Autothermal Reforming of Methane: A Thermodynamic Study on the Use of Air and Pure Oxygen as Oxidizing Agents in Isothermal and Adiabatic Systems
Abstract
:1. Introduction
2. Methodology
2.1. Gibbs Energy Minimization: Isothermal Reactors
2.2. Entropy Maximization: Adiabatic Reactors
3. Results and Discussion
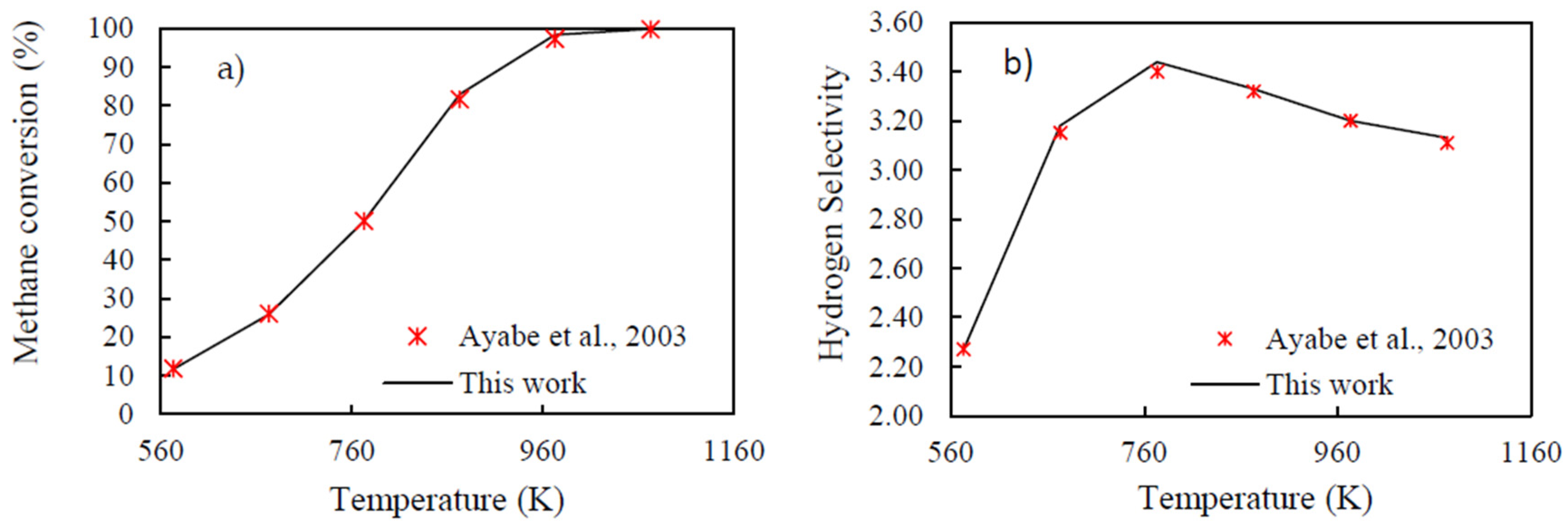
3.1. Gibbs Energy Minimization Methodology Validation
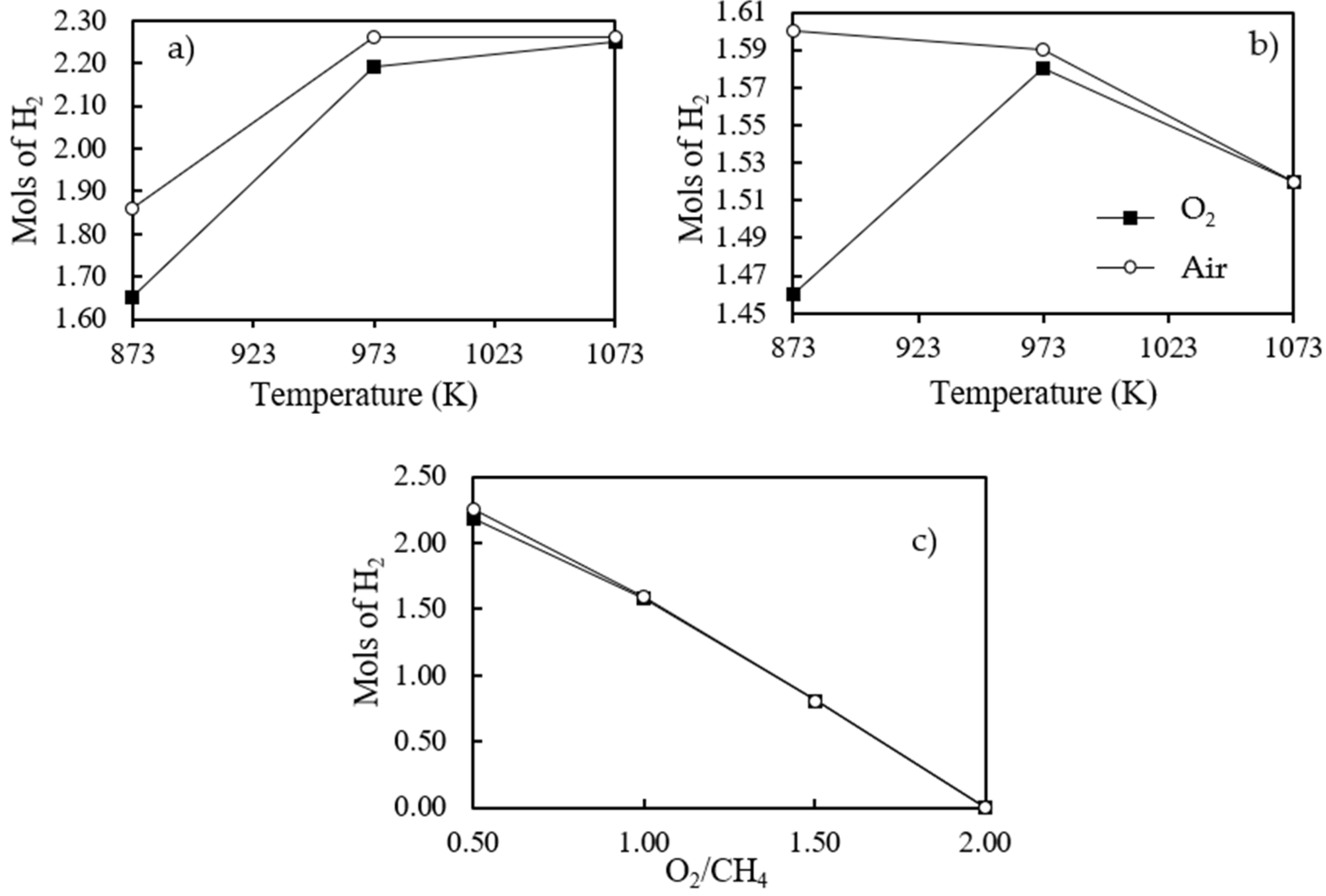
3.2. Thermodynamic Analysis for Isothermal System Reaction
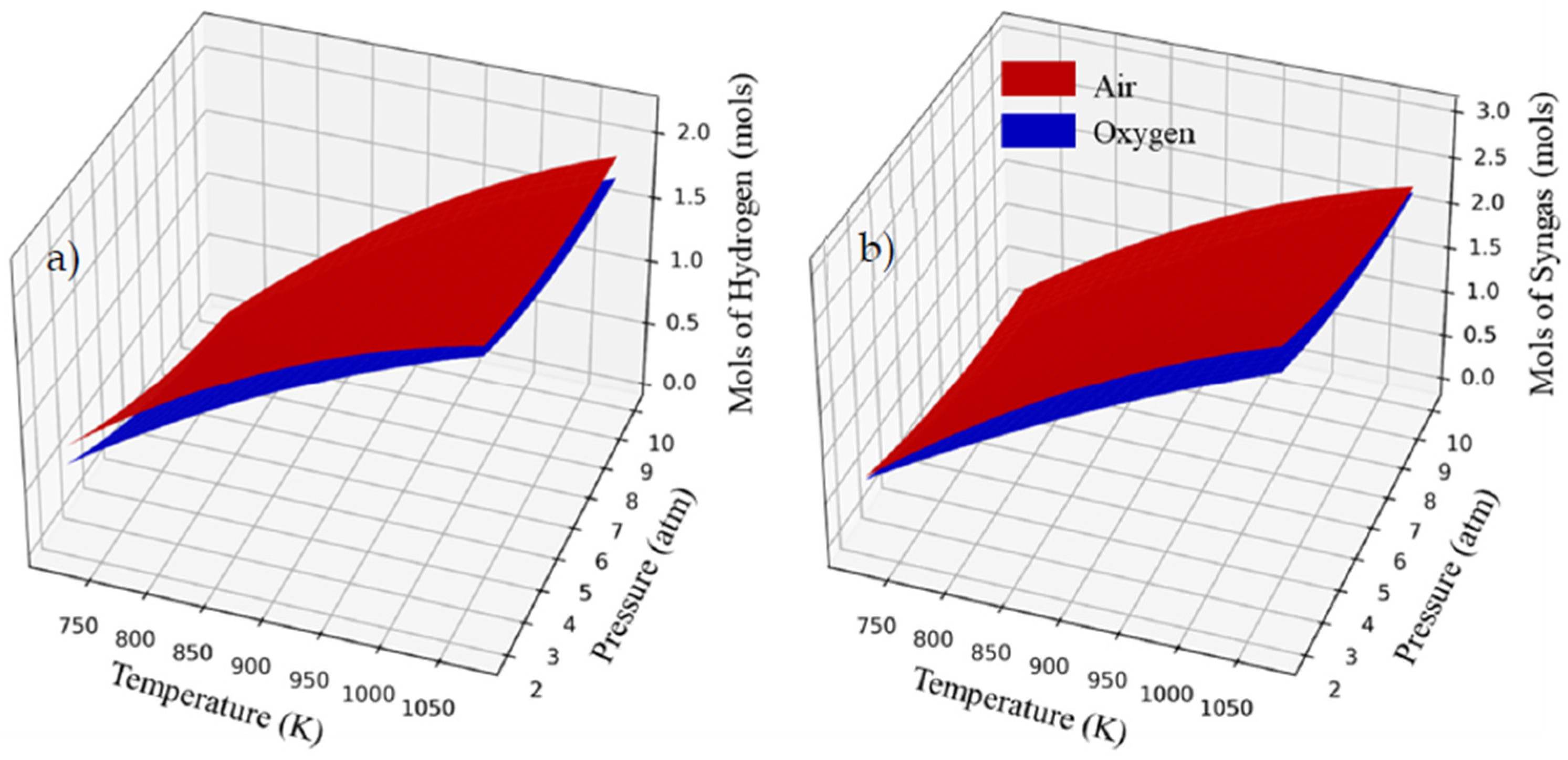
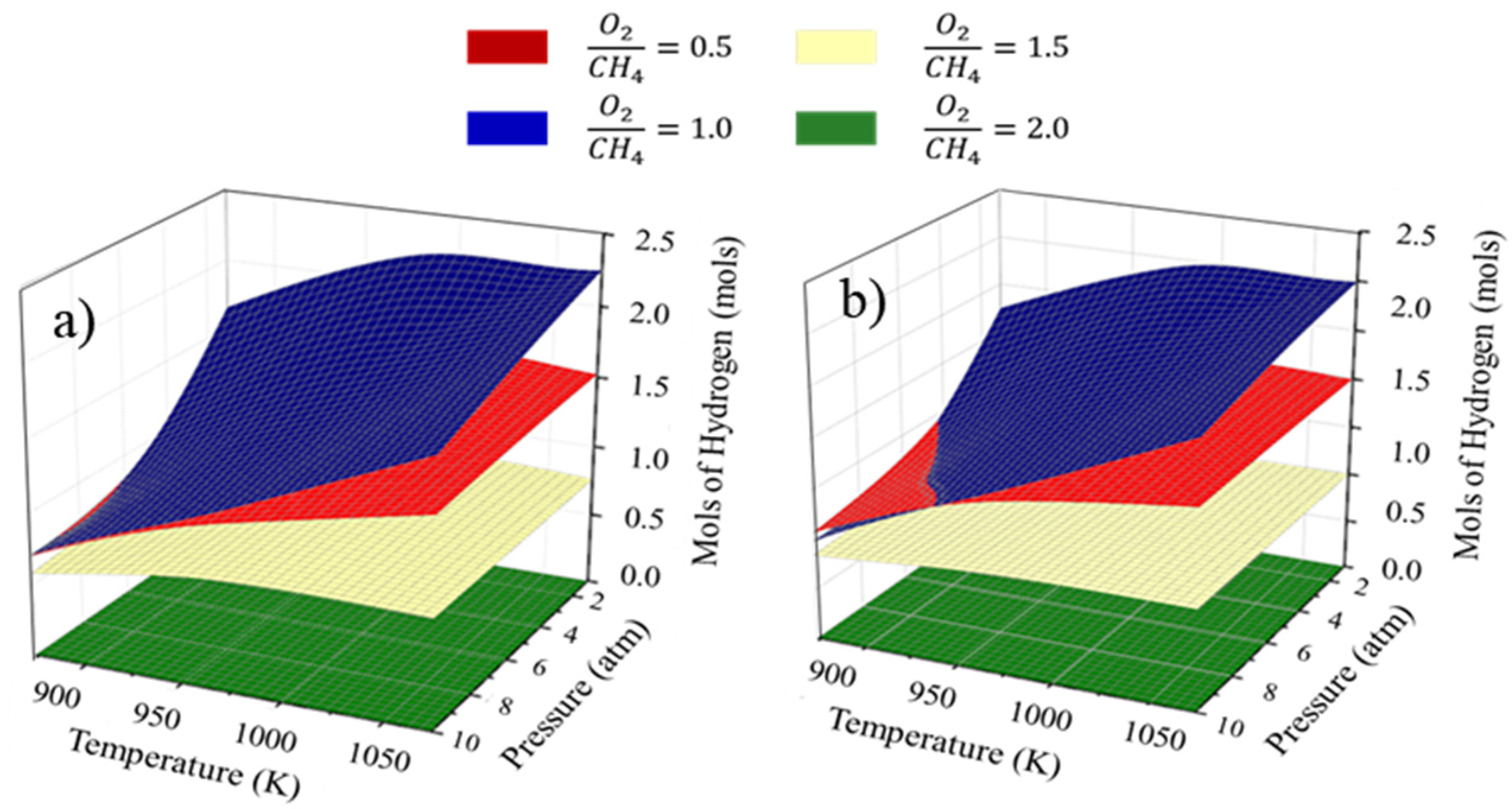
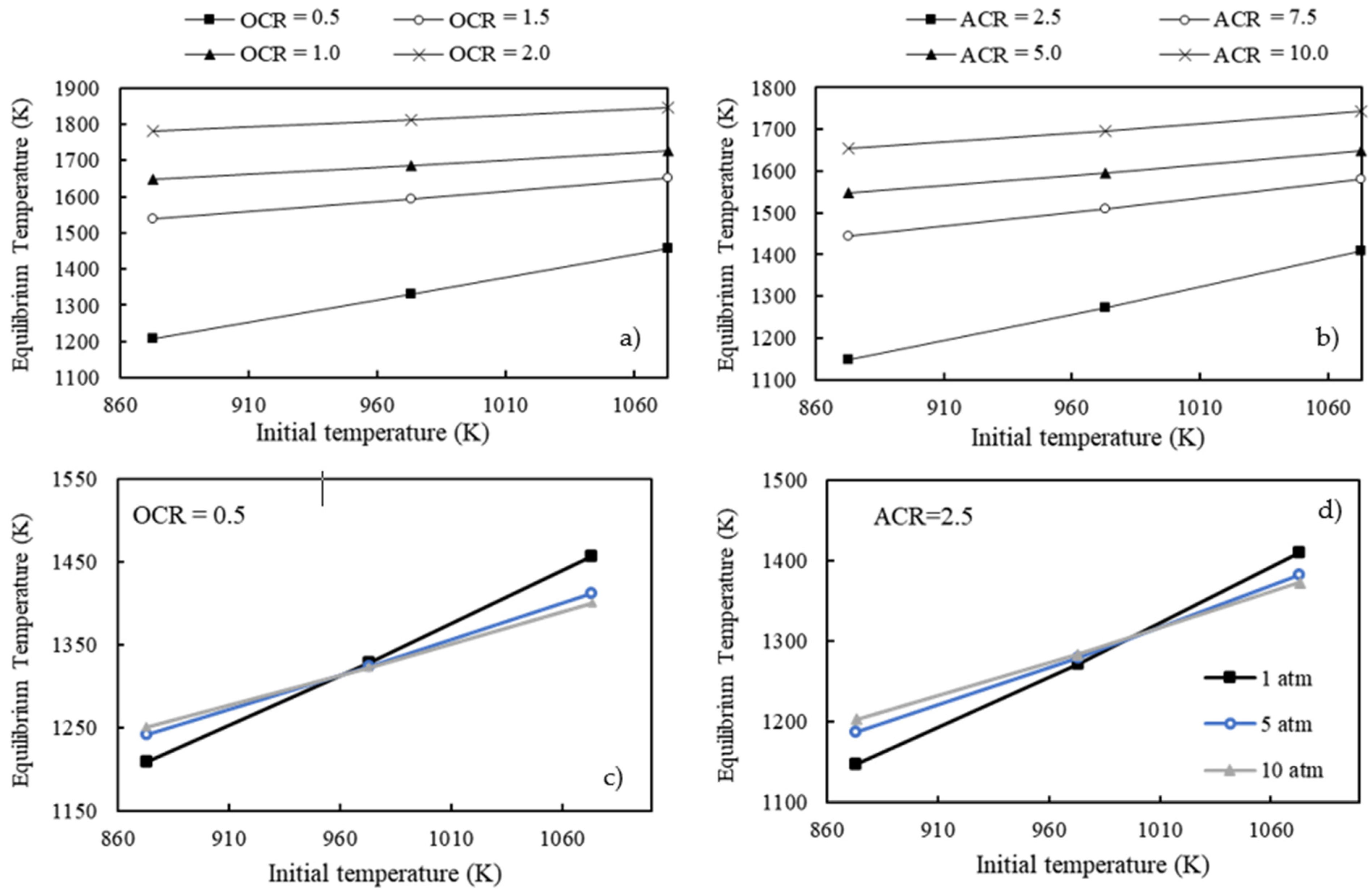
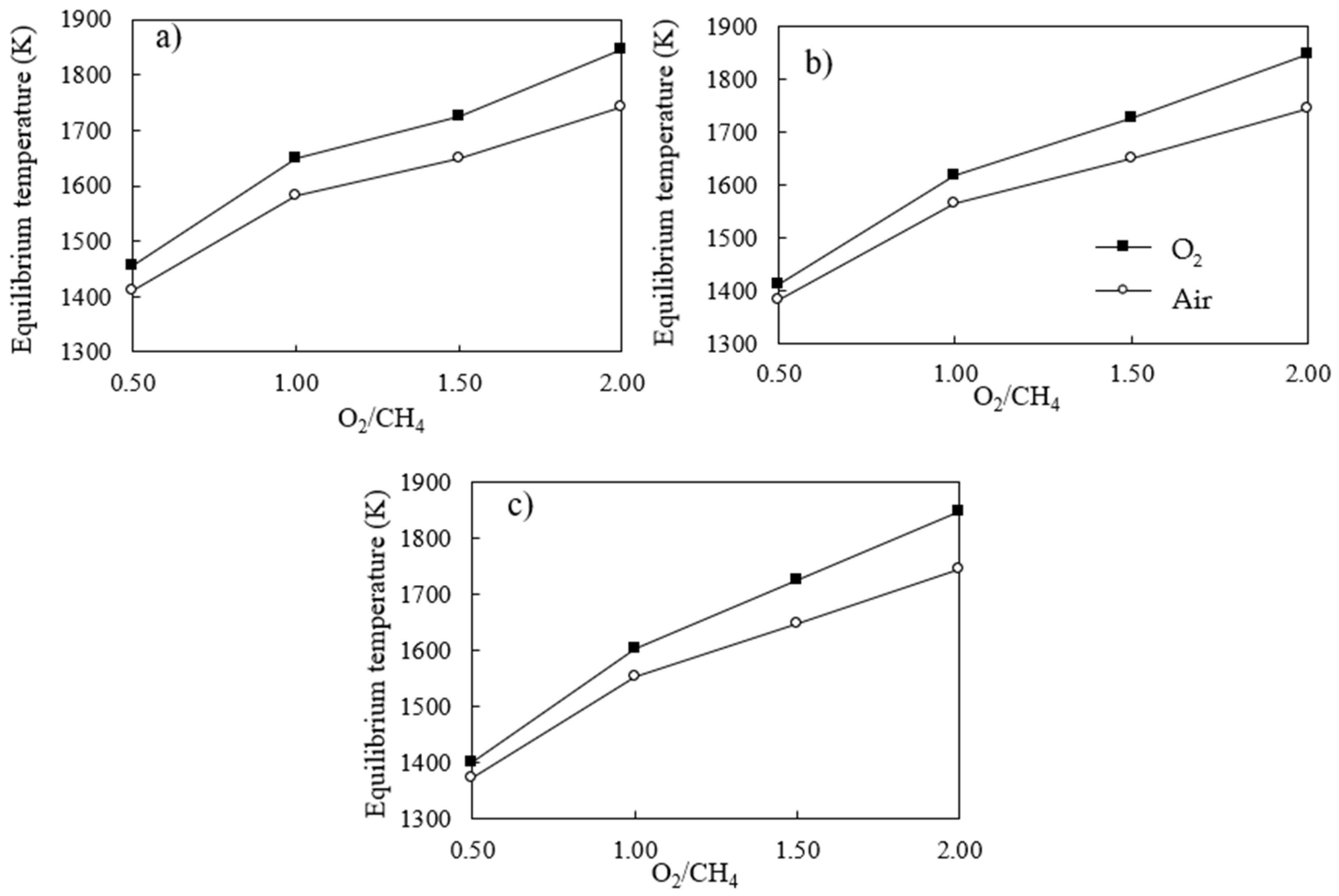
3.3. Thermodynamic Analysis for Adiabatic System Operation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Second coefficient of the virial | |
| Second coefficient of the virial for mixture | |
| Fugacity coefficient of component i | |
| Fugacity coefficient of component i in mixture | |
| Universal gas constant | |
| Gibbs energy | |
| Enthalpy of component i in phase k | |
| Enthalpy of component i in the standard state | |
| Total enthalpy | |
| Temperature | |
| Pressure | |
