Recent Advances in the Use of Controlled Nanocatalysts in Methane Conversion Reactions
Abstract
1. Introduction
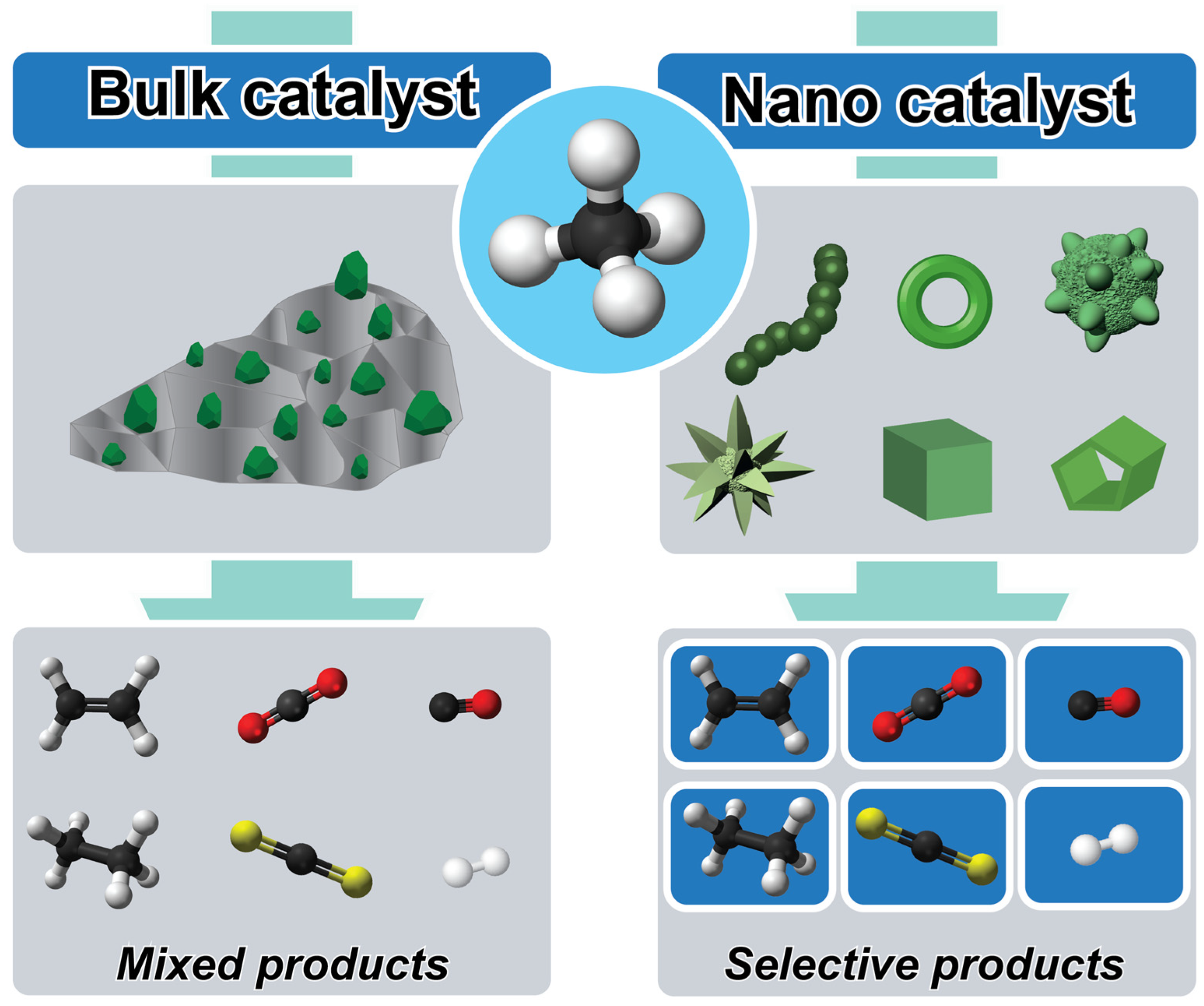
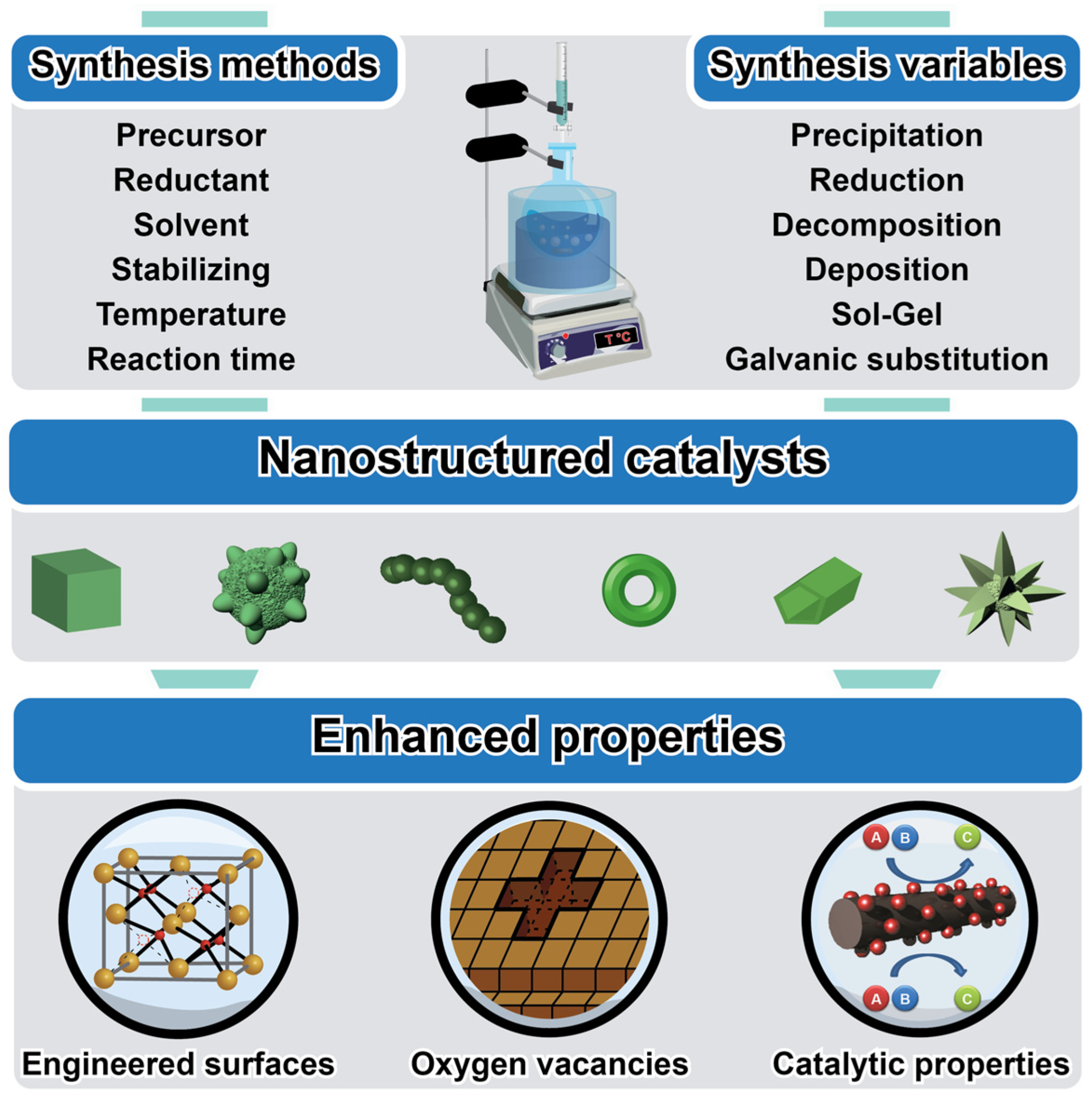
2. Controlled Nanocatalysts: A Comprehensive Overview
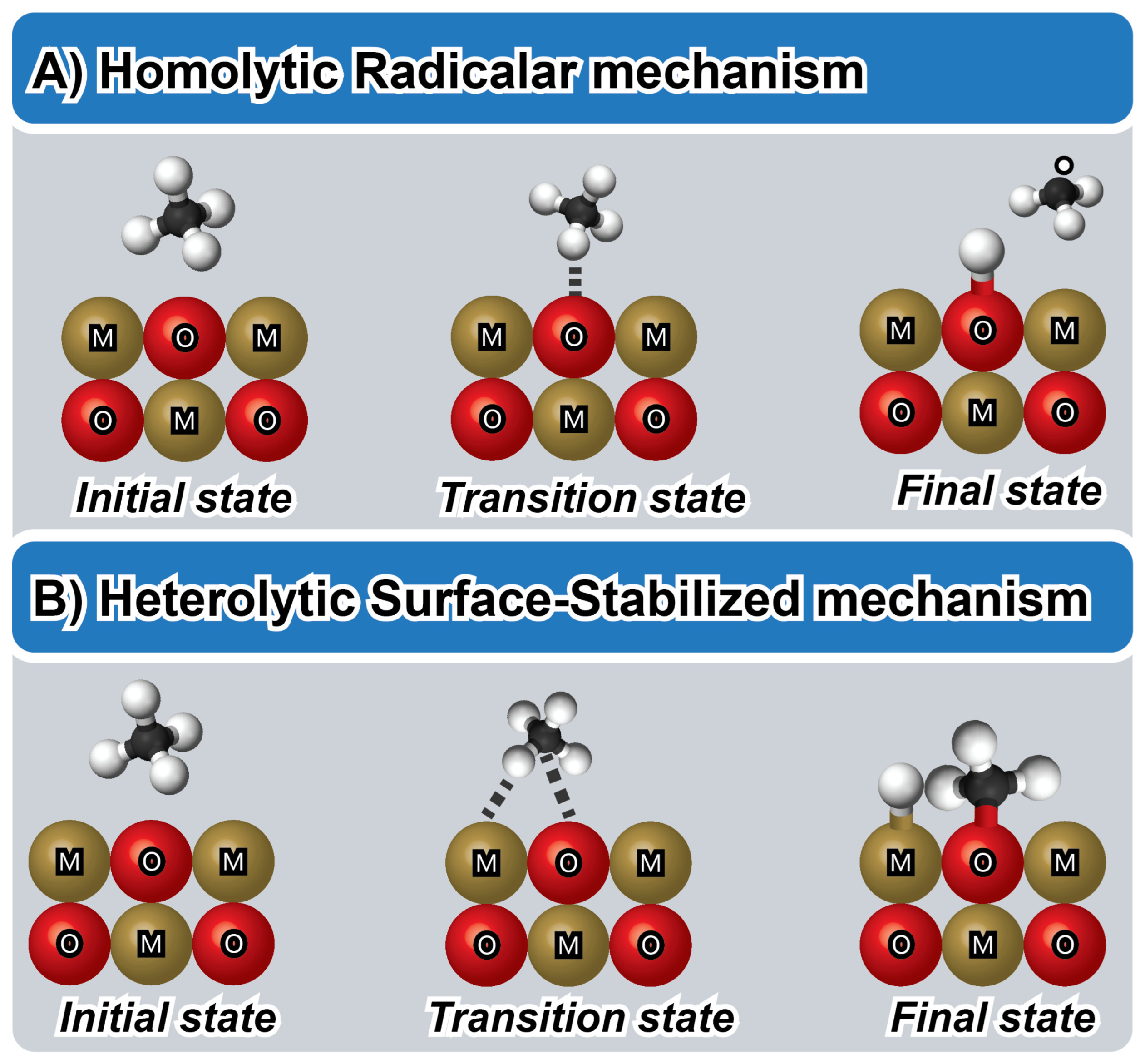
3. Methane Activation Strategies
4. Reaction Mechanisms and Kinetics of Methane in Sustainable Energy Production
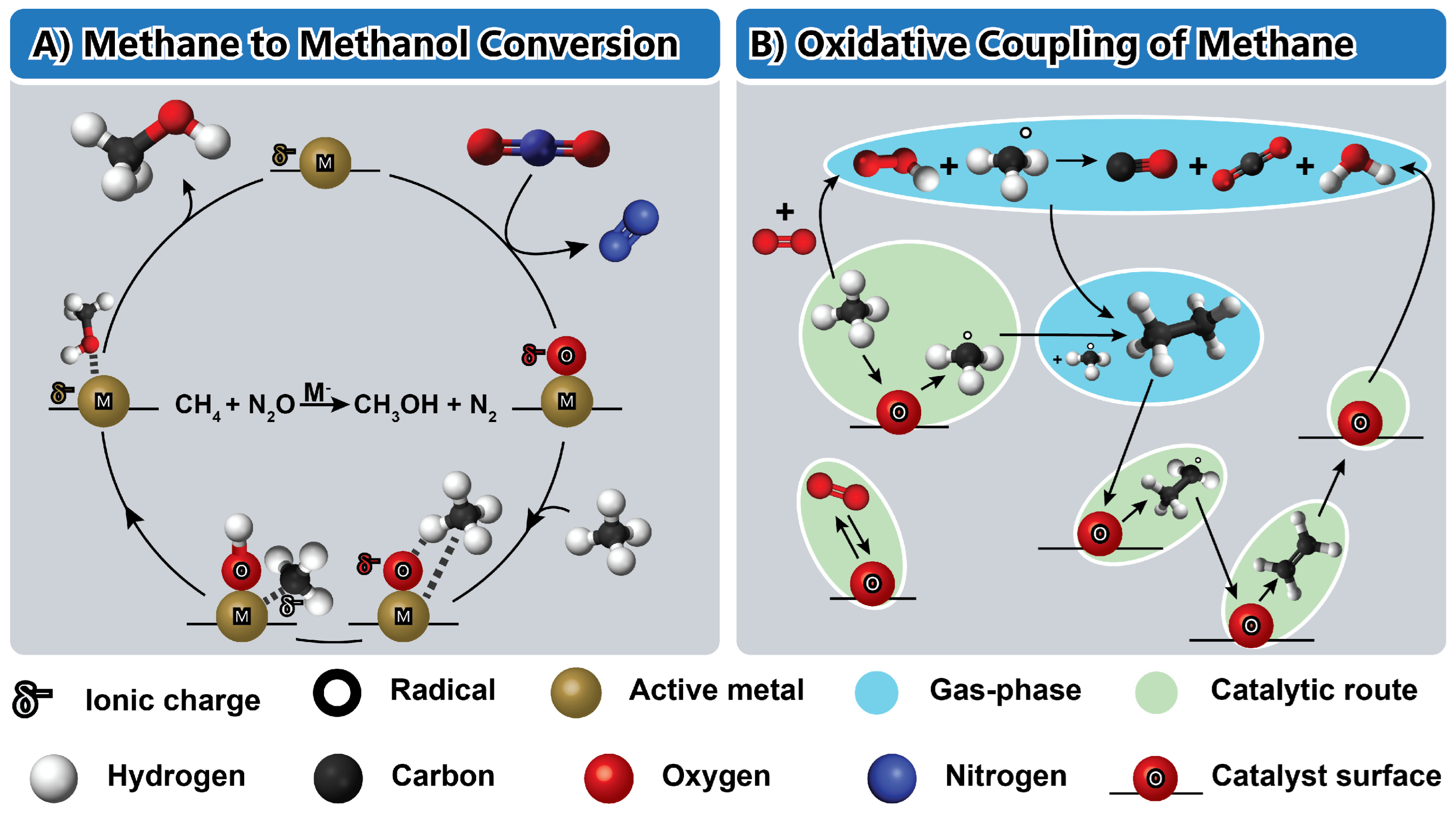
4.1. Methane-to-Methanol
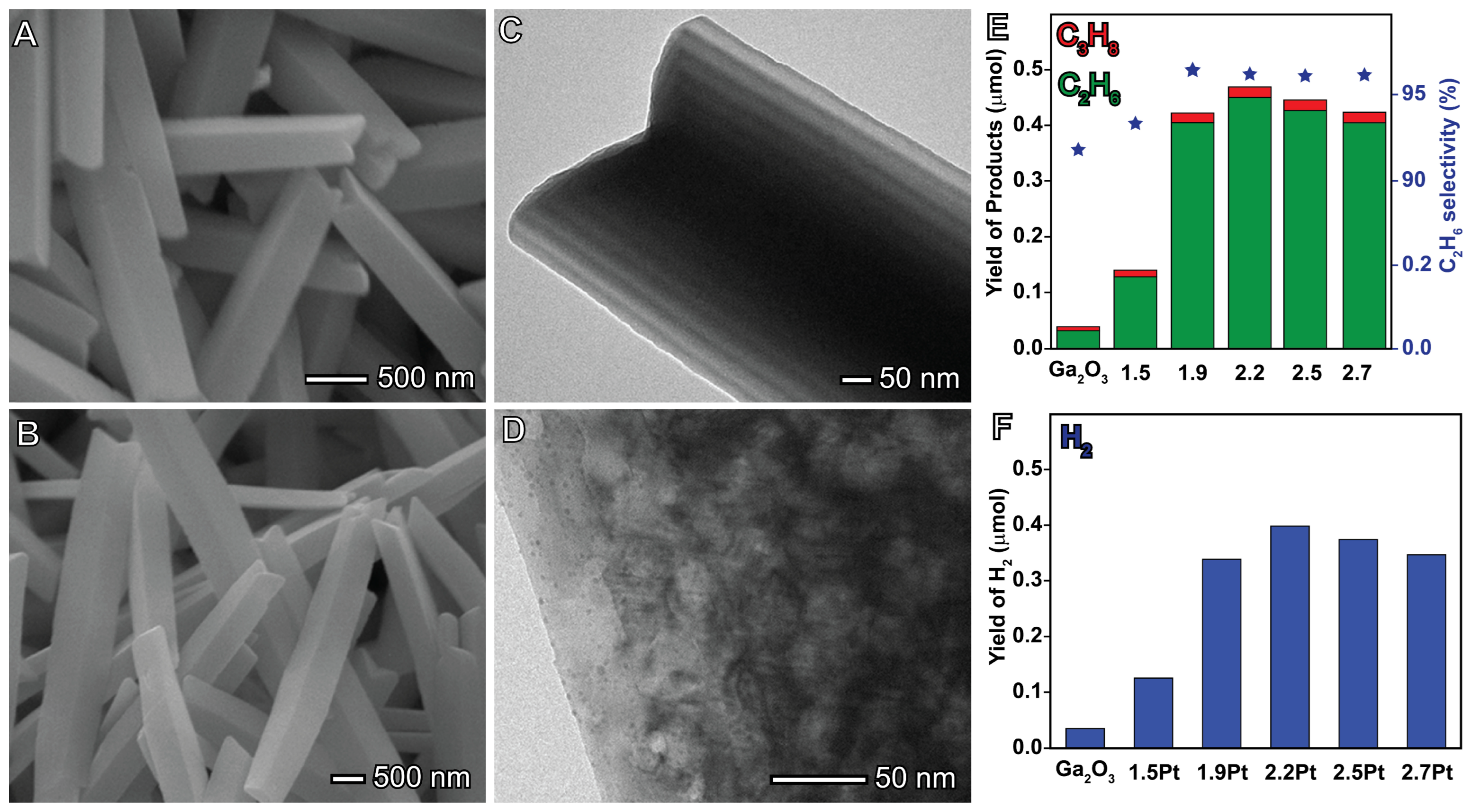
4.2. Oxidative Coupling of Methane (OCM)
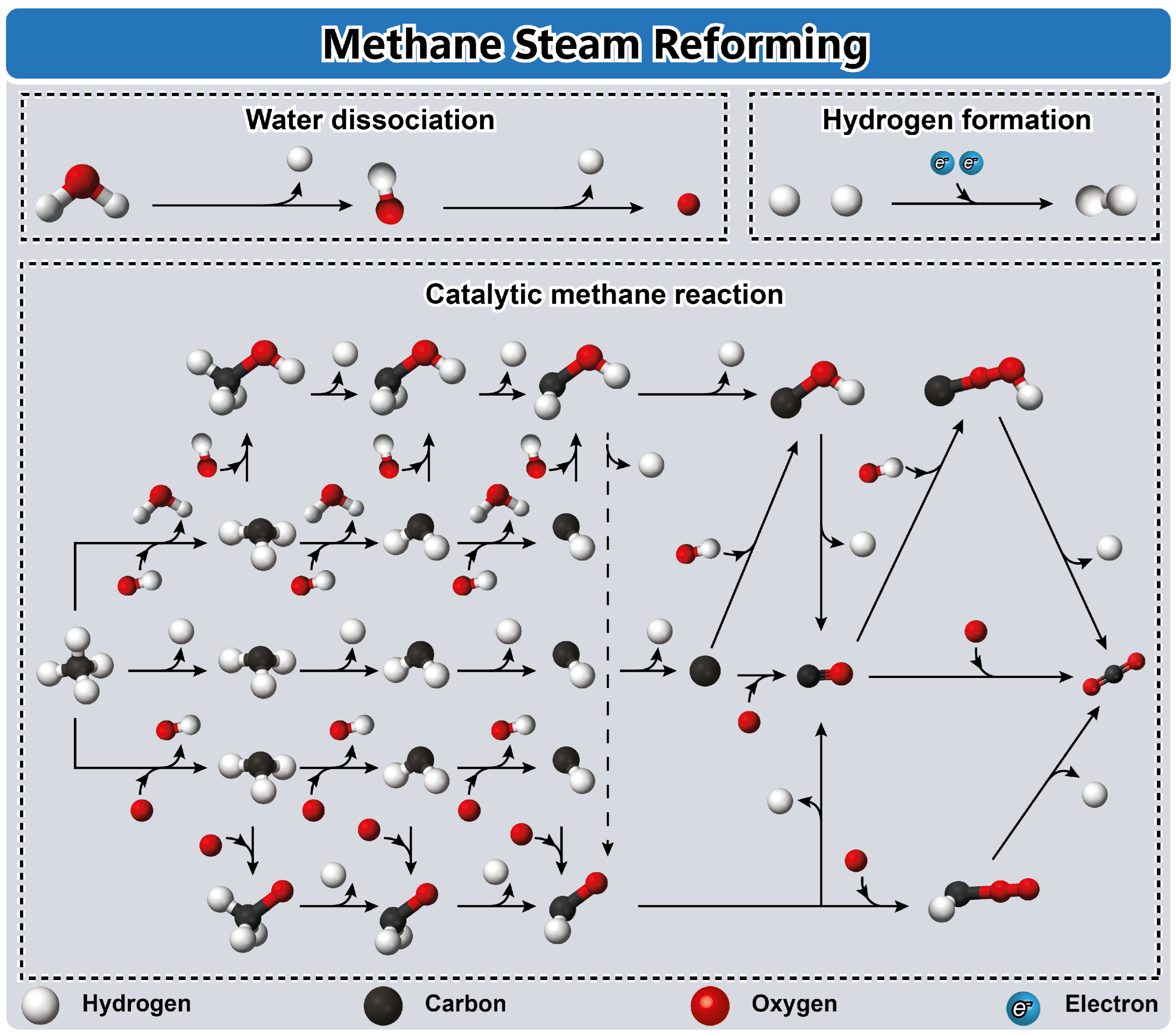
4.3. Steam Reforming of Methane
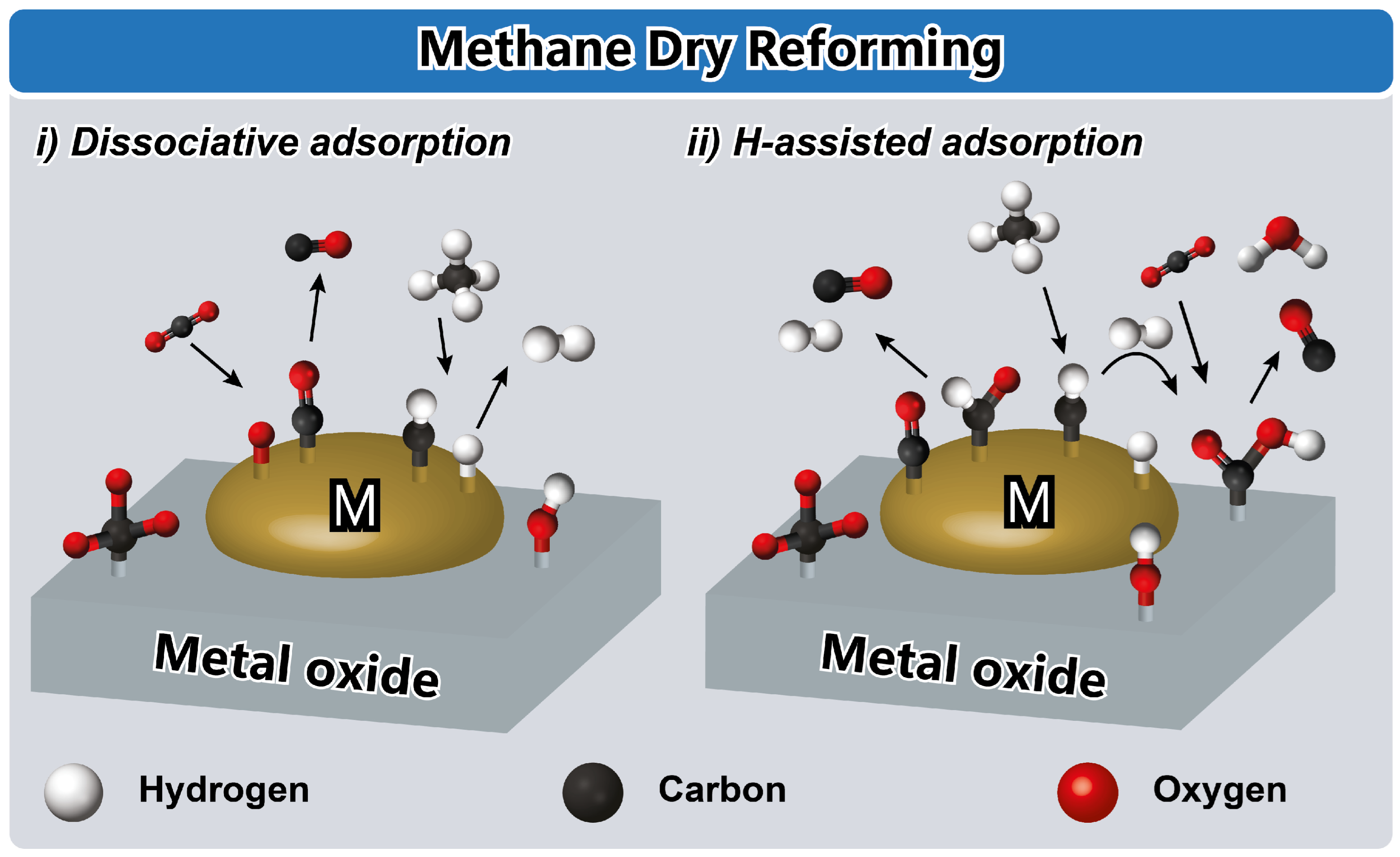
4.4. Dry Reforming of Methane
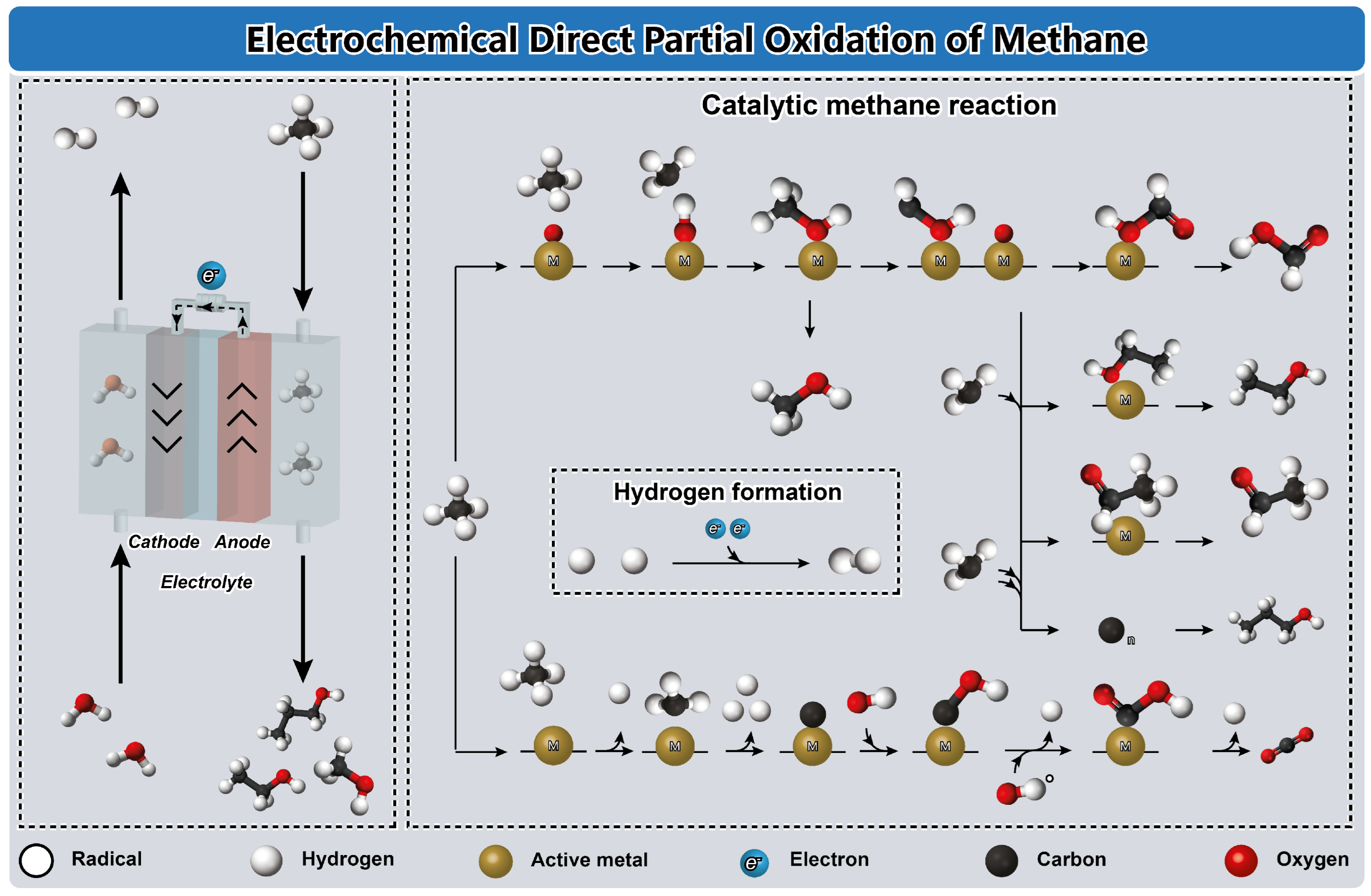
4.5. Electrochemical Direct Partial Oxidation of Methane
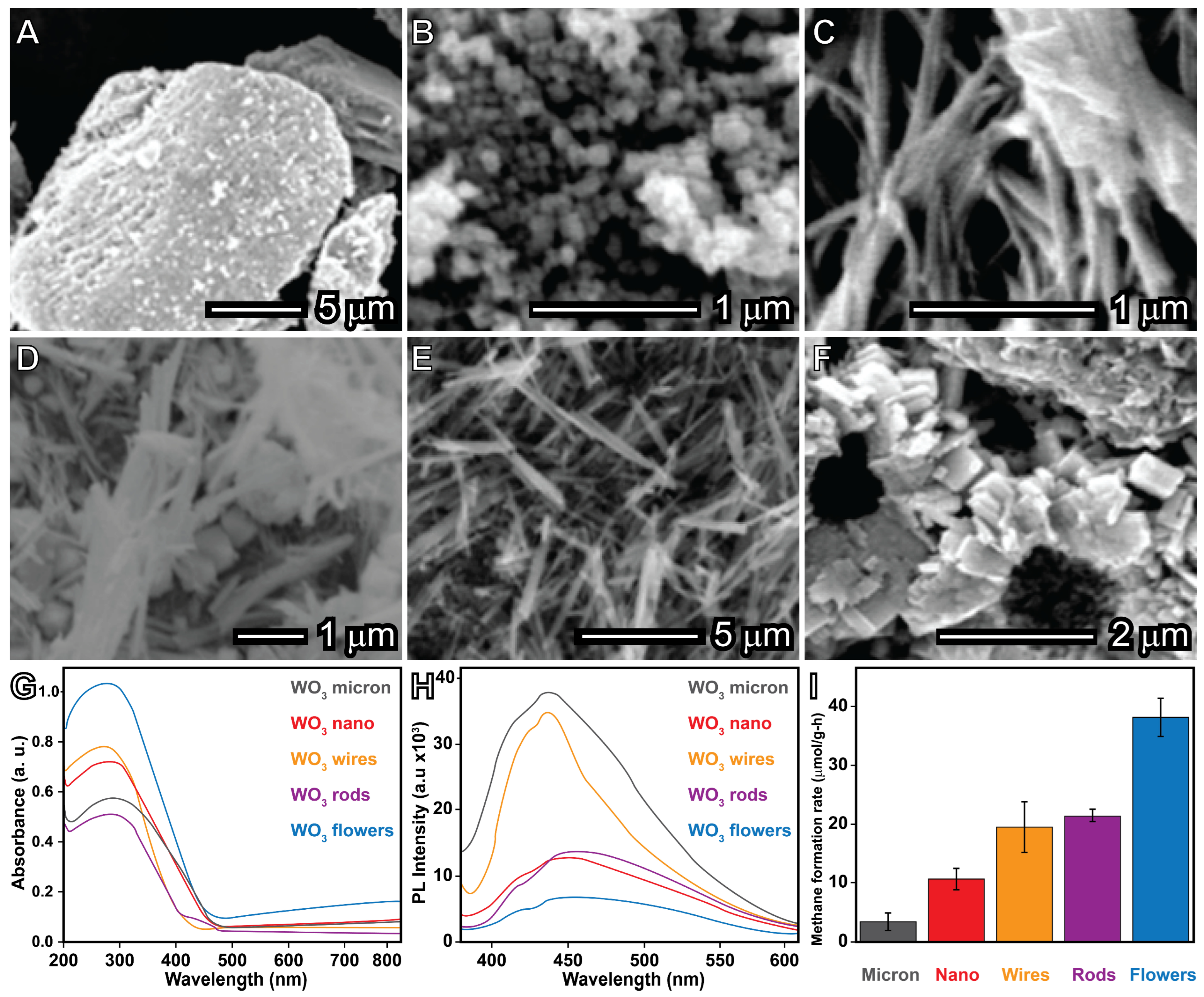
4.6. Photocatalytic Direct Partial Oxidation of Methane
5. Comparative Analysis with Traditional Catalysts
6. Conclusions: Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, R.; Weng, G. Sustainable Energy Resources for Driving Methane Conversion. Adv. Energy Mater. 2023, 13, 2301734. [Google Scholar] [CrossRef]
- Hameed, S.; Comini, E. Methane Conversion for Hydrogen Production: Technologies for a Sustainable Future. Sustain. Energy Fuels 2024, 8, 670–683. [Google Scholar] [CrossRef]
- Trovarelli, A.; Llorca, J. Ceria Catalysts at Nanoscale: How Do Crystal Shapes Shape Catalysis? ACS Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Gao, X.; Wen, Y.; Tan, R.; Huang, H.; Kawi, S. A Review of Catalyst Modifications for a Highly Active and Stable Hydrogen Production from Methane. Int. J. Hydrogen Energy 2023, 48, 6204–6232. [Google Scholar] [CrossRef]
- Latimer, A.A.; Aljama, H.; Kakekhani, A.; Yoo, J.S.; Kulkarni, A.; Tsai, C.; Garcia-Melchor, M.; Abild-Pedersen, F.; Nørskov, J.K. Mechanistic Insights into Heterogeneous Methane Activation. Phys. Chem. Chem. Phys. 2017, 19, 3575–3581. [Google Scholar] [CrossRef] [PubMed]
- Kopp Alves, A.; Bergmann, C.P.; Berutti, F.A. Novel Synthesis and Characterization of Nanostructured Materials; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-41274-5. [Google Scholar]
