The Evaluation of Citrus bergamia Phytochemicals as Potential Cholesterol-Lowering Agents against HMG-CoA Reductase: An In Silico Molecular Docking Study †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Protein
2.2. Preparation of Ligands
2.3. Molecular Docking
3. Results
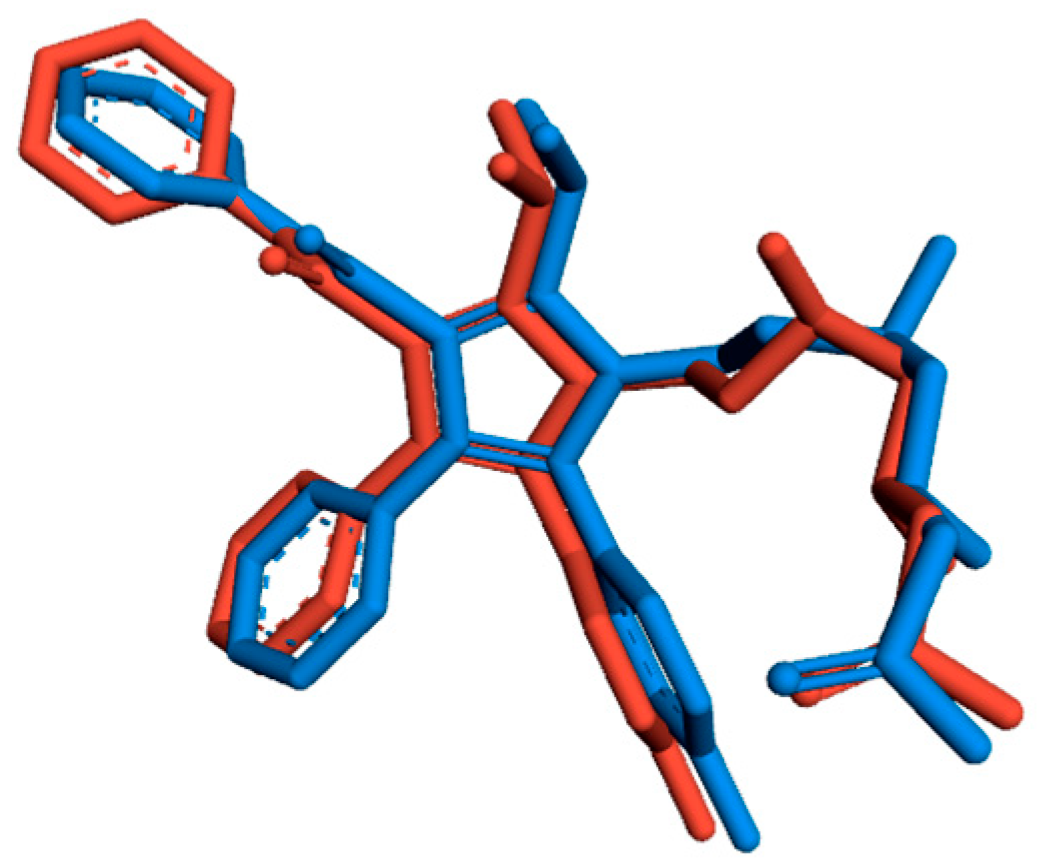
3.1. Validation of the Docking Protocol
3.2. Binding Affinities of the Phytochemicals
3.3. Protein–Ligand Interactions of the Top Ligands
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jalaja, R.; Leela, S.G.; Mohan, S.; Nair, M.S.; Gopalan, R.K.; Somappa, S.B. Anti-hyperlipidemic potential of natural product based labdane-pyrroles via inhibition of cholesterol and triglycerides synthesis. Bioorg. Chem. 2021, 108, 104664. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Baek, A.; Sakkiah, S.; Park, C.; John, S.; Lee, K.W. Exploration of virtual candidates for human HMG-CoA reductase inhibitors using pharmacophore modeling and molecular dynamics simulations. PLoS ONE 2013, 8, e83496. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, S.; Raghunath, A.; Raghunath, S. Statin therapy: Review of safety and potential side effects. Acta Cardiol. Sin. 2016, 32, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tocmo, R.; Nauman, M.C.; Haughan, M.A.; Johnson, J.J. defining the cholesterol lowering mechanism of bergamot (Citrus Bergamia) extract in HepG2 and Caco-2 Cells. Nutrients 2021, 13, 3156. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Arigò, A.; Calabrò, M.L.; Farnetti, S.; Mondello, L.; Dugo, P. Bergamot (Citrus bergamia Risso) as a source of nutraceuticals: Limonoids and flavonoids. J. Funct. Foods 2016, 20, 10–19. [Google Scholar] [CrossRef]
- Baron, G.; Altomare, A.; Mol, M.; Garcia, J.L.; Correa, C.; Raucci, A.; Mancinelli, L.; Mazzotta, S.; Fumagalli, L.; Trunfio, G.; et al. Analytical profile and antioxidant and anti-inflammatory activities of the enriched polyphenol fractions isolated from bergamot fruit and leave. Antioxidants 2021, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Miceli, N.; Mondello, M.R.; Monforte, M.T.; Sdrafkakis, V.; Dugo, P.; Crupi, M.L.; Taviano, M.F.; De Pasquale, R.; Trovato, A. Hypolipidemic effects of Citrus Bergamia Risso et Poiteau juice in rats fed a hypercholesterolemic diet. J. Agric. Food Chem. 2007, 55, 10671–10677. [Google Scholar] [CrossRef] [PubMed]
