids from the Leaves of Euphorbia Neriifolia. Nat. Prod. Commun. 2012, 7, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Antiviral Activities of Oleanolic Acid and Its Analogues. Molecules 2018, 23, 2300. [Google Scholar] [CrossRef] [PubMed]
- Pawełczyk, A.; Zaprutko, L. Anti-COVID Drugs: Repurposing Existing Drugs or Search for New Complex Entities, Strategies and Perspectives. Future Med. Chem. 2020, 12, 1743–1757. [Google Scholar] [CrossRef] [PubMed]
- Sampangi-Ramaiah, M.H.; Vishwakarma, R.; Shaanker, R.U. Molecular Docking Analysis of Selected Natural Products from Plants for Inhibition of SARS-CoV-2 Main Protease. Curr. Sci. 2020, 118, 1087–1092. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhir, G.; Shukla, S.K.; Sharma, M.; Tyagi, P.; Bhushan, A.; Rathore, M. Identification of Phytochemical Inhibitors against Main Protease of COVID-19 Using Molecular Modeling Approaches. J. Biomol. Struct. Dyn. 2021, 39, 3760–3770. [Google Scholar] [CrossRef]
- Fuzo, C.A.; Martins, R.B.; Fraga-Silva, T.F.C.; Amstalden, M.K.; Canassa De Leo, T.; Souza, J.P.; Lima, T.M.; Faccioli, L.H.; Okamoto, D.N.; Juliano, M.A.; et al. Celastrol: A Lead Compound That Inhibits SARS-CoV-2 Replication, the Activity of Viral and Human Cysteine Proteases, and Virus-induced IL-6 Secretion. Drug Dev. Res. 2022, 83, 1623–1640. [Google Scholar] [CrossRef]
- Veerasamy, R.; Karunakaran, R. Molecular Docking Unveils the Potential of Andrographolide Derivatives against COVID-19: An in Silico Approach. J. Genet. Eng. Biotechnol. 2022, 20, 58. [Google Scholar] [CrossRef]
- Zou, Z.; Shan, H.; Sun, D.; Xia, L.; Shi, Y.; Wan, J.; Zhou, A.; Wu, Y.; Xu, H.; Lei, H.; et al. Parthenolide Reveals an Allosteric Mode to Inhibit the DeISGylation Activity of SARS-CoV-2 Papain-like Protease. Acta Biochim. Biophys. Sin. 2022, 54, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gálvez, M.Á.; Rafael-Pita, C.; Fernández, N.; Baixinho, J.; Anastácio, J.D.; Cankar, K.; Bosch, D.; Nunes dos Santos, C. Targeting Proteases Involved in the Viral Replication of SARS-CoV-2 by Sesquiterpene Lactones from Chicory (Cichorium intybus L.). Food Funct. 2022, 13, 8977–8988. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Tsai, F.-J.; Hsu, Y.-M.; Ho, T.-J.; Wang, G.-K.; Chiu, Y.-J.; Ha, H.-A.; Yang, J.-S. Study of Baicalin toward COVID-19 Treatment: In Silico Target Analysis and In Vitro Inhibitory Effects on SARS-CoV-2 Proteases. Biomed. Hub 2021, 6, 122–137. [Google Scholar] [CrossRef]
- Pitsillou, E.; Liang, J.; Ververis, K.; Lim, K.W.; Hung, A.; Karagiannis, T.C. Identification of Small Molecule Inhibitors of the Deubiquitinating Activity of the SARS-CoV-2 Papain-like Protease: In Silico Molecular Docking Studies and In Vitro Enzymatic Activity Assay. Front. Chem. 2020, 8, 623971. [Google Scholar] [CrossRef]
- Montone, C.M.; Aita, S.E.; Arnoldi, A.; Capriotti, A.L.; Cavaliere, C.; Cerrato, A.; Lammi, C.; Piovesana, S.; Ranaldi, G.; Lagana, A.; et al. Characterization of the Trans-Epithelial Transport of Green Tea (C. sinensis) Catechin Extracts with In Vitro Inhibitory Effect against the SARS-CoV-2 Papain-like Protease Activity. Molecules 2021, 26, 6744. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.; Badshah, S.L.; Kubra, B.; Emwas, A.-H.; Jaremko, M. Alkaloids as Potential Antivirals. A Comprehensive Review. Nat. Prod. Bioprospect. 2023, 13, 4. [Google Scholar] [CrossRef]
- Yang, L.; Stöckigt, J. Trends for Diverse Production Strategies of Plant Medicinal Alkaloids. Nat. Prod. Rep. 2010, 27, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.T.; Wong, A.Y.L.; Kaewpreedee, P.; Sia, S.F.; Chen, D.; Hui, K.P.Y.; Chu, D.K.W.; Chan, M.C.W.; Cheung, P.P.H.; Huang, X.; et al. Remdesivir, Lopinavir, Emetine, and Homoharringtonine Inhibit SARS-CoV-2 Replication In Vitro. Antiviral Res. 2020, 178, 104786. [Google Scholar] [CrossRef]