| Component i entropy in phase k | |
| Entropy of component i in the standard state | |
| Number of moles of component i in phase k | |
| Number of moles in standard state | |
| Number of atoms of element i in component m | |
| Number of components | |
| Number of elements | |
| Chemical potential of component i in phase k | |
| Molar fraction of gases | |
| Methane conversion | |
| Literature value | |
| Calculated value | |
| Hydrogen selectivity | |
| Number of moles of CH4 in feed stream | |
| Number of moles of CH4 in outlet stream | |
| Number of moles of H2 on outlet stream | |
| Number of moles of CO on outlet stream | |
| Number of moles of CO2 on outlet stream | |
| Superscripts | |
| Gas phase | |
| Liquid phase | |
| Solid phase | |
References
- Mitoura dos Santos Junior, J.; Gomes, J.G.; de Freitas, A.C.D.; Guirardello, R. An Analysis of the Methane Cracking Process for CO2-Free Hydrogen Production Using Thermodynamic Methodologies. Methane 2022, 1, 243–261. [Google Scholar] [CrossRef]
- Liu, F.; Deng, H.; Ding, H.; Kazempoor, P.; Liu, B.; Duan, C. Process-intensified protonic ceramic fuel cells for power generation, chemical production, and greenhouse gas mitigation. Joule 2023, 7, 1308–1332. [Google Scholar] [CrossRef]
