Thermal Stability of Blending Soybean Oil with Coconut Oil During Continuous Deep Frying of Banana Chips †
Abstract
1. Introduction
2. Methodology
2.1. Materials
2.2. Oil Blending Preparation
2.3. Banana Preparation
2.4. Deep Frying Procedure
2.5. Physicochemical Analysis
2.5.1. Free Fatty Acid Content
2.5.2. Peroxide Value
2.5.3. P-Anisidine Value
2.5.4. TOTOX Value
2.5.5. Analysis of Oil and Fried Banana Color
2.6. Determination of Lipid Content in Fried Banana
2.7. Statistical Analysis
3. Results and Discussion
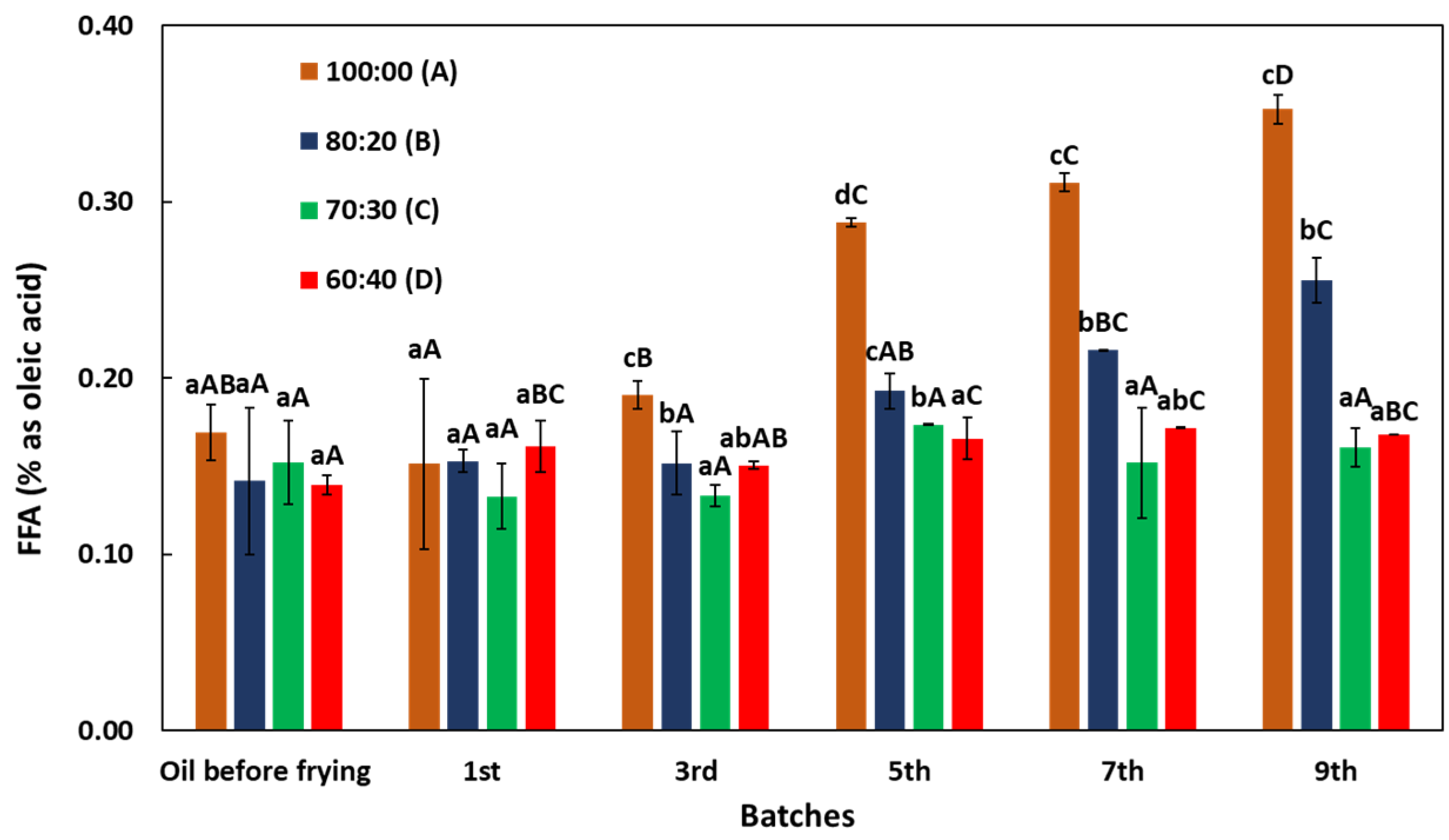
3.1. Changes in Free Fatty Acid (FFA) Content
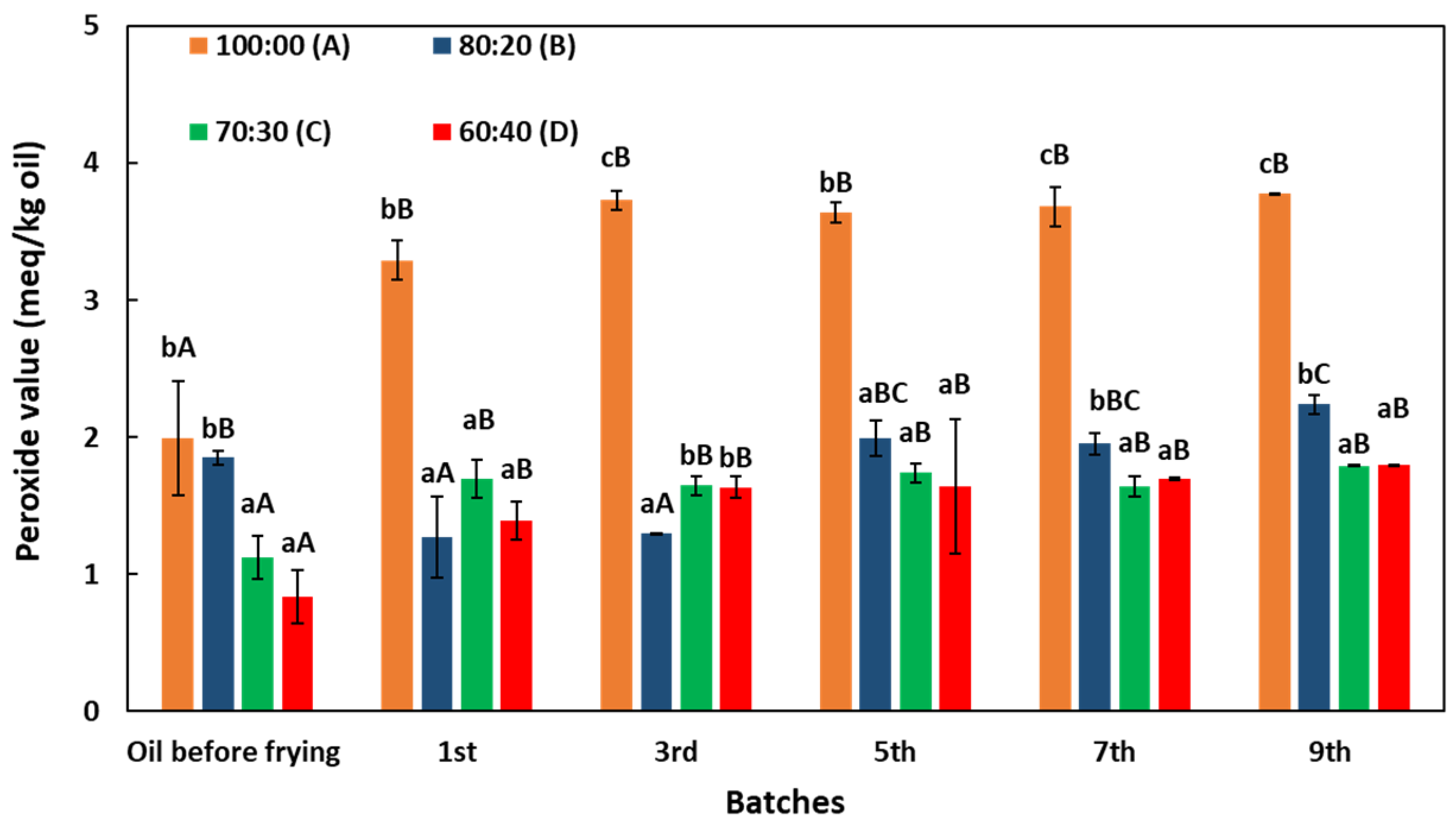
3.2. Changes in Peroxide Value (PV)
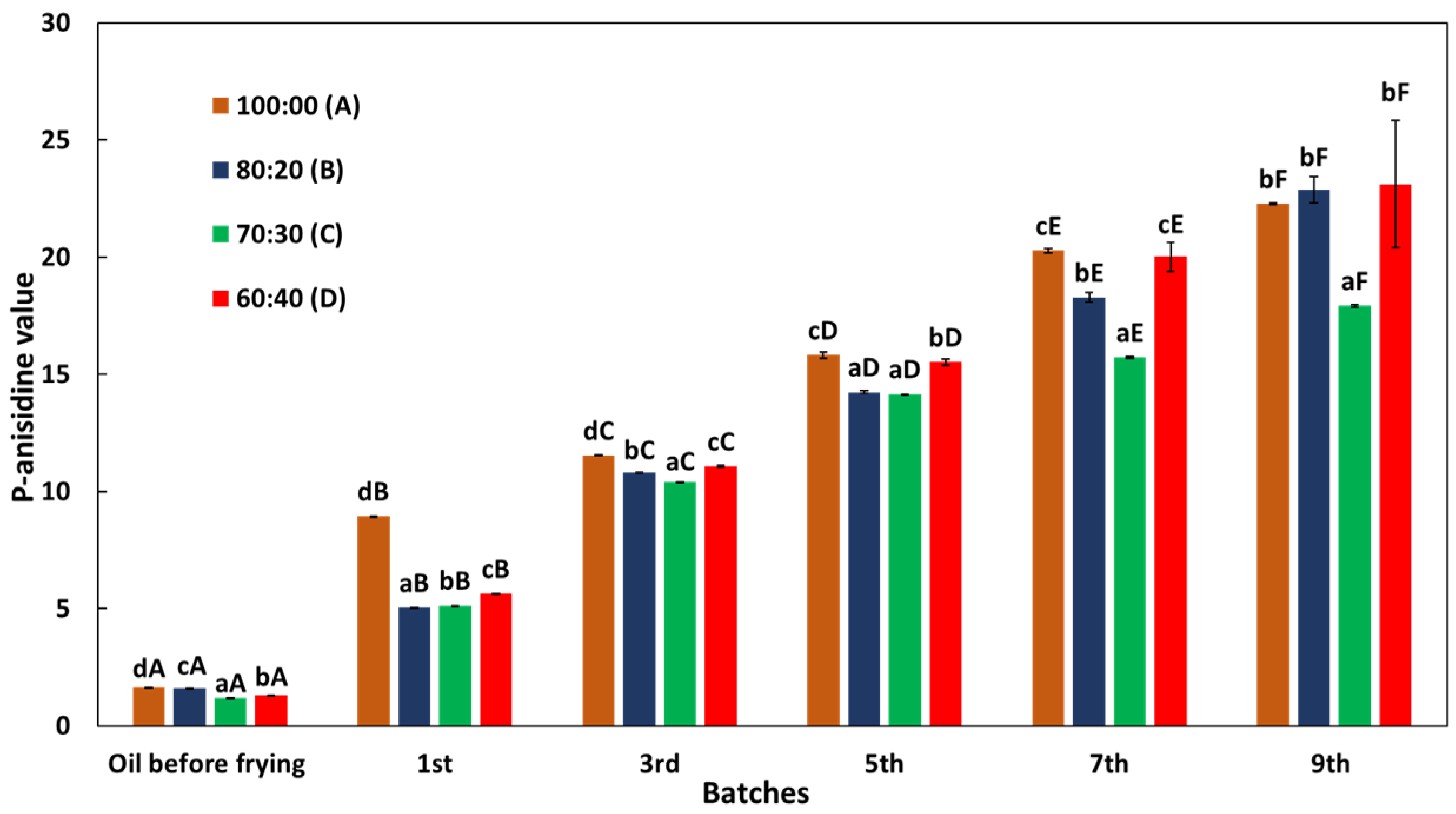
3.3. Changes in p-Anisidine Value (p-AV)
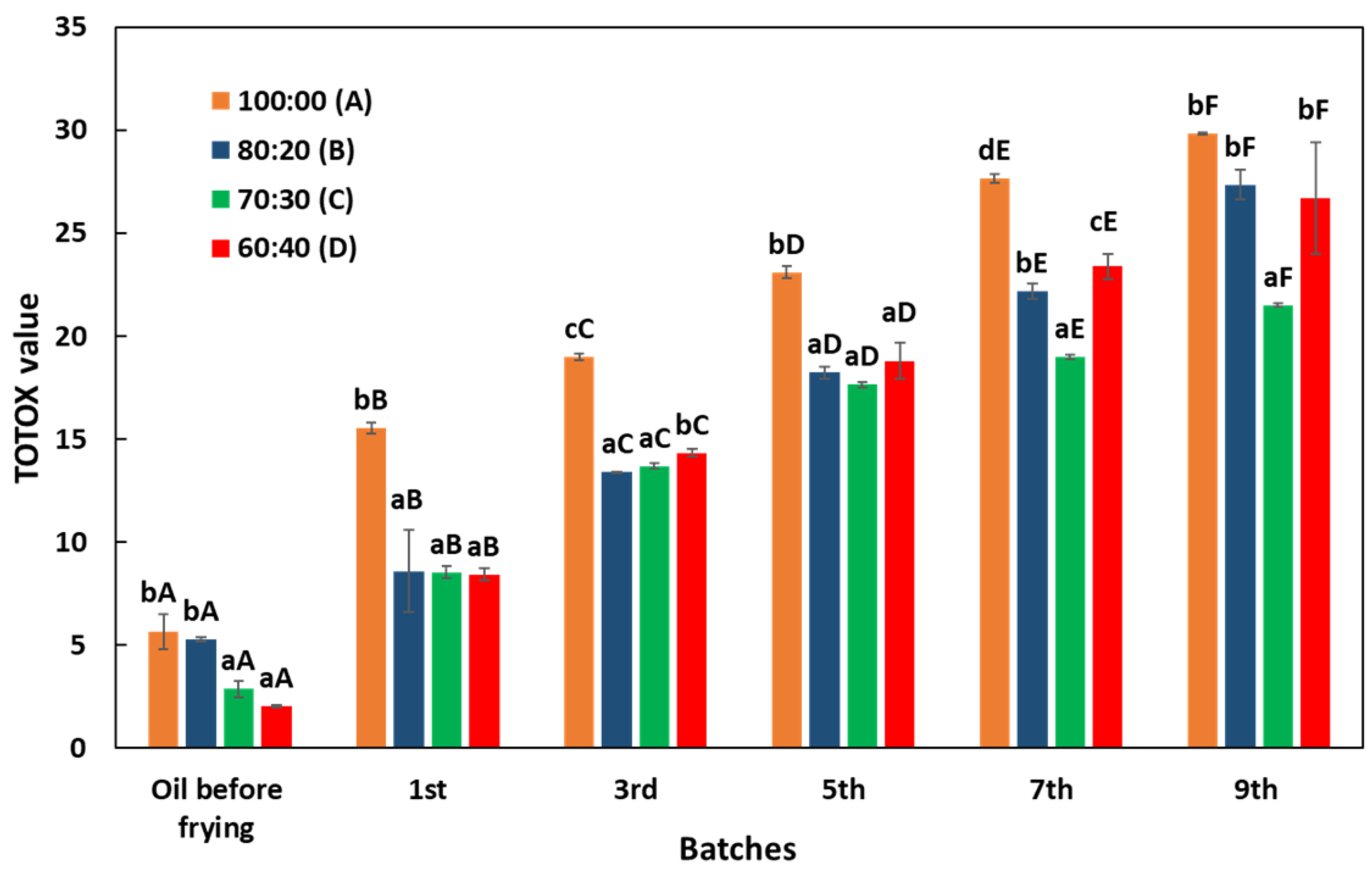
3.4. Changes in TOTOX Value
3.5. Changes in Color Value of Oils
3.6. Color of Fried Banana Chips After Deep-Frying Process
3.7. Lipid Content of Fried Banana Chips After Deep-Frying Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onal-Ulusoy, B.; White, P.; Hammond, E. Effects of Linalyl Oleate on Soybean Oil Flavor and Quality in a Frying Application. JAOCS J. Am. Oil Chem. Soc. 2007, 84, 157–163. [Google Scholar] [CrossRef]