- Gendrot, M.; Andreani, J.; Boxberger, M.; Jardot, P.; Fonta, I.; Le Bideau, M.; Duflot, I.; Mosnier, J.; Rolland, C.; Bogreau, H.; et al. Antimalarial Drugs Inhibit the Replication of SARS-CoV-2: An In Vitro Evaluation. Travel Med. Infect. Dis. 2020, 37, 101873. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kashyap, P.; Chowdhury, S.; Kumar, S.; Panwar, A.; Kumar, A. Identification of Phytochemicals as Potential Therapeutic Agents That Binds to Nsp15 Protein Target of Coronavirus (SARS-CoV-2) That Are Capable of Inhibiting Virus Replication. Phytomedicine 2021, 85, 153317. [Google Scholar] [CrossRef] [PubMed]
- Mamkulathil Devasia, R.; Altaf, M.; Fahad Alrefaei, A.; Manoharadas, S. Enhanced Production of Camptothecin by Immobilized Callus of Ophiorrhiza mungos and a Bioinformatic Insight into Its Potential Antiviral Effect against SARS-CoV-2. J. King Saud Univ. Sci. 2021, 33, 101344. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Afsar, M.; Khandelwal, N.; Chander, Y.; Riyesh, T.; Dedar, R.K.; Gulati, B.R.; Pal, Y.; Barua, S.; Tripathi, B.N.; et al. Emetine Suppresses SARS-CoV-2 Replication by Inhibiting Interaction of Viral MRNA with EIF4E. Antiviral Res. 2021, 189, 105056. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Brognaro, H.; Prabhu, P.R.; de Souza, E.E.; Günther, S.; Reinke, P.Y.A.; Lane, T.J.; Ginn, H.; Han, H.; Ewert, W.; et al. Antiviral Activity of Natural Phenolic Compounds in Complex at an Allosteric Site of SARS-CoV-2 Papain-like Protease. Commun. Biol. 2022, 5, 805. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.S.M.; Ho, J.; Wills, S.; Kawall, A.; Sharma, A.; Chavada, K.; Ebert, M.C.C.J.C.; Evoli, S.; Singh, A.; Rayalam, S.; et al. Aloin Isoforms (A and B) Selectively Inhibits Proteolytic and Deubiquitinating Activity of Papain like Protease (PLpro) of SARS-CoV-2 In Vitro. Sci. Rep. 2022, 12, 2145. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Nehul, S.; Choudhary, S.; Upadhyay, A.; Kumar Sharma, G.; Kumar, P.; Tomar, S. Revealing and Evaluation of Antivirals Targeting Multiple Druggable Sites of RdRp Complex in SARS-CoV-2. bioRxiv 2023. [Google Scholar] [CrossRef]
- Chen, Z.; Cui, Q.; Cooper, L.; Zhang, P.; Lee, H.; Chen, Z.; Wang, Y.; Liu, X.; Rong, L.; Du, R. Ginkgolic Acid and Anacardic Acid Are Specific Covalent Inhibitors of SARS-CoV-2 Cysteine Proteases. Cell Biosci. 2021, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Mahanta, S.; Tanti, B.; Tag, H.; Hui, P.K. Identification of Phytocompounds from Houttuynia Cordata Thunb. as Potential Inhibitors for SARS-CoV-2 Replication Proteins through GC-MS/LC-MS Characterization, Molecular Docking and Molecular Dynamics Simulation. Mol. Divers. 2022, 26, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Kandeil, A.; Mostafa, A.; Kutkat, O.; Moatasim, Y.; Al-karmalawy, A.A.; Rashad, A.A.; Kayed, A.E.; Kayed, A.E.; El-Shesheny, R.; Kayali, G.; et al. Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2. Pathogens 2021, 10, 758. [Google Scholar] [CrossRef]
- Cheng, F.J.; Huynh, T.K.; Yang, C.S.; Hu, D.W.; Shen, Y.C.; Tu, C.Y.; Wu, Y.C.; Tang, C.H.; Huang, W.C.; Chen, Y.; et al. Hesperidin Is a Potential Inhibitor against Sars-CoV-2 Infection. Nutrients 2021, 13, 2800. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Hesperidin and Diosmin for Treatment of COVID-19. Available online: https://clinicaltrials.gov/study/NCT04452799 (accessed on 25 December 2022).
