Methane Removal from Air: Challenges and Opportunities
Abstract
:1. Introduction: The Necessity of Methane Removal from Air
2. Challenges in Methane Removal from Air
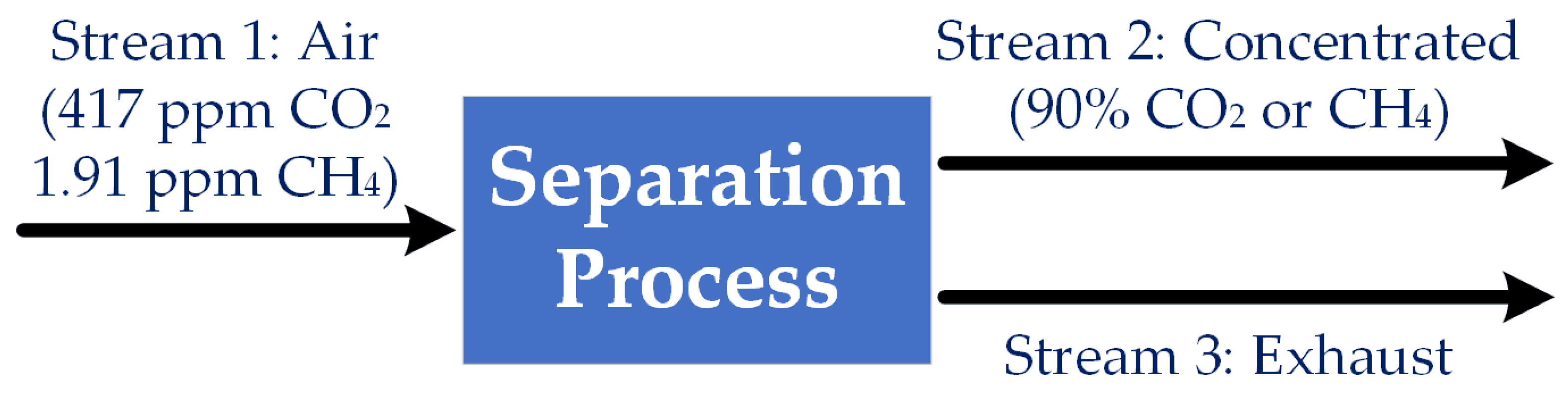
2.1. Minimum Thermodynamic Work for Separation
2.2. The Scale of the Problem: Volume of Air to Be Processed
2.3. Methane Conversion vs. Capture
2.4. Consideration in Methane Oxidation
3. State-of-the-Art for Methane Removal from the Air
3.1. Natural Methane Sinks
3.2. Potential Technological Methane Sinks
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA Global Methane Tracker. 2023. Available online: https://www.iea.org/reports/global-methane-tracker-2023 (accessed on 29 August 2023).
- Theo Stein Greenhouse Gases Continued to Increase Rapidly in 2022. Available online: https://www.noaa.gov/news-release/greenhouse-gases-continued-to-increase-rapidly-in-2022 (accessed on 29 August 2023).
- EPA Understanding Global Warming Potentials. Available online: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials (accessed on 29 August 2023).
- Buis, A. The Atmosphere: Getting a Handle on Carbon Dioxide. Available online: https://climate.nasa.gov/news/2915/the-atmosphere-getting-a-handle-on-carbon-dioxide/ (accessed on 29 August 2023).
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I. Climate Change 2021: The Physical Science Basis. Contrib. Work. Group I Sixth Assess. Rep. Intergov. Panel Clim. Chang. 2021, 2. Available online: https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_FrontMatter.pdf (accessed on 11 September 2023).