- Goburdhun, D.; Jhurree, B. Effect of Deep-Fat Frying on Fat Oxidation in Soybean Oil. Int. J. Food Sci. Nutr. 1995, 46, 363–371. [Google Scholar] [CrossRef]
- Ali, M.A.; Islam, M.A.; Othman, N.H.; Noor, A.M.; Ibrahim, M. Effect of Rice Bran Oil Addition on the Oxidative Degradation and Fatty Acid Composition of Soybean Oil during Heating. Acta Sci. Pol. Technol. Aliment. 2019, 18, 427–438. [Google Scholar] [PubMed]
- Wang, S.N.; Sui, X.N.; Wang, Z.J.; Qi, B.K.; Jiang, L.Z.; Li, Y.; Wang, R.; Wei, X. Improvement in Thermal Stability of Soybean Oil by Blending with Camellia Oil during Deep Fat Frying. Eur. J. Lipid Sci. Technol. 2016, 118, 524–531. [Google Scholar] [CrossRef]
- Lee, Y.J.; Khor, Y.P.; Kadir, N.S.A.; Lan, D.; Wang, Y.; Tan, C.P. Deep-Fat Frying Using Soybean Oil-Based Diacylglycerol-Palm Olein Oil Blends: Thermo-Oxidative Stability, 3-MCPDE and Glycidyl Ester Formation. J. Oleo Sci. 2023, 72, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Wason, S.; Meena, P.L.; Chopra, R.; Sadhu, S.D.; Dhyani, A. Effect of Frying on Physicochemical Properties of Sesame and Soybean Oil Blend. J. Appl. Nat. Sci. 2021, 13, 820–829. [Google Scholar] [CrossRef]
- Olagunju, A.I.; Adelakun, O.S.; Olawoyin, M.S. The Effect of Rice Bran Extract on the Quality Indices, Physicochemical Properties and Oxidative Stability of Soybean Oil Blended with Various Oils. Meas. Food 2022, 6, 100032. [Google Scholar] [CrossRef]