- U.S. National Library of Medicine. Study of Hesperidin Therapy on COVID-19 Symptoms (HESPERIDIN) (Hesperidin). Available online: https://clinicaltrials.gov/study/NCT04715932 (accessed on 25 December 2022).
- Yi, Y.; Zhang, M.; Xue, H.; Yu, R.; Bao, Y.O.; Kuang, Y.; Chai, Y.; Ma, W.; Wang, J.; Shi, X.; et al. Schaftoside Inhibits 3CLpro and PLpro of SARS-CoV-2 Virus and Regulates Immune Response and Inflammation of Host Cells for the Treatment of COVID-19. Acta Pharm. Sin. B 2022, 12, 4154–4164. [Google Scholar] [CrossRef] [PubMed]
- Kanchanapoom, T.; Kasai, R.; Chumsri, P.; Hiraga, Y.; Yamasaki, K. Canthin-6-One and Beta-Carboline Alkaloids from Eurycoma harmandiana. Phytochemistry 2001, 56, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Tahir ul Qamar, M.; Maryam, A.; Muneer, I.; Xing, F.; Ashfaq, U.A.; Khan, F.A.; Anwar, F.; Geesi, M.H.; Khalid, R.R.; Rauf, S.A.; et al. Computational Screening of Medicinal Plant Phytochemicals to Discover Potent Pan-Serotype Inhibitors against Dengue Virus. Sci. Rep. 2019, 9, 1433. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Liu, Z.; Yan, G.; Liu, X.; Liu, X.; Wang, Y.; Chen, Y. A Robust High-Throughput Fluorescence Polarization Assay for Rapid Screening of SARS-CoV-2 Papain-like Protease Inhibitors. Virology 2022, 574, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Y.; Wang, D.-Y.; Li, Y.-P.; Deyrup, S.T.; Zhang, H.-J. Plant-Derived Lignans as Potential Antiviral Agents: A Systematic Review. Phytochem. Rev. 2022, 21, 239–289. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.d.R.; Caetano, B.C.; de Almeida Filho, J.L.; Martins, J.S.C.d.C.; de Oliveira, M.G.P.; Sousa, T.d.C.; Horta, M.A.P.; Siqueira, M.M.; Fernandez, J.H. Identification of Hypericin as a Candidate Repurposed Therapeutic Agent for COVID-19 and Its Potential Anti-SARS-CoV-2 Activity. Front. Microbiol. 2022, 13, 828984. [Google Scholar] [CrossRef]
- Romeo, A.; Iacovelli, F.; Falconi, M. Targeting the SARS-CoV-2 Spike Glycoprotein Prefusion Conformation: Virtual Screening and Molecular Dynamics Simulations Applied to the Identification of Potential Fusion Inhibitors. Virus Res. 2020, 286, 198068. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.F.; Anhlan, D.; Schöfbänker, M.; Schreiber, A.; Classen, N.; Hensel, A.; Hempel, G.; Scholz, W.; Kühn, J.; Hrincius, E.R.; et al. Hypericum perforatum and Its Ingredients Hypericin and Pseudohypericin Demonstrate an Antiviral Activity against SARS-CoV-2. Pharmaceuticals 2022, 15, 530. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Gao, H.-M.; Wang, S.; Zhang, M.; Chen, K.; Zhang, Y.-Q.; Wang, H.-D.; Han, B.-Y.; Xu, L.-L.; Song, T.-Q.; et al. Dissection of the General Two-Step Di-C-Glycosylation Pathway for the Biosynthesis of (Iso)Schaftosides in Higher Plants. Proc. Natl. Acad. Sci. USA 2020, 117, 30816–30823. [Google Scholar] [CrossRef] [PubMed]