- Harmsen, M.; van Vuuren, D.P.; Bodirsky, B.L.; Chateau, J.; Durand-Lasserve, O.; Drouet, L.; Fricko, O.; Fujimori, S.; Gernaat, D.E.H.J.; Hanaoka, T. The Role of Methane in Future Climate Strategies: Mitigation Potentials and Climate Impacts. Clim. Chang. 2020, 163, 1409–1425. [Google Scholar] [CrossRef]
- Ocko, I.B.; Naik, V.; Paynter, D. Rapid and Reliable Assessment of Methane Impacts on Climate. Atmos. Chem. Phys. 2018, 18, 15555–15568. [Google Scholar] [CrossRef]
- Ocko, I.B.; Sun, T.; Shindell, D.; Oppenheimer, M.; Hristov, A.N.; Pacala, S.W.; Mauzerall, D.L.; Xu, Y.; Hamburg, S.P. Acting Rapidly to Deploy Readily Available Methane Mitigation Measures by Sector Can Immediately Slow Global Warming. Environ. Res. Lett. 2021, 16, 054042. [Google Scholar] [CrossRef]
- Collins, W.J.; Webber, C.P.; Cox, P.M.; Huntingford, C.; Lowe, J.; Sitch, S.; Chadburn, S.E.; Comyn-Platt, E.; Harper, A.B.; Hayman, G. Increased Importance of Methane Reduction for a 1.5 Degree Target. Environ. Res. Lett. 2018, 13, 054003. [Google Scholar] [CrossRef]
- Jackson, R.B.; Solomon, E.I.; Canadell, J.G.; Cargnello, M.; Field, C.B. Methane Removal and Atmospheric Restoration. Nat. Sustain. 2019, 2, 436–438. [Google Scholar] [CrossRef]
- Abernethy, S.; O’Connor, F.M.; Jones, C.D.; Jackson, R.B. Methane Removal and the Proportional Reductions in Surface Temperature and Ozone. Philos. Trans. R. Soc. A 2021, 379, 20210104. [Google Scholar] [CrossRef]
- Alvarez, R.A.; Pacala, S.W.; Winebrake, J.J.; Chameides, W.L.; Hamburg, S.P. Greater Focus Needed on Methane Leakage from Natural Gas Infrastructure. Proc. Natl. Acad. Sci. USA 2012, 109, 6435–6440. [Google Scholar] [CrossRef]
- Rogelj, J.; Meinshausen, M.; Schaeffer, M.; Knutti, R.; Riahi, K. Impact of Short-Lived Non-CO2 Mitigation on Carbon Budgets for Stabilizing Global Warming. Environ. Res. Lett. 2015, 10, 075001. [Google Scholar] [CrossRef]
- Nisbet, E.G.; Fisher, R.E.; Lowry, D.; France, J.L.; Allen, G.; Bakkaloglu, S.; Broderick, T.J.; Cain, M.; Coleman, M.; Fernandez, J. Methane Mitigation: Methods to Reduce Emissions, on the Path to the Paris Agreement. Rev. Geophys. 2020, 58, e2019RG000675. [Google Scholar] [CrossRef]
- Malozyomov, B.V.; Golik, V.I.; Brigida, V.; Kukartsev, V.V.; Tynchenko, Y.A.; Boyko, A.A.; Tynchenko, S.V. Substantiation of Drilling Parameters for Undermined Drainage Boreholes for Increasing Methane Production from Unconventional Coal-Gas Collectors. Energies 2023, 16, 4276. [Google Scholar] [CrossRef]
- Jackson, R.B.; Abernethy, S.; Canadell, J.G.; Cargnello, M.; Davis, S.J.; Féron, S.; Fuss, S.; Heyer, A.J.; Hong, C.; Jones, C.D. Atmospheric Methane Removal: A Research Agenda. Philos. Trans. R. Soc. A 2021, 379, 20200454. [Google Scholar] [CrossRef]
- Warszawski, L.; Kriegler, E.; Lenton, T.M.; Gaffney, O.; Jacob, D.; Klingenfeld, D.; Koide, R.; Costa, M.M.; Messner, D.; Nakicenovic, N. All Options, Not Silver Bullets, Needed to Limit Global Warming to 1.5 °C: A Scenario Appraisal. Environ. Res. Lett. 2021, 16, 064037. [Google Scholar] [CrossRef]
- Nisbet-Jones, P.B.R.; Fernandez, J.M.; Fisher, R.E.; France, J.L.; Lowry, D.; Waltham, D.A.; Woolley Maisch, C.A.; Nisbet, E.G. Is the Destruction or Removal of Atmospheric Methane a Worthwhile Option? Philos. Trans. R. Soc. A 2022, 380, 20210108. [Google Scholar] [CrossRef] [PubMed]
- Lackner, K.S. Practical Constraints on Atmospheric Methane Removal. Nat. Sustain. 2020, 3, 357. [Google Scholar] [CrossRef]
- He, L.; Groom, J.D.; Wilson, E.H.; Fernandez, J.; Konopka, M.C.; Beck, D.A.C.; Lidstrom, M.E. A Methanotrophic Bacterium to Enable Methane Removal for Climate Mitigation. Proc. Natl. Acad. Sci. USA 2023, 120, e2310046120. [Google Scholar] [CrossRef]
- Lü, R.; Lin, J. A Interpretation of Stepwise Bond Dissociation Energies of CH4. Comput. Theor. Chem. 2014, 1037, 10–13. [Google Scholar] [CrossRef]
- NIST NIST Chemistry WebBook, SRD 69. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=74-82-8 (accessed on 6 September 2023).