- Chen, J.; Zhang, Y.; Zhang, Z.; Hou, D.; Bai, F.; Han, Y.; Zhang, C.; Zhang, Y.; Hu, J. Metal–Support Interactions for Heterogeneous Catalysis: Mechanisms, Characterization Techniques and Applications. J. Mater. Chem. A Mater. 2023, 11, 8540–8572. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Qian, K.; Huang, W. Metal–Support Interactions in Metal/Oxide Catalysts and Oxide–Metal Interactions in Oxide/Metal Inverse Catalysts. ACS Catal. 2022, 12, 1268–1287. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Tang, J. Methane Transformation by Photocatalysis. Nat. Rev. Mater. 2022, 7, 617–632. [Google Scholar] [CrossRef]
- Li, C.; Clament Sagaya Selvam, N.; Fang, J. Shape-Controlled Synthesis of Platinum-Based Nanocrystals and Their Electrocatalytic Applications in Fuel Cells. Nanomicro Lett. 2023, 15, 83. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, B.; Jiang, Y.-J.; Zhou, Y.; Sun, X.; Wang, Y.; Zhu, W. Photocatalytic Conversion of Methane: Current State of the Art, Challenges, and Future Perspectives. ACS Environ. Au 2023, 3, 252–276. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Moura, A.B.L.; e Silva, F.A.; Candido, E.G.; da Silva, A.G.M.; de Oliveira, D.C.; Quiroz, J.; Camargo, P.H.C.; Bergamaschi, V.S.; Ferreira, J.C.; et al. Ni Supported Ce0.9Sm0.1O2-δ Nanowires: An Efficient Catalyst for Ethanol Steam Reforming for Hydrogen Production. Fuel 2019, 237, 1244–1253. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; da Silva, A.G.M.; Camargo, P.H.C. Nanocatalysis by Noble Metal Nanoparticles: Controlled Synthesis for the Optimization and Understanding of Activities. J. Mater. Chem. A Mater. 2019, 7, 5857–5874. [Google Scholar] [CrossRef]
- Huo, D.; Kim, M.J.; Lyu, Z.; Shi, Y.; Wiley, B.J.; Xia, Y. One-Dimensional Metal Nanostructures: From Colloidal Syntheses to Applications. Chem. Rev. 2019, 119, 8972–9073. [Google Scholar] [CrossRef] [PubMed]
- Qamar, O.A.; Jamil, F.; Hussain, M.; Bae, S.; Inayat, A.; Shah, N.S.; Waris, A.; Akhter, P.; Kwon, E.E.; Park, Y.-K. Advances in Synthesis of TiO2 Nanoparticles and Their Application to Biodiesel Production: A Review. Chem. Eng. J. 2023, 460, 141734. [Google Scholar] [CrossRef]