- Lamiquiz-Moneo, I.; Giné-González, J.; Alisente, S.; Bea, A.M.; Pérez-Calahorra, S.; Marco-Benedí, V.; Baila-Rueda, L.; Jarauta, E.; Cenarro, A.; Civeira, F.; et al. Effect of bergamot on lipid profile in humans: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 60, 3133–3143. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S.; et al. hypoglycemic and hypolipemic effects of a new lecithin formulation of bergamot polyphenolic fraction: A double blind, randomized, placebo-controlled study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Gesto, D.S.; Pereira, C.M.S.; Cerqueira, N.M.F.S.; Sousa, S.F. An atomic-level perspective of HMG-CoA-reductase: The targe enzyme to treat hypercholesterolemia. Molecules 2020, 25, 3891. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, D.; Caballero, J. Is it reliable to take the molecular docking top scoring position as the best solution without considering available structural data? Molecules 2018, 23, 1038. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, P.L.; Bonvin, A.M.J.J. On the binding affinity of macromolecular interactions: Daring to ask why proteins interact. J. R. Soc. Interface 2013, 10, 20120835. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Liu, X.; Russell, P.; Li, J.; Pan, W.; Fu, J.; Zhang, A. Evaluation of the binding performance of flavonoids to estrogen receptor alpha by Autodock, Autodock Vina and Surflex-Dock. Ecotoxicol. Environ. Saf. 2022, 233, 113323. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, Y.; Xia, Y.; Ai, S.; Liang, J.; Sang, P.; Ji, X.; Liu, S. Insights into protein-ligand interactions: Mechanisms, models, and methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Cholesterol Lowering Drugs. In Endotext [Internet]; P MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK395573/ (accessed on 27 August 2024).