- Jose, R.J.; Manuel, A. COVID-19 Cytokine Storm: The Interplay between Inflammation and Coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Q.; Zhang, C.; Zhang, N.; Cui, Z.; Huang, L.; Xiao, P. An Ethnopharmacological Investigation of Medicinal Salvia Plants (Lamiaceae) in China. Acta Pharm. Sin. B 2013, 3, 273–280. [Google Scholar] [CrossRef]
- Ren, J.; Fu, L.; Nile, S.H.; Zhang, J.; Kai, G. Salvia miltiorrhiza in Treating Cardiovascular Diseases: A Review on Its Pharmacological and Clinical Applications. Front. Pharmacol. 2019, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zheng, J.; Huang, G.; Xiang, Y.; Lang, C.; Li, B.; Huang, D.; Sun, Q.; Luo, Y.; Zhang, Y.; et al. Xuebijing Injection in the Treatment of COVID-19: A Retrospective Case-Control Study. Ann. Palliat. Med. 2020, 9, 3235–3248. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, Critical Pharmacological Components in Salvia miltiorrhiza. Front. Pharmacol. 2019, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Liang, J.; Wang, X.; Xu, T.; Hua, L.; Shang, R.; Liu, Y.; Xing, Y. Anti-Influenza A Virus Effect of Hypericum perforatum L. Extract. Virol. Sin. 2009, 24, 19–27. [Google Scholar] [CrossRef]
- Shih, C.-M.; Wu, C.-H.; Wu, W.-J.; Hsiao, Y.-M.; Ko, J.-L. Hypericin Inhibits Hepatitis C Virus Replication via Deacetylation and Down-Regulation of Heme Oxygenase-1. Phytomedicine 2018, 46, 193–198. [Google Scholar] [CrossRef]
- Chen, H.; Muhammad, I.; Zhang, Y.; Ren, Y.; Zhang, R.; Huang, X.; Diao, L.; Liu, H.; Li, X.; Sun, X.; et al. Antiviral Activity against Infectious Bronchitis Virus and Bioactive Components of Hypericum perforatum L. Front. Pharmacol. 2019, 10, 1272. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabeen, J.; Ahmed, N.; Shahzad, Z.; Shahid, M.; Ahmad, T. Insights for Future Pharmacology: Exploring Phytochemicals as Potential Inhibitors Targeting SARS-CoV-2 Papain-like Protease. Future Pharmacol. 2024, 4, 510-540. https://doi.org/10.3390/futurepharmacol4030029
Jabeen J, Ahmed N, Shahzad Z, Shahid M, Ahmad T. Insights for Future Pharmacology: Exploring Phytochemicals as Potential Inhibitors Targeting SARS-CoV-2 Papain-like Protease. Future Pharmacology. 2024; 4(3):510-540. https://doi.org/10.3390/futurepharmacol4030029
Chicago/Turabian StyleJabeen, Jawaria, Nabeel Ahmed, Zunaira Shahzad, Maida Shahid, and Taseer Ahmad. 2024. "Insights for Future Pharmacology: Exploring Phytochemicals as Potential Inhibitors Targeting SARS-CoV-2 Papain-like Protease" Future Pharmacology 4, no. 3: 510-540. https://doi.org/10.3390/futurepharmacol4030029
APA StyleJabeen, J., Ahmed, N., Shahzad, Z., Shahid, M., & Ahmad, T. (2024). Insights for Future Pharmacology: Exploring Phytochemicals as Potential Inhibitors Targeting SARS-CoV-2 Papain-like Protease. Future Pharmacology, 4(3), 510-540. https://doi.org/10.3390/futurepharmacol4030029