- e Silva, F.; Salim, V.; Rodrigues, T. Controlled Nickel Nanoparticles: A Review on How Parameters of Synthesis Can Modulate Their Features and Properties. AppliedChem 2024, 4, 86–106. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Yadav, A.B.; Sharma, N.; Bawarig, S.; García-Betancourt, M.-L.; Karatutlu, A.; Bechelany, M.; Barhoum, A. Cutting-Edge Advances in Tailoring Size, Shape, and Functionality of Nanoparticles and Nanostructures: A Review. J. Taiwan. Inst. Chem. Eng. 2023, 149, 105010. [Google Scholar] [CrossRef]
- França, M.C.; Ferreira, R.M.; dos Santos Pereira, F.; Silva, F.A.; Silva, A.C.A.; Cunha, L.C.S.; Varela Júnior, J.d.J.G.; de Lima Neto, P.; Takana, A.A.; Rodrigues, T.S.; et al. Galvanic Replacement Managing Direct Methanol Fuel Cells: AgPt Nanotubes as a Strategy for Methanol Crossover Effect Tolerance. J. Mater. Sci. 2022, 57, 8225–8240. [Google Scholar] [CrossRef]
- Xie, C.; Niu, Z.; Kim, D.; Li, M.; Yang, P. Surface and Interface Control in Nanoparticle Catalysis. Chem. Rev. 2020, 120, 1184–1249. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.V.; Fajardo, H.V.; Rodrigues, T.S.; e Silva, F.A.; Bergamaschi, V.S.; Dias, A.; Siqueira, K.P.F. Synthesis of NiMoO4 Ceramics by Proteic Sol-Gel Method and Investigation of Their Catalytic Properties in Hydrogen Production. Mater. Chem. Phys. 2021, 262, 124301. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, Y.; Chowdhury, P.R.; Wu, Z.; Ma, T.; Chen, J.Z.; Wan, G.; Kim, T.-H.; Jing, D.; He, P.; et al. Direct Methane Activation by Atomically Thin Platinum Nanolayers on Two-Dimensional Metal Carbides. Nat. Catal. 2021, 4, 882–891. [Google Scholar] [CrossRef]
- Rangel de Melo Rodrigues, M.; Machado Ferreira, R.; dos Santos Pereira, F.; Anchieta e Silva, F.; César Azevedo Silva, A.; Aguilar Vitorino, H.; de Jesus Gomes Varela Júnior, J.; Atsushi Tanaka, A.; Aurélio Suller Garcia, M.; Silva Rodrigues, T. Application of AgPt Nanoshells in Direct Methanol Fuel Cells: Experimental and Theoretical Insights of Design Electrocatalysts over Methanol Crossover Effect. ChemCatChem 2022, 14, 2200605. [Google Scholar] [CrossRef]