- Sayar, A.; Eskin, N. Experimental and theoretical analysis of a monolith type auto-thermal reforming reactor. Int. J. Hydrogen Energy 2019, 44, 10232–10249. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Howarth, R.W.; Jacobson, M.Z. How green is blue hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Ugbeh-Johnson, J.; Okeke, N.E.; Ogbonnaya, C. Present and Projected Developments in Hydrogen Production: A Technological Review. Carbon Capture Sci. Technol. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Bodhankar, P.; Patnaik, S.; Kale, G.R. Thermodynamic analysis of autothermal steam-reforming of methane for ammonia production. Int. J. Energy Res. 2021, 45, 6943–6957. [Google Scholar] [CrossRef]
- Oni, A.O.; Anaya, K.; Giwa, T.; Di Lullo, G.; Kumar, A. Comparative assessment of blue hydrogen from steam methane reforming, autothermal reforming, and natural gas decomposition technologies for natural gas-producing regions. Energy Convers. Manag. 2022, 254, 115245. [Google Scholar] [CrossRef]
- Kayfeci, M.; Keçebaş, A.; Bayat, M. Hydrogen production. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–83. [Google Scholar] [CrossRef]
- Rau, F.; Herrmann, A.; Krause, H.; Fino, D.; Trimis, D. Efficiency of a pilot-plant for the autothermal reforming of biogas. Int. J. Hydrogen Energy 2019, 44, 19135–19140. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.; Zhang, L. Properties of thermodynamic equilibrium-based methane autothermal reforming to generate hydrogen. Int. J. Hydrogen Energy 2013, 38, 15744–15750. [Google Scholar] [CrossRef]
- Cherif, A.; Lee, J.-S.; Nebbali, R.; Lee, C.-J. Novel design and multi-objective optimization of autothermal steam methane reformer to enhance hydrogen production and thermal matching. Appl. Therm. Eng. 2022, 217, 119140. [Google Scholar] [CrossRef]
- Hu, S.; Cui, X.; Yang, L. Thermal matching characteristics in an autothermal methanol reforming microchannel reactor for hydrogen production. Chem. Eng. Sci. 2023, 280, 118987. [Google Scholar] [CrossRef]
- Freitas, A.C.D.; Guirardello, R. Oxidative reforming of methane for hydrogen and synthesis gas production: Thermodynamic equilibrium analysis. J. Nat. Gas Chem. 2012, 21, 571–580. [Google Scholar] [CrossRef]