- Bhatnagar, A.S.; Prasanth Kumar, P.K.; Hemavathy, J.; Gopala Krishna, A.G. Fatty Acid Composition, Oxidative Stability, and Radical Scavenging Activity of Vegetable Oil Blends with Coconut Oil. JAOCS J. Am. Oil Chem. Soc. 2009, 86, 991–999. [Google Scholar] [CrossRef]
- Sharanke, K.; Sivakanthan, S. Comparative Study on Stability of Coconut Oil, Sunflower Oil and Palm Oil during Continuous Deep Frying. Ceylon J. Sci. 2022, 51, 359–368. [Google Scholar] [CrossRef]
- Mekonnen, M.F.; Desta, D.T.; Alemayehu, F.R.; Kelikay, G.N.; Daba, A.K. Evaluation of Fatty Acid-Related Nutritional Quality Indices in Fried and Raw Nile Tilapia, (Oreochromis Niloticus), Fish Muscles. Food Sci. Nutr. 2020, 8, 4814–4821. [Google Scholar] [CrossRef]
- Patil, R.S.; Waghmare, J.; Annapure, U. Comparative Assessment of the Frying Performance of Palm Olein and Sunflower Oil during Deep-Fat Frying of Indian Battered Food Products. J. Agric. Food Res. 2023, 14, 100778. [Google Scholar] [CrossRef]
- Khan, M.I.; Asha, M.R.; Bhat, K.K.; Khatoon, S. Studies on Quality of Coconut Oil Blends after Frying Potato Chips. JAOCS J. Am. Oil Chem. Soc. 2008, 85, 1165–1172. [Google Scholar] [CrossRef]