- Vieira, L.H.; Rasteiro, L.F.; Santana, C.S.; Catuzo, G.L.; da Silva, A.H.M.; Assaf, J.M.; Assaf, E.M. Noble Metals in Recent Developments of Heterogeneous Catalysts for CO2 Conversion Processes. ChemCatChem 2023, 15, 2300493. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Patel, N.; Fakeeha, A.H.; Alotibi, M.F.; Alreshaidan, S.B.; Kumar, R. Reforming of Methane: Effects of Active Metals, Supports, and Promoters. Catal. Rev. 2023, 5, 1–99. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Han, N.; Orooji, Y. Hydrogen Production through Methane Reforming Processes Using Promoted-Ni/Mesoporous Silica: A Review. J. Ind. Eng. Chem. 2022, 107, 20–30. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Qi, Y.; Dam, A.H.; Wang, H.; Zhu, Y.-A.; Holmen, A.; Ran, J.; Chen, D. New Mechanism Insights into Methane Steam Reforming on Pt/Ni from DFT and Experimental Kinetic Study. Fuel 2020, 266, 117143. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Wang, Y.; Zhang, C.; Chu, L.; Yang, L.; Fan, X. Mechanism Insights into Sorption Enhanced Methane Steam Reforming Using Ni-Doped CaO for H2 Production by DFT Study. Fuel 2022, 319, 123849. [Google Scholar] [CrossRef]
- Wittich, K.; Krämer, M.; Bottke, N.; Schunk, S.A. Catalytic Dry Reforming of Methane: Insights from Model Systems. ChemCatChem 2020, 12, 2130–2147. [Google Scholar] [CrossRef]
- Kumar, G.; Lau, S.L.J.; Krcha, M.D.; Janik, M.J. Correlation of Methane Activation and Oxide Catalyst Reducibility and Its Implications for Oxidative Coupling. ACS Catal. 2016, 6, 1812–1821. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Xie, Z.; Song, W.; Liu, B.; Zhao, Z. Rational Design of the Catalysts for the Direct Conversion of Methane to Methanol Based on a Descriptor Approach. Catalysts 2023, 13, 1226. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Ohba, T. Size-Dependent Catalytic Hydrogen Production via Methane Decomposition and Aromatization at a Low-Temperature Using Co, Ni, Cu, Mo, and Ru Nanometals. Phys. Chem. Chem. Phys. 2022, 24, 28794–28803. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Esmaeilkhanian, A.; Bazli, L.; Eskandarinezhad, S.; Khaksar, S.; Shafiee, P.; Yusuf, M.; Abdullah, B.; Salahshour, P.; Sadeghi, F. A Review on Recent Advances in Dry Reforming of Methane over Ni- and Co-Based Nanocatalysts. Int. J. Hydrogen Energy 2022, 47, 42213–42233. [Google Scholar] [CrossRef]