- Freitas, A.C.D.; Guirardello, R. Comparison of several glycerol reforming methods for hydrogen and syngas production using Gibbs energy minimization. Int. J. Hydrogen Energy 2014, 39, 17969–17984. [Google Scholar] [CrossRef]
- Dos Santos, J.M.; De Sousa, G.F.B.; Vidotti, A.D.S.; De Freitas, A.C.D.; Guirardello, R. Optimization of glycerol gasification process in supercritical water using thermodynamic approach. Chem. Eng. Trans. 2021, 86, 847–852. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Lippmann, D.Z.; Curl, R.F.; Huggins, C.M.; Petersen, D.E. The Volumetric and Thermodynamic Properties of Fluids. II. Compressibility Factor, Vapor Pressure and Entropy of Vaporization. J. Am. Chem. Soc. 1955, 77, 3433–3440. [Google Scholar] [CrossRef]
- Tsonopoulos, C. An empirical correlation of second virial coefficients. AIChE J. 1974, 20, 263–272. [Google Scholar] [CrossRef]
- Ayabe, S.; Omoto, H.; Utaka, T.; Kikuchi, R.; Sasaki, K.; Teraoka, Y.; Eguchi, K. Catalytic autothermal reforming of methane and propane over supported metal catalysts. Appl. Catal. A Gen. 2003, 241, 261–269. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’connell, J.P. Properties of Gases and Liquids; McGraw-Hill Education: New York, NY, USA, 2001. [Google Scholar]