- Lannung, A.; Gjaldbaek, J.C. The Solubility of Methane in Hydrocarbons, Alcohols, Water and Other Solvents. Acta Chem. Scand. 1960, 14, 1124–1128. [Google Scholar] [CrossRef]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Jackson, R.B.; Saunois, M.; Bousquet, P.; Canadell, J.G.; Poulter, B.; Stavert, A.R.; Bergamaschi, P.; Niwa, Y.; Segers, A.; Tsuruta, A. Increasing Anthropogenic Methane Emissions Arise Equally from Agricultural and Fossil Fuel Sources. Environ. Res. Lett. 2020, 15, 071002. [Google Scholar] [CrossRef]
- Li, Q.; Meidan, D.; Hess, P.; Añel, J.A.; Cuevas, C.A.; Doney, S.; Fernandez, R.P.; van Herpen, M.; Höglund-Isaksson, L.; Johnson, M.S.; et al. Global Environmental Implications of Atmospheric Methane Removal through Chlorine-Mediated Chemistry-Climate Interactions. Nat. Commun. 2023, 14, 4045. [Google Scholar] [CrossRef] [PubMed]
- Ming, T.; Li, W.; Yuan, Q.; Davies, P.; De Richter, R.; Peng, C.; Deng, Q.; Yuan, Y.; Caillol, S.; Zhou, N. Perspectives on Removal of Atmospheric Methane. Adv. Appl. Energy 2022, 5, 100085. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Pan, X.; Cortie, D.; Huang, X.; Yi, Z. Photocatalytic Oxidation of Methane over Silver Decorated Zinc Oxide Nanocatalysts. Nat. Commun. 2016, 7, 12273. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Pan, Z.; Jiao, X.; Zheng, K.; Li, L.; Shao, W.; Zu, X.; Hu, J.; Zhu, J. Efficient Photooxidation of Methane to Liquid Oxygenates over ZnO Nanosheets at Atmospheric Pressure and near Room Temperature. Nano Lett. 2021, 21, 4122–4128. [Google Scholar] [CrossRef] [PubMed]
- Thampi, K.R.; Kiwi, J.; Grätzel, M. Room Temperature Photo-Activation of Methane on TiO2 Supported Molybdena. Catal. Lett. 1988, 1, 109–116. [Google Scholar] [CrossRef]
- Ming, T.; de Richter, R.; Oeste, F.D.; Tulip, R.; Caillol, S. A Nature-Based Negative Emissions Technology Able to Remove Atmospheric Methane and Other Greenhouse Gases. Atmos. Pollut. Res. 2021, 12, 101035. [Google Scholar] [CrossRef]
- Oeste, F.D.; de Richter, R.; Ming, T.; Caillol, S. Climate Engineering by Mimicking Natural Dust Climate Control: The Iron Salt Aerosol Method. Earth Syst. Dyn. 2017, 8, 1–54. [Google Scholar] [CrossRef]
- Bracco, S.; Piga, D.; Bassanetti, I.; Perego, J.; Comotti, A.; Sozzani, P. Porous 3D Polymers for High Pressure Methane Storage and Carbon Dioxide Capture. J. Mater. Chem. A Mater. 2017, 5, 10328–10337. [Google Scholar] [CrossRef]
- Kim, J.; Maiti, A.; Lin, L.-C.; Stolaroff, J.K.; Smit, B.; Aines, R.D. New Materials for Methane Capture from Dilute and Medium-Concentration Sources. Nat. Commun. 2013, 4, 1694. [Google Scholar] [CrossRef]
- Smith, P.; Goulding, K.W.; Smith, K.A.; Powlson, D.S.; Smith, J.U.; Falloon, P.; Coleman, K. Enhancing the Carbon Sink in European Agricultural Soils: Including Trace Gas Fluxes in Estimates of Carbon Mitigation Potential. Nutr. Cycl. Agroecosyst 2001, 60, 237–252. [Google Scholar] [CrossRef]
- Price, S.J.; Sherlock, R.R.; Kelliher, F.M.; McSeveny, T.M.; Tate, K.R.; Condron, L.M. Pristine New Zealand Forest Soil Is a Strong Methane Sink. Glob. Chang. Biol. 2004, 10, 16–26. [Google Scholar] [CrossRef]