- Gangarajula, Y.; Hong, F.; Li, Q.; Jiang, X.; Liu, W.; Akri, M.; Su, Y.; Zhang, Y.; Li, L.; Qiao, B. Operando Induced Strong Metal-Support Interaction of Rh/CeO2 Catalyst in Dry Reforming of Methane. Appl. Catal. B 2024, 343, 123503. [Google Scholar] [CrossRef]
- Szécsényi, Á.; Li, G.; Gascon, J.; Pidko, E.A. Unraveling Reaction Networks behind the Catalytic Oxidation of Methane with H2O2 over a Mixed-Metal MIL-53(Al,Fe) MOF Catalyst. Chem. Sci. 2018, 9, 6765–6773. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Wang, D.Z.; Yang, Y.; Koel, B.E. Interactions of Incident H Atoms with Metal Surfaces. Surf. Sci. Rep. 2018, 73, 153–189. [Google Scholar] [CrossRef]
- Latimer, A.A.; Kulkarni, A.R.; Aljama, H.; Montoya, J.H.; Yoo, J.S.; Tsai, C.; Abild-Pedersen, F.; Studt, F.; Nørskov, J.K. Understanding Trends in C-H Bond Activation in Heterogeneous Catalysis. Nat. Mater. 2017, 16, 225–229. [Google Scholar] [CrossRef]
- Su, J.; Zou, X.; Chen, J.-S. Self-Modification of Titanium Dioxide Materials by Ti3+ and/or Oxygen Vacancies: New Insights into Defect Chemistry of Metal Oxides. RSC Adv. 2014, 4, 13979–13988. [Google Scholar] [CrossRef]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Tsunekawa, S.; Wang, J.-T.; Kawazoe, Y. Lattice Constants and Electron Gap Energies of Nano- and Subnano-Sized Cerium Oxides from the Experiments and First-Principles Calculations. J. Alloys Compd. 2006, 408–412, 1145–1148. [Google Scholar] [CrossRef]
- Zhou, X.-D.; Huebner, W. Size-Induced Lattice Relaxation in CeO2 Nanoparticles. Appl. Phys. Lett. 2001, 79, 3512–3514. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Sahara, R.; Kawazoe, Y.; Ishikawa, K. Lattice Relaxation of Monosize CeO2−x Nanocrystalline Particles. Appl. Surf. Sci. 1999, 152, 53–56. [Google Scholar] [CrossRef]
- Aschauer, U.; Pfenninger, R.; Selbach, S.M.; Grande, T.; Spaldin, N.A. Strain-Controlled Oxygen Vacancy Formation and Ordering in CaMnO3. Phys. Rev. B 2013, 88, 054111. [Google Scholar] [CrossRef]
- Quantum Confinement Effects in Semiconductors. In Applied Nanophotonics; Cambridge University Press: Cambridge, UK, 2018; pp. 52–91.
