Efficient Storage of Methane in Hydrate Form Using Soybean Powder
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials Needed for the Formation of Hydrate
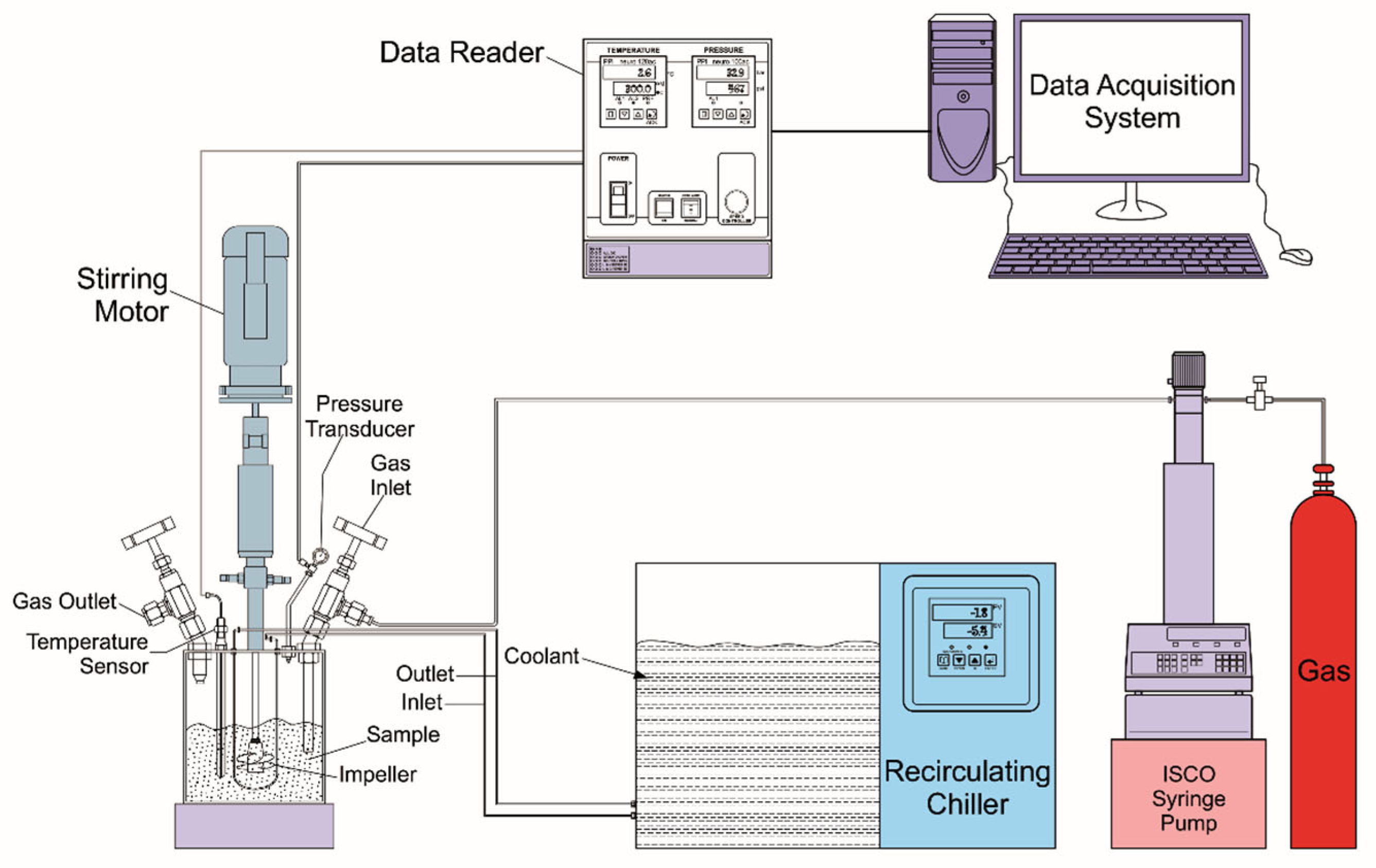
2.2. The Equipment and Steps Used to Form the Hydrate
2.3. Equations Used for Calculating and Inferring the Data
2.3.1. The Formula for Estimating How Much Gas Is Consumed during Hydrate Formation
2.3.2. The Equation for Calculating the Gas Uptake (v/v)
3. Results and Discussion
3.1. Methane Hydrate Formation
3.2. Methane Gas Uptake Capacity
3.3. Methane Gas Consumption Kinetics
3.4. Reusability and Foaming of Sample Solution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faramawy, S.; Zaki, T.; Sakr, A.A.E. Natural gas origin, composition, and processing: A review. J. Nat. Gas Sci. Eng. 2016, 34, 34–54. [Google Scholar] [CrossRef]
- Makogon, Y.F. Natural gas hydrates—A promising source of energy. J. Nat. Gas Sci. Eng. 2010, 2, 49–59. [Google Scholar] [CrossRef]
- Pinnelli, S.R.P.; Burla, S.K. Feasibility of hydrate technology for natural gas storage and transportation. Curr. Sci. 2022, 122, 513–514. [Google Scholar]
- Bhattacharjee, G.; Veluswamy, H.P.; Kumar, A.; Linga, P. Stability analysis of methane hydrates for gas storage application. Chem. Eng. J. 2021, 415, 128927. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Hao, W.F.; Wang, J.Q.; Fan, S.S.; Hao, W.B. Evaluation and analysis method for natural gas hydrate storage and transportation processes. Energy Convers. Manag. 2008, 49, 2546–2553. [Google Scholar] [CrossRef]
- Thomas, S.; Dawe, R.A. Review of ways to transport natural gas energy from countries which do not need the gas for domestic use. Energy 2003, 28, 1461–1477. [Google Scholar] [CrossRef]