- Cáceres, M.; Dorado, A.D.; Gentina, J.C.; Aroca, G. Oxidation of Methane in Biotrickling Filters Inoculated with Methanotrophic Bacteria. Environ. Sci. Pollut. Res. 2017, 24, 25702–25712. [Google Scholar] [CrossRef]
- La, H.; Hettiaratchi, J.P.A.; Achari, G.; Dunfield, P.F. Biofiltration of Methane. Bioresour. Technol. 2018, 268, 759–772. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Tang, J. Methane Transformation by Photocatalysis. Nat. Rev. Mater. 2022, 7, 617–632. [Google Scholar] [CrossRef]
- Snyder, B.E.R.; Vanelderen, P.; Bols, M.L.; Hallaert, S.D.; Böttger, L.H.; Ungur, L.; Pierloot, K.; Schoonheydt, R.A.; Sels, B.F.; Solomon, E.I. The Active Site of Low-Temperature Methane Hydroxylation in Iron-Containing Zeolites. Nature 2016, 536, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Lawton, T.J.; Rosenzweig, A.C. Methane-Oxidizing Enzymes: An Upstream Problem in Biological Gas-to-Liquids Conversion. J. Am. Chem. Soc. 2016, 138, 9327–9340. [Google Scholar] [CrossRef]
- Lebrero, R.; López, J.C.; Lehtinen, I.; Pérez, R.; Quijano, G.; Muñoz, R. Exploring the Potential of Fungi for Methane Abatement: Performance Evaluation of a Fungal-Bacterial Biofilter. Chemosphere 2016, 144, 97–106. [Google Scholar] [CrossRef]
- Scheutz, C.; Kjeldsen, P.; Bogner, J.E.; de Visscher, A.; Gebert, J.; Hilger, H.A.; Huber-Humer, M.; Spokas, K. Microbial Methane Oxidation Processes and Technologies for Mitigation of Landfill Gas Emissions. Waste Manag. Res. 2009, 27, 409–455. [Google Scholar] [CrossRef]
- Nikiema, J.; Brzezinski, R.; Heitz, M. Elimination of Methane Generated from Landfills by Biofiltration: A Review. Rev. Environ. Sci. Biotechnol. 2007, 6, 261–284. [Google Scholar] [CrossRef]
- Limbri, H.; Gunawan, C.; Thomas, T.; Smith, A.; Scott, J.; Rosche, B. Coal-Packed Methane Biofilter for Mitigation of Green House Gas Emissions from Coal Mine Ventilation Air. PLoS ONE 2014, 9, e94641. [Google Scholar] [CrossRef]
- Nikiema, J.; Heitz, M. The Use of Inorganic Packing Materials during Methane Biofiltration. Int. J. Chem. Eng. 2010, 2010, 573149. [Google Scholar] [CrossRef]
- Melse, R.W.; van der Werf, A.W. Biofiltration for Mitigation of Methane Emission from Animal Husbandry. Environ. Sci. Technol. 2005, 39, 5460–5468. [Google Scholar] [CrossRef]
- Prenafeta-Boldú, F.X.; Illa, J.; van Groenestijn, J.W.; Flotats, X. Influence of Synthetic Packing Materials on the Gas Dispersion and Biodegradation Kinetics in Fungal Air Biofilters. Appl. Microbiol. Biotechnol. 2008, 79, 319–327. [Google Scholar] [CrossRef]
- Chanton, J.P.; Powelson, D.K.; Green, R.B. Methane Oxidation in Landfill Cover Soils, Is a 10% Default Value Reasonable? J. Env. Qual. 2009, 38, 654–663. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, J.; Hilliard, M. Culture Systems and Methods of Using Same. U.S. Patent No. 11,339,360, 24 May 2022. [Google Scholar]