- Carapellucci, R.; Giordano, L. Steam, dry and autothermal methane reforming for hydrogen production: A thermodynamic equilibrium analysis. J. Power Sources 2020, 469, 228391. [Google Scholar] [CrossRef]
- Lutz, A.; Bradshaw, R.; Keller, J.; Witmer, D. Thermodynamic analysis of hydrogen production by steam reforming. Int. J. Hydrogen Energy 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Peng, X.; Jin, Q. Molecular simulation of methane steam reforming reaction for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 7569–7585. [Google Scholar] [CrossRef]
- S G Adiya, Z.I.; Dupont, V.; Mahmud, T. Effect of hydrocarbon fractions, N2 and CO2 in feed gas on hydrogen production using sorption enhanced steam reforming: Thermodynamic analysis. Int. J. Hydrogen Energy 2017, 42, 21704–21718. [Google Scholar] [CrossRef]
- de Souza, T.L.; da Silva, C.D.; Alonso, C.G.; Guirardello, R.; Cabral, V.F.; Fernandes-Machado, N.R.; Specchia, S.; Zabaloy, M.S.; Cardozo-Filho, L. Thermodynamic analysis of autothermal reforming of methane via entropy maximization: Hydrogen production. Int. J. Hydrogen Energy 2014, 39, 8257–8270. [Google Scholar] [CrossRef]