- Gudmundsson, J.-S.; Parlaktuna, M.; Khokhar, A.A. Storage of Natural Gas as Frozen Hydrate. SPE Prod. Facil. 1994, 9, 69–73. [Google Scholar] [CrossRef]
- Kanda, H. Economic study on natural gas transportation with natural gas hydrate (Ngh) pellets. In Proceedings of the 23rd World Gas Conference, Amsterdam, The Netherlands, 5–9 June 2006. [Google Scholar]
- Kelland, M.A. Challenges with gas hydrate formation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 700, 012057. [Google Scholar] [CrossRef]
- Khurana, M.; Yin, Z.; Linga, P. A Review of Clathrate Hydrate Nucleation. ACS Sustain. Chem. Eng. 2017, 5, 11176–11203. [Google Scholar] [CrossRef]
- Xia, Z.; Zhao, Q.; Chen, Z.; Li, X.; Zhang, Y.; Xu, C.; Yan, K. Review of methods and applications for promoting gas hydrate formation process. J. Nat. Gas Sci. Eng. 2022, 101, 104528. [Google Scholar] [CrossRef]
- Nasir, Q.; Suleman, H.; Elsheikh, Y.A. A review on the role and impact of various additives as promoters/inhibitors for gas hydrate formation. J. Nat. Gas Sci. Eng. 2020, 76, 103211. [Google Scholar] [CrossRef]
- Sai Kiran, B.; Sowjanya, K.; Prasad, P.S.R.; Yoon, J.-H. Experimental investigations on tetrahydrofuran–methane–water system: Rapid methane gas storage in hydrates. Oil Gas Sci. Technol.—Revue d’IFP Energies Nouvelles 2019, 74, 12. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.-T.; Chen, C.; Zhang, G.-D.; Chao, K.; Lin, Y.; Wang, F. Surfactant-based promotion to gas hydrate formation for energy storage. J. Mater. Chem. A 2019, 7, 21634–21661. [Google Scholar] [CrossRef]
- Mohammadi, A.; Babakhanpour, N.; Javidani, A.M.; Ahmadi, G. Corn’s dextrin, a novel environmentally friendly promoter of methane hydrate formation. J. Mol. Liq. 2021, 336, 116855. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Chen, F.-L.; Yu, S.-J.; Wang, F. Biopromoters for Gas Hydrate Formation: A Mini Review of Current Status. Front. Chem. 2020, 8, 514. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, Y.; Cai, Y.; Niu, X.; Zhu, Z.; Lei, D.; Wang, W. Chinese herbs: Treasure troves for the discovery of environmentally friendly promoters for methane hydrate formation. Sustain. Energy Fuels 2020, 4, 5947–5951. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Z.; Chen, H.; Tan, Z.; Chen, C.; Sun, L. Methane hydrates with a high capacity and a high formation rate promoted by biosurfactants. Chem. Commun. 2012, 48, 11638–11640. [Google Scholar] [CrossRef]
- Fakharian, H.; Ganji, H.; Naderi Far, A.; Kameli, M. Potato starch as methane hydrate promoter. Fuel 2012, 94, 356–360. [Google Scholar] [CrossRef]
- Garcia, M.C.; Torre, M.; Marina, M.L.; Laborda, F. Composition and characterization of soyabean and related products. Crit. Rev. Food Sci. Nutr. 1997, 37, 361–391. [Google Scholar] [CrossRef]
- Etiosa, O.; Chika, N.; Benedicta, A. Mineral and Proximate Composition of Soya Bean. Asian J. Phys. Chem. Sci. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Kiran, B.S.; Bhavya, T.; Prasad, P.S.R. Synergistic and antagonistic effects of amino acids in clathrate hydrates of greenhouse gases. Chem. Eng. J. Adv. 2021, 7, 100117. [Google Scholar] [CrossRef]
- Bhavya, T.; Sai Kiran, B.; Prasad, P.S.R. The Role of Stirring and Amino Acid Mixtures to Surpass the Sluggishness of CO2 Hydrates. Energy Fuels 2021, 35, 13937–13944. [Google Scholar] [CrossRef]
- Prasad, P.S.R.; Kiran, B.S. Synergistic effects of amino acids in clathrates hydrates: Gas capture and storage applications. Chem. Eng. J. Adv. 2020, 3, 100022. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B.; Chen, Y.; Zhang, S.; Guo, W.; Cai, Y.; Tan, B.; Wang, W. Methane Storage in a Hydrated Form as Promoted by Leucines for Possible Application to Natural Gas Transportation and Storage. Energy Technol. 2015, 3, 815–819. [Google Scholar] [CrossRef]