- Tiwari, M.R.; Tiwari, K.K.; Toliwal, S.D. Studies on Thermal Stability of Palm-Sesame Oil Blends during Deep Fat Frying. J. Sci. Ind. Res. 2014, 73, 153–156. [Google Scholar]
- Venkatachalam, K. The Different Concentrations of Citric Acid on Inhibition of Longkong Pericarp Browning during Low Temperature Storage. Int. J. Fruit Sci. 2015, 15, 353–368. [Google Scholar] [CrossRef]
- Khattab, R. Physicochemical Quality and Thermal Stability of Vegetable Oils during Deep-Fat Frying of Potato Chips. Curr. Nutr. Food Sci. 2022, 20, 337–348. [Google Scholar] [CrossRef]
- Serjouie, A.; Tan, C.P.; Mirhosseini, H.; Man, Y.B.C. Effect of Vegetable-Based Oil Blends on Physicochemical Properties of Oils during Deep-Fat Frying. Am. J. Food Technol. 2010, 5, 310–323. [Google Scholar] [CrossRef]
- Hu, B.; Wu, R.; Sun, J.; Shi, H.; Jia, C.; Liu, R.; Rong, J. Monitoring the Oxidation Process of Soybean Oil during Deep-Frying of Fish Cakes with 1H Nuclear Magnetic Resonance. Food Chem. X 2023, 17, 100587. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Methods Ce 2-66 Preparation of Methyl Esters of Fatty Acids; Official Methods and Recommended practices of AOCS; AOCS: Urbana, IL, USA, 1997. [Google Scholar]