- Kusakabe, K.; Sotowa, K.-I.; Eda, T.; Iwamoto, Y. Methane steam reforming over Ce–ZrO2-supported noble metal catalysts at low temperature. Fuel Process. Technol. 2004, 86, 319–326. [Google Scholar] [CrossRef]





| Technology | Capital Cost (M$) | H2 Production Cost ($/kg) |
|---|---|---|
| SMR with CCS | 226.4 | 2.27 |
| ATR with CCS | 180.7 | 2.08 |
| CH4 ATR with CCS | 183.8 | 1.48 |
| Parameters | ||||
|---|---|---|---|---|
| Condition 1 OCR = 0.4; P = 1 atm; SCR = 3.0 | Ti (K) | (K) | (K) | Deviation (%) |
| 723 | 906 | 894 | 1.3 | |
| Condition 2 OCR = 0.5; P = 1 atm; SCR = 3.83 | Ti (K) | (K) | (K) | Deviation (%) |
| 723 | 1031 | 945 | 8.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalcante, M.H.S.; Maccari Zelioli, Í.A.; Guimarães Filho, E.É.X.; Júnior, J.M.d.S.; Souza Vidotti, A.D.; Daltro de Freitas, A.C.; Guirardello, R. Autothermal Reforming of Methane: A Thermodynamic Study on the Use of Air and Pure Oxygen as Oxidizing Agents in Isothermal and Adiabatic Systems. Methane 2023, 2, 389-403. https://doi.org/10.3390/methane2040026