- Ferrarese, I.; Lupo, M.G.; Rossi, I.; Sut, S.; Loschi, F.; Allegrini, P.; Riva, A.; Ferri, N.; Dall’Acqua, S. Bergamot (Citrus bergamia) peel extract as new hypocholesterolemic agent modulating PCSK9 expression. J. Funct. Foods 2023, 108, 105724. [Google Scholar] [CrossRef]
- Gliozzi, M.; Walker, R.; Muscoli, S.; Vitale, C.; Gratteri, S.; Carresi, C.; Musolino, V.; Russo, V.; Janda, E.; Ragusa, S.; et al. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int. J. Cardiol. 2013, 170, 140–145. [Google Scholar] [CrossRef] [PubMed]



| Ligand Name | PubChem ID | Phytochemical Class | Binding Affinity (kcal/mol) |
|---|---|---|---|
| Eriocitrin | 83489 | Flavanone | −10.0 |
| Narirutin | 442431 | Flavanone | −9.9 |
| Scolymoside | 5282152 | Flavone | −9.8 |
| Neodiosmin | 69964214 | Flavone | −9.8 |
| Brutieridin | 45279710 | Flavanone | −9.7 |
| Neohesperidin | 442439 | Flavanone | −9.7 |
| Rhoifolin | 5282150 | Flavone | −9.6 |
| Naringin | 442428 | Flavone | −9.5 |
| Melitidin | 168011951 | Flavanone | −8.7 |
| Vitexin | 5280441 | Flavone | −8.7 |
| Scoparin | 20055255 | Flavone | −8.6 |
| Diosmetin-8-C-glucoside | — | Flavone | −8.6 |
| Stellarin 2 | 44258167 | Flavone | −8.3 |
| Lucenin 2 | 44257937 | Flavone | −8.2 |
| Diosmetin-6,8-di-C-glucoside | — | Flavone | −8.1 |
| Vicenin 2 | 442664 | Flavone | −8.0 |
| Isovitexin | 162350 | Flavone | −7.8 |
| Bergamottin | 5471349 | Coumarin | −7.7 |
| Bergapten | 2355 | Coumarin | −7.3 |
| Sinensetin | 145659 | Flavone | −7.1 |
| Atorvastatin * | — | — | −9.2 |
| Ligand Name | Binding Affinity (kcal/mol) | Amino Acid Residues Involved in Interaction | ||
|---|---|---|---|---|
| Hydrogen Bond | Hydrophobic Interactions | Electrostatic Interactions | ||
| Eriocitrin | −10.0 | Gly 560A, Asn 755A, Arg 590B, Met 655B, Lys 691B, Lys 692B, Asp 690B, Ala 751A | Met 655B, Gly 806B | Glu 559A |
| Narirutin | −9.9 | Gly 560A, Lys 735A, Asn 755A, Arg 590B, Lys 691B, Lys 692B, Asp 690B, Val 805B, Glu 559A | Met 655B, Gly 806B | Glu 559A |
| Scolymoside | −9.8 | Gly 560A, Arg 590B, Gly 765B | Met 655B | Glu 559A, Asp 767B |
| Neodiosmin | −9.8 | Gly 560A, Arg 590B, Lys 691B, Asp 690B | Met 655B, Gly 806B | Glu 559A |
| Brutieridin | −9.7 | Asn 755A, Arg 590B, Met 655B, Gly 656B, Ser 684B, Lys 691B, Lys 692B, Asn 658B, Asp 690B | Met 655B, Ala 654B | None |
| Neohesperidin | −9.7 | Gly 560A, Arg 590B | Met 655B | Glu 559A |
| Rhoifolin | −9.6 | Gly 560A, Arg 590B, Glu 559A, Asp 690B | Met 655B, Gly 806B, Met 657B | Glu 559A |
| Naringin | −9.5 | Asn 755A, Arg 590B, Lys 691B, Asp 767B, Gly 765B, Gly 560A | Met 655B, Gly 806B | Glu 559A, Asp 767B |
| Atorvastatin * | −9.2 | Ser 565A, Ser 661B, Lys 735A, His 752A, Asn 755A, Arg 590B, Lys 692B, Asp 690B | Ala 856A, Leu 853A, Val 683B, Cys 561A, Ala 564A | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agosto, N.J.; Alambatin, P.B.; Bacalso, J.; Cabisada, J.; Carating, B.D. The Evaluation of Citrus bergamia Phytochemicals as Potential Cholesterol-Lowering Agents against HMG-CoA Reductase: An In Silico Molecular Docking Study. Biol. Life Sci. Forum 2024, 35, 7. https://doi.org/10.3390/blsf2024035007
Agosto NJ, Alambatin PB, Bacalso J, Cabisada J, Carating BD. The Evaluation of Citrus bergamia Phytochemicals as Potential Cholesterol-Lowering Agents against HMG-CoA Reductase: An In Silico Molecular Docking Study. Biology and Life Sciences Forum. 2024; 35(1):7. https://doi.org/10.3390/blsf2024035007
Chicago/Turabian StyleAgosto, Nesteve John, Patricia Blanch Alambatin, Joemer Bacalso, JC Cabisada, and Beatriz Danica Carating. 2024. "The Evaluation of Citrus bergamia Phytochemicals as Potential Cholesterol-Lowering Agents against HMG-CoA Reductase: An In Silico Molecular Docking Study" Biology and Life Sciences Forum 35, no. 1: 7. https://doi.org/10.3390/blsf2024035007