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A Review on Oxygen Storage Capacity of CeO2-Based Materials: Influence Factors, Measurement Techniques, and Applications in Reactions Related to Catalytic Automotive Emissions Control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Zhao, G.; Yang, F.; Chen, Z.; Liu, Q.; Ji, Y.; Zhang, Y.; Niu, Z.; Mao, J.; Bao, X.; Hu, P.; et al. Metal/Oxide Interfacial Effects on the Selective Oxidation of Primary Alcohols. Nat. Commun. 2017, 8, 14039. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.C.; Park, E.D. Gas-Phase Selective Oxidation of Methane into Methane Oxygenates. Catalysts 2022, 12, 314. [Google Scholar] [CrossRef]
- Karakaya, C.; Zhu, H.; Loebick, C.; Weissman, J.G.; Kee, R.J. A Detailed Reaction Mechanism for Oxidative Coupling of Methane over Mn/Na2WO4/SiO2 Catalyst for Non-Isothermal Conditions. Catal. Today 2018, 312, 10–22. [Google Scholar] [CrossRef]
- Postma, R.S.; Mendes, P.S.F.; Pirro, L.; Banerjee, A.; Thybaut, J.W.; Lefferts, L. Modelling of the Catalytic Initiation of Methane Coupling under Non-Oxidative Conditions. Chem. Eng. J. 2023, 454, 140273. [Google Scholar] [CrossRef]
- Dummer, N.F.; Willock, D.J.; He, Q.; Howard, M.J.; Lewis, R.J.; Qi, G.; Taylor, S.H.; Xu, J.; Bethell, D.; Kiely, C.J.; et al. Methane Oxidation to Methanol. Chem. Rev. 2023, 123, 6359–6411. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Chen, P.; Xia, S.; Zheng, M.; Song, D.; Lin, Y.; Liu, A.; Wang, X.; Zhao, K.; Zheng, A. Advances in Oxidative Coupling of Methane. Atmosphere 2023, 14, 1538. [Google Scholar] [CrossRef]
- Alkathiri, R.; Alshamrani, A.; Wazeer, I.; Boumaza, M.; Hadj-Kali, M.K. Optimization of the Oxidative Coupling of Methane Process for Ethylene Production. Processes 2022, 10, 1085. [Google Scholar] [CrossRef]
- Zhang, T. Recent Advances in Heterogeneous Catalysis for the Nonoxidative Conversion of Methane. Chem. Sci. 2021, 12, 12529–12545. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Guan, N.; Li, L. Methane Activation and Utilization: Current Status and Future Challenges. Energy Technol. 2020, 8, 1900826. [Google Scholar] [CrossRef]
- Zhao, G.; Drewery, M.; Mackie, J.; Oliver, T.; Kennedy, E.M.; Stockenhuber, M. The Catalyzed Conversion of Methane to Value-Added Products. Energy Technol. 2020, 8, 1900665. [Google Scholar] [CrossRef]
- Wang, J.; Rao, Z.; Huang, Z.; Chen, Y.; Wang, F.; Zhou, Y. Recent Progress of Metal-Oxide-Based Catalysts for Non-Oxidative Coupling of Methane to Ethane and Hydrogen. Catalysts 2023, 13, 719. [Google Scholar] [CrossRef]
- Ogawa, Y.; Xu, Y.; Zhang, Z.; Ma, H.; Yamamoto, Y. Development of Catalysts for Direct Non-Oxidative Methane Aromatization. Resour. Chem. Mater. 2022, 1, 80–92. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, X.; Luo, X.; Shi, K.; Yang, G.; Wu, T. Catalytic Conversion of Methane at Low Temperatures: A Critical Review. Energy Technol. 2020, 8, 1900750. [Google Scholar] [CrossRef]
- Konnov, S.V. Direct Non-Oxidative Conversion of Methane over Metal-Containing Zeolites: Main Strategies for Shifting the Thermodynamic Equilibrium (A Review). Pet. Chem. 2022, 62, 280–290. [Google Scholar] [CrossRef]
- Kang, J.; Park, E.D. Liquid-Phase Selective Oxidation of Methane to Methane Oxygenates. Catalysts 2024, 14, 167. [Google Scholar] [CrossRef]
- Li, D.; Xu, R.; Gu, Z.; Zhu, X.; Qing, S.; Li, K. Chemical-Looping Conversion of Methane: A Review. Energy Technol. 2020, 8, 1900925. [Google Scholar] [CrossRef]
- Wu, L.; Fan, W.; Wang, X.; Lin, H.; Tao, J.; Liu, Y.; Deng, J.; Jing, L.; Dai, H. Methane Oxidation over the Zeolites-Based Catalysts. Catalysts 2023, 13, 604. [Google Scholar] [CrossRef]
- Kumar, P.; Al-Attas, T.A.; Hu, J.; Kibria, M.G. Single Atom Catalysts for Selective Methane Oxidation to Oxygenates. ACS Nano 2022, 16, 8557–8618. [Google Scholar] [CrossRef]
- Anggoro, D.D.; Chamdani, F.T.; Buchori, L. One Step Catalytic Oxidation Process of Methane to Methanol at Low Reaction Temperature: A Brief Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012056. [Google Scholar] [CrossRef]
- Newton, M.A.; Knorpp, A.J.; Sushkevich, V.L.; Palagin, D.; van Bokhoven, J.A. Active Sites and Mechanisms in the Direct Conversion of Methane to Methanol Using Cu in Zeolitic Hosts: A Critical Examination. Chem. Soc. Rev. 2020, 49, 1449–1486. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, S.; Liu, Y.; Huang, W.; Guan, G.; Zuo, Z. Quasicatalytic and Catalytic Selective Oxidation of Methane to Methanol over Solid Materials: A Review on the Roles of Water. Catal. Rev. 2020, 62, 313–345. [Google Scholar] [CrossRef]
- Sharma, R.; Poelman, H.; Marin, G.B.; Galvita, V.V. Approaches for Selective Oxidation of Methane to Methanol. Catalysts 2020, 10, 194. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, W.; Zhang, Y.; Pan, C.; Xu, J.; Zhu, Y.; Lou, Y. Research Progress on Methane Conversion Coupling Photocatalysis and Thermocatalysis. Carbon. Energy 2021, 3, 519–540. [Google Scholar] [CrossRef]
- Cruchade, H.; Medeiros-Costa, I.C.; Nesterenko, N.; Gilson, J.-P.; Pinard, L.; Beuque, A.; Mintova, S. Catalytic Routes for Direct Methane Conversion to Hydrocarbons and Hydrogen: Current State and Opportunities. ACS Catal. 2022, 12, 14533–14558. [Google Scholar] [CrossRef]
- Arinaga, A.M.; Ziegelski, M.C.; Marks, T.J. Alternative Oxidants for the Catalytic Oxidative Coupling of Methane. Angew. Chem. Int. Ed. 2021, 60, 10502–10515. [Google Scholar] [CrossRef]
- Liu, J.; Yue, J.; Lv, M.; Wang, F.; Cui, Y.; Zhang, Z.; Xu, G. From Fundamentals to Chemical Engineering on Oxidative Coupling of Methane for Ethylene Production: A Review. Carbon. Resour. Convers. 2022, 5, 1–14. [Google Scholar] [CrossRef]
- Kim, K.-J.; Kim, K.Y.; Rhim, G.B.; Youn, M.H.; Lee, Y.-L.; Chun, D.H.; Roh, H.-S. Nano-Catalysts for Gas to Liquids: A Concise Review. Chem. Eng. J. 2023, 468, 143632. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Huang, X.; Feng, T.; Mu, M. Sorption-Enhanced Reaction Process Using Advanced Ca-Based Sorbents for Low-Carbon Hydrogen Production. Process Saf. Environ. Prot. 2021, 155, 325–342. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Torimoto, M.; Sekine, Y. Effects of Alloying for Steam or Dry Reforming of Methane: A Review of Recent Studies. Catal. Sci. Technol. 2022, 12, 3387–3411. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Jagadeesh, P.; Varun, Y.; Himajaa Reddy, B.; Sreedhar, I.; Singh, S.A. A Short Review on Recent Advancements of Dry Reforming of Methane (DRM) over Pyrochlores. Mater. Today Proc. 2023, 72, 361–369. [Google Scholar] [CrossRef]