- Nayak, P.K.; Dash, U.; Rayaguru, K.; Krishnan, K.R. Physio-Chemical Changes During Repeated Frying of Cooked Oil: A Review. J. Food Biochem. 2016, 40, 371–390. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 19th ed.; AOAC Official Methods of Analysis; AOCS: Urbana, IL, USA, 2012. [Google Scholar]
- Matthäus, B. Oxidation in Foods and Beverages and Antioxidant Applications. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Elsevier: Amsterdam, The Netherlands, 2010; Volume 2, pp. 183–238. [Google Scholar]
- Subajiny, S.; Dilini, B.; Terrence, M. A Comparative Study on Stability of Different Types of Coconut (Cocos Nucifera) Oil against Autoxidation and Photo-Oxidation. Afr. J. Food Sci. 2018, 12, 216–229. [Google Scholar] [CrossRef]
- Ramroudi, F.; Yasini Ardakani, S.A.; Dehghani-Tafti, A.; Khalili Sadrabad, E. Investigation of the Physicochemical Properties of Vegetable Oils Blended with Sesame Oil and Their Oxidative Stability during Frying. Int. J. Food Sci. 2022, 2022, 3165512. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Preedy, V.R. Oxidative Stress and Dietary Antioxidants in Neurological Diseases; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, 5. [Google Scholar] [CrossRef] [PubMed]