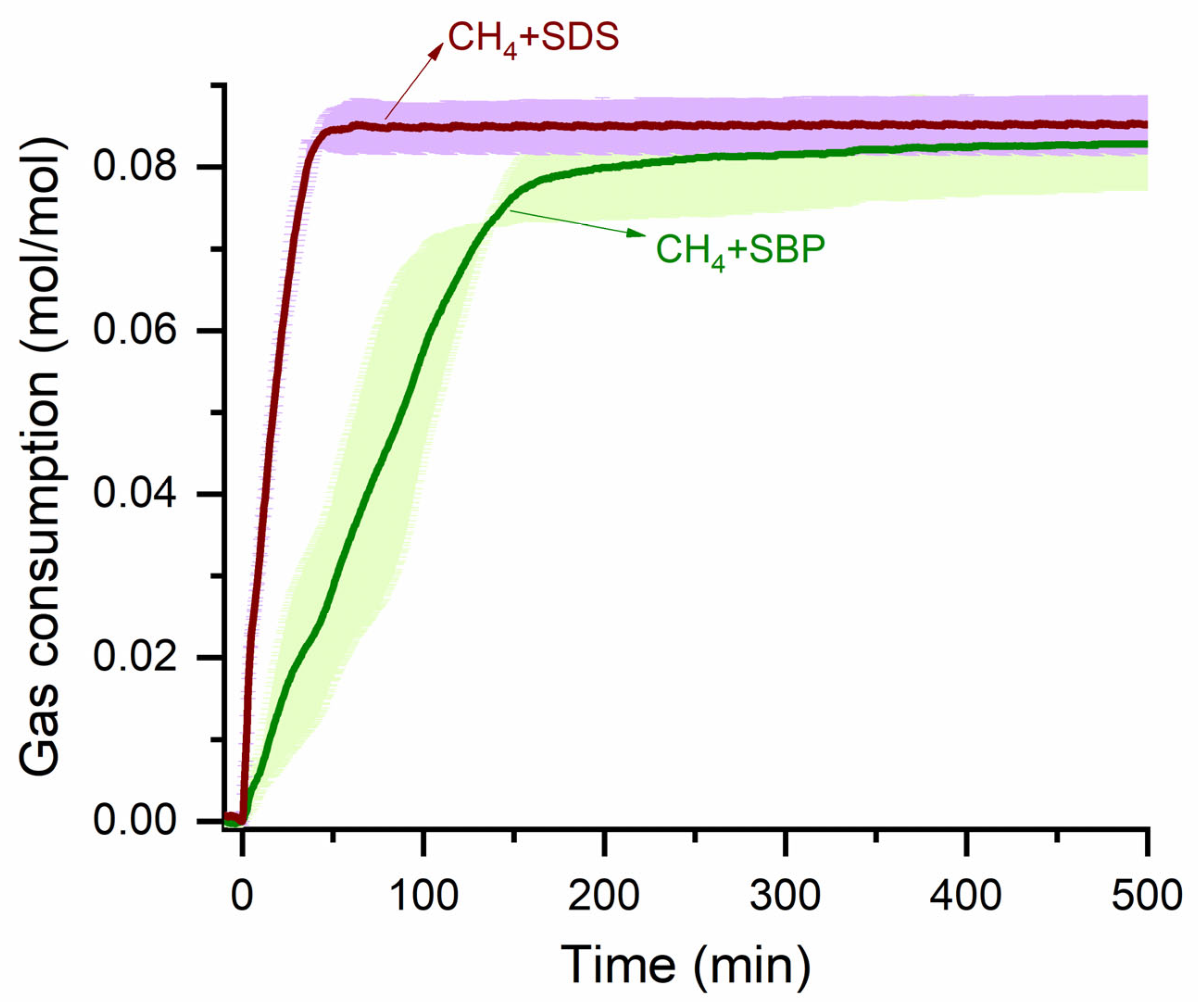

| Sample | Tformation (K) | Pformation (kPa) | Subcooling (K) | T90 Uptake Kinetics (min) | Hydrate Yield (v/v) |
|---|---|---|---|---|---|
| SBP | 277.8 ± 3.2 | 7050.9 ± 76.2 | 5.1 ± 3.1 | 144 ± 4.2 | 94.2 ± 4.5 |
| SDS | 277.2 ± 0.3 | 7446.3 ± 5.7 | 6.4 ± 0.3 | 31 ± 5.2 | 92.4 ± 4.6 |
| S.No | Amino Acid | Content (g/100 g Protein) |
|---|---|---|
| 1 | Cysteine | 1.1 |
| 2 | Tryptophan | 1.1 |
| 3 | Methionine | 1.2 |
| 4 | Histidine | 2.3 |
| 5 | Threonine | 3.3 |
| 6 | Tyrosine | 3.3 |
| 7 | Glycine | 3.7 |
| 8 | Alanine | 3.8 |
| 9 | Isoleucine | 4.3 |
| 10 | Valine | 4.4 |
| 11 | Proline | 4.5 |
| 12 | Phenylalanine | 4.6 |
| 13 | Serine | 4.6 |
| 14 | Lysine | 5.5 |
| 15 | Arginine | 6.7 |
| 16 | Leucine | 7.2 |
| 17 | Aspartic acid | 10.2 |
| 18 | Glutamic acid | 16.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganteda, R.R.; Burla, S.K.; Boggu, J.M.R.; Prasad, P.S.R. Efficient Storage of Methane in Hydrate Form Using Soybean Powder. Methane 2022, 1, 201-209. https://doi.org/10.3390/methane1030016
Ganteda RR, Burla SK, Boggu JMR, Prasad PSR. Efficient Storage of Methane in Hydrate Form Using Soybean Powder. Methane. 2022; 1(3):201-209. https://doi.org/10.3390/methane1030016
Chicago/Turabian StyleGanteda, Rama Rao, Sai Kiran Burla, Jagan Mohan Reddy Boggu, and Pinnelli S. R. Prasad. 2022. "Efficient Storage of Methane in Hydrate Form Using Soybean Powder" Methane 1, no. 3: 201-209. https://doi.org/10.3390/methane1030016
APA StyleGanteda, R. R., Burla, S. K., Boggu, J. M. R., & Prasad, P. S. R. (2022). Efficient Storage of Methane in Hydrate Form Using Soybean Powder. Methane, 1(3), 201-209. https://doi.org/10.3390/methane1030016