- Xu, Z.; Park, E.D. Recent Advances in Coke Management for Dry Reforming of Methane over Ni-Based Catalysts. Catalysts 2024, 14, 176. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Hamid, M.Y.S. A Review on Catalyst Development for Dry Reforming of Methane to Syngas: Recent Advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Kanitkar, S.; Spivey, J.J. A Review on Dry Reforming of Methane over Perovskite Derived Catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A Review of Recent Efforts to Promote Dry Reforming of Methane (DRM) to Syngas Production via Bimetallic Catalyst Formulations. Appl. Catal. B 2021, 296, 120210. [Google Scholar] [CrossRef]
- Teh, L.P.; Setiabudi, H.D.; Timmiati, S.N.; Aziz, M.A.A.; Annuar, N.H.R.; Ruslan, N.N. Recent Progress in Ceria-Based Catalysts for the Dry Reforming of Methane: A Review. Chem. Eng. Sci. 2021, 242, 116606. [Google Scholar] [CrossRef]
- Torrez-Herrera, J.J.; Korili, S.A.; Gil, A. Recent Progress in the Application of Ni-Based Catalysts for the Dry Reforming of Methane. Catal. Rev. 2023, 65, 1300–1357. [Google Scholar] [CrossRef]
- Hussien, A.G.S.; Polychronopoulou, K. A Review on the Different Aspects and Challenges of the Dry Reforming of Methane (DRM) Reaction. Nanomaterials 2022, 12, 3400. [Google Scholar] [CrossRef]
- Sher Shah, M.S.A.; Oh, C.; Park, H.; Hwang, Y.J.; Ma, M.; Park, J.H. Catalytic Oxidation of Methane to Oxygenated Products: Recent Advancements and Prospects for Electrocatalytic and Photocatalytic Conversion at Low Temperatures. Adv. Sci. 2020, 7, 2001946. [Google Scholar] [CrossRef]
- Wang, Q.; Kan, M.; Han, Q.; Zheng, G. Electrochemical Methane Conversion. Small Struct. 2021, 2, 2100037. [Google Scholar] [CrossRef]
- Xie, S.; Lin, S.; Zhang, Q.; Tian, Z.; Wang, Y. Selective Electrocatalytic Conversion of Methane to Fuels and Chemicals. J. Energy Chem. 2018, 27, 1629–1636. [Google Scholar] [CrossRef]
- Abdelkader Mohamed, A.G.; Zahra Naqviab, S.A.; Wang, Y. Advances and Fundamental Understanding of Electrocatalytic Methane Oxidation. ChemCatChem 2021, 13, 787–805. [Google Scholar] [CrossRef]
- Liu, F.; Yan, Y.; Chen, G.; Wang, D. Recent Advances in Ambient Electrochemical Methane Conversion to Oxygenates Using Metal Oxide Electrocatalysts. Green. Chem. 2024, 26, 655–677. [Google Scholar] [CrossRef]
- Kishore, M.R.A.; Lee, S.; Yoo, J.S. Fundamental Limitation in Electrochemical Methane Oxidation to Alcohol: A Review and Theoretical Perspective on Overcoming It. Adv. Sci. 2023, 10, 2301912. [Google Scholar] [CrossRef]
- Yan, L.; Jiang, L.; Qian, C.; Zhou, S. Electrocatalytic Conversion of Methane: Recent Progress and Future Prospects. Energy Rev. 2024, 3, 100065. [Google Scholar] [CrossRef]
- Shi, T.; Sridhar, D.; Zeng, L.; Chen, A. Recent Advances in Catalyst Design for the Electrochemical and Photoelectrochemical Conversion of Methane to Value-Added Products. Electrochem. Commun 2022, 135, 107220. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, L.; Han, Z.; Tang, Y.; Sun, Y.; Wan, P.; Chen, Y.; Argyle, M.D.; Fan, M. Direct Conversion of Methane to Methanol by Electrochemical Methods. Green. Energy Environ. 2022, 7, 1132–1142. [Google Scholar] [CrossRef]
- Wang, P.; Shi, R.; Zhao, J.; Zhang, T. Photodriven Methane Conversion on Transition Metal Oxide Catalyst: Recent Progress and Prospects. Adv. Sci. 2024, 11, 2305471. [Google Scholar] [CrossRef]
- Lin, X.-Y.; Li, J.-Y.; Qi, M.-Y.; Tang, Z.-R.; Xu, Y.-J. Methane Conversion over Artificial Photocatalysts. Catal. Commun. 2021, 159, 106346. [Google Scholar] [CrossRef]
- Li, Q.; Ouyang, Y.; Li, H.; Wang, L.; Zeng, J. Photocatalytic Conversion of Methane: Recent Advancements and Prospects. Angew. Chem. 2022, 134, 2108069. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Xie, S.; Wang, Y. Photocatalytic Conversion of Methane: Catalytically Active Sites and Species. Chem. Catal. 2023, 3, 100437. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, E.; Tang, J. Photocatalytic Methane Conversion to High-Value Chemicals. Carbon. Future 2024, 1, 9200004. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, S.; Fan, Y.; Tang, Z. Best Practices for Experiments and Reports in Photocatalytic Methane Conversion. Angew. Chem. 2024, 4, 2404658. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, Y.; Li, S.; Tang, Z. Photocatalytic Methane Conversion: Insight into the Mechanism of C(Sp3 )–H Bond Activation. CCS Chem. 2023, 5, 30–54. [Google Scholar] [CrossRef]
- Sader, S.; Miliordos, E. Methane to Methanol Conversion Facilitated by Anionic Transition Metal Centers: The Case of Fe, Ni, Pd, and Pt. J. Phys. Chem. A 2021, 125, 2364–2373. [Google Scholar] [CrossRef]
- Vali, S.A.; Markeb, A.A.; Moral-Vico, J.; Font, X.; Sánchez, A. Recent Advances in the Catalytic Conversion of Methane to Methanol: From the Challenges of Traditional Catalysts to the Use of Nanomaterials and Metal-Organic Frameworks. Nanomaterials 2023, 13, 2754. [Google Scholar] [CrossRef]
- Bobadilla, L.F.; Garcilaso, V.; Centeno, M.A.; Odriozola, J.A. Monitoring the Reaction Mechanism in Model Biogas Reforming by In Situ Transient and Steady-State DRIFTS Measurements. ChemSusChem 2017, 10, 1193–1201. [Google Scholar] [CrossRef]
- Blaylock, D.W.; Ogura, T.; Green, W.H.; Beran, G.J.O. Computational Investigation of Thermochemistry and Kinetics of Steam Methane Reforming on Ni(111) under Realistic Conditions. J. Phys. Chem. C 2009, 113, 4898–4908. [Google Scholar] [CrossRef]
- González-Castaño, M.; Dorneanu, B.; Arellano-García, H. The Reverse Water Gas Shift Reaction: A Process Systems Engineering Perspective. React. Chem. Eng. 2021, 6, 954–976. [Google Scholar] [CrossRef]
- Chen, L.; Filot, I.A.W.; Hensen, E.J.M. Elucidation of the Reverse Water–Gas Shift Reaction Mechanism over an Isolated Ru Atom on CeO2(111). J. Phys. Chem. C 2023, 127, 20314–20324. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, G.; Cheng, H.; Liu, J.; Li, G. Kinetics and Mechanistic Studies of Methane Dry Reforming over Ca Promoted 1Co–1Ce/AC-N Catalyst. Int. J. Hydrogen Energy 2021, 46, 531–542. [Google Scholar] [CrossRef]
- Yu, Y.; Ji, P.; Zhang, W.; Yang, K.; Zhang, M. Theoretical Study Coupling DFT Calculations and KMC Simulation of CO Methanation on Ni(111) and Ni3Fe(111). New J. Chem. 2023, 47, 17923–17936. [Google Scholar] [CrossRef]
- Zhang, Q.; Bown, M.; Pastor-Pérez, L.; Duyar, M.S.; Reina, T.R. CO2 Conversion via Reverse Water Gas Shift Reaction Using Fully Selective Mo–P Multicomponent Catalysts. Ind. Eng. Chem. Res. 2022, 61, 12857–12865. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.F.; Bresciani, A.E.; Ferreira, N.L.; Bassani, G.S.; Alves, R.M.B. Carbon Dioxide Conversion via Reverse Water-Gas Shift Reaction: Reactor Design. J. Environ. Manag. 2023, 345, 118822. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Jin, B.J.; Li, P.; Jung, M.S.; Kim, J.I.; Cho, Y.; Kim, S.; Moon, J.H.; Park, J.H. Ultrahigh Electrocatalytic Conversion of Methane at Room Temperature. Adv. Sci. 2017, 4, 1700379. [Google Scholar] [CrossRef]