- Negroni, M.; D’Agostina, A.; Arnoldi, A. Effects of Olive, Canola, and Sunflower Oils on the Formation of Volatiles from the Maillard Reaction of Lysine with Xylose and Glucose. J. Agric. Food Chem. 2001, 49, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Mittal, G.S. Regulating the Use of Degraded Oil/Fat in Deep-Fat/Oil Food Frying. Crit. Rev. Food Sci. Nutr. 1997, 37, 635–662. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Rastogi, N.K.; Gopala Krishna, A.G.; Lokesh, B.R. Effect of Frying Cycles on Physical, Chemical and Heat Transfer Quality of Rice Bran Oil during Deep-Fat Frying of Poori: An Indian Traditional Fried Food. Food Bioprod. Process. 2012, 90, 249–256. [Google Scholar] [CrossRef]
- Kittipongpittaya, K.; Panya, A.; Prasomsri, T.; Sueaphet, P. Tropical Oil Blending and Their Effects on Nutritional Content and Physicochemical Properties during Deep Fat Frying. J. Nutr. Sci. Vitaminol. 2020, 66, S206–S214. [Google Scholar] [CrossRef]
- Isabel Minguez-Mosquera, M.; Rejano-Navarro, L.; Gandul-Rojas, B.; SanchezGomez, A.H.; Garrido-Fernandez, J. Color-Pigment Correlation in Virgin Olive Oil. J. Am. Oil Chem. Soc. 1991, 68, 332–336. [Google Scholar] [CrossRef]
- Aida, S.A.; Noriza, A.; Haswani, M.M.; Mya, S.M.Y. A Study on Reducing Fat Content of Fried Banana Chips Using a Sweet Pretreatment Technique. Int. Food Res. J. 2016, 23, 68–71. [Google Scholar]