Cavalcante MHS, Maccari Zelioli ÍA, Guimarães Filho EÉX, Júnior JMdS, Souza Vidotti AD, Daltro de Freitas AC, Guirardello R. Autothermal Reforming of Methane: A Thermodynamic Study on the Use of Air and Pure Oxygen as Oxidizing Agents in Isothermal and Adiabatic Systems. Methane. 2023; 2(4):389-403. https://doi.org/10.3390/methane2040026
Chicago/Turabian StyleCavalcante, Matheus Henrique Silva, Ícaro Augusto Maccari Zelioli, Emílio Émerson Xavier Guimarães Filho, Julles Mitoura dos Santos Júnior, Annamaria Dória Souza Vidotti, Antonio Carlos Daltro de Freitas, and Reginaldo Guirardello. 2023. "Autothermal Reforming of Methane: A Thermodynamic Study on the Use of Air and Pure Oxygen as Oxidizing Agents in Isothermal and Adiabatic Systems" Methane 2, no. 4: 389-403. https://doi.org/10.3390/methane2040026
APA StyleCavalcante, M. H. S., Maccari Zelioli, Í. A., Guimarães Filho, E. É. X., Júnior, J. M. d. S., Souza Vidotti, A. D., Daltro de Freitas, A. C., & Guirardello, R. (2023). Autothermal Reforming of Methane: A Thermodynamic Study on the Use of Air and Pure Oxygen as Oxidizing Agents in Isothermal and Adiabatic Systems. Methane, 2(4), 389-403. https://doi.org/10.3390/methane2040026