- Özdemir, H.; Çiftçioğlu, E.; Faruk Öksüzömer, M.A. Lanthanum Based Catalysts for Oxidative Coupling of Methane: Effect of Morphology and Structure. Chem. Eng. Sci. 2023, 270, 118520. [Google Scholar] [CrossRef]
- Bresciani, L.; Stülp, S. Electrochemical Deposition of Pt and Pd on TiO2 Nanotubes for Application in the Photoelectrocatalytic Conversion of Biomethane and Biogas for Hydrogen Generation. Electrocatalysis 2024, 15, 70–86. [Google Scholar] [CrossRef]
- Özdemir, H. Detailed Investigation of Sm2O3 Catalysts with Different Morphologies for Oxidative Coupling of Methane. ChemistrySelect 2021, 6, 7999–8006. [Google Scholar] [CrossRef]
- Kim, I.; Lee, G.; Na, H.B.; Ha, J.-M.; Jung, J.C. Selective Oxygen Species for the Oxidative Coupling of Methane. Mol. Catal. 2017, 435, 13–23. [Google Scholar] [CrossRef]
- Premachandra, D.; Heagy, M.D. Morphology-Controlled WO3 for the Photocatalytic Oxidation of Methane to Methanol in Mild Conditions. Methane 2023, 2, 103–112. [Google Scholar] [CrossRef]
- Chen, J.; Pham, H.N.; Mon, T.; Toops, T.J.; Datye, A.K.; Li, Z.; Kyriakidou, E.A. Ni/CeO2 Nanocatalysts with Optimized CeO2 Support Morphologies for CH4 Oxidation. ACS Appl. Nano Mater. 2023, 6, 4544–4553. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; e Silva, F.A.; Candido, E.G.; da Silva, A.G.M.; Geonmonond, R.d.S.; Camargo, P.H.C.; Linardi, M.; Fonseca, F.C. Ethanol Steam Reforming: Understanding Changes in the Activity and Stability of Rh/MxOy Catalysts as Function of the Support. J. Mater. Sci. 2019, 54, 11400–11416. [Google Scholar] [CrossRef]
- Ma, J.; Tan, X.; Zhang, Q.; Wang, Y.; Zhang, J.; Wang, L. Exploring the Size Effect of Pt Nanoparticles on the Photocatalytic Nonoxidative Coupling of Methane. ACS Catal. 2021, 11, 3352–3360. [Google Scholar] [CrossRef]
- Kiss, J.; Kukovecz, Á.; Kónya, Z. Beyond Nanoparticles: The Role of Sub-Nanosized Metal Species in Heterogeneous Catalysis. Catal. Lett. 2019, 149, 1441–1454. [Google Scholar] [CrossRef]
- Zhu, M.; Aikens, C.M.; Hollander, F.J.; Schatz, G.C.; Jin, R. Correlating the Crystal Structure of A Thiol-Protected Au25 Cluster and Optical Properties. J. Am. Chem. Soc. 2008, 130, 5883–5885. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Jiang, D.; Mann, A.K.P.; Mullins, D.R.; Qiao, Z.-A.; Allard, L.F.; Zeng, C.; Jin, R.; Overbury, S.H. Thiolate Ligands as a Double-Edged Sword for CO Oxidation on CeO2 Supported Au25 (SCH2CH2Ph)18 Nanoclusters. J. Am. Chem. Soc. 2014, 136, 6111–6122. [Google Scholar] [CrossRef]
- László, B.; Baán, K.; Varga, E.; Oszkó, A.; Erdőhelyi, A.; Kónya, Z.; Kiss, J. Photo-Induced Reactions in the CO2-Methane System on Titanate Nanotubes Modified with Au and Rh Nanoparticles. Appl. Catal. B 2016, 199, 473–484. [Google Scholar] [CrossRef]
- Gualteros, J.A.D.; Garcia, M.A.S.; da Silva, A.G.M.; Rodrigues, T.S.; Cândido, E.G.; e Silva, F.A.; Fonseca, F.C.; Quiroz, J.; de Oliveira, D.C.; de Torresi, S.I.C.; et al. Synthesis of Highly Dispersed Gold Nanoparticles on Al2O3, SiO2, and TiO2 for the Solvent-Free Oxidation of Benzyl Alcohol under Low Metal Loadings. J. Mater. Sci. 2019, 54, 238–251. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/Gold Nanocomposites. Effect of Metal Particle Size on the Fermi Level Equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.; Bahnemann, D.W.; Bannat, I.; Wark, M. Gold Nanoparticles on Mesoporous Interparticle Networks of Titanium Dioxide Nanocrystals for Enhanced Photonic Efficiencies. J. Phys. Chem. C 2009, 113, 7429–7435. [Google Scholar] [CrossRef]
- Sheng, K.; Luan, D.; Jiang, H.; Zeng, F.; Wei, B.; Pang, F.; Ge, J. NixCoy Nanocatalyst Supported by ZrO2 Hollow Sphere for Dry Reforming of Methane: Synergetic Catalysis by Ni and Co in Alloy. ACS Appl. Mater. Interfaces 2019, 11, 24078–24087. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Peres, L.; Collière, V.; Philippot, K.; Lecante, P.; Chen, Y.; Yan, N. Oxidation of Methane to Methanol over Pd@Pt Nanoparticles under Mild Conditions in Water. Catal. Sci. Technol. 2021, 11, 3493–3500. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, L.; Qi, Y.; Yang, J.; Zhu, Y.-A.; Chen, D. Insight into Size- and Metal-Dependent Activity and the Mechanism for Steam Methane Re-Forming in Nanocatalysis. J. Phys. Chem. C 2020, 124, 2501–2512. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Liu, J.-H.; Lv, C.-Q.; Ren, R.-R.; Wang, G.-C. Dry Reforming of Methane on Ni(1 1 1) Surface with Different Mo Doping Ratio: DFT-Assisted Microkinetic Study. Appl. Surf. Sci. 2022, 581, 152310. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Zhang, H.-L.; An, P.; Wu, H.-S.; Jia, J.-F. Effect of Potassium on Methanol Steam Reforming on the Cu(111) and Cu(110) Surfaces: A DFT Study. J. Phys. Chem. C 2021, 125, 20905–20918. [Google Scholar] [CrossRef]
- Yadavalli, S.S.; Jones, G.; Stamatakis, M. DFT Benchmark Studies on Representative Species and Poisons of Methane Steam Reforming on Ni(111). Phys. Chem. Chem. Phys. 2021, 23, 15601–15612. [Google Scholar] [CrossRef]
- Xu, L.; Papanikolaou, K.G.; Lechner, B.A.J.; Je, L.; Somorjai, G.A.; Salmeron, M.; Mavrikakis, M. Formation of Active Sites on Transition Metals through Reaction-Driven Migration of Surface Atoms. Science 2023, 380, 70–76. [Google Scholar] [CrossRef]
- Tsuji, Y.; Yoshida, M.; Yoshizawa, K.; Kamachi, T. Concepts of Computational Approach to Explore Heterogeneous Catalysts for Direct Methane Conversion. ChemCatChem 2023, 15, 2201488. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
e Silva, F.A.; Rodrigues, T.S. Recent Advances in the Use of Controlled Nanocatalysts in Methane Conversion Reactions. Methane 2024, 3, 359-379. https://doi.org/10.3390/methane3020020
e Silva FA, Rodrigues TS. Recent Advances in the Use of Controlled Nanocatalysts in Methane Conversion Reactions. Methane. 2024; 3(2):359-379. https://doi.org/10.3390/methane3020020
Chicago/Turabian Stylee Silva, Felipe Anchieta, and Thenner Silva Rodrigues. 2024. "Recent Advances in the Use of Controlled Nanocatalysts in Methane Conversion Reactions" Methane 3, no. 2: 359-379. https://doi.org/10.3390/methane3020020
APA Stylee Silva, F. A., & Rodrigues, T. S. (2024). Recent Advances in the Use of Controlled Nanocatalysts in Methane Conversion Reactions. Methane, 3(2), 359-379. https://doi.org/10.3390/methane3020020