- Krokida, M.K.; Oreopoulou, V.; Maroulis, Z.B.; Marinos-Kouris, D. Colour Changes during Deep Fat Frying. J. Food Eng. 2001, 48, 219–225. [Google Scholar] [CrossRef]
- Kita, A.; Lisińska, G. The Influence of Oil Type and Frying Temperatures on the Texture and Oil Content of French Fries. J. Sci. Food Agric. 2005, 85, 2600–2604. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, S.; Kong, S.; Say, M.; Tan, R. Thermal Stability of Blending Soybean Oil with Coconut Oil During Continuous Deep Frying of Banana Chips. Biol. Life Sci. Forum 2024, 40, 33. https://doi.org/10.3390/blsf2024040033
Yi S, Kong S, Say M, Tan R. Thermal Stability of Blending Soybean Oil with Coconut Oil During Continuous Deep Frying of Banana Chips. Biology and Life Sciences Forum. 2024; 40(1):33. https://doi.org/10.3390/blsf2024040033
Chicago/Turabian StyleYi, Sopheaktra, Sela Kong, Manit Say, and Reasmey Tan. 2024. "Thermal Stability of Blending Soybean Oil with Coconut Oil During Continuous Deep Frying of Banana Chips" Biology and Life Sciences Forum 40, no. 1: 33. https://doi.org/10.3390/blsf2024040033
APA StyleYi, S., Kong, S., Say, M., & Tan, R. (2024). Thermal Stability of Blending Soybean Oil with Coconut Oil During Continuous Deep Frying of Banana Chips. Biology and Life Sciences Forum, 40(1), 33. https://doi.org/10.3390/blsf2024040033