- Morales, M.; Bonnefond, H.; Bernard, O. Rotating Algal Biofilm versus Planktonic Cultivation: LCA Perspective. J. Clean. Prod. 2020, 257, 120547. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Liu, T. Biofilm Based Attached Cultivation Technology for Microalgal Biorefineries—A Review. Bioresour. Technol. 2017, 244, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Mascarenhas, V.; Wen, Z. Evaluating Algal Growth Performance and Water Use Efficiency of Pilot-scale Revolving Algal Biofilm (RAB) Culture Systems. Biotechnol. Bioeng. 2015, 112, 2040–2050. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Wen, Z. Yearlong Evaluation of Performance and Durability of a Pilot-Scale Revolving Algal Biofilm (RAB) Cultivation System. Bioresour. Technol. 2014, 171, 50–58. [Google Scholar] [CrossRef]
- Gross, M.; Henry, W.; Michael, C.; Wen, Z. Development of a Rotating Algal Biofilm Growth System for Attached Microalgae Growth with in Situ Biomass Harvest. Bioresour. Technol. 2013, 150, 195–201. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, Y. Solar Updraft Tower Power Generation. Sol. Energy 2016, 128, 95–125. [Google Scholar] [CrossRef]
- Schlaich, J.R.; Bergermann, R.; Schiel, W.; Weinrebe, G. Design of Commercial Solar Updraft Tower Systems—Utilization of Solar Induced Convective Flows for Power Generation. J. Sol. Energy Eng. 2005, 127, 117–124. [Google Scholar] [CrossRef]
- Ming, T.; Davies, P.; Liu, W.; Caillol, S. Removal of Non-CO2 Greenhouse Gases by Large-Scale Atmospheric Solar Photocatalysis. Prog. Energy Combust. Sci. 2017, 60, 68–96. [Google Scholar]
- Ming, T.; Caillol, S.; Liu, W. Fighting Global Warming by GHG Removal: Destroying CFCs and HCFCs in Solar-Wind Power Plant Hybrids Producing Renewable Energy with No-Intermittency. Int. J. Greenh. Gas Control 2016, 49, 449–472. [Google Scholar]
- Terlouw, T.; Bauer, C.; Rosa, L.; Mazzotti, M. Life Cycle Assessment of Carbon Dioxide Removal Technologies: A Critical Review. Energy Environ. Sci. 2021, 14, 1701–1721. [Google Scholar] [CrossRef]

| Technology Pathway | Sub-Pathway | Approach | Reaction Locus | Gas Flow | Sample Reference |
|---|---|---|---|---|---|
| Chemical conversion | Photocatalytic oxidation | TiO2 or Ag-ZnO | Gas–solid interface | Active or passive | [28,29,30] |
| Photochemical oxidation | Iron-salt aerosols | Gas | Passive | [31,32] | |
| Adsorption–oxidation | Zeolites or Porous Polymer Network | Gas–solid interface | Active or passive | [10,33,34] | |
| Biological conversion | Biofilter | Within cell | Active or passive | [35,36] | |
| Soil amendments | Within cell | Passive | [37,38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; He, Q.P. Methane Removal from Air: Challenges and Opportunities. Methane 2023, 2, 404-414. https://doi.org/10.3390/methane2040027
Wang J, He QP. Methane Removal from Air: Challenges and Opportunities. Methane. 2023; 2(4):404-414. https://doi.org/10.3390/methane2040027
Chicago/Turabian StyleWang, Jin, and Qinghua Peter He. 2023. "Methane Removal from Air: Challenges and Opportunities" Methane 2, no. 4: 404-414. https://doi.org/10.3390/methane2040027
APA StyleWang, J., & He, Q. P. (2023). Methane Removal from Air: Challenges and Opportunities. Methane, 2(4), 404-414. https://doi.org/10.3390/methane2040027







